Abstract
Background
Transcatheter aortic valve implantation is an effective alternative to surgical treatment of severe aortic stenosis in patients who are inoperable or at high surgical risk.
Objectives
To report the immediate and follow-up clinical and echocardiographic results of the initial experience of transcatheter aortic valve implantation.
Methods
From 2009 June to 2013 February, 112 patients underwent transcatheter aortic valve implantation.
Results
Mean age was 82.5 ± 6.5 years, and the logistic EuroSCORE was 23.6 ± 13.5. Procedural success was 84%. After the intervention, a reduction in the mean systolic gradient was observed (pre: 54.7 ± 15.3 vs. post: 11.7 ± 4.0 mmHg; p < 0.01). Cerebrovascular accidents occurred in 3.6%, vascular complications in 19% and permanent pacemaker was required by 13% of the patients. Thirty-day mortality and at follow-up of 16 ± 11 months was 14% and 8.9% respectively. The presence of chronic obstructive pulmonary disease was the only predictor of mortality at 30 days and at follow-up. During follow up, aortic valve area and mean systolic gradient did not change significantly.
Conclusions
Transcatheter aortic valve implantation is an effective and safe procedure for the treatment of aortic stenosis in high-surgical risk or inoperable patients. The presence of chronic obstructive pulmonary disease was the only independent predictor of mortality identified both in the first month post-intervention and at follow-up.
Keywords: Aortic valve stenosis/surgery, Heart valve prosthesis, Aged, Risk factors
Introduction
Aortic stenosis is the most common acquired heart valve disease in industrialized countries1, affecting, in its severe form, 2% of the population older than 65 years2. Data from the Brazilian Institute of Geography and Statistics (IBGE) indicate that aging of the Brazilian population in the next decade will increase from 7.4% of the population older than 65 years to 13.3% in 2030. Surgical aortic valve replacement is the treatment of choice for patients with severe symptomatic aortic stenosis, not only reducing symptoms, but also prolonging survival3.
However, as it is a common condition among elderly patients that sometimes accompanies other important comorbidities, frequently surgery is not suggested as the therapeutic option, due to the high inherent surgical risks among these individuals1. In recent years, the implantation of transcatheter aortic prosthesis has emerged as the treatment of choice in patients with symptomatic severe aortic stenosis with surgical contraindication4 and as an effective alternative in patients with high surgical risk5.
However, the relatively high cost of this new technology makes its incorporation into the routine of most hospitals in Brazil to be slow, despite its important benefits in the short and middle-term6-9.
The present study aimed at showing immediate and middle-term clinical and echocardiographic outcomes of the initial experience of transcatheter implantation of an aortic prosthesis in two institutions in the State of São Paulo. The primary objective of this analysis was to determine the rates of immediate procedural success, as well as establish the incidence of periprocedural mortality and cerebrovascular accident (CVA) in the middle-term. Second, the occurrence of vascular and renal complications, as well as the durability and hemodynamics of the valve prosthesis was evaluated in middle-term follow-up.
Methods
Subjects and procedure
Between June 2009 and February 2013, a total of 182 symptomatic patients with severe aortic stenosis considered to be high surgical risk, was referred for evaluation.
The clinical pre-procedure evaluation of this population was performed through the analysis of comorbidities, degree of patient frailty, calculation of risk scores (EuroSCORE and STS) and laboratory exams. The assessment of aortic stenosis severity, left ventricular function, total aorta and coronary arteries was performed by computed angiotomography of the heart, aorta and iliac arteries, transthoracic and / or transesophageal echocardiography and cardiac catheterization. According to the results of these tests and after discussion with the local heart team the most appropriate treatment was chosen, as well as the access route, type and diameter of the prosthesis.
Three types of prostheses were used: CoreValve® (Medtronic, by transfemoral, transubclavian or transaortic access), SAPIEN XT® (Edwards Lifesciences, by transfemoral or transapical access) and Acurate TF® (Symetis, by transfemoral access). The choice of prosthesis used in the procedure involved clinical and anatomic criteria, such as diameter, calcification and tortuosity of the iliac-femoral arteries, as well as prosthesis availability at the time of the procedure.
Definitions
The surgical risk and possible postoperative complications of the patients were defined by calculating the STS10 score and logistic EuroSCORE11.
All study complications and outcomes followed the criteria established by the Valve Academic Research Consortium-2 (VARC-2)12.
The procedure was considered successful when a single prosthetic device was released properly and the end result, assessed by transesophageal echocardiography, showed absence of prosthesis-patient mismatch, mean aortic transvalvular gradient < 20 mmHg or peak velocity < 3m/s without aortic regurgitation ≥ moderate.
Procedural safety was also analyzed, according to mortality from all causes and complications within 30 days after the procedure.
CVA was defined as focal or global neurological deficit lasting > 24 hours or presence of new area of cerebral infarction by imaging techniques, regardless of symptom duration.
Hemorrhagic complications were divided into:
Life-threatening bleeding: fatal, or evident bleeding in vital organ, or that could lead to hypovolemic shock or severe hypotension that required vasopressor agent or surgery; or evident bleeding with decrease in hemoglobin ≥ 5 g/dL or need for transfusion of four or more units of packed red blood cells;
major bleeding: evident bleeding with decrease in hemoglobin ≥ 3 g/dL or need for transfusion of two or three units of packed red blood cells, or need for hospitalization or surgery not meeting the criteria for life-threatening bleeding;
minor bleeding: any bleeding worthy mentioning (e.g., hematoma at the puncture site).
All vascular complications were analyzed and defined according to criteria established by VARC-212 and subdivided into:
major vascular complications: aortic dissection; aortic rupture; left ventricular perforation; apical pseudoaneurysm or vascular injury related to the puncture site leading to death; major or life-threatening bleeding; visceral ischemia or neurologic impairment; noncerebral distal embolization requiring surgery; need for surgery or percutaneous intervention leading to death; major bleeding, visceral ischemia, or neurologic impairment; or any documented ipsilateral ischemia;
minor vascular complications: vascular injury related to the puncture site not meeting the major criteria; noncerebral distal embolization treated with embolectomy or thrombectomy; need for surgical or percutaneous intervention not meeting the major criteria and the percutaneous closure system failure.
Acute kidney injury was defined according to the AKIN13 system, and was evaluated on the seventh day post-implant. The AKIN classification stratifies renal function in three stages, according to serum creatinine and urine volume measurement:
stage 1: increase of 0.3 mg/dL or increase of 150 to 200% of the baseline serum creatinine level or urine output < 0.5 mL/kg/h for 6 hours;
stage 2: increase > 200 to 300% of baseline serum creatinine or urine output < 0.5 mL/kg/h for > 12 hours;
stage 3: increase > 300% of baseline serum creatinine or serum creatinine ≥ 4.0 mg/dL with acute increase of at least 0.5 mg/dL or one diuresis < 0.3 mL/kg/h for 24 hours or anuria for 12 hours.
The clinical data and the additional information during follow-up assessments were collected during medical visits or through phone calls, 30 days after the procedure and every 6 months thereafter.
Statistical Analysis
Continuous variables are shown as mean ± standard deviation and categorical variables as frequencies (number and percentage), having been compared using chi-square test, Student's t test, ANOVA or nonparametric Mann-Whitney test, depending on the distribution. The logistic regression model was used to determine independent predictors of death within 30 days post-implantation, and a Cox regression model was used to determine independent predictors of death during follow-up. The Kaplan-Meier survival curve was used to estimate the rate of event-free survival of this population.
Data were analyzed using the Statistical Package for Social Sciences (SPSS) release 16 (Chicago, Illinois). An alpha error of 5% was established and p values ≤ 0.05 were considered significant.
Results
Study population
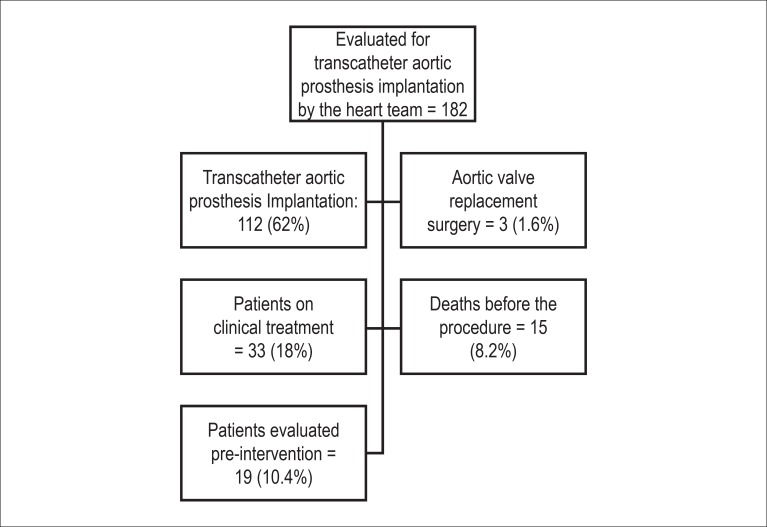
Between June 2009 and February 2013, a total of 182 patients with severe symptomatic aortic stenosis were assessed for transcatheter aortic prosthesis implantation. Of these, 112 (62%) patients underwent transcatheter implantation of an aortic prosthesis. Of the remainder, 33 (18%) patients were maintained on medical treatment, 3 (1.6%) were referred for surgical aortic valve replacement, 15 (8.2%) died before the procedure and 19 (10.4%) patients are undergoing pre intervention assessment (Figure 1).
Figure 1.
Study flow chart showing the total number of patients evaluated for transcatheter implantation of an aortic prosthesis by the heart teams of Instituto de Cardiologia Dante Pazzanese and Hospital do Coração.
Clinical and echocardiographic baseline characteristics of 112 patients included in this sample are described in Tables 1 and 2, respectively.
Table 1.
Baseline clinical characteristics
| Variable | Assessed population (112 patients) n (%) |
|---|---|
| Age, mean ± SD | 82.5 ± 6.5 |
| Female sex | 66 (58.9) |
| Diabetes mellitus | 39 (34.8) |
| Hypertension | 91 (81.3) |
| NYHA II Functional Class | 24 (21.4) |
| NYHA III-IV Functional Class | 88 (78.6) |
| Coronary artery disease | 63 (56.3) |
| Previous PCI | 19 (17.0) |
| Previous CABG | 24 (21.4) |
| POAD | 33 (29.5) |
| Carotid disease | 19 (17.0) |
| COPD | 14 (12.5) |
| CKD | 78 (69.6) |
| Pulmonary hypertension | 37 (33.0) |
| Logistic EuroSCORE, mean ± SD | 23.6 ± 13.5 |
SD: standard deviation; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; CABG: coronary-artery bypass graft; POAD: peripheral obstructive arterial disease; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease (clearance of creatinine < 60 mL/min).
Table 2.
Pre-procedural echocardiographic findings
| Variable | Assessed population (112 patients) |
|---|---|
| Left ventricular ejection fraction % | 57.1 ± 13.2 |
| Left Ventricular End-Diastolic Diameter, mm | 51.4 ± 7.1 |
| Left Ventricular End-Systolic Diameter, mm | 34.4 ± 9.6 |
| Left atrium, mm | 44.3 ± 5.3 |
| Septal diameter do, mm | 12.5 ± 1.5 |
| Posterior wall diameter, mm | 12.1 ± 1.5 |
| Maximum systolic gradient, mmHg | 89.0 ± 23.5 |
| Mean systolic gradient, mmHg | 54.7 ± 15.2 |
| Aortic valve area, mm2 | 0.66 ± 0.15 |
| Aortic annulus diameter, mm | 22.0 ± 3.3 |
| Pulmonary artery systolic pressure, mmHg | 49.8 ± 12.7 |
| Moderate /severe aortic regurgitation, n (%) | 11 (9.8) |
| Moderate/severe mitral regurgitation, n (%) | 19 (17) |
| Moderate/severe tricuspid regurgitation, n (%) | 14 (12.5) |
The mean age of patients was 82.5 ± 6.5 years and 66 (59%) were females. The mean logistic EuroSCORE was 23.6 ± 13.5. One third of patients (33%) had severe pulmonary hypertension, 33 (29%) patients had significant peripheral vascular disease, 24 (21%) had a history of previous heart surgery and 88 (79%) were in Chronic Heart Failure (CHF) functional class III-IV (NYHA).
The maximum transaortic gradient before the procedure was 88.0 ± 24.3 mmHg and the mean gradient was 54.7± 15.3 mmHg, whereas the mean aortic valve area was 0.66 ± 0.14 cm2 and the mean left ventricular ejection fraction was 57.1 ± 13.2%.
Procedure data
All procedures were performed under general anesthesia and guided by transesophageal echocardiography.
The femoral access was the predominant route used in 91% of patients. The transapical (6%), transaortic (2%) and subclavian (1%) access routes were also used.
The CoreValve® prosthesis was used in 76 patients (68%), whereas Sapien XT® was used in 21 (19%) and Acurate TF(tm) was used in 15 (13%).
Device success was attained in 94 patients (84%). Two patients died during the procedure (1.8%) and in five cases (4.5%), a second prosthesis had to be implanted due to inadequate positioning of the first one. Moderate paravalvular aortic regurgitation (PAR) was seen in 11 patients (9.8%).
There was a significant decrease in the mean systolic gradient (pre = 54.7 ± 15.3 mmHg vs. post = 11.7± 4.0 mmHg; p < 0.01) and gain of aortic valve area (pre = 0.6± 0.2 cm2 vs. post = 1.8 ± 0.3 cm2; p < 0.01) immediately after prosthesis implantation. Doppler echocardiography showed no severe PAR in any of the patients.
Acute complications (up to 30 days)
The main complications in the first 30 days post-implantation are shown in Table 3.
Table 3.
Complications occurring within the first 30 days after the procedure (VARC 2)
| Variable | Value n (%) |
|---|---|
| CVA | 4 (3.6) |
| Hemorrhagic complications | 46 (41.1) |
| Minor bleeding | 14 (12.5) |
| Major bleeding | 26 (23.2) |
| Life-threatening bleeding | 6 (5.3) |
| Vascular complications | 21 (18.7) |
| Minor vascular complication | 12 (10.7) |
| Major vascular complication | 9 (8.0) |
| Acute kidney injury | 29 (25.9) |
| Stage 1 | 16 (14.3) |
| Stage 2 | 2 (1.8) |
| Stage 3 | 11 (9.8) |
| Need for definitive pacemaker | 15 (13.4) |
| Prosthesis embolization | 3 (2.7) |
| Need for a second prosthesis | 5 (4.5) |
| Death (any cause) | 16 (14.3) |
VARC-2: Valve Academic Research Consortium-2; CVA: cerebrovascular accident
In this initial series, mortality from all causes in the first 30 days was 14%. When the first 40 patients are excluded, mortality decreases to 8.1%. The only independent predictor of death at 30 days was chronic obstructive pulmonary disease (OR = 4.7 [CI: 1.2 to 18.6], p = 0.025).
Cerebrovascular accident occurred in four patients (3.6%), three of which were of the ischemic type. Bleeding complications occurred in 46 patients (41%), of which 5.5% were classified as life-threatening. Complications related to the vascular access route were detected in 19% of cases, the majority (11%) minor vascular complications. Fifteen patients (13.4%) required the implantation of a permanent pacemaker.
Twenty-nine patients (27%) had acute renal failure after the procedure, with most (14%) at stage 1, according to the AKIN classification.
Late clinical follow-up
A mean follow-up of 16 ± 11 months was attained in 100% of the treated population. After the first 30 days, mortality from all causes was 8.9%, of which two cases were heart-related, four were due to noncardiac causes and four were due to undetermined causes. Figure 2 shows the probability and survival of this population.
Figure 2.
Kaplan-Meier survival curve (death from any cause).
There were no differences in mortality according to gender, presence of peripheral vascular disease, presence of pulmonary hypertension, history of coronary artery bypass surgery and chronic renal failure (Figure 3).
Figure 3.
Study of subgroups: Kaplan-Meier survival curves. (A) Survival according to gender. (B) Survival according to the presence of chronic obstructive pulmonary disease. (C) Survival according to the presence of peripheral vascular disease. (D) Survival according to the presence of pulmonary hypertension. (E) Survival according to history of CABG. (F) Survival according to the presence of chronic renal failure.
Moreover, during the clinical follow-up, chronic obstructive pulmonary disease was the only independent predictor of mortality (OR = 6.37 [2.14 to 19.0], p = 0.001).
Major complications during the follow-up after the first 30 days of the procedure were: need to implant a permanent pacemaker (3.6%), major bleeding complications (1.8%) and CVA (0.9%). There were no cases of prosthesis dysfunction, and none of the patients needed a new valve intervention during follow-up.
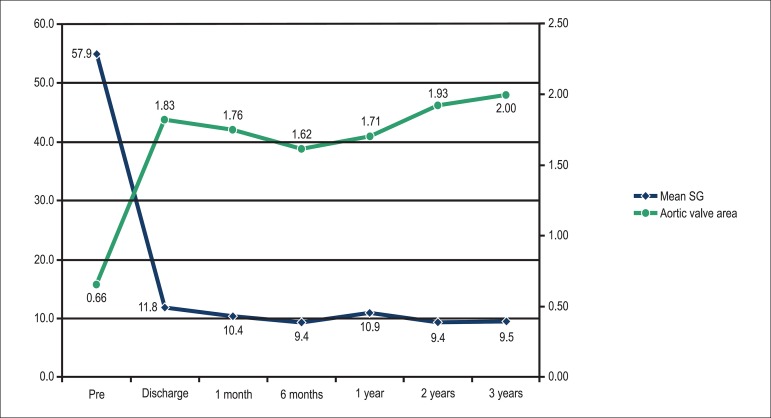
The hemodynamic results of prostheses assessed by Doppler echocardiography, were maintained for up to 3 years, as shown below: prosthetic valve area (1 year = 1.7± 0.5 cm2; 2 years = 1.9± 0.3 cm2 and 3 years = 2.0 ± 0.1 cm2; p = 0.3) and mean systolic gradient (1 year = 10.9± 5.3 mmHg; 2 years = 9.4 ± 4.4 mmHg and 3 years = 9.5 ± 2.1 mmHg, p = 0.1) (Figure 4).
Figure 4.
Hemodynamic results assessed by Doppler echocardiography according to the mean systolic gradient (mmHg) and prosthetic valve area (cm2).
Discussion
The results of this study confirm that implantation of transcatheter aortic valve prosthesis in patients with high surgical risk or with contraindications to conventional valve replacement surgery is a feasible and effective therapeutic procedure with high incidence of immediate success and maintenance of outcome in the middle term.
The present study represents, together with the registry by Albert Einstein Hospital6, the initial national experience with percutaneous implantation of aortic valve prosthesis, being the largest Brazilian series by the same group, using three different types of prosthesis and different access routes.
Although the patients included here have high risk of morbidity and mortality, with a mean EuroScore of 23%, mortality from all causes was 14%, which is similar to or lower than that reported in other national and international case series6,7,14,15. It is noteworthy that mortality is also related to the learning curve of the multidisciplinary team, as when the first 40 patients were excluded, the mortality rate decreased to 8.1%. Similar data were reported by Gurvitch et al16, who observed a mortality of 13.5% among the first 135 patients treated by them, and 5.9% in the 135 patients that followed.
The middle-term survival of these patients, regardless of a mean age of 82 years, was 77% at 16 ± 11 months of follow-up. These numbers are similar to those published in the randomized PARTNER study4,5.
Several studies have attempted to identify predictors of early and late mortality in patients treated with transcatheter aortic prosthesis implantation17-20. This study identified the presence of chronic obstructive pulmonary disease as the only independent predictor of mortality, both in the first month after the procedure, as well as in the late follow-up. In patients with chronic obstructive pulmonary disease, the probability of survival after 1 year was 50%, whereas in patients without chronic obstructive pulmonary disease it was 85%. These results suggest that a careful evaluation of pulmonary function in patients with suspected pulmonary disease is required before referring them for transcatheter implantation of an aortic prosthesis.
The immediate results observed in this series of patients are in agreement with the results of several international series14,15,21,22. Device success was attained in 84% of cases, which is in agreement with other current series6,23. Initially, the definition of device success was focused on the implantation of a single prosthesis in the correct anatomic position and without postoperative mortality, whereas the new definition proposed by VARC-2 includes the latter, as well as the hemodynamic function of the prosthetic valve. The main cause of device failure was the presence of PAR greater than or equal to moderate, present in approximately 10% of our patients, an incidence similar to that found in the randomized PARTNER study (cohorts A and B)4,5, in which this complication occurred in 12.2 and 11.8%, respectively.
Regarding the occurrence of bleeding complications, the incidence of major bleeding in this study was higher than that described in the first studies on this technique, including the cohort B of the PARTNER study, in which only 16.8% of said major bleeding was observed. However, it is noteworthy the fact that bleeding complications were inconsistently reported in the literature until the publication of the VARC definitions12,24.
Recent meta-analysis by Généreuxe et al25, using definitions standardized by that consensus, showed an estimated incidence of major bleeding of 22.3%, which is similar to that found in the discussed series. Here, it is also worth noting the importance of the learning curve, in addition to improvement in current sheath introducers and percutaneous valve delivery systems. If the authors consider the occurrence of bleeding in the latest 80 patients, the incidence of major bleeding is decreased to 18%.
Previous studies have also demonstrated that acute kidney injury is a common complication after transcatheter aortic prosthesis implantation, occurring in up to 28% of cases26. Despite associated comorbidities and the need for contrast use during the procedure, acute kidney injury, stages 2 and 3, occurred in 2 and 11% of cases, respectively.
In this study, it was necessary to implant a permanent pacemaker in 15 patients (13.3%). As reported in several series27,28, the need to implant a permanent pacemaker in the group of patients treated with CoreValve® prosthesis was higher (13 in 77 cases, 17.1%) than in patients with Sapien XT ® (2 in 21 patients, 9.5%), or Accurate prosthesis (no cases).
Finally, regarding the transcatheter prosthesis durability, several studies, as well as the current one, evaluated immediate echocardiographic outcomes, as well as during late follow-up, confirming prosthesis hemodynamic maintenance7,19,29.
Conclusions
This initial experience indicated that transcatheter aortic prosthesis implantation is an effective and safe therapeutic procedure for the treatment of symptomatic aortic stenosis in high surgical-risk or inoperable patients. The presence of chronic obstructive pulmonary disease was the only independent predictor of mortality identified both in the first month post-intervention and at the late follow-up.
Footnotes
Author contributions
Conception and design of the research: Lluberas S, Abizaid A, Siqueira S, Costa Jr. JR,Ramos A, Arrais M, Le Bihan D, Sousa A, Sousa JE; Acquisition of data: Lluberas S, Abizaid A, Siqueira S, Ramos A, Costa Jr. JR, Arrais M, Kambara A, Le Bihan D, Sousa A, Sousa JE; Analysis and interpretation of the data: Lluberas S, Abizaid A, Ramos A, Costa Jr. JR, Sousa JE; Statistical analysis: Lluberas S; Writing of the manuscript: Lluberas S, Ramos A, Costa Jr. JR; Critical revision of the manuscript for intellectual content: Lluberas S, Abizaid A, Ramos A, Costa Jr. JR, Sousa JE.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341(3):142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 3.Tarasoutchi F, Montera MW, Grinberg M, Barbosa MR, Piñeiro DJ, Sánchez CR, et al. Diretriz Brasileira de Valvopatias - SBC 2011 / I Diretriz Interamericana de Valvopatias - SIAC 2011. Arq Bras Cardiol. 2011;97(5) supl. 1:1–67. doi: 10.1590/s0066-782x2011002000001. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. PARTNER Trial Investigators Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 5.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Brito FS, Junior, Abizaid A, Almeida BO, Caixeta A, Tarasoutchi F, Grube E, et al. Implante por cateter de bioprótese valvar para tratamento da estenose aórtica: experiência de três anos. Arq Bras Cardiol. 2012;99(2):697–705. doi: 10.1590/s0066-782x2012005000063. [DOI] [PubMed] [Google Scholar]
- 7.Ghandour MS, Marchini JF, Rocha AM, Neto, Erudilho A, Carnieto NM, Silva BS, et al. Resultados imediatos e seguimento clínico dos pacientes submetidos a implante valvar aórtico transcateter. Rev Bras Cardiol Invasiva. 2012;20(3):260–266. [Google Scholar]
- 8.Gaia DF, Palma JH, Ferreira CB, Souza JA, Agreli G, Guilhen JC, et al. Implante transapical de valva aórtica: resultados de uma nova prótese brasileira. Rev Bras Cir Cardiovasc. 2010;25(3):293–302. doi: 10.1590/s0102-76382010000300004. [DOI] [PubMed] [Google Scholar]
- 9.Gaia DF, Palma JH, Ferreira CB, Souza JA, Gimenes MV, Macedo MT, et al. Implante transcateter de valva aórtica: resultados atuais do desenvolvimento e implante de um nova prótese brasileira. Rev Bras Cir Cardiovasc. 2011;26(3):338–347. doi: 10.5935/1678-9741.20110007. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. Society of Thoracic Surgeons Quality Measurement Task Force The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2 - isolated valve surgery. Ann Thorac Surg. 2009;88(1) Suppl:S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 11.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 12.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33(19):2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 13.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31–R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, et al. FRANCE Registry Investigators Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32(2):191–197. doi: 10.1093/eurheartj/ehq261. [DOI] [PubMed] [Google Scholar]
- 15.Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein S, et al. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation. 2009;119(23):3009–3016. doi: 10.1161/CIRCULATIONAHA.108.837807. [DOI] [PubMed] [Google Scholar]
- 16.Gurvitch R, Tay EL, Wijesinghe N, Ye J, Nietlispach F, Wood DA, et al. Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv. 2011;78(7):977–984. doi: 10.1002/ccd.22961. [DOI] [PubMed] [Google Scholar]
- 17.Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Osten M, et al. Long-term outcomes after transcatheter aortic valve implantation: insights on prognostic factors and valve durability from the Canadian multicenter experience. J Am Coll Cardiol. 2012;60(19):1864–1875. doi: 10.1016/j.jacc.2012.08.960. [DOI] [PubMed] [Google Scholar]
- 18.Toggweiler S, Humphries KH, Lee M, Binder RK, Moss RR, Freeman M, et al. 5-year outcome after transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61(4):413–419. doi: 10.1016/j.jacc.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. PARTNER Trial Investigators Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 20.Zahn R, Gerckens U, Linke A, Sievert H, Kahlert P, Hambrecht R, et al. German Transcatheter Aortic Valve Interventions-Registry Investigators Predictors of one-year mortality after transcatheter aortic valve implantation for severe symptomatic aortic stenosis. Am J Cardiol. 2013;112(2):272–279. doi: 10.1016/j.amjcard.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55(11):1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O, et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention. 2008;4(2):242–249. doi: 10.4244/eijv4i2a43. [DOI] [PubMed] [Google Scholar]
- 23.Gurvitch R, Toggweiler S, Willson AB, Wijesinghe N, Cheung A, Wood DA, et al. Outcomes and complications of transcatheter aortic valve replacement using a balloon expandable valve according to the Valve Academic Research Consortium (VARC) guidelines. EuroIntervention. 2011;7(1):41–48. doi: 10.4244/EIJV7I1A10. [DOI] [PubMed] [Google Scholar]
- 24.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized end-point definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32(2):205–217. doi: 10.1093/eurheartj/ehq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59(25):2317–2326. doi: 10.1016/j.jacc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Aregger F, Wenaweser P, Hellige GJ, Kadner A, Carrel T, Windecker S, et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009;24(7):2175–2179. doi: 10.1093/ndt/gfp036. [DOI] [PubMed] [Google Scholar]
- 27.Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, et al. German Transcatheter Aortic Valve Interventions-Registry Investigators Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. 2011;32(2):198–204. doi: 10.1093/eurheartj/ehq339. [DOI] [PubMed] [Google Scholar]
- 28.Chieffo A, Buchanan GL, Van Mieghem NM, Tchetche D, Dumonteil N, Latib A, et al. Transcatheter aortic valve implantation with the Edwards SAPIEN versus the Medtronic CoreValve Revalving system devices: a multicenter collaborative study: the PRAGMATIC Plus Initiative (Pooled-Rotterd Am-Milano-Toulouse In Collaboration) J Am Coll Cardiol. 2013;61(8):830–836. doi: 10.1016/j.jacc.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 29.Ussia GP, Barbanti M, Petronio AS, Tarantini G, Ettori F, Colombo A, et al. CoreValve Italian Registry Investigators Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J. 2012;33(8):969–976. doi: 10.1093/eurheartj/ehr491. [DOI] [PubMed] [Google Scholar]