Abstract
Surgical techniques have emerged as a viable therapeutic option in patients with drug refractory epilepsy. Pre-surgical evaluation of epilepsy requires a comprehensive, multiparametric, and multimodal approach for precise localization of the epileptogenic focus. Various non-invasive techniques are available at the disposal of the treating physician to detect the epileptogenic focus, which include electroencephalography (EEG), video-EEG, magnetic resonance imaging (MRI), functional MRI including blood oxygen level dependent (BOLD) techniques, single photon emission tomography (SPECT), and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET). Currently, non-invasive high-resolution MR imaging techniques play pivotal roles in the preoperative detection of the seizure focus, and represent the foundation for successful epilepsy surgery. BOLD functional magnetic resonance imaging (fMRI) maps allow for precise localization of the eloquent cortex in relation to the seizure focus. This review article focuses on the clinical utility of BOLD (fMRI) in the pre-surgical work-up of epilepsy patients.
Keywords: Blood oxygen dependent level, epilepsy, functional magnetic resonance imaging
Introduction
Epilepsy is a chronic, neurologic disorder caused by excessive and abnormal electrical discharges, with a prevalence of 0.4-1% of population.[1,2,3] Broadly speaking, epilepsies are classified into focal (partial) and generalized subtypes. Partial seizures originate from a focal area in the brain, whereas generalized seizures originate from both hemispheres. Partial seizures are subclassified into two forms – simple partial, without loss of any consciousness; and complex partial, with loss of consciousness. Partial seizures can spread and become secondary generalized seizures. It has been noted that approximately 30% of partial seizures are refractory to antiepileptic medications.[4,5] Magnetic resonance imaging (MRI) is an excellent and potent tool for detecting anatomical and structural anomalies in the cerebral hemispheres. The advent of high-resolution 3 T scanners, which are capable of producing three-dimensional volumetric datasets with submillimeter resolution, has further enhanced the potency of MR imaging in detecting epileptogenic foci. However, it must be noted that in patients suffering from idiopathic generalized epilepsy, MR imaging is of little or no benefit.[6] In epilepsy surgery, high-resolution MR imaging at 3 T plays a critical role in detecting the epileptogenic focus, pre-surgical mapping of the focus in relation to the eloquent cortex, and in post-surgical mapping of treatment bed to evaluate for complications, assess the treatment margins for failure of surgery, and look for residual and or recurrent disease. MR imaging data cannot be used in isolation, and it is vital to correlate this data and assess for concordance with clinical and electrophysiological data.
Background of Functional Magnetic Resonance Imaging
Functional magnetic resonance (fMRI) imaging is a qualitative and indirect measuring tool used to detect and localize brain activity. Detection of MR signal changes allows the mapping of neural networks involved in a particular task. The foundation of fMRI is based on the principle that neuronal activation and cerebral blood flow (CBF) are coupled, and an increase in neuronal activity is associated with a localized increase in the regional blood flow, accompanied by an increase in the amount of deoxyhemoglobin formed at the venous end of the capillary network. This synergistic effect originating from neuronal activation produces rapid changes in the MR signal, resulting in T2 shortening and the blood oxygen level dependent (BOLD) signal, and is called the hemodynamic response (HDR). Critically, HDR lags the triggering neuronal activity by approximately 1-2 sec and rises rapidly to its peak at about 4-6 sec after the activity. If neuronal activity persists, the HDR and BOLD signal would tend to plateau, and if it stops, the HDR and BOLD signal would regress to fall below the original levels. Hence, the HDR response would typically last for at least 10 sec. Logothetis[7] suggested that the majority of the BOLD signal is derived from synaptic activity. In fMRI studies, TR is the principal parameter, and can vary from very short (500 msec) to very long (3 sec) times. Current MR imaging techniques at 3 T allow for whole-brain acquisition with submillimeter resolution, but most studies have a voxel size near 4-5 mm. The temporal resolution of the present-day scanners does not allow for assessment of interaction between individual regions in a particular network, but only helps to identify the broad distribution of regions involved in the neural network governing a particular task.
Structural MR Imaging Technique
Neuroimaging is not required in all patients with epilepsy. On MR imaging, identification of epileptogenic lesions can pose a significant diagnostic challenge, and may result in the generation of MR negative reports. Solving this problem necessitates the implementation of a dedicated high-resolution MR imaging epilepsy protocol. Illustration 1 shows the high-resolution MR imaging epilepsy protocol at 3 T used at our institution.
Illustration 1.
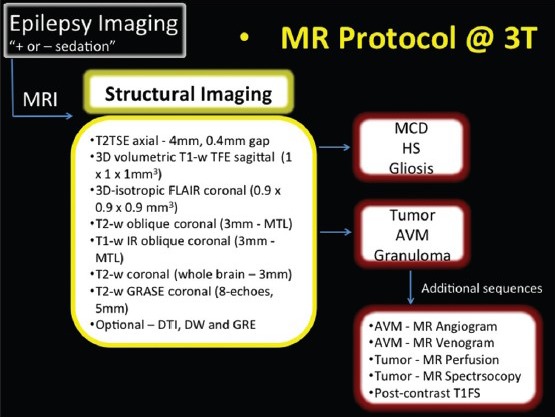
MR imaging protocol at 3 T
MR Imaging of Epileptogenic Lesions
Focal (partial) epilepsy is caused by a broad subset of etiologies, which may be classified into five major categories: hippocampal sclerosis [HS]; malformations of cortical development (MCD); neoplasms; vascular abnormalities; and miscellaneous pathologies which include intracortical scarring and infections.
Hippocampal sclerosis
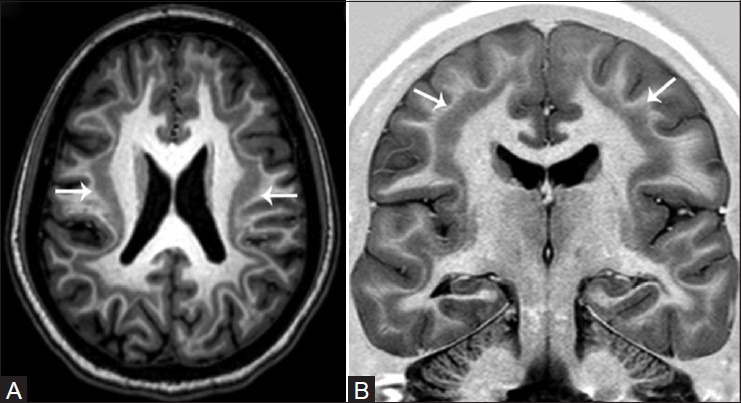
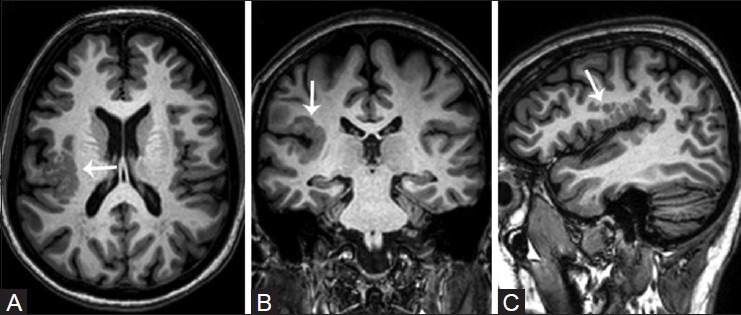
In patients with epilepsy, the hippocampal complex is the most vulnerable and frequently affected structure and represents the most frequently observed abnormality in temporal lobe epilepsy (TLE).[8,9,10] Small minorities of adult patients with newly diagnosed epilepsy have HS.[6,11] In children with refractory focal epilepsy, focal cortical dysplasia and tumors are the most common etiologies, rather than HS. The most striking changes are visualized in CA1, also known as the “Sommer's sector,” in which neuronal loss is always present. Similar, but less severe changes may also be visualized in CA3, hilus of the dentate gyrus, and CA2,[12] and are often accompanied by severe astrogliosis. MR imaging features of hippocampal sclerosis includes hippocampal atrophy, which is the most common feature, increased T2W signal, and loss of internal structure [Figure 1]. Associated findings include: widened hippocampal and choroidal fissures, enlarged ipsilateral temporal horn, reduced gray–white matter differentiation in the ipsilateral anterior temporal lobe [which may be due to an underlying type I focal cortical dysplasia (FCD)], ipsilateral temporal lobe atrophy, ipsilateral amygdala atrophy and increased T2W signal, abnormalities involving the entorhinal cortex, and atrophy of the ipsilateral mammillary body and posterior fornix. Unilateral hippocampal sclerosis may diffusely involve the hippocampal complex or may be localized to the anterior region affecting only the head, and is a less frequent cause of refractory focal TLE. In many patients, the contralateral hippocampal complex may be involved, though the changes may be asymmetric. Bilateral involvement has been reported in about 10% of adult patients with medically refractory epilepsy. These patients are more prone for secondary generalized seizures, and have associated cognitive deficits [Figure 2]. Hippocampal sclerosis may co-exist with a second pathology, which may critically impact prognostication of seizure outcome in patients who undergo surgery [Figure 3]. This often overlooked fact highlights the need for obtaining high-resolution scans at 3 T to exclude or detect a second pathology in patients with hippocampal sclerosis.
Figure 1(A and B).
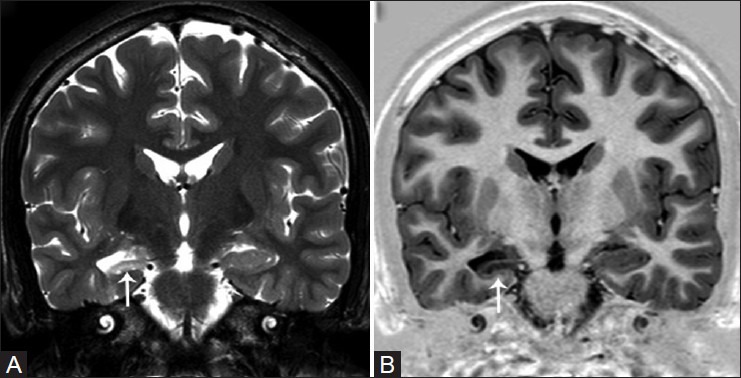
Unilateral right hippocampal sclerosis. Oblique T2W (A) and T1W inversion recovery (B) coronal images reveal diffuse hippocampal atrophy, increased T2W signal, and loss of internal architecture (white arrows)
Figure 2(A and C).
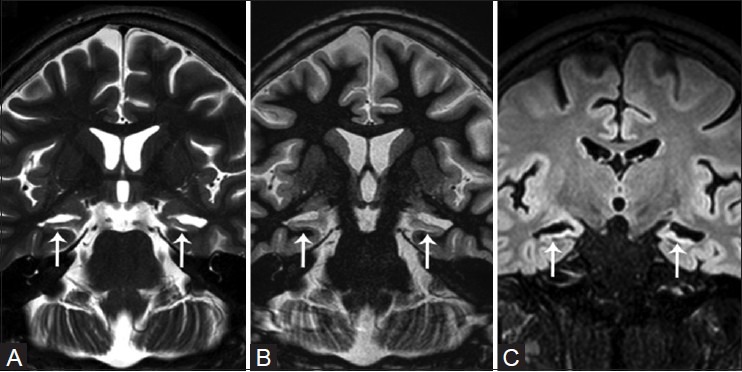
Post-encephalitic bilateral hippocampal sclerosis. Oblique T2W (A) and T1W inversion recovery (B) coronal, and reconstructed coronal FLAIR (C) images demonstrate bilateral diffuse hippocampal atrophy, increased T2W signal, and loss of internal architecture (white arrows)
Figure 3(A and C).
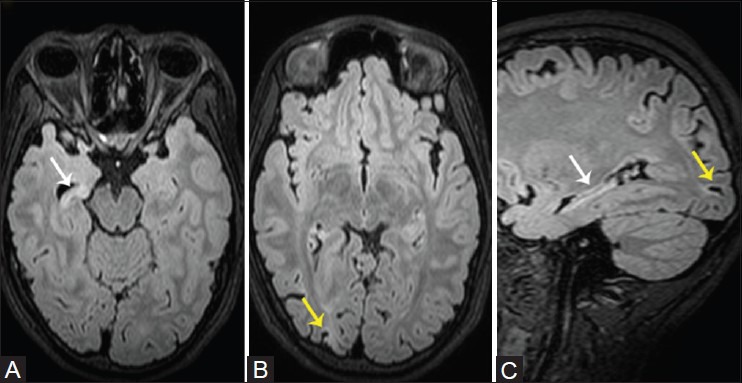
Unilateral right hippocampal sclerosis with ipsilateral occipital intracortical scar. Reconstructed axial (A, B) and sagittal (C) 3D FLAIR images demonstrate diffuse right hippocampal atrophy with increased T2W signal and loss of internal architecture (white arrows), associated with an intracortical scar and subcortical gliosis in the right occipital lobe (yellow arrows)
Malformations of cortical development
Development of the cerebral cortex is a complex process, and can be divided into three major but partially overlapping steps, which are cell proliferation, cell migration, and cortical organization. Disruption of these sequential and critical steps produces characteristic structural abnormalities with typical sulcation and gyral abnormalities, which are described as MCDs. MCDs are a major cause of drug-resistant epilepsy, and the role of surgical treatment of refractory epilepsy due to localized MCD is well established. Certain functional aspects of MCDs are poorly understood, including the reason for preservation of function within the dysplastic cortex or the cortical reorganization that results in shift of functions outside the MCDs. MCDs may be diffuse or focal; the diffuse form is seen in cases such as lissencephaly which are often associated with generalized seizures and cognitive decline and are poor candidates for elective surgery; the focal form is seen in cases of focal cortical dysplasia (FCD), heterotopia, and polymicrogyria which are amenable to surgery. However, it must be noted that the goal of surgery is to resect the epileptogenic zone or the ictal origin zone, and not only epileptogenic lesion.
Focal cortical dysplasia (FCD) – Taylor et al.,[13] first described FCD as a distinct subtype of MCD. FCD represents a broad spectrum of abnormalities involving the laminar structure of the cerebral cortex. Tassi et al.,[14] recognized three major histopathologic patterns of FCD including architectural dysplasia, cytoarchitectural dysplasia, and Taylor-type cortical dysplasia. Palmini et al.,[15] first described the pathological classification of FCDs and broadly segregated FCDs into Type I and II forms [Table 1]. In 2011, the ad hoc task force of the International League against Epilepsy (ILAE) proposed a consensus classification system for FCD [Table 2].[16] Patients with mild or type I FCDs may or may not have epilepsy. Type I FCDs can be subtle or undetectable on high-resolution MR imaging at 3 T [Figure 4]. Characteristic findings of type II FCDs include the presence of subcortical T2 hyperintensity extending to the ventricle, focal cortical thickening, blurring of the gray matter-white matter junction, and gray matter T2 hyperintensity [Figures 5 and 6]. Dysembryoplastic neuroepithelial tumors (DNETs) [Figure 7] and gangliogliomas [Figure 8] are benign neoplasms, which are often associated with medically refractory partial seizures, and may represent the extreme end of the FCD spectrum. These tumors are associated with abnormal cytoarchitecture and dysmorphic neurons and glial cells in the adjacent cortices.[17,18] FCD has been associated with dual pathologies including hippocampal sclerosis.
Heterotopia represents the presence of clusters of normal neurons in abnormal locations. Heterotopias are classified into three major groups including periventricular heterotopia, subcortical heterotopia (laminar or nodular), and leptomeningeal heterotopia. Periventricular heterotopia may either be unilateral or bilateral, and is located along the margins of the lateral ventricle [Figure 9]. The overlying cortex is mostly normal, but occasionally abnormal cortical folding may result if the heterotopia is located close to the depth of a sulcus. Subcortical nodular heterotopia represents focal accumulations of gray matter nodules extending from the ventricular margin to the cortex, with thinning of the overlying cortex. Subcortical band heterotopia [Figure 10], also called “double cortex syndrome,” is a form of diffuse gray matter heterotopia. The thickness of the band of gray matter predicts the configuration and thickness of the overlying cerebral cortex and white matter.
Polymicrogyria is characterized by excessive number of abnormally small gyri that produce a characteristic “bumpy” cortical surface [Figure 11]. Polymicrogyria may be associated with various genetic mutations, metabolic disorders, congenital cytomegalovirus (CMV) infection, and multiple congenital anomaly syndromes. Polymicrogyria may be localized to a discrete region of a hemisphere and, in such cases, the abnormal cortex tends to retain its functionality [Figure 12]. When the polymicrogyric cortex involves a large portion of or an entire hemisphere, the involved hemisphere is often hypoplastic, with a high incidence of cortical reorganization.
Table 1.
Palmini's classification of focal cortical dysplasia (2004)
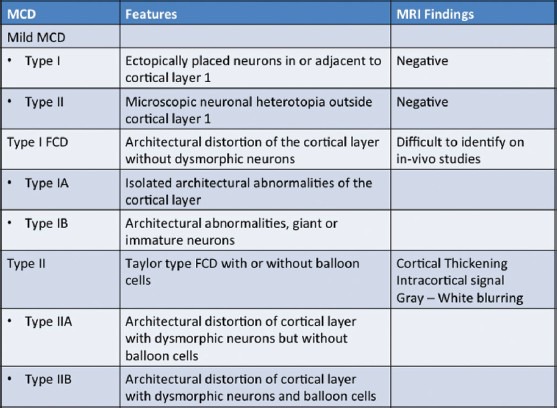
Table 2.
ILAE classification of focal cortical dysplasia (2011)
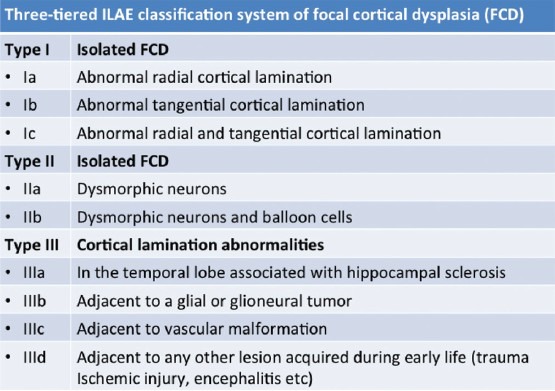
Figure 4(A-H).
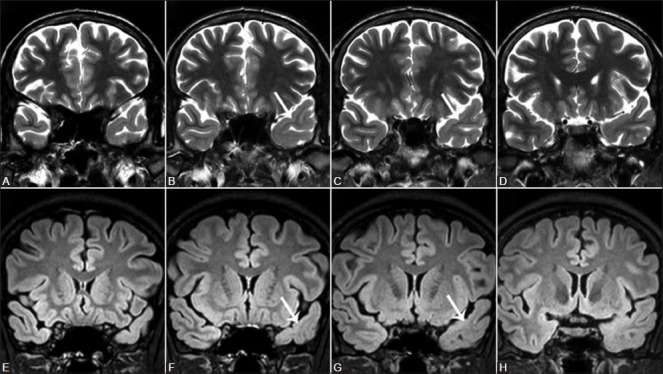
Focal cortical dysplasia Type I. Oblique T2W (A-D) and FLAIR (E-H) coronal images demonstrate subtle hyperintense signal changes in the left anterior temporal white matter, with blurring of the cortical-subcortical interphase (white arrows)
Figure 5(A-C).
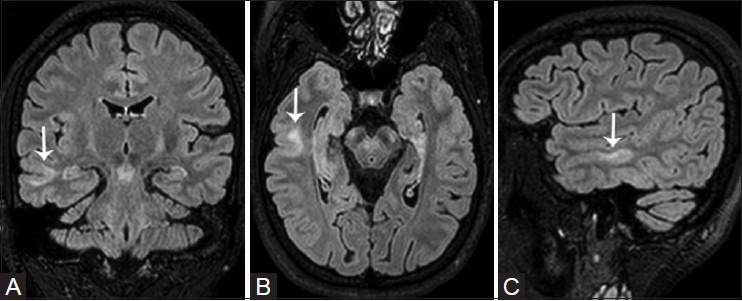
Focal cortical dysplasia Type IIB of transmantle subtype in a 26-year-old woman with complex partial seizures. Focal cortical thickening with intracortical hyperintense signal and blurring of the cortical–subcortical interphase is visualized within the middle temporal gyrus (white arrows). Note a wedge-shaped tag of FLAIR hyperintense signal extending through the temporal white matter to the lateral margin of the temporal horn, confirming the transmantle subtype
Figure 6(A-F).
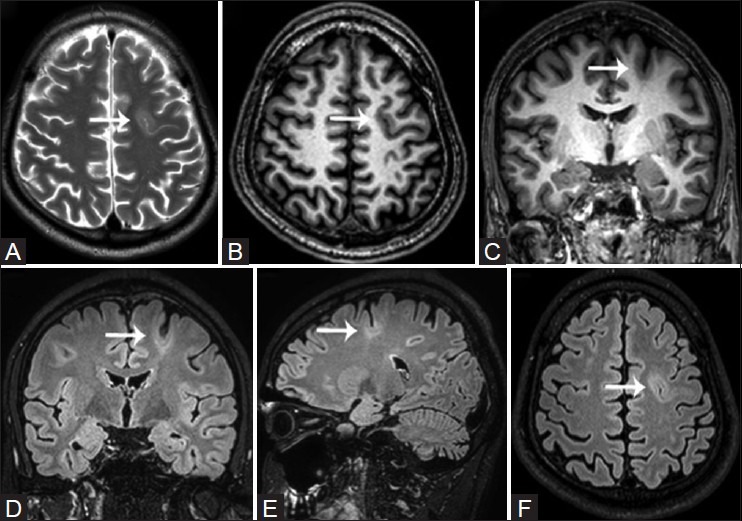
Focal cortical dysplasia Type IIB (bottom of sulcus dysplasia) in a 26-year-old patient with complex partial seizures. Cortical thickening with intracortical hyperintense signal and blurring of the cortical-subcortical interphase is visualized along the depth of the left superior frontal sulcus (white arrows). Note a thin comet tail of gray matter coursing through the frontal white matter between the dysplastic cortex and the ventricular margin
Figure 7(A-F).
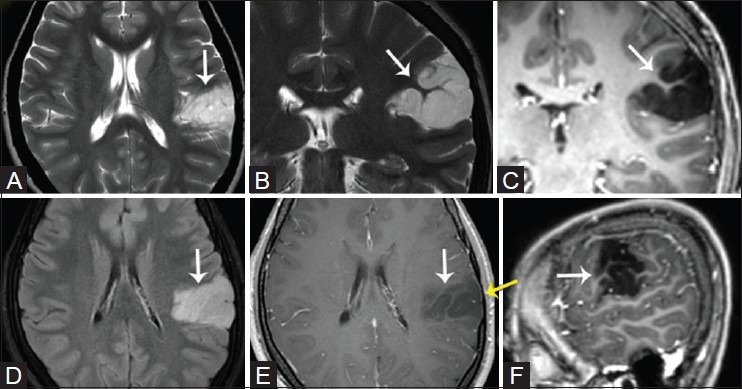
Dysembryoblastic neuroepithelial tumor (DNET) in a 17-year-old patient. Axial (A) and coronal (B) T2W, post-contrast FLAIR (D), T1W coronal (C), axial (E), and sagittal (F) images demonstrate a non-enhancing left intracortical lesion with cortical thickening and a “soap bubble appearance” located within the postero-lateral cortex of the left frontal lobe involving also the fronto-parietal posterior operculum cortex (white arrows). Note subtle scalloping of the overlying inner table (yellow arrow)
Figure 8(A-F).
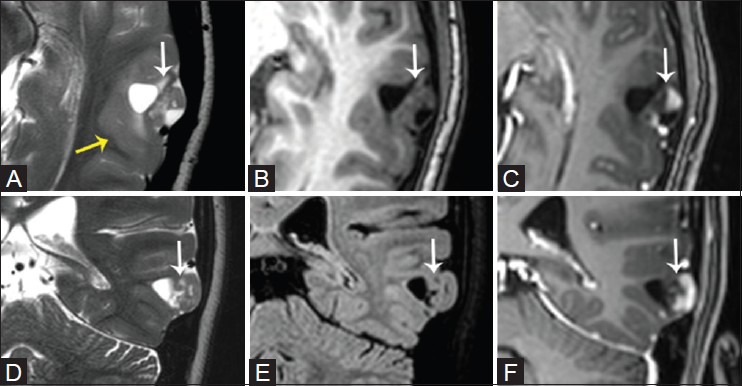
Ganglioglioma in a 21-year-old patient. T2W (A), T1W (B) and post-contrast T1W axial images, T2W (D), FLAIR (E), and post-contrast T1W coronal (F) images reveal a discrete complex intracortical lesion with the posterior temporal lobe located within the middle temporal gyrus, causing smooth scalloping of the inner table (white arrows). The lesion consists of intracortical cysts and small enhancing nodular structures, and is associated with thickening of the adjacent cortex, suggestive of a co-existent cortical dysplasia (yellow arrow)
Figure 9.
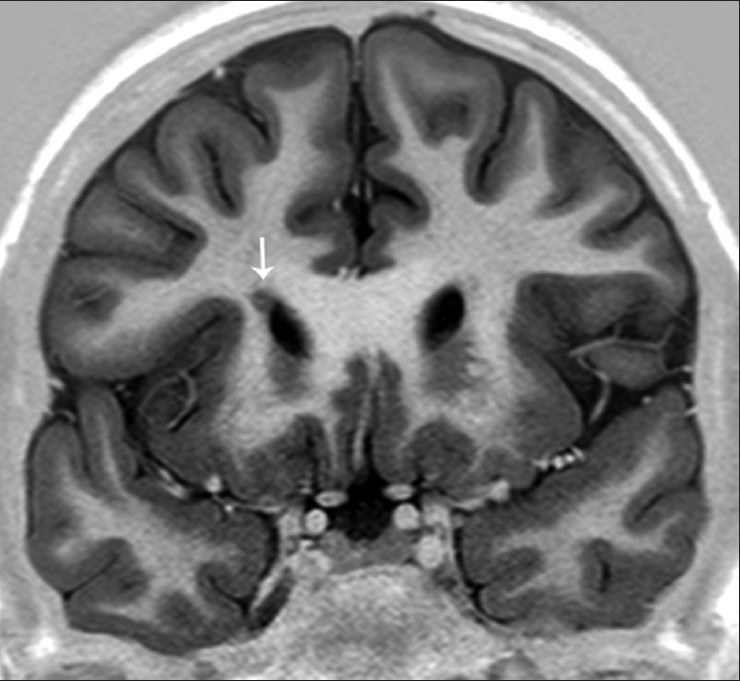
An 18-year-old with seizures since 3 years and abnormal behavior with subependymal heterotopia. Video-EEG showed right frontal spiking with ictal onset zone. Oblique T1W inversion recovery coronal image demonstrates a nodular structure isointense with the cerebral cortex, along the anterior tip of the frontal horn of the right lateral ventricle (white arrows)
Figure 10(A and B).
Double cortex syndrome in a 19-year-old patient with generalized seizures since 6 years (complex partial seizures with secondary generalization). Axial 3D T1W (A) and oblique coronal T1W inversion recovery (B) images demonstrate an abnormal continuous band of heterotopic gray matter in both cerebral hemispheres (white arrows), interposed between the cortex and ventricular margins and separated by bands of white matter
Figure 11(A-C).
Polymicrogyria in a 39-year-old subject. Multiplanar 3D T1W gradient echo sequence reconstructed in the axial (A), coronal (B), and sagittal (C) planes demonstrates a polymicrogyric cortex involving the right posterior perisylvian temporo-parietal cortex extending into the posterior and mid insular cortex (white arrows)
Figure 12(A-F).
Right hemispheric perisylvian polymicrogyria, with preservation of cortical functionality. The left-hand motor task (A) involved repetitive passive clenching of the fingers, and elicited activation in the superolateral aspect of the polymicrogyric cortex (pink blob). Tongue movements (D) generated bilateral cortical activations, with activation located just beneath, and there was a subtle overlap with the left-hand task activation (red blob). (B-C) and (E-F) show overlay images of left hand motor and tongue activations over the polymicrogyric cortex
Neoplasms
Neoplasms often manifest clinically as seizures, and constitute 2-4% of epileptogenic substrates in the general epilepsy population. A particular subset of neoplasms referred to as epilepsy-associated developmental tumors, including ganglioglioma, gangliocytoma, desmoplastic infantile ganglioglioma (DIG), DNET, and pleomorphic xanthoastrocytoma (PXA), are cortical based. These entities represent World Health Organization (WHO) grade I benign/low-grade tumors, contain varying amounts of glial and neural elements, and have low proliferation index, without perilesional edema or mass effect, and may remodel the calvarium. DNET and ganglioglioma represent an extreme form of FCD. The role of fMRI imaging in the pre- and postoperative assessment of CNS neoplasms is beyond the scope of this article.
Vascular abnormalities
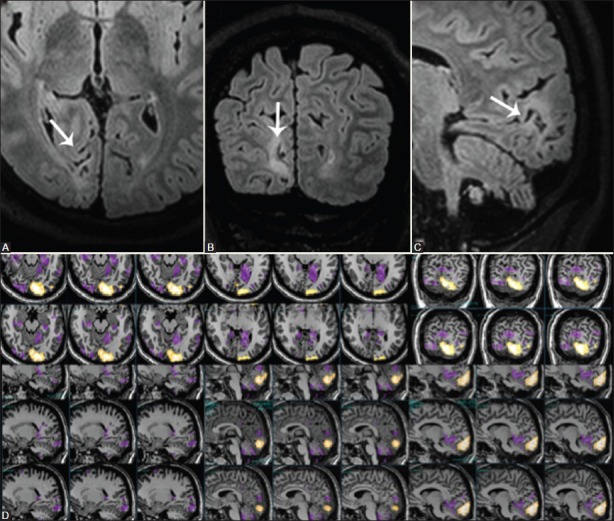
Arteriovenous malformations (AVMs) and cavernomas are the most common vascular malformations causing seizures in epilepsy patients. In AVMs, various mechanisms for the development of seizures have been postulated which include focal cerebral ischemia from steal phenomena due to shunting, and gliosis and hemorrhage in the parenchyma.[19] Cavernomas are well-circumscribed vascular spaces with the absence of intervening neural tissue within the lesion [Figure 13]. In patients with congenital or acquired vascular anomalies, the intensity of the acquired BOLD fMRI signal may be diminished in the eloquent cortex adjacent to the epileptogenic lesion. This is an epiphenomenon, which is secondary to rapid shunting of blood from the eloquent cortex, and may result in misinterpretation of the functional data. In all our patients with vascular malformations, we perform perfusion studies to assess alterations in regional blood flow parameters prior to interpreting the BOLD fMRI results.
Figure 13(A-L).
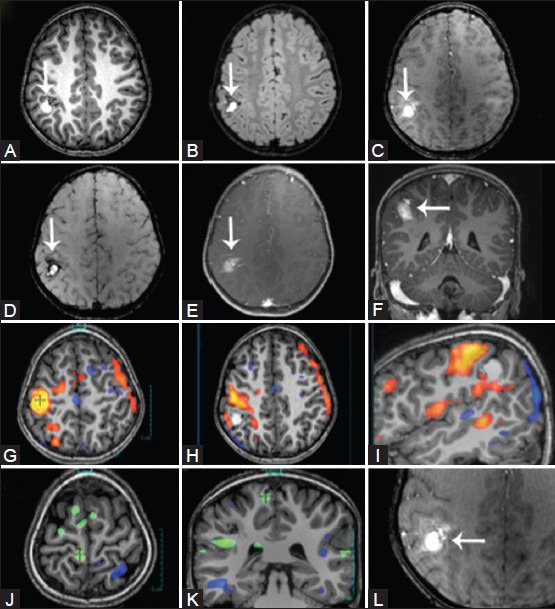
Right parietal lobe cavernoma. Axial T2W (A), 3D T1W (B), TOF MR angiogram (C), VENBOLD (D), post-contrast T1W (E), and coronal post-contrast T1W (F) images demonstrate a cavernoma and an accompanying DVA in the right superior parietal lobule (white arrows). During the left-hand task (sequential finger tapping), activation is seen in the vicinity of the hand knob (orange blob), which is separated from the lesion by one complete gyrus. During the left-foot task (clenching of toes), activations are identified in the right paracentral lobule (green blob)
Miscellaneous
These include a broad spectrum of pathologies including intracortical gliosis [Figure 14], perinatal insults [Figure 15], post-traumatic epilepsy, infections, Rasmussen's encephalitis, neurocutaneous syndromes (tuberous sclerosis) [Figure 16], and Sturge–Weber syndrome. Intracortical gliosis is the end result of a wide spectrum of pathologies, including head injury, infarcts, and infection. MR imaging findings include intracortical and subcortical white matter T2 fluid attenuated inversion recovery (FLAIR) hyperintensity, penciling of the cortex, and localized volume loss with sulcal widening, with or without ventricular enlargement. Typical perinatal injuries arising from either ischemic, hypoglycemic, or a combination of both entities produce typical patterns of brain damage, which depend on the timing and severity of the insult. Post-traumatic epilepsy is considered a specialized form of epilepsy, and results from shearing injuries with or without hemorrhagic transformation. The common sites of injury are along the regions of the brain in close proximity to the skull base, especially the basifrontal and inferior temporal lobes and the fronto-temporal poles.
Figure 14(A-E).
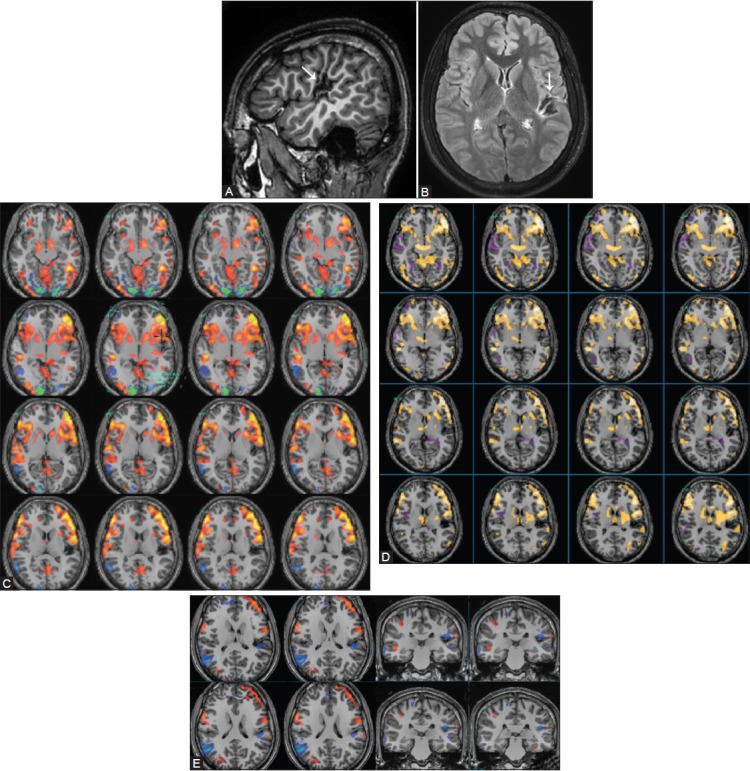
A 22-year-old male with left posterior perisylvian intracortical scar. 3D sagittal T1W (A) and axial FLAIR (B) images demonstrate a localized intracortical gliotic scar in the left lateral, posterior perisylvian cortex and the lateral cortical surface of the superior temporal, angular, and supramarginal gyri (white arrows). Note that the scarring in the posterior insular region is located close to but does not involve Heschl's gyrus and also distorts the central sulcus. fMRI studies of the language networks revealed dominant left lateralization of the language networks on the silent verb (C), word generation (D), and comprehension (E) tasks. Activations were identified in the left inferior frontal gyrus, bilateral ventrolateral prefrontal cortex, and lateral frontal association areas, and right > left activations were identified in the posterior temporal and left parietal cortex. The language activations in the left parietal lobe are in proximity with the posterior margin of the scar
Figure 15(A and B).
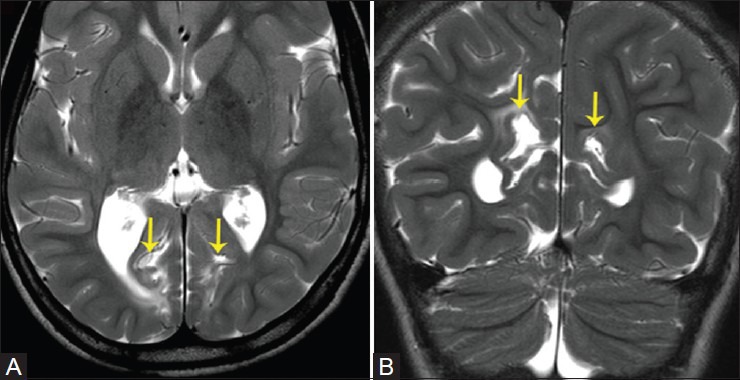
A 7-year-old patient with global developmental delay. T2W axial (A) and coronal (B) images demonstrate sequelae of hypoglycemic injury, with asymmetric cortical scarring involving bilateral medial occipital cortices, large on right, and subcortical white matter injury and gliosis in the occipital white matter (yellow arrows)
Figure 16(A-G).
A 1-year-old patient with tuberous sclerosis. Axial FLAIR images reveal numerous cortical tubers with subcortical hyperintensity in bilateral frontal and temporal and left occipital lobes. Axial T1W images reveal tiny high-signal subependymal hamartomas around the caudothalamic grooves and margins of the atria of lateral ventricles (yellow arrows)
fMRI Imaging Paradigms
Task designs are either block- or event-related paradigms. Both designs are based on the subtraction paradigm, which assumes that conditions differ in only the cognitive process of interest and differences in the BOLD signal between these two conditions reflects the differing cognitive process. The choice of selecting the task and control conditions is critical as a selected stimulus must be sufficient to elicit a HDR. Cognitive abnormalities are common in patients suffering from medically refractory epilepsy, and task selection plays a vital role in eliciting a good HDR. This is exemplified to a great extent in our country due to vast regional differences in culture and language. Most studies employ a block design, in which the patients are required to regulate the BOLD signal for usually 15-30 sec followed by a rest block of similar duration. Block designs are less sensitive to delays arising from task switching and slow BOLD response. Various statistical methods are available to evaluate intravoxel changes in the BOLD signal between the task and rest phases; however, many of these methods use extremely rigid thresholds, which result in deletion or addition of regions of brain activation involved in a particular network. Of note, clinically applicable thresholds vary among patient subsets, the type of paradigms used, and needs to assessed on an individual basis with due relevance for the surgical intervention which has been planned for the patient.
Motor and sensory tasks
The current gold standard to detect cortical functional organization is electrical cortical stimulation (ECS). However, ECS is an imprecise technique, disrupts cortical function, and may trigger seizures. Other techniques to map the sensorimotor strip without disrupting the cortical function include somatosensory evoked potentials, fMRI, and electrocorticography (ECoG). Wray et al.,[20] compared all four techniques for pre-surgical localization of the sensorimotor cortex in pediatric patients undergoing epilepsy surgery. They concluded from their study results that though these modalities produced concordant localization of cortical sensorimotor function in children, ECoG was most likely to localize the sensorimotor cortex, whereas ECS was more likely to fail to localize the sensorimotor cortex. Various studies have attempted to compare fMRI with ECoG, and these have depicted a good correlation between the two modalities.[21,22]
Sensorimotor tasks are the most reliable and reproducible of fMRI imaging paradigms.[23,24,25,26] These paradigms are a block design of either motor movement or sensory stimulation compared with rest. The motor strip may be identified with sequential or non-sequential finger counting, finger tapping, tongue wiggling or rolling, and lip smacking. In patients with hemiparesis or MCDs such as polymicrogyria, fine finger movements may be difficult to perform and may result in motion-induced artifacts. Alternatively, in such cases, active or passive clenching of the hand or toes may be performed. The supplementary motor area also needs to be identified in the paracentral lobule. Brushing or manual stroking of these areas may help to identify the sensory strip. The visual task is an extremely reliable task, with flashing checkerboards shown during the activation period and a blank screen shown during the rest period [Figure 17]. In our experience, it is extremely critical to identify sensorimotor cortex when planning extratemporal neocortical resection for tumor [Figure 18], cortical dysplasia [Figure 19], intracortical scar [Figure 20], or vascular malformation resection.
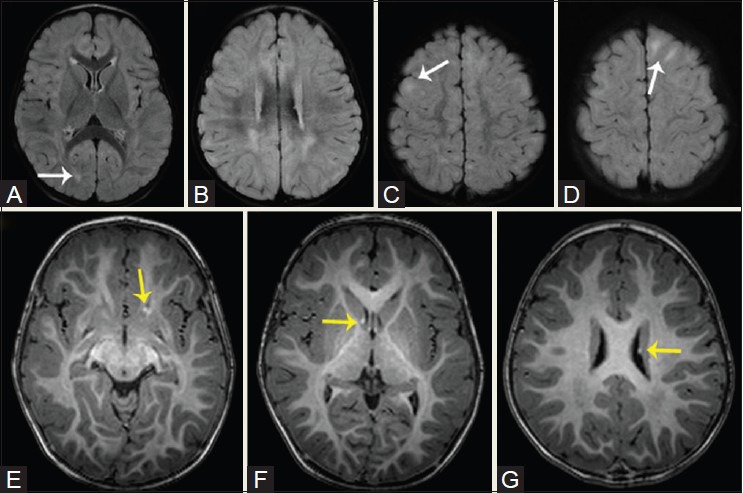
Figure 17(A-D).
A 22-year-old patient with hypoglycemia-induced asymmetric occipital scarring. Reconstructed 3D FLAIR sequence in axial (A), coronal (B), and sagittal (C) planes reveals asymmetric scarring in the occipital cortices, right more than left. fMRI was performed to assess the visual networks using flashing checkerboard stimulation. Activations were identified in the left occipital lobe, including the cuneus and lingual gyri, and within the calcarine sulcus and along its banks. No activation was seen in the right occipital lobe
Figure 18(A-I).
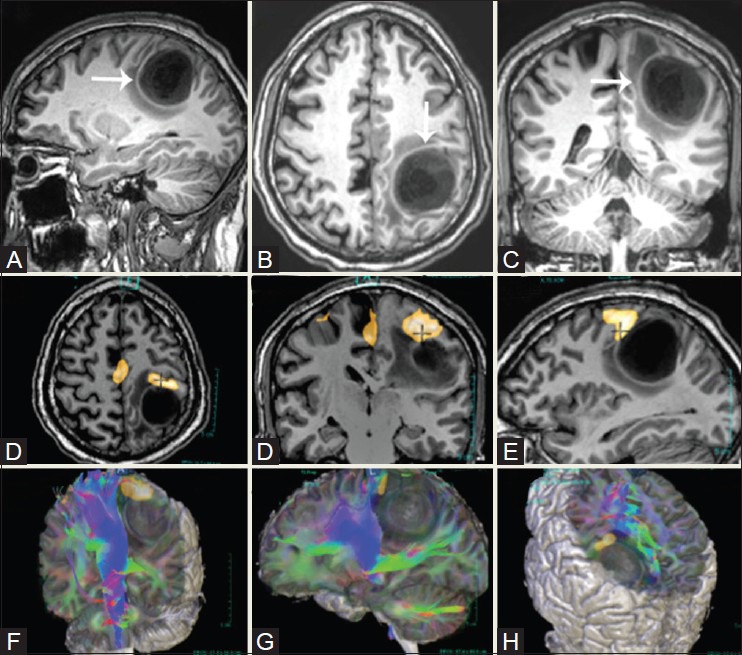
A 61-year-old patient with left post-central gyrus anaplastic oligodendroglioma. Multiplanar 3D T1W sagittal (A), axial (B), and coronal (C) images reveal a large, sharply marginated lesion in the left parietal lobe involving the post-central gyrus and distorting the central sulcus. Right-hand finger tapping demonstrates robust activation in the left sensorimotor strip, along the anterior boundary of the tumor (D-F). Diffusion tensor imaging (DTI) maps reveal ventral displacement of the ipsilateral corticospinal tracts (G-I)
Figure 19(A-E).
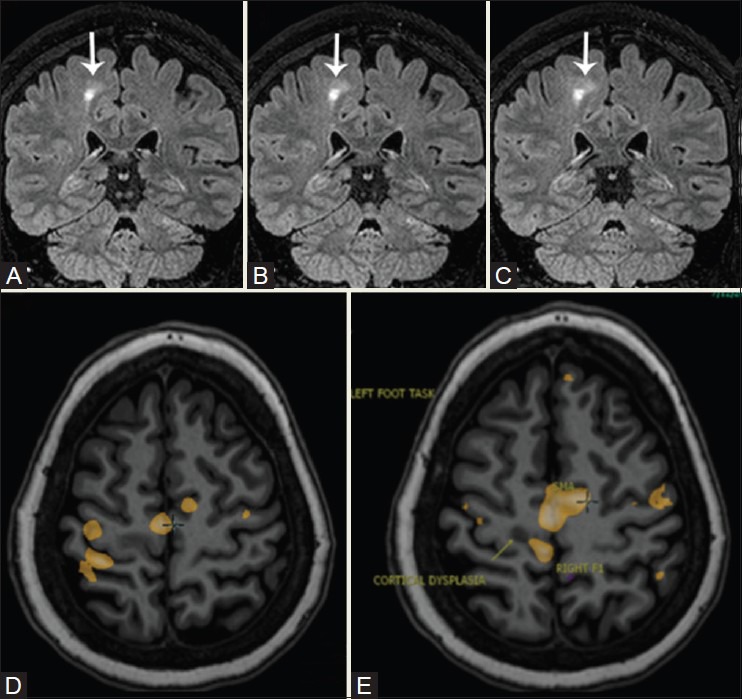
A 28-year-old patient with refractory seizures. 3D FLAIR sequence shows focal cortical dysplasia in the medial cortex of the right frontal lobe, just anterior to the pars marginalis. Toe clenching demonstrates activation in right SM1 (yellow blob) immediately postero-medial to the dysplasia, separated by a shallow sulcus. The supplementary motor area [SMA] activation is located anterior to the dysplastic cortex. Finger tapping resulted in activation lateral to the dysplastic cortex in the hand knob (yellow blob)
Figure 20(A-D).
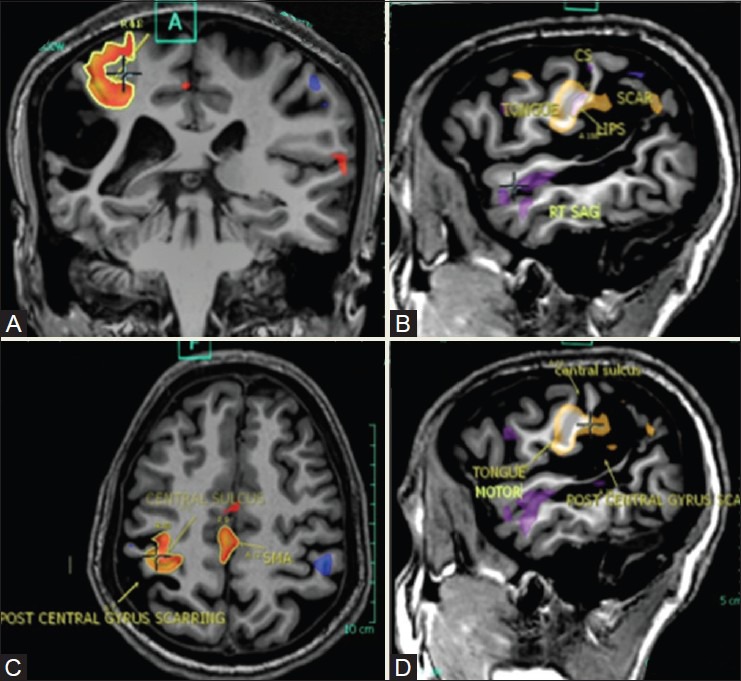
A 33-year-old patient with a large cortical-subcortical scar in the right post-central gyrus. Left-hand clasping task generated activations in the right central sulcus, anteromedial to the scar, and in the SMA (red blobs). Lip puckering and tongue movement tasks generated activations anterior to the scar, with overlap between both activations, the tongue appearing lower (yellow blobs)
Language tasks
Language-related functions were among the first to be localized to a specific location in the human brain. In the 1940s, Juan Wada's work established the role of intracarotid injection of sodium amytal (IAT) for selective hemispheric anesthetization. His pioneering work was used to minimize the cognitive side effects of ECT by preventing bilateral generalization of ECT-related seizure activity through temporary anesthetization of the language-dominant hemisphere. Over time, both IAT and clinical neuropsychological assessment have become the current mainstay of pre-surgical evaluation of patients with medically refractory epilepsy. IAT has numerous limitations, including its invasiveness, potential for complications, invalidation of studies due to anatomic or developmental variants, extreme time sensitivity, lack of standardization across centers, and lack of lateralization in some individuals. In view of these limitations, alternative options to IAT are being investigated, including event-related potentials, transcranial magnetic stimulation, 15O-PET, and functional MRI.
The fMRI has been used for well over a decade to investigate the cortical regions involved in language processing. In patients with TLE, the need for precise pre-surgical lateralization and localization of the language networks is critical, so as to assess the surgical feasibility and define the surgical margins. In right-handed individuals, the left hemisphere is the dominant hemisphere for language. Many studies show some degree of bilateral activation even in normal right-handed individuals.[27,28] However, in patients with epilepsy, atypical lateralization or bilateral representation may be present due to network reorganization. Using fMRI, Yuan et al., have demonstrated neuroplasticity of language function in pediatric epilepsy, which suggests that the recurrent ictus causes redistribution of language function in the developing brain to compensate for injury to the dominant areas or their neural networks.[29] In left-handed individuals, fMRI experiments have demonstrated increased prevalence of right hemispheric dominance.[30,31] Non-dominant hemispheric activation may be related to the task complexity or the non-verbal aspects of language.
Based on the initial “language maps,” in right-handed individuals, the Broca's area (left inferior frontal lobe activation area) and the Wernicke's area (left postero-superior temporal gyrus activation area), and their communications were considered as the principal language-related centers. Numerous studies including fMRI experiments have shown the following: frontal lobe activation extends beyond the Broca's area to include the dorsolateral and medial prefrontal cortex, and anterior cingulate gyrus; comprehension extends beyond the Wernicke's area to involve the left temporo-parietal regions and even the frontal lobe; and Wernicke's area is not the primary center for comprehension, with involvement of other areas including the middle temporal gyrus, angular gyrus, and supramarginal gyrus. Compared with IAT, fMRI is extremely useful to assess non-dominant hemispheric activation. Currently, the role of fMRI is not only restricted to lateralize language-based function to a particular hemisphere, but also to assess and localize the complex language networks. It is of note that fMRI does not differentiate between critical areas governing a particular function and those which only form a small link in the neural network and can be compensated by other parts of the network. Hence, to determine these links, it is vital to evaluate the fMRI data at low but statistically significant thresholds. The results published by Desmond et al.,[32] and Binder et al.,[27] have shown complete agreement between language lateralization and high concordance between IAT and fMRI. Benbadis et al.,[33] questioned the data from Binder et al., and they found that the laterality index calculated solely on IAT speech arrest is not a valid indicator for lateralization of language dominance. Yetkin et al.,[34] found a 100% concordance between fMRI data and IAT results in 13 patients with complex partial seizures in whom a word-generation task was used for language lateralization. However, a contrary view also exists as elucidated by Worthington et al., who demonstrated poor concordance between fMRI and IAT language lateralization.[35]
There are two major considerations while designing the language-based tasks – the modality of stimulus presentation, whether it is visual or auditory; and the mode of patient response, whether it is covert or overt. Auditory-based tasks result in activation of bilateral auditory cortices and posterior superior temporal gyrus, which represents the receptive language cortex. However, these seem to be less useful to detect language dominance, and subtraction models used to remove the primary auditory cortices also tend to remove activation areas, which may have been part of the broader language network. On the other hand, reading-based tasks tend to localize comprehension, processing, and verbal memory areas. The most common tasks used are the visual reading tasks, but in the case of young children and illiterates, pictures can be used instead of written stimuli.
The response to a stimulus can be covert wherein the subject “thinks” of the answer in the mind without verbalizing it. The advantage of this method of responding is that the subjects often report no performance anxiety, but the disadvantage is that subjects may not respond at all. On the other hand, the advantage of an overt “push button” response is that the subject can be monitored continually to check if they are responding, and also, the appropriateness of their responses can be checked.
A wide spectrum of robust visual language tasks is available at the clinician's disposal as follows:
Silent word generation: During this task activation, the subjects are instructed to generate as many words as they can that begin with the letter seen. Activation is typically visualized in the left inferior frontal gyrus, bilateral premotor cortices (left more than right), and left posterior temporal region.
Silent verb generation (word or picture cues): In this task, the subject generates a verb for the noun seen (e.g. for the noun “chair,” the subject has to think of the action verb “sit”). For young children or illiterates, pictures can be used instead of words and a picture of a “chair” is the stimulus instead of the word. Activation is typically identified in the left inferior frontal gyrus, bilateral premotor cortices (left more than right), and left posterior temporal region
Semantic decision: This task involves decision making regarding the stimulus and uses an overt push button response of either “yes” or “no.” Thus, for example, a category and its example may be presented. If the example belongs to the category, the subject pushes the right button indicating a “yes,” but if the wrong example is presented, then the subject presses the left button indicating “no.” This is primarily a frontal language processing task, with activations typically visualized in the left inferior frontal gyrus, left prefrontal, lateral, and ventral left temporal, left posterior parietal, and visual word form regions in the fusiform gyrus.
Sentence completion/comprehension: The reading activation blocks consist of a visual display of simple incomplete sentences with a blank space at the end, which requires completion based on the semantic meaning of the sentences. Subjects read each sentence and attempt to retrieve a word to complete the sentence. Activations are seen in the left fusiform gyrus (visual word form), postero-superior temporal gyrus and superior temporal sulcus, and inferior frontal gyrus.
So how do we do it? Our protocol
We use visual tasks, words, as well as picture stimuli, and responses are either covert (think the answer in your mind) or overt (push the right button to indicate “yes” and the left button to indicate “no”). We have created the tasks in four languages – English, Hindi, Marathi, and Guajarati. At our center, the subject is trained for the specific tasks by a neuropsychologist and it is critical that the task be performed in the language the subject is most proficient in. We would typically perform three language tasks, which include the “silent word generation,” “silent verb generation,” and the “sentence comprehension” tasks. In cases of subjects with diminished intellect, we would attempt only the “silent word generation” and the “picture verb generation” tasks.
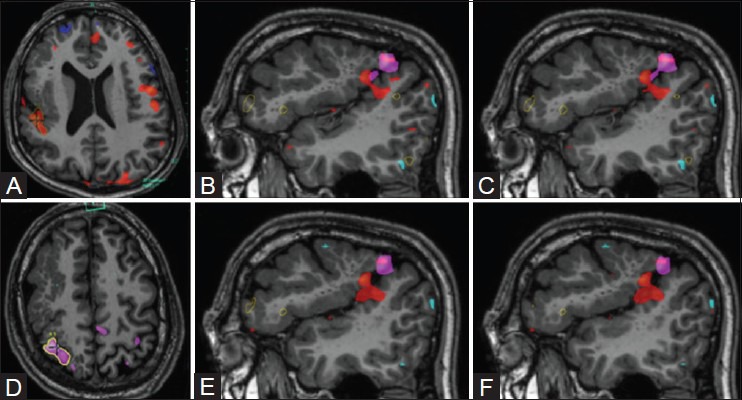
At times, we are asked, “Isn't one language task enough to assess the language networks?” The answer is categorically no. Of note, reading-based language tasks may result in different areas of activation and dominance, which may suggest the presence of language reorganization [Figure 21]. Moddel et al.,[36] coined a term “bilateral dependent” in Wada testing, which indicates that both sides jointly participate in performing a given task and there is no evidence of functional segregation. However, in certain cases, language reorganization may be noted, with a subset of functions in one hemisphere and others relocated to the contralateral lobe. Rasmussen et al.,[37] have reported similar functional dissociation using the Wada test, and Wilke et al.[38] have reported similar findings in pediatric subjects with epilepsy. Anecdotally, the authors have seen similar results in language tasks performed on few subjects. At our institution, we primarily train our subjects to undergo the “silent verb generation” and the “sentence completion/comprehension” tasks. Additionally, we overlay multiple language datasets to obtain voxel-by-voxel correlation between activation maps and identify the most statistically significant regions involved in the language networks [Figure 22].
Figure 21(A-D).
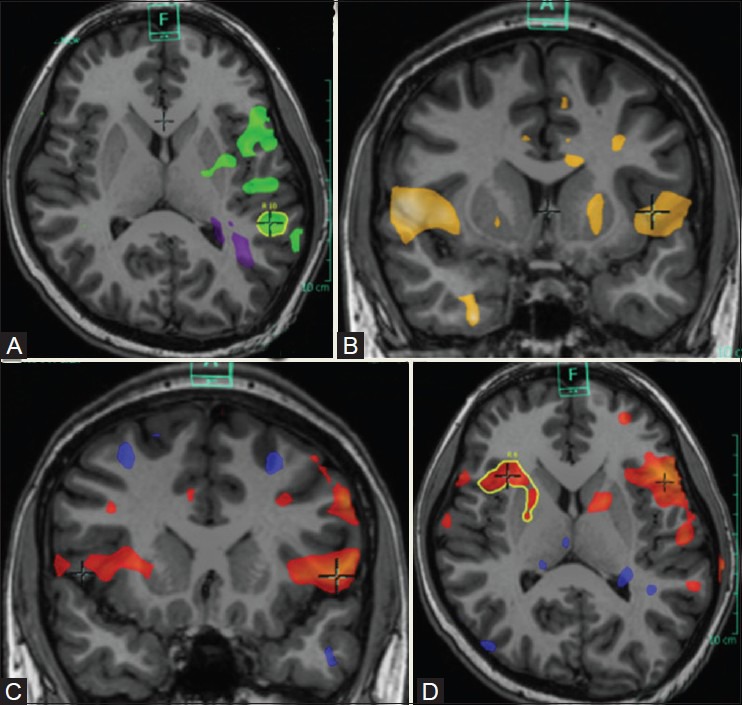
Functional dissociation of language networks. Semantic task (A) demonstrates lateralization to left inferior frontal gyrus and postero-inferior temporal lobe (green blobs). Silent word generation task (B) demonstrates no obvious dominance, with activations in the inferior frontal gyri, right middle temporal gyrus, and left superior and inferior temporal gyri. Silent verb generation task (C) demonstrates bilateral activations, left more than right (red blobs). Sentence completion task (D) demonstrate lateralization to left inferior frontal gyrus and postero-superior temporal gyrus and sulcus
Figure 22(A-C).
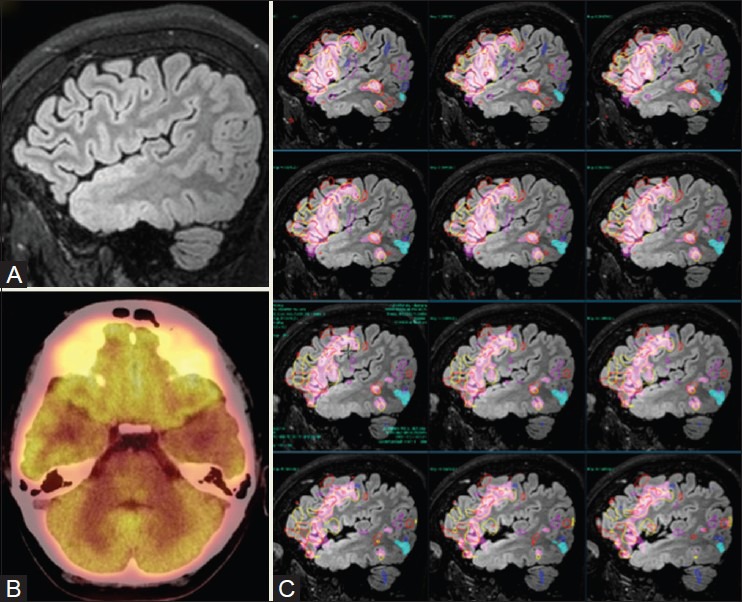
A 16-year-old patient with poorly controlled seizures, scholastic difficulties, and behavioral issues. 3D sagittal FLAIR (A) image shows left anterior temporal polar cortical dysplasia extending into the superior cortical surface and entorhinal cortex. PET (B) demonstrates hypometabolism in the left anterior temporal lobe. Language fMRI tasks showed clear-cut left dominance. Overlay of activation maps generated from multiple tasks demonstrated no activation in the anterior temporal pole, with the posterior temporal activations located further from the dysplastic cortex
The incidence of atypical language lateralization is increased in patients with focal epilepsy. Initial studies suggested that shifts in language dominance are likely when epileptogenic foci are in close proximity to the language-eloquent areas rather than in more remote regions such as the hippocampus [Figure 23]. Contradictory to the initial reports, Weber et al.,[39] demonstrated that patients with left hippocampal sclerosis showed less left lateralized language representations than subjects with left frontal or temporal lobe lesions, which suggested that the hippocampus seems to play an important role in the establishment of language dominance. Davies et al.,[40] stated that the risk of naming decline following anterior temporal resection is lower in patients with hippocampal sclerosis vis-à-vis those with a normal hippocampus. Hamberger et al.,[41] in their study, suggest that preserved naming ability in postoperative hippocampal sclerosis patients following anterior temporal resection might reflect cortical reorganization possibly due to intrahemispheric reorganization of language.
Figure 23(A-F).
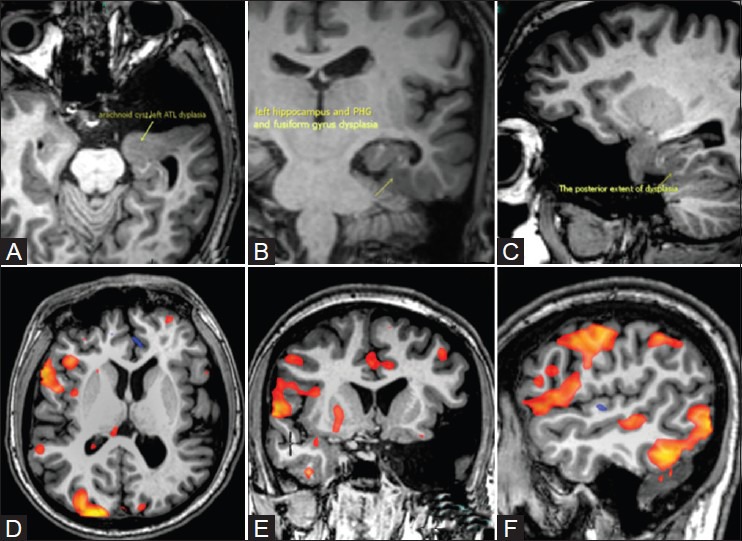
Neuroplasticity and language reorganization in a left-handed 21-year-old patient with left anterior temporal, mesial temporal, parahippocampal, and fusiform cortical dysplasia. Covert verb generation task was performed in Gujarati language from visually presented color pictures. Activations were identified in the right hemisphere in the inferior frontal gyrus, inferior aspect of middle frontal gyrus, posterior aspect of superior and middle temporal gyri, and, in the visual receptive and perceptive areas (red blobs). The fMRI results showed language lateralization to the right hemisphere
The interpretation of language fMRI results in the pediatric population represents an extreme challenge for the following reasons:
Many children with epilepsy have co-existent developmental delay and cognitive decline and are on long-term antiepileptic medications, which may diminish their capacity to undergo fMRI language tests
In healthy toddlers, language representation is still more bilateral.[42] Various authors have also shown an increasing trend toward lateralization of the networks to the left perisylvian region until 18 years.[29,43,44] Hence, a left-sided lesion occurring early in life may have diminished propensity to produce speech disturbance
In subjects with early brain lesions, cortical reorganization seems to occur, resulting in bilateral or right hemispheric dominance. However, this finding is more often seen in patients with gliosis or neoplasms, rather than in MCDs, which have a tendency to retain language networks.
Memory tasks
Numerous diagnostic tools have been used to predict the likely possibility of development of postoperative memory decline following a standard temporal lobectomy. In the last decade, fMRI techniques were used to evaluate the neural networks involved in memory encoding, especially focusing on contributions from the medial temporal lobe and amygdala. The development of memory paradigms has lagged behind that of language paradigms, primarily due to various challenges including lack of an ideal memory task with high specificity, variable paradigms, and discrepancies in analysis and interpretation. Memory is complex and diverse, and is broadly classified as explicit or implicit. Most fMRI studies are based on explicit-based paradigms, with only a few studies based on implicit-based paradigms.[45] Forms of explicit or declarative memory include episodic memory, semantic memory, and working memory. Procedural memory may either be explicit or implicit. Most pre-surgical memory studies utilize encoding tasks to understand episodic memory. Golby et al.,[46] used fMRI to assess lateralization of the encoding processes. It was found in this study that sentence completion tasks activated the inferior prefrontal cortex and medial temporal lobe more on the left, pattern encoding more on the right, and faces and scene encoding symmetrically on both sides. A study conducted by Powell et al.,[47] reported that activation was left-lateralized for word encoding, bilateral for picture encoding, and right-lateralized for face encoding. Detre et al., used scene memory task in nine TLE patients and demonstrated 100% concordance between IAT and fMRI results, with clinically relevant activation asymmetries in the parahippocampal region in patients with TLE.[48] The contrary view also exists, in which Dupont et al., demonstrate striking discordance between fMRI and IAT, with only 48% of their 25 patients showing concordance between both tests; however, the study noted that fMRI was better than IAT in accurately predicting postoperative memory changes.[49] Memory decline following anterior temporal lobe surgery is a major concern, and current fMRI language paradigms have shown promise in predicting postoperative verbal memory decline after anterior temporal lobe surgery. Newer memory encoding paradigms, which produce greater activation of the anterior part of the mesial temporal lobe, would be more useful, when compared with current encoding paradigms, which result in activation of the mid and posterior aspects of the mesial temporal lobes. Though encoding tasks are useful, based on the current data, fMRI cannot be used in isolation to predict postoperative memory changes.
Challenges
In clinical practice, performing pediatric fMRI studies is a challenge and may require the use of sedation. The aim of sedation is to have very limited or no effect on cerebrovascular response and neuronal metabolism. fMRI studies have been performed in pediatric patients with passive tasks, such as auditory and visual stimuli, and few authors have demonstrated drug-dependent anatomical variations in activation. More research is required to understand the effects of sedation on the neural networks and fMRI-generated activation maps. Anecdotally, we have attempted passive sensorimotor tasks under sedation using short-acting agents such as propofol and have repeatedly found inconsistent or poor results. Presently, we do not perform fMRI under sedation for sensorimotor tasks
Progressive cognitive impairment is frequent in epilepsy patients. In adults, cognitive decline appears as attention deficits, memory disturbances, and mental slowing. In children, it is associated with behavioral decline, language difficulties, and poor academic outcome. This represents a serious challenge to train and elicit an adequate response from these patients. At our center, we train all our patients in advance with the help of a neuropsychologist or a physician. Post-procedural review is performed in each patient to ascertain task performance
Physiological artifacts arising from blood vessels and pulsatile cerebrospinal fluid (CSF) flow contaminate the echo planar imaging (EPI) BOLD signal at a miniscule and acceptable level. However, head motion results in serious contamination of the EPI-BOLD signal and often leads to the data being discarded. Our third party vendor has extremely stringent criteria for fMRI data processing, with a 5-mm cut-off for head motion in any one plane. Various techniques are available to restrict motion; however, in our practice, the most useful technique represents a combination of the following: performing fMRI tasks before structural MR studies, use of clinically relevant lesion-centric tasks which limit scan time, pre-procedural patient training, and also post-procedural reward-based system, especially for children. We also perform each task twice, but not in succession, to avoid repetition and conditioning
Careful assessment of post-surgical outcome data would only be able to ascertain what the gold standard for language localization. At present, fMRI has the potential to replace IAT as the gold standard for “lateralization of language dominance”. However, it must be noted that fMRI has still not achieved reliability to dethrone ECS from identifying surgical margins. Neuroplasticity and language reorganization is known in pediatric epilepsy, especially in left-handed individuals with mesial temporal or frontal pathologies. In relation to temporal surgeries, few caveats regarding fMRI still exist, and these include the following: different language tasks produce variable and inconsistent activation maps; inability to produce activation in the anterior temporal lobe; and poor understanding of the specificity of activation on fMRI studies. These suggest that further advances in development of such paradigms are needed, followed by a greater understanding of the patterns of activation
Unlike the West, India is a multilingual nation, reflected by the numerous regional variations in each dialect. The patient background and level of education also affect language fMRI results. Hence, the performance of language-based tasks is an extreme challenge in our subset of patients. To overcome this, we perform each of our language studies as reading-based tasks in the primary language of our patients, with the texts available in English, Hindi, Marathi, and Gujarati
fMRI activation maps are principally dependent on fluctuations in BOLD signal achieved with neuronal activation. In the presence of a pathological entity such as a neoplasm or an AVM, alteration in the locoregional hemodynamics may produce a decrease or increase in the BOLD fMRI signal and lead to false-negative or false-positive fMRI results. In our practice, patients with intracranial vascular malformations or neoplasms always undergo MR perfusion to assess fluctuations in regional cerebral blood volume (CBV), CBF, and mean transit time (MTT). Alternatively, patients with extracranial pathologies, such as carotid artery stenosis or tumors of the carotid space, may diminish the intracranial blood flow and BOLD response. In such patients, proper clinical history and pre-test assessment including a dedicated study of the neck or angiogram may be pertinent.
Conclusion
Pre-surgical mapping of the epileptogenic lesion and zone is vital to achieve seizure-free status. Currently, high-resolution MR imaging with 3 T using tailor-made epilepsy protocols has become a critical component in the routine clinical work-up of epilepsy patients. The addition of fMR imaging techniques, including sensorimotor, language, and memory tasks, has resulted in clinically robust pre-surgical mapping of the neural networks and eloquent cerebral cortex and in prediction of post-surgical deficits. However, presently, fMRI tests are not used in isolation in clinical practice, and supplementation via results obtained from IAT (Wada test), ECoG, or ECS tests is needed prior to surgical intervention. Improved understanding of data concordance achieved between these structural and functional techniques is important, and may pave the way for better treatment approaches. In future, the development of faster high-resolution magnets, better task designs, and stringent but clinically requisite post-processing algorithms may result in fMRI becoming a vital and possibly irreplaceable element in the algorithm governing pre- and post-surgical work-up of epilepsy patients.
Acknowledgment
The authors would like to sincerely thank Dr. Urvashi Shah for rendering her contribution to this article.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bell GS, Sander JW. The epidemiology of epilepsy: The size of the problem. Seizure. 2001;16:165–70. doi: 10.1053/seiz.2001.0584. [DOI] [PubMed] [Google Scholar]
- 2.Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165–70. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 3.Bernal B, Altman N. Evidence-based medicine: Neuroimaging of seizures. Neuroimaging Clin N Am. 2003;13:211–24. doi: 10.1016/s1052-5149(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie M. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo S. Evaluation of drug-resistant epilepsy. Rev Neurol. 2000;30:881–9. [PubMed] [Google Scholar]
- 6.King MA, Newton MR, Jackson GD, Fitt GJ, Mitchell LA, Silvapulle MJ, et al. Epileptology of the first-seizure presentation: A clinical, electro- encephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998;352:1007–11. doi: 10.1016/S0140-6736(98)03543-0. [DOI] [PubMed] [Google Scholar]
- 7.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–71. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corsellis JA. The neuropathology of temporal lobe epilepsy. Mod Trends Neurol. 1970;5:254–70. [PubMed] [Google Scholar]
- 9.Jensen I, Klinken L. Temporal lobe epilepsy and neuropathology. Histological findings in resected temporal lobes correlated to surgical results and clinical aspects. Acta Neurol Scand. 1976;54:391–414. doi: 10.1111/j.1600-0404.1976.tb04372.x. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol. 1993;52:433–43. doi: 10.1097/00005072-199309000-00001. Cross Reference. [DOI] [PubMed] [Google Scholar]
- 11.Van Paesschen W, Duncan JS, Stevens JM, Connelly A. Etiology and early prognosis of newly diagnosed partial seizures in adults: A quantitative hippocampal MRI study. Neurology. 1997;49:753–7. doi: 10.1212/wnl.49.3.753. [DOI] [PubMed] [Google Scholar]
- 12.Van Paesschen W, Revesz T, Duncan JS, King MD, Connelly A. Quantitative neuropathology and quantitative magnetic resonance imaging of the hippocampus in temporal lobe epilepsy. Ann Neurol. 1997;42:756–66. doi: 10.1002/ana.410420512. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–87. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tassi L, Colombo N, Garbelli R, Francione S, Lo Russo G, Mai R, et al. Focal cortical dysplasia: Neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125:1719–32. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- 15.Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- 16.Blumcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. The clinicopathologic spectrum of focal cortical dysplasias: A consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–74. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf HK, Muller MB, Spanle M, Zentner J, Schramm J, Wiestler OD. Ganglioglioma: A detailed histopathological and immunocytochemical analysis of 61 cases. Acta Neuropathol. 1994;88:166–73. doi: 10.1007/BF00294510. [DOI] [PubMed] [Google Scholar]
- 18.Prayson RA, Khajavi K, Comair YG. Cortical architectural abnormalities and MIB1 immunoreactivity in gangliogliomas: A study of 60 patients with intracranial tumors. J Neuropathol Exp Neurol. 1995;54:513–20. doi: 10.1097/00005072-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Friedland R, Bronen R. Magnetic resonance imaging of neoplastic, vascular, and indeterminate substrates. In: Cascino G, Jack CJ, editors. Neuroimaging in epilepsy: Principles and practice. Newton (MA): Butter-worth-Heinemann; 1996. pp. 29–50. [Google Scholar]
- 20.Wray CD, Blakely TM, Poliachik SL, Poliakov A, McDaniel SS, Novotny EJ, et al. Multimodality localization of the sensorimotor cortex in pediatric patients undergoing epilepsy surgery. J Neurosurg Pediatr. 2012;10:1–6. doi: 10.3171/2012.3.PEDS11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehericy S, Duffau H, Cornu P, Capelle L, Pidoux B, Carpentier A, et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: Comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg. 2000;92:589–98. doi: 10.3171/jns.2000.92.4.0589. [DOI] [PubMed] [Google Scholar]
- 22.Puce A, Constable RT, Luby ML, McCarthy G, Nobre AC, Spencer DD, et al. Functional magnetic resonance imaging of sensory and motor cortex: Comparison with electrophysiological localization. J Neurosurg. 1995;83:262–70. doi: 10.3171/jns.1995.83.2.0262. [DOI] [PubMed] [Google Scholar]
- 23.Hammeke TA, Yetkin FZ, Mueller WM, Morris GL, Haughton VM, Rao SM, et al. Functional magnetic resonance imaging of somatosensory stimulation. Neurosurgery. 1994;35:677–81. doi: 10.1227/00006123-199410000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Kim SG, Ashe J, Georgopoulos AP, Merkle H, Ellermann JM, Menon RS, et al. Functional imaging of human motor cortex at high magnetic field. J Neurophysiol. 1993;69:297–302. doi: 10.1152/jn.1993.69.1.297. [DOI] [PubMed] [Google Scholar]
- 25.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin YZ, Jesmanowicz A, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–8. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- 27.Binder JR, Rao SM, Hammeke TA, Morris JL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology. 1996;46:978–84. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 28.Hertz-Pannier L, Galliard WD, Mott SH, Cuenoid CA, Bookheimer SY, Weinstein S, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: A preliminary study. Neurology. 1997;48:1003–12. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- 29.Yuan W, Szaflarski JP, Schmithorst VJ, Schapiro M, Byars AW, Strawsburg RH, et al. FRMI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47:593–600. doi: 10.1111/j.1528-1167.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pujol J, Deus J, Losilla JM, Capdevilla A. Cerebral lateralization of language in normal and left handed people studied by functional MRI. Neurology. 1999;52:1038–43. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- 31.Szaflarski JP, Binder JR, Possing ET, McKeirnan KA, Ward BD, Hammeke TA. Language lateralization in left handed and ambidextrous people. fMRI data. Neurology. 2002;59:238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 32.Desmond JE, Sum JM, Wagner AD, Demb JB, Shear PK, Glover GH, et al. Functional MRI assessment of language lateralization in Wada-tested patients. Brain. 1995;118:1411–9. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- 33.Benbadis SR, Binder JR, Swanson SJ, Fischer M, Hammeke TA, Morris GL, et al. Is speech arrest during Wada testing a valid method for determining hemispheric representation of language? Brain Lang. 1998;65:441–6. doi: 10.1006/brln.1998.2018. [DOI] [PubMed] [Google Scholar]
- 34.Yetkin FZ, Swanson S, Fischer M, Akansel G, Morris G, Mueller W, et al. Functional MR of frontal lobe activation: Comparison with Wada language results. Am J Neuroradiol. 1998;19:1095–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Worthington C, Vincent DJ, Bryant AE, Roberts DR, Vera CL, Ross DA, et al. Comparison of functional magnetic resonance imaging for language localization and intracarotid speech amytal testing in presurgical evaluation for intractable epilepsy. Preliminary results. Stereotact Funct Neurosurg. 1997;69:197–201. doi: 10.1159/000099874. [DOI] [PubMed] [Google Scholar]
- 36.Moddel G, Linewaever T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50:1505–16. doi: 10.1111/j.1528-1167.2008.02000.x. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann NY Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilke M, Pieper T, Lindner K, Dushe T, Holthausen H, Krageloh-Mann I. Why one task is not enough: Functional MRI for atypical language organization in two children. Eur J Paediatr Neurol. 2010;14:474–8. doi: 10.1016/j.ejpn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, et al. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–51. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- 40.Davies K, Bell B, Bush A, Hermann B, Dohan FC, Japp AS. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39:407–19. doi: 10.1111/j.1528-1157.1998.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 41.Hamberger MJ, Seidel WT, Goodman RR, Williams A, Perrine J, Devinsky O, et al. Evidence of cortical reorganization in patients with hippocampal sclerosis. Brain. 2007;130:2942–50. doi: 10.1093/brain/awm187. [DOI] [PubMed] [Google Scholar]
- 42.Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev Sci. 2008;11:237–52. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- 43.Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- 44.Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15:2575–8. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber B, Kugler F, Elger CE. Comparison of implicit memory encoding paradigms for the activation of mediotemporal structures. Epilepsy Behav. 2007;10:442–8. doi: 10.1016/j.yebeh.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, et al. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124:1841–54. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- 47.Powell HW, Koepp MJ, Symms MS. Material-specific lateralization of memory encoding in the medial temporal lobe: Blocked versus event-related design. Neuroimage. 2005;27:231–9. doi: 10.1016/j.neuroimage.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 48.Detre JA, Maccotta L, King D, Alsop DC, Glosser D, D’Esposito M, et al. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998;50:926–32. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- 49.Dupont S, Duron E, Samson S. Functional MR imaging or wada test: Which is the better predictor of individual postoperative memory outcome. Radiology. 2010;255:128–34. doi: 10.1148/radiol.09091079. [DOI] [PubMed] [Google Scholar]


