Abstract
Background:
Functional magnetic resonance imaging (fMRI), a non-invasive technique with high spatial resolution and blood oxygen level dependent (BOLD) contrast, has been applied to localize and map cognitive functions in the clinical condition of chronic intractable epilepsy.
Purpose:
fMRI was used to map the language and memory network in patients of chronic intractable epilepsy pre- and post-surgery.
Materials and Methods:
After obtaining approval from the institutional ethics committee, six patients with intractable epilepsy with an equal number of age-matched controls were recruited in the study. A 1.5 T MR scanner with 12-channel head coil, integrated with audio-visual fMRI accessories was used. Echo planar imaging sequence was used for BOLD studies. There were two sessions in TLE (pre- and post-surgery).
Results:
In TLE patients, BOLD activation increased post-surgery in comparison of pre-surgery in inferior frontal gyrus (IFG), middle frontal gyrus (MFG), and superior temporal gyrus (STG), during semantic lexical, judgment, comprehension, and semantic memory tasks.
Conclusion:
Functional MRI is useful to study the basic concepts related to language and memory lateralization in TLE and guide surgeons for preservation of important brain areas during ATLR. This will help in understanding future directions for the diagnosis and treatment of such disease.
Keywords: Cognition, functional magnetic resonance imaging, intractable epilepsy
Introduction
Epilepsy is a widely prevalent neurological disorder affecting an estimated 50 million people worldwide.[1] Estimates on the incidence of epilepsy also suggest that at least 5000 persons with epilepsy would go on to have medical intractability each year.[2] In a developing country like India, the burden of epilepsy occurs in 1-5% of the population and about 20-30% of these are medically refractory.[3] Intractable epilepsy occurs with an approximate incidence of 6/100,000/year and with a prevalence of 2-3 per 1000 (>1 episode/year). A comprehensive meta-analysis of all published and unpublished studies on the Indian prevalence of epilepsy reveals an overall prevalence rate of epilepsy in India at 5.59/1000.[3] Prevalence rates of epilepsy based on zone- and community-based surveys in India have revealed (per 1000 population) an incidence of 2.5 in Kashmir, 3.6 among the Parsis of Bombay, 4.4 in Bangalore, and 4.9 in Kerala.[4] Epilepsy is a non-communicable neurological condition characterized by recurrent seizures. Seizures occur due to erratic neuronal electrical activity (which may remain localized or spread) and lead to involuntary movements of the whole body (generalized) or some part of the body (partial).
However, hippocampal sclerosis (HS) is a unique pathological condition characterized by specific neuropathological features and is the most common cause of temporal lobe epilepsy (TLE). The minimal criteria for the diagnosis of HS are summarized in the International League against Epilepsy (ILAE) commission report and include selective neuronal cell loss and gliosis in CA1 and endfolium.[5] Other alterations in HS include dispersion of the granule cell layer of the dentate gyrus (DG), neurogenesis of granule cells, and synaptic reorganization of the mossy fibers (mossy fiber sprouting).[6] Intractable TLE is treated by a variety of surgical techniques including tailored anterior temporal lobectomy guided by intraoperative electrocorticography (ECoG), standardized anterior temporal lobectomy with or without ECoG, and amygdalohippocampectomy with sparing of the lateral temporal neocortex.[7] All these approaches provide for an extensive resection of the mesial temporal lobe structures, particularly the hippocampus. For amygdalohippocampectomies, the extent of hippocampal resection varies among surgeons, most of whom believe that maximizing the extent of hippocampal removal will improve seizure outcome. Although epileptic seizures can be extratemporal or outside the temporal lobe, originating in the frontal, parietal, or occipital lobes, they may still affect some of the language and memory components, but the extent of damage to the cognitive capability differs from patient to patient.[8]
The most widely studied clinical application of functional magnetic resonance imaging (fMRI) in intractable epilepsy has been in pre-surgical language and memory lateralization, providing results comparable to intracarotid amobarbital testing.[9] fMRI has surpassed the status of being a novice and has come a long way since it was first demonstrated by Seigi Ogawa.[10] Also, a comparatively competent spatial resolution has warranted its longevity as a globally accepted investigative tool. fMRI enables efficient quantification of neural activity of different regions of the brain. Blood-oxygenation level dependent (BOLD) imaging uses the endogenous MRI contrast, based upon the paramagnetic property of de-oxyhemoglobin, wherein the activity resultant change in oxygen utilization acts as the source of BOLD contrast that is detected by fMRI.[11] During language task, the nondominant hemisphere could play a dominant role in language function in patients with epilepsy, though it depends on localization of the lesion, than it does in neurologically intact subjects.[12] However, epilepsy patients exhibited left hemispheric dominance for language, though significantly lower than that in healthy subjects, indicating atypical lateralization of language.[13]
We explore the possibility that neural changes as observed with functional imaging in TLE may contribute toward refining the diagnostic or surgical management. The objective of the discussion is to corroborate the findings of the study performed by us and to understand the role of fMRI in evaluating the brain function in pre- and post-surgery chronic intractable epilepsy.
Materials and Methods
Subjects’ recruitment details
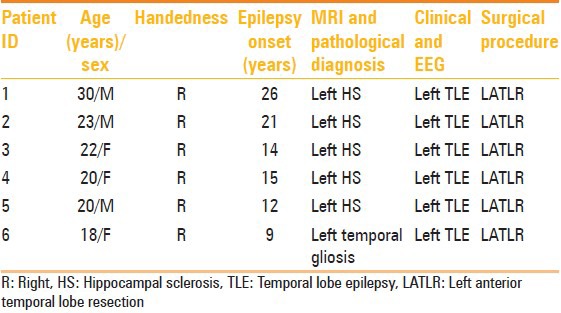
The study was approved by the institutional ethics committee. Six consecutive adult patients (3 males and 3 females, n = 6, mean age: ±22.16 years, SD: 4.2) with medically intractable epilepsy, who could read, understand, and comprehend Hindi and were cleared for epilepsy surgery following Epilepsy Surgery Case Conference (unit 1 Neurology) were recruited from our neurology clinics, after obtaining their informed consent. Five out of six patients had left mesial temporal sclerosis (MTS; demonstrated by MRI) and the other one had left temporal gliosis lesion [Demographic data, Table 1]. Six healthy controls (5 males and 1 female, n = 6; mean age: ±31.4 years, SD: 3.80) with no history of neurological disorder and who were relatives of the patients were recruited after getting their informed consent. The Edinburgh Handedness Inventory[14] was used to assess the dominance in hand activities. All patients and controls were right handed, and standard diagnostic inclusion and exclusion criteria were followed. Patients with metallic implant or any other contraindications for MRI and suffering from any disease other than epilepsy were excluded from the study. All subjects underwent a comprehensive epilepsy presurgery work-up, including video-EEG (VEEG), MRI, single-photon emission computed tomography (SPECT), positron emission tomography (PET), and neuropsychological and psychiatric evaluations. The location of the epileptogenic zone was determined by VEEG and SPECT.
Table 1.
Demographic and clinical details of temporal lobe epilepsy patients
Paradigm and MR acquisition
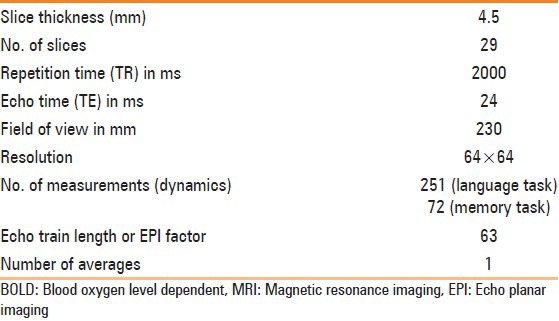
The experiment was carried out using 1.5 T MR scanner (Magnetom Avanto; M/s. Siemens, Erlangen, Germany) with a 12-channel head coil. Three-dimensional magnetization prepared rapid acquisition gradient echo (3D MPRAGE) was used to rule out anatomical deficits and for overlaying BOLD results. Single-shot echo planar imaging (EPI) sequence was used for the BOLD studies, with the parameters listed in Table 2.
Table 2.
MRI parameters used during BOLD image acquisition in the study
The language task consisted of lexical reading, semantic judgment, semantic reading, and comprehension syntactic–semantic components, and the visual cues were presented using MR-compatible binocular goggles (Nordic Neuro Lab, Bergen, Norway). Subjects were instructed to read simple semantic words and sentences during the task. For semantic judgment task, they were instructed to choose the correct synonym or antonym of the noun among the four options provided, using a four-button response pad (Lumina LP 400; Cedrus Inc., San Pedro, CA, USA). During comprehension syntactic–semantic task, subjects were requested to correct the syntax of sentences and read out. Each patient underwent fMRI scan twice, on recruitment (pre-surgery) and 6 months post-surgery. Before entering into the MRI, the subjects were instructed and trained to execute the tasks by using visual cues. For the semantic memory task, a standardized story in Hindi[15] using SuperLab presentation software (Cedrus Inc., San Pedro, CA, USA) was provided to the subjects using MR-compatible auditory interface system (Nordic Neuro Lab, Bergen, Norway). After the story, patients were instructed to verbally answer the questions corresponding to the semantic memory task during fMRI scan (recorded using MR-compatible microphone).
Statistical analysis
The fMRI data were analyzed by Statistical Parametric Mapping (SPM) software (Wellcome trust, London, UK; version SPM 8) using Matlab (Mathworks Inc., Natick, MA, USA). BOLD whole-brain series for each subject was corrected for motion during the study to the first obtained EPI volume using 3D rigid body transformation. Paired t-test was used for group pre- and post-surgery data analysis (P < 0.005, cluster threshold 5, Z score >5, one voxel is 2 × 2 × 2 mm3). One-way analysis of variance (ANOVA) test was performed for the control group. Brain activation pattern was overlaid onto the whole brain T1-weighted normalized MPRAGE images. Coordinates of local maxima of threshold (P value uncorrected: 0.005) SPM maps were converted from MNI frame to standard Talairach coordinates using an automated procedure of assigning an anatomic label to these coordinates by searching for the label associated with the nearest gray matter coordinate (Research Imaging Institute, University of Texas, San Antonio, Texas, USA, www.brainmap.org).
Results
BOLD activation during language and memory task
Patients with left TLE (n = 6; mean age: ±22.16 years, SD: 4.2) and healthy controls (n = 6; mean age: ±31.4 years, SD: 3.80) were recruited for fMRI language and memory task. All the six subjects complied with the instructions, and language and memory tasks were carried out pre- and post-surgery in all of them. During lexical reading task [Figure 1A-C], BOLD activation was observed in bilateral superior temporal gyrus (STG) and left middle temporal gyrus (MTG) in the controls [Figure 1A] and in post-surgery patients compared to the pre-surgery patient group. During semantic judgment task, bilateral precentral gyrus and left MTG were activated in post-surgery patients in comparison to the pre-surgery group [Figure 1D-F]. In controls, the task invoked activation in the right hemispheric inferior frontal gyrus (IFG), middle frontal gyrus (MFG), superior frontal gyrus (SFG), and left hemispheric supramarginal gyrus. During semantic reading task [Figure 1G-I], left cerebral post central gyrus, fusiform gyrus, and MTG brain areas were activated post-surgery in comparison to pre-surgery. In the control group, BOLD activation was observed in the left hemispheric MTG, post central gyrus, and bilateral STG. In semantic syntax task [Figure 1J-L], BOLD activation was invoked in bilateral precentral gyrus, left cerebral STG, MTG, and lingual gyrus in the post-surgery patients, whereas the control group showed activation in left hemispheric SFG, precuneus, IFG, MTG, and STG. During memory task [Figure 2], activation was observed in left MFG, right cerebral STG, bilateral insula, and right cerebral parahippocampal gyrus in post-surgery patient group in comparison to pre-surgery group. In controls, important BOLD activation was observed in bilateral MFG, right STG, right hemispheric MFG, and bilateral insula [Figure 2A].
Figure 1(A-L).
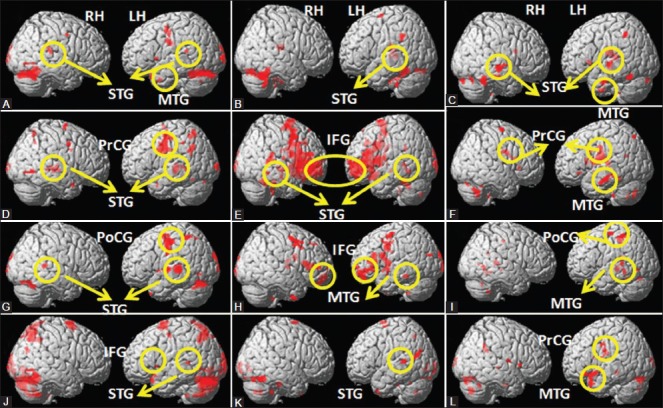
The BOLD activation on group analysis in controls (A, D, G, J), pre-surgery TLE group (B, E, H, K), and post-surgery TLE group (C, F, I, L) during (i) semantic lexical reading (A,B,C); (ii) semantic judgment task (D,E,F); (iii) semantic reading (simple sentence) task (G, H, I); and (iv) semantic syntactic reading (jumbled sentences) task (J, K, L). (LH: Left hemisphere, RH: Right hemisphere, STG: Superior temporal gyrus, MTG: Middle temporal gyrus, IFG: Inferior frontal gyrus, PoCG: Post central gyrus, PrCG: Precentral gyrus)
Figure 2(A-C).
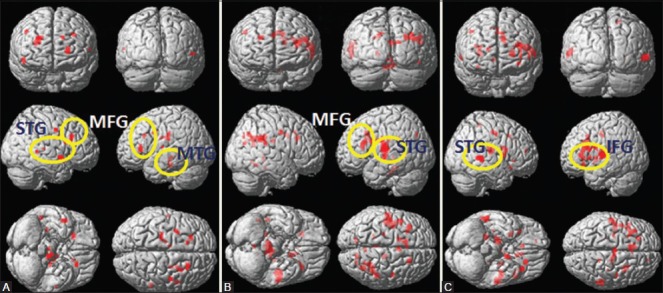
BOLD activation during verbal memory task in (A) healthy control (B) pre-surgery TLE, and (C) post-surgery TLE (STG: Superior temporal gyrus, MTG: Middle temporal gyrus, IFG: Inferior frontal gyrus, MFG: Middle frontal gyrus)
Discussion
Neuroimaging methods, such as fMRI, have the ability to map, identify, and assist in preservation of important brain areas involved in corresponding task during presurgical evaluation. Functional recovery is attributed to reorganization processes in the damaged brain. When damage to a functional system is partial, the recovery process can be self-regulated by within-system reorganization.[16] The neurons would find a way to communicate with each other even after any injury; they do this to compensate for the lost function and it can be very well ascertained using fMRI. The cortical reorganization in epileptics can efficiently be mapped with fMRI, further strengthening the status of fMRI as a potent tool in neuroscience research. In left temporal lobe epilepsy (LTLE) patient group, absence of left temporal lobe activation suggests a deficit in these patients during simple semantic reading task, and may be the result of left temporal lobe abnormality due to intractable seizure discharges and pathological disturbances. However, recruitment of left STG during simple semantic task after anterior temporal lobe resection (ATLR) suggests a reorganization of language areas in LTLE patients [Figure 1]. Increasing language complexity invoked activation in the left temporal cortex, indicating that Wernicke's area along with left hemispheric post central gyrus, superior parietal lobule, and insula, right hemispheric fusiform gyrus, and middle frontal gyrus may still be intact for higher language functions after ATLR [Table 3].[17] This may suggest that after ATLR, reorganization may involve multiple components of language function.[18] However, similar studies have reported involvement of STG and the frontal operculum brain areas interlinked with syntactic processing and subserved by the ventral pathway, whereas complex grammatical structures involve the Wernicke's and Broca's areas, that are connected via the dorsal pathway.[19,20] We observed activation in IFG during all tasks suggesting its involvement in the search for and retrieval of semantic information. Closed activation of this area might thus indicate that weak performing patients have a greater demand for such search-and-retrieval operations.[21,22] These findings suggest that epilepsy affects the cortical language networks in TLE patients. During comprehension syntactic task, BOLD-activated clusters were found in lesser amount in post-surgery LTLE group in comparison to the control group. In semantic judgment comprehension, processing of language is involved and semantic reading task engages motor speech area. In the present study, activation was mainly observed in the IFG and temporal lobes at the level of superior and middle temporal gyri, a region identified as Wernicke's area seems reconnected strongly in the post-surgery follow-up cases. It suggests the processing of neurons in IFG and STG were not precisely interlinked at cellular level due to dysfunction of seizure mechanism in presurgical session which is in agreement with previous functional neuroimaging studies.[23,24,25,26] BOLD activation in MTG and STG is involved in integration of semantic and syntactic information and is particularly responsive to meaningful sentences during semantic reading task.[27,28,22] Patients with left hippocampal sclerosis usually showed atypical language lateralization, which may suggest hippocampal involvement in semantic and lexical information during targeted word retrieval.[29] LTLE patients showed decreased BOLD activation in the left hemisphere during language task (naming, verb generation, and fluency) compared to healthy controls, similar to earlier reports.[22,30]
Table 3.
Comparison of BOLD activation (number of voxels) in temporal lobe epilepsy patients post-surgery with respect to pre-surgery and controls for different tasks
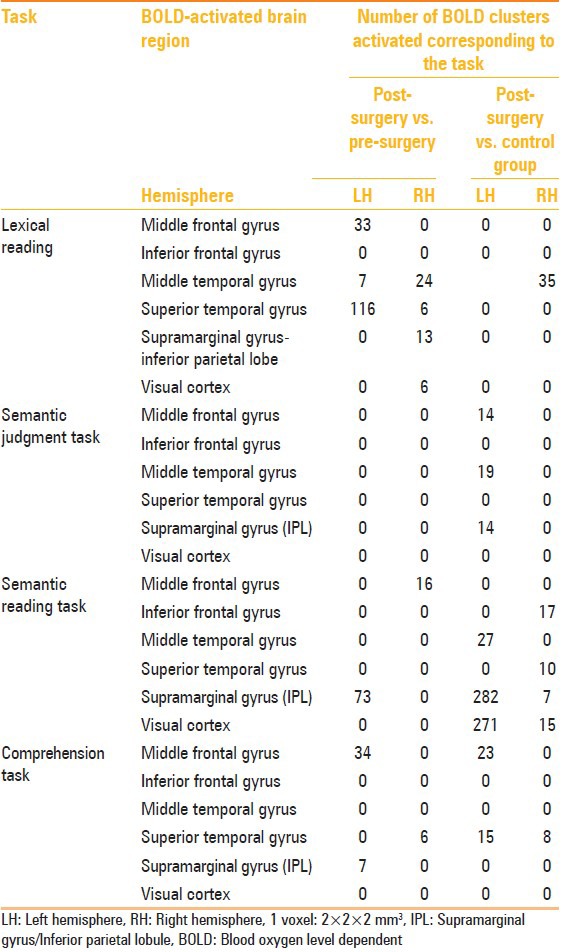
In memory task, deactivation of left insular cortex in the LTLE group (with respect to the control group) suggests that cognitive processing is affected in LTLE patients. Medial temporal abnormality may affect semantic memory network in LTLE subjects. The BOLD activation in bilateral fusiform gyrus suggests extra effort in decision making during the semantic memory task in LTLE patients group [Figure 2]. Poorer performance in verbal memory tasks has been associated with left insular damage, suggesting that the insula may be part of a functional network that mediates verbal memory, switching between networks, conscious awareness, etc.[26,31]
These observations indicate the undoubted contextual relevance of fMRI in assessing the extent of reorganization, restoration, and regeneration of neural functions in chronic intractable epilepsy.[30,31] These findings support our hypothesis that the cortical organization of language and memory processing is affected in TLE subjects in comparison to healthy brain, and may be restored after ATLR.
Combining two or more modalities like EEG/magnetoencephalography (MEG) or EEG/fMRI signals would give a more comprehensive diagnostic and scientific knowledge about epilepsy and cognitive function with an understanding of the basic structure and dynamics of neuronal networks.[31] Though we had acquired SPECT, PET, and VEEG data, we could not correlate the same in this study. The subjects were unwilling to participate in the study post-surgery, limiting the sample size (to six), since relevance of the fMRI study is yet to be established.
Conclusion
The fMRI is an important neuroscience tool capable of investigating brain functions and helps in understanding the basic concepts related to brain remodeling and reorganization in chronic intractable epilepsy patients. Various fMRI language tasks (lexical reading, semantic decision, semantic–syntactic processing) can cover important language components and guide surgeons for preservation of important functional brain areas during ATLR.
Footnotes
Source of Support: DST-CSI, New Delhi (TLE study)
Conflict of Interest: None declared.
References
- 1.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7:154–64. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen CC, Chen LS, Yen MF, Chen HH, Liou HH. Geographic variation in the age- and gender-specific prevalence and incidence of epilepsy: Analysis of Taiwanese National Health Insurance-based data. Epilepsia. 2012;53:283–90. doi: 10.1111/j.1528-1167.2011.03332.x. [DOI] [PubMed] [Google Scholar]
- 3.Sridharan R, Murthy BN. Prevalence and pattern of epilepsy in India. Epilepsia. 1999;40:631–6. doi: 10.1111/j.1528-1157.1999.tb05566.x. [DOI] [PubMed] [Google Scholar]
- 4.Koul R, Razdan S, Mott A. Prevalence and patterns of epilepsy in rural Kashmir in India. Epilepsia. 1988;29:116–22. doi: 10.1111/j.1528-1157.1988.tb04406.x. [DOI] [PubMed] [Google Scholar]
- 5.Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 6.Thom M. Recent advances in the neuropathology of focal lesions in epilepsy. Expert Rev Neurother. 2004;4:973–84. doi: 10.1586/14737175.4.6.973. [DOI] [PubMed] [Google Scholar]
- 7.Chandra SP, Bal CS, Sarkar C, Tripathi M. Intraoperative coregistration of magnetic resonance imaging, positron emission tomography, and electrocorticographic data for neocortical lesional epilepsies may improve the localization of the epileptogenic focus: A pilot study. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.02.057. pii: S1878-8750(13):00343.4. [DOI] [PubMed] [Google Scholar]
- 8.Lippincott CE. An investigation of extra-temporal deficits in temporal lobe epilepsy. Ph.D Thesis. 2010 [Google Scholar]
- 9.Detre A. fMRI Applications in Epilepsy. Epilepsia. 2004;45:26–31. doi: 10.1111/j.0013-9580.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith K. fMRI 2.0. Nature. 2012;484:24–6. doi: 10.1038/484024a. [DOI] [PubMed] [Google Scholar]
- 11.Herholz K, Heiss W. Functional imaging correlates of recovery after stroke in humans. J Cereb Blood Flow Metab. 2000;20:1619–31. doi: 10.1097/00004647-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Yetkin FZ, Swanson S, Fischer M, Akansel G, Morris G, Mueller W, et al. Functional MR of Frontal Lobe Activation: Comparison with Wada Language Results. AJNR Am J Neuroradiol. 1998;19:1095–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Cousin E, Baciu M, Pichat C, Kahane P, Le Bas JF. Functional MRI evidence for language plasticity in adult epileptic patients: Preliminary results. Neuropsychiatr Dis Treat. 2008;4:235–46. doi: 10.2147/ndt.s2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 15.Mukundan CR, Rao SL, Jain VK, Jayakumar PN, Shailaja K. Neuropsychological assessment: a cross validation study with neuroradiological/operative findings in patients with cerebral hemisphere lesions. Pharmacopsychoecologia. 1991;4:33–9. [Google Scholar]
- 16.Feydy A, Carlier R, Brami AR, Bussel B, Cazalis F, Pierot L, et al. Longitudinal study of motor recovery after stroke recruitment and focusing of brain activation. Stroke. 2002;33:1610–7. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 17.Wong SW, Jong L, Bandur D, Bihari F, Yen YF, Takahashi AM, et al. Cortical reorganization following anterior temporal lobectomy in patients with temporal lobe epilepsy. Neurology. 2009;73:518–25. doi: 10.1212/WNL.0b013e3181b2a48e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JH, Lee JM, Kang E, Kim JS, Song IC, Chung CK. Functional reorganization associated with semantic language processing in temporal lobe epilepsy patients after anterior temporal lobectomy: A longitudinal functional magnetic resonance image study. J Korean Neurosurg Soc. 2010;47:17–25. doi: 10.3340/jkns.2010.47.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:46. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Weber B, Wellmer J, Schür S, Dinkelacker V, Ruhlmann J, Mormann F, et al. Presurgical language fMRI in patients with drug-resistant epilepsy: Effects of task performance. Epilepsia. 2006;47:880–6. doi: 10.1111/j.1528-1167.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 22.Rosazza C, Ghielmetti F, Minati L, Vitali P, Giovagnoli AR, Deleo F, et al. Preoperative language lateralization in temporal lobe epilepsy (TLE) predicts peri-ictal, pre- and post-operative language performance: An fMRI study. Neuroimage Clin. 2013;3:73–83. doi: 10.1016/j.nicl.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–51. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Oh YM, Koh EJ. Language lateralization in patients with temporal lobe epilepsy: A comparison between volumetric analysis and the Wada test. J Korean Neurosurg Soc. 2009;45:329–35. doi: 10.3340/jkns.2009.45.6.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benke T, Visa P, Willmes K. Language lateralization in TLE: A comparison between FMRI and the Wada test. Epilepsia. 2006;47:1308–19. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 26.Appel S, Duke ES, Martinez AR, Khan OI, Dustin IM, Reeves-Tyer P, et al. Cerebral blood flow and fMRI BOLD auditory language activation in temporal lobe epilepsy. Epilepsia. 2012;53:631–8. doi: 10.1111/j.1528-1167.2012.03403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–49. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manes F, Springer J, Jorge R, Robinson RG. Verbal memory impairment after left insular cortex infarction. J Neurol Neurosurg Psychiatry. 1999;67:532–4. doi: 10.1136/jnnp.67.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haberg A, Unsgard G. Preoperative blood oxygen level dependent functional magnetic resonance imaging in patients with primary brain tumors: Clinical application and outcome. Neurosurgery. 2004;54:902–14. doi: 10.1227/01.neu.0000114510.05922.f8. [DOI] [PubMed] [Google Scholar]
- 30.Wong SW, Jong L, Bandur D, Bihari F, Yen YF, Takahashi AM, et al. Cortical reorganization following anterior temporal lobectomy in patients with temporal lobe epilepsy. Neurology. 2009;73:518–25. doi: 10.1212/WNL.0b013e3181b2a48e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefan H, Lopes da Silva FH. Epileptic neuronal networks: methods of identification and clinical relevance. Front Neurol. 2013;4:8. doi: 10.3389/fneur.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]


