Abstract
Background
Subclinical cardiovascular disease is prevalent in patients with Metabolic Syndrome (MetSyn). Left ventricular (LV) circumferential strain (εCC) and longitudinal strain (εLL), assessed by Speckle Tracking Echocardiography (STE), are indices of systolic function: shortening is indicated by negative strain, and thus, the more negative the strain, the better the LV systolic function. They have been used to demonstrate subclinical ventricular dysfunction in several clinical disorders.
Objective
We hypothesized that MetSyn is associated with impaired myocardial function, as assessed by STE.
Methods
We analyzed Multi-Ethnic Study of Atherosclerosis (MESA) participants who underwent STE and were evaluated for all MetSyn components.
Results
Among the 133 participants included [women: 63%; age: 65 ± 9 years (mean ± SD)], the prevalence of MetSyn was 31% (41/133). Individuals with MetSyn had lower εCC and lower εLL than those without MetSyn (-16.3% ± 3.5% vs. -18.4% ± 3.7%, p < 0.01; and -12.1% ± 2.5% vs. -13.9% ± 2.3%, p < 0.01, respectively). The LV ejection fraction (LVEF) was similar in both groups (p = 0.09). In multivariate analysis, MetSyn was associated with less circumferential myocardial shortening as indicated by less negative εCC (B = 2.1%, 95%CI:0.6 3.5, p < 0.01) even after adjusting for age, ethnicity, LV mass, and LVEF). Likewise, presence of MetSyn (B = 1.3%, 95%CI:0.3 2.2, p < 0.01) and LV mass (B = 0.02%, 95% CI: 0.01-0.03, p = 0.02) were significantly associated with less longitudinal myocardial shortening as indicated by less negative εLL after adjustment for ethnicity, LVEF, and creatinine.
Conclusion
Left ventricular εCC and εLL, markers of subclinical cardiovascular disease, are impaired in asymptomatic individuals with MetSyn and no history of myocardial infarction, heart failure, and/or LVEF < 50%.
Keywords: Atherosclerosis, Metabolic X Syndrome, Diabetes Mellitus / mortality, Ventricular Dysfunction / physiopathology, Ethnic Group
Introduction
Metabolic syndrome (MetSyn) is characterized by a cluster of cardiovascular (CV) risk factors including atherogenic dyslipidemia, abdominal obesity, hyperglycemia, elevated blood pressure, and proinflammatory and prothrombotic state1. It affects about 25% of the population, being associated with an increased risk of developing diabetes, CV morbidity and mortality2.
Previous studies using carotid intima-media thickness measured with ultrasound, coronary artery calcium, Tei index, and tissue Doppler have shown the presence of subclinical CV disease (CVD) in participants with MetSyn3-13.
Recently, speckle tracking echocardiography (STE) has been introduced as a new non-invasive method for assessing left ventricular (LV) myocardial shortening or strain. The method is angle-independent, does not require contrast agents, and has been validated against sonomicrometry and magnetic resonance imaging (MRI)14-17. It has been used to demonstrate subclinical ventricular dysfunction in various clinical disorders18-22.
The purpose of this study was to evaluate the use of STE to assess myocardial strain as a marker of LV systolic function in an asymptomatic population with MetSyn and LV ejection fraction (LVEF) ≥ 50%. We hypothesized that MetSyn is associated with impaired LV circumferential strain (εCC), as well as with LV longitudinal strain (εLL).
Methods
Study population
The purpose of the Multi-Ethnic Study of Atherosclerosis (MESA), a multicenter population-based study, is to investigate mechanisms underlying the development and progression of subclinical CVD in asymptomatic men and women from four different ethnic groups - Caucasian, African-American, Hispanic, and Chinese23. Between July/2008 and June/2009, during MESA follow-up evaluation, 162 consecutive participants in the Baltimore cohort of MESA were invited to undergo an echocardiographic study. We included participants who underwent echocardiography, tagged MRI (reference method for εCC), and evaluation for all MetSyn components. Participants who had more than two LV segments not analyzable on STE in short-axis- or 4-chamber view were excluded from the strain analysis. Exclusion criteria also included a history of myocardial infarction (MI), heart failure (HF) and/or LVEF < 50%. The institutional review board of the Johns Hopkins University approved the study, and participants provided written informed consent.
Metabolic syndrome definition
Metabolic syndrome was defined according to revised National Cholesterol Education Program Adult Treatment Panel (NCEP ATP) III criteria24. Participants having at least three of the following criteria were considered as having MetSyn: fasting plasma glucose (FPG) ≥ 100 mg/dL; triglycerides ≥ 150 mg/dL; HDL-cholesterol < 40 mg/dL in men and < 50 mg/dL in women; abdominal obesity [waist circumference (WC) ≥ 102 cm in men and ≥ 88 cm in women]; and blood pressure ≥ 130/85 mm Hg. The revised NCEP definition still includes patients being treated for dyslipidemia, systemic hypertension, or hyperglycemia.
Risk factors
Participants completed standardized medical history questionnaires ascertaining medication use and previous diagnoses and provided samples for quantification of FPG, lipids, and creatinine25. Waist circumference was measured at the level of the umbilicus using a standard tape measure. Resting blood pressure was measured three times in a seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL, USA). The average of the last two measurements was used in the analysis. Arterial hypertension was diagnosed using the VII Joint National Committee criteria26. Diabetes was defined as a FPG ≥126 g/mL or use of insulin or of an oral hypoglycemic agent. Smoking status was defined as current smoking or no smoking.
Echocardiography and speckle tracking
Examinations were performed by expert sonographers, and reviewed off-line by one reader (ALCA). Two-dimensional echocardiograms were recorded during breath-holds using an Artida scanner (Toshiba Medical Systems Corp, Tochigi, Japan) at the time of MESA follow-up evaluation. The images were acquired from the short-axis view of the left ventricle at the papillary muscle level and from the apical 4-chamber view, and stored digitally. The images were recorded using B-mode harmonic images and adjusting transducer frequencies (1.7-3.5 MHz), frame rate (40-80 frames per second), focus (midventricular), sector width (as narrow as possible), sector depth (minimal) and gain in order to optimize myocardial image quality. The echocardiographic recordings were analyzed with STE software (Toshiba 2D Wall Motion Tracking software, Toshiba Medical Systems). The manual counterclockwise tracing of endocardial and epicardial borders in the mid-LV short-axis image at end-systole was followed by automatic drawing of a mid-wall tracking line14. A similar approach was made in the 4-chamber recording, starting at the lateral corner of the mitral annulus, at the end-diastole. STE software uses the 'sum of squared differences' method to find the most similar speckle pattern of the 2D template in two subsequent frames27. Strain (ε, %) was calculated as the change in regional length relative to its end-diastolic length; ε = (L(t) - L0)x100/L0, where Lt is the length at time t, and L0 is the segment length at the onset of QRS. Global εCC (Figure 1) and εLL (Figure 2) are represented by the peak of average strain. As ε reflects myocardial shortening, a more negative value indicates more shortening. The mid-ventricular short axis level and the 4-chamber view of the left ventricle were divided according to the American Society of Echocardiography recommendation28. Segments were excluded from analyses due to dropouts, motion artifacts, reverberations, or bad tracking quality due to manual assessment.
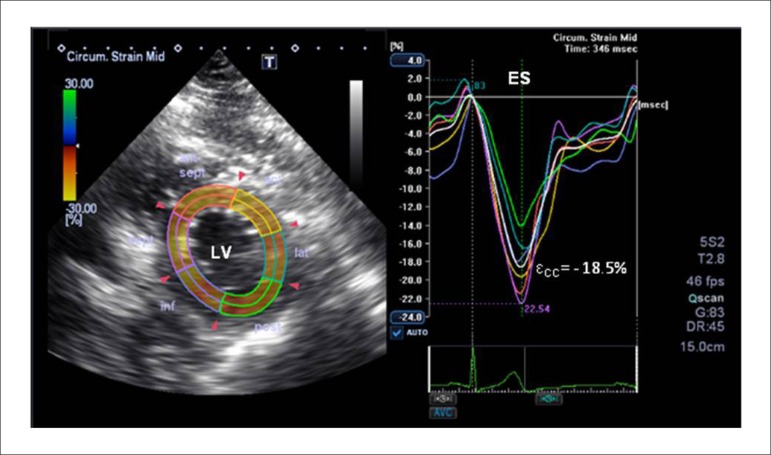
Figure 1.
Representative LV εCC curve from a participant without MetSyn. Different colors depict different myocardial segments. The white strain curve represents the global circumferential peak strain. ES: end-systole; εCC: circumferential strain; LV: left ventricle.
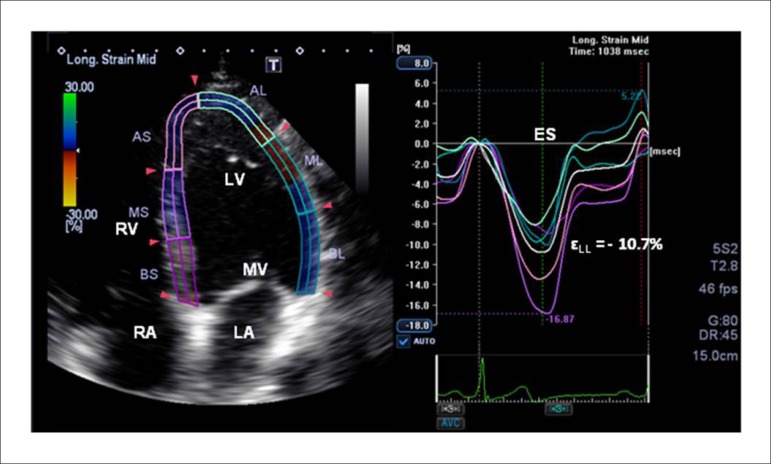
Figure 2.
Representative LV εLL curve from a participant with MetSyn. Different colors depict different myocardial segments. The white strain curve represents the global longitudinal peak strain. ES: end-systole; εLL: longitudinal strain; LV: left ventricle; LA: Left atrium; RV: Right ventricle; RA: Right atrium; MV: Mitral valve
Magnetic Resonance Imaging: Tagged MRI protocol and strain measurement
Participants underwent MRI and echocardiography on the same day. Cardiac MRI scan was acquired using 1.5T MR scanners [Signa LX or CVi (GE Medical Systems, Waukesha, WI, USA)]. Images were obtained using a segmented k-space and ECG-triggered SPGR pulse sequence. After acquisition of standard scout images, 2- and 4-chamber cine MR images were acquired. Short-axis cine images were then obtained with retrospective gating and temporal resolution of ≤ 50 msec, from above the mitral valve plane to the LV apex29. Left ventricular structural parameters (end-systolic and end diastolic volumes, LV mass) and LVEF were measured using standard commercially available software (MASS 4.2, MEDIS, Leiden, The Netherlands), as previously described29. Left ventricular mass index was defined as LV mass divided by body surface area. After completing the standard protocol, three tagged short-axis slices (base to apex) were obtained. Parallel striped tags were prescribed in two orthogonal orientations (0° and 90°) using ECG-triggered fast gradient echo sequence with spatial modulation of magnetization. The parameters for tagged images were as follows: field of view, 40 cm; slice thickness, 7 to 8 mm; repetition time, 6 ms; echo time, 3.0 ms; flip angle, 10° to 12°; phase encoding views, 128 with six phase encoding views per segment; temporal resolution, 35 msec; and tag spacing, 7 mm. Circumferential strain was measured using HARP method embedded in MATLAB software (The MathWorks, Natick, MA, USA)30.
Reproducibility
Thirty studies were randomly selected for the assessment of intra- and inter-observer variability of εCC and εLL on STE. To test intra-observer variability, a single observer (ALCA) analyzed the data twice with a minimal interval of 30 days. To test inter-observer variability, a second observer (EYC) analyzed the data without knowing the first observer's measurements.
Statistical analysis
Data are shown as mean ± standard deviation (SD) for continuous variables and as proportions for categorical variables. Student's t test was used to assess differences in continuous variables between the groups studied, whereas chi-square test was used for categorical analysis. Univariate analysis was performed to assess the relationship of εCC and εLL with the variables of interest. Multiple linear regression (MLR) analysis was performed to study the association between εCC assessed on echocardiography and variables with a p value < 0.10 in the univariate analysis, and variables with biological plausibility to interfere with LV strain. The same approach was used for εLL measured on echocardiography and for εCC measured on tagged MRI. In addition, models of MLR analysis were created, using the stepwise variable selection procedures, to evaluate the potential association of εCC and εLL on STE and individual MetSyn components, as well as all the possible term interactions between the MetSyn components. Intra observer and inter-observer variability was assessed by using intraclass correlation coefficient. A two-tailed p value < 0.05 was considered statistically significant for all analyses. Statistical analyses were performed using the SPSS 18 statistical software package (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
Echocardiography was obtained in 162 consecutive MESA participants. Compared to the entire MESA cohort, this study cohort had higher HDL-cholesterol (57.3 ± 19.0 mg/dL versus 52.7 ± 15.6 mg/dL, p < 0.01) and a greater percentage of females (63% versus 47%, p < 0.05). Due to enrollment characteristics of the study site, only black and white study participants were included.
Twenty-six (16%) participants were excluded from the analysis because of poor echocardiographic image quality, and three others (1.9%) because of missing data about at least one of the MetSyn components. No participant was excluded because of a history of MI, HF, or LVEF < 50%. The final study population consisted of 133 participants (women: 63%, mean age = 65 ± 9 years).
The prevalence of MetSyn in this sample was 31% (41 MetSyn in 133 participants). Table 1 lists the clinical and demographic characteristics of the participants according to the MetSyn status. The LVEF measured by use of MRI was 61 ± 5%.
Table 1.
Clinical and demographic data of participants according to the MetSyn status
| Variable | Without MetSyn (n = 92) Mean ± SD | With MetSyn (n = 41) Mean ± SD | p value |
|---|---|---|---|
| Age (year) | 65 ± 8 | 66 ± 9 | 0.27 |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.15 |
| Gender (Female, %) | 63% | 63% | 0.97 |
| Ethnicity (Caucasian, %) | 60% | 44% | 0.09 |
| Smoking (current, %) | 8% | 10% | 0.69 |
| Weight (Kg) | 77 ± 17 | 91 ± 18 | < 0.01 |
| BMI (Kg/m2) | 28 ± 5 | 32 ± 4 | < 0.01 |
| Total cholesterol (mg/dL) | 197 ± 44 | 177 ± 39 | 0.01 |
| LDL-cholesterol (mg/dL) | 116 ± 39 | 101 ± 36 | < 0.05 |
| Any lipid-lowering medication (Yes, %) | 28% | 47% | < 0.05 |
| Waist circumference (cm)* | 93 ± 13 | 107 ± 14 | < 0.01 |
| Glucose (mg/dL)* | 93 ± 16 | 107 ± 24 | < 0.01 |
| Triglycerides (mg/dL)* | 94 ± 46 | 151 ± 71 | < 0.01 |
| HDL-cholesterol (mg/dL)* | 62 ± 20 | 46 ± 11 | < 0.01 |
| Hypertension (Yes, %)* | 42% | 80% | < 0.01 |
MetSyn: Metabolic Syndrome; SD: standard deviation; BMI: body mass index; HDL: high-density lipoprotein; LDL: low-density lipoprotein
Metabolic Syndrome component.
Data reproducibility
The inter-observer and intra-observer intraclass correlation coefficients (ICC) for εLL were 0.84 (p < 0.01) and 0.87 (p < 0.01), respectively. Those for εCC were 0.86 (p < 0.01) and 0.85 (p < 0.01), respectively.
Metabolic syndrome and LV circumferential strain on STE
Table 2 shows the echocardiographic and MRI data of participants according to the MetSyn status.
Table 2.
Echocardiography and MRI data of participants according to the MetSyn status
| Variable | Without MetSyn (n = 92) Mean ± SD | With MetSyn (n = 41) Mean ± SD | p value |
|---|---|---|---|
| εCC (%) [STE] | -18.4 ± 3.7 | -16.3 ± 3.5 | < 0.01 |
| εCC (%) [MRI] | -17.6 ± 2.3 | -15.8 ± 3.3 | < 0.01 |
| εLL (%) [STE] | -13.9 ± 2.3 | -12.1 ± 2.5 | < 0.01 |
| LVEF (%) [MRI] | 61 ± 5 | 59 ± 4 | 0.09 |
| LV mass (g) [MRI] | 120 ± 28 | 136 ± 35 | < 0.01 |
| LV mass index (g/m2) [MRI] | 65 ± 11 | 68 ± 13 | 0.18 |
| LVID (mm) [Echo] | 46 ± 6 | 44 ± 5 | 0.15 |
εCC:circumferential strain; εLL: longitudinal strain; LV: left ventricle; LVEF: LV ejection fraction; LVID: LV internal diameter; MetSyn: Metabolic Syndrome; MRI: magnetic resonance imaging; STE: speckle tracking echocardiography.
The LVEF was similar in both groups (p = 0.09). However, individuals with MetSyn had lower εCC on STE than those without MetSyn (εCC = -16.3% ± 3.5% versus -18.4% ± 3.7, p < 0.01). Men with MetSyn had lower εCC on STE as compared with those without MetSyn (-15.5% versus -18.6%, p < 0.01). Women with MetSyn tended to have lower εCC (-16.8% versus -18.3%), but with no significance (p = 0.11). The εCC was similar in women and men with MetSyn (p > 0.05).
In univariate analysis, εCC on STE was significantly related to the presence of MetSyn (p < 0.01), weight (p < 0.01), body mass index (BMI) (p < 0.05), and WC (p < 0.01) (Table 3). The MLR analysis included variables that were significant in the univariate analysis (MetSyn), as well as variables with biological plausibility to change εCC, such as LVEF, LV mass, age, and ethnicity. Weight, BMI, and WC were excluded from the model because they are directly or indirectly related to the MetSyn definition. In this model, MetSyn was significantly related to εCC even after adjustment for covariates (B = 2.1%; 95% CI: 0.6-3.5; p < 0.01) (Table 3).
Table 3.
Univariate and multivariate analysis – Dependent variable: εCC on STE
| Independent variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| B coeff (%) | 95%CI | p value | B coeff (%) | 95%CI | p value | |
| Presence of MetSyn | 2.07 | (0.72;3.42) | < 0.01 | 2.06 | (0.59;3.52) | < 0.01 |
| Weight (Kg) | 0.05 | (0.01;0.08) | < 0.01 | - | - | - |
| Waist circumference (cm) | 0.07 | (0.03;0.11) | < 0.01 | - | - | - |
| BMI (Kg/m2) | 0.15 | (0.03;0.26) | < 0.05 | - | - | - |
| Age (years) | 0.02 | (-0.06;0.09) | 0.60 | 0.01 | (-0.07;0.08) | > 0.05 |
| Ethnicity (Caucasian) | -0.57 | (-1.86;0.72) | 0.60 | -0.87 | (-2.17;0.43) | > 0.05 |
| LV mass (g) | 0.02 | (-0.01;0.04) | 0.17 | 0.01 | (-0.01;0.03) | > 0.05 |
| LVEF (%) | -0.03 | (-0.13;0.07) | 0.51 | -0.01 | (-0.11;0.10) | > 0.05 |
| Gender (female) | 0.17 | (-1.16;1.51) | 0.80 | - | - | - |
| Creatinine (mg/dL) | -0.18 | (-2.97;2.62) | 0.90 | - | - | - |
| Smoking status (current) | -0.38 | (-2.73;1.97) | 0.75 | - | - | - |
| Hypertension (Yes) | 0.16 | (-1.14;1.47) | 0.81 | - | - | - |
| Diabetes (Yes) | 0.80 | (-1.00;2.60) | 0.38 | - | - | - |
| FPG (mg/dL) | 0.03 | (-0.01;0.06) | 0.09 | - | - | - |
| Total cholesterol (mg/dL) | 0.01 | (-0.01;0.02) | 0.95 | - | - | - |
| LDL-cholesterol (mg/dL) | -0.01 | (-0.02;0.01) | 0.72 | - | - | - |
| HDL-cholesterol (mg/dL) | -0.01 | (-0.04;0.03) | 0.87 | - | - | - |
| Triglycerides (mg/dL) | 0.01 | (-0.01;0.02) | 0.10 | - | - | - |
BMI: body mass index; εCC: circumferential strain; FPG: fasting plasma glucose; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LV: left ventricle; LVEF: LV ejection fraction; MetSyn: Metabolic Syndrome; MRI: magnetic resonance imaging.
In another model of MLR analysis, LVEF, LV mass, age, and ethnicity were maintained, and the MetSyn variable was replaced for all five components of MetSyn. In that case, only WC remained significantly associated with εCC (B = 2.0%; 95% CI: 0.7-3.3; p < 0.01, stepwise method). Again, using all the MetSyn components and all the possible interaction terms between them, only WC maintained significance (B = 2.0%; 95% CI: 0.7-3.3; p < 0.01).
The εCC measured on echocardiography changed from -18.7% in participants with ≤ one MetSyn component to -18.0% in participants with two MetSyn components and to -16.3% in those with ≥ three MetSyn criteria (p < 0.01).
The WC component was present in 95.1% of the participants with MetSyn, while blood pressure, HDL-cholesterol, FPG, and triglycerides were present in 87.8%, 61.0%, 46.3%, and 43.9%, respectively. On the other hand, the WC component was present in 45% of the participants without MetSyn.
Metabolic syndrome and LV circumferential strain on tagged MRI
Similarly to STE results, individuals with MetSyn had lower εCC on tagged MRI than those without MetSyn (εCC = -15.8% ± 3.3% versus -17.6% ± 2.3%, p < 0.01) (Table 2).
On univariate analysis, the presence of MetSyn, weight, WC, LVEF, and LV mass were significantly related to εCC on tagged MRI. On MLR analysis, both the presence of MetSyn (B = 1.3%; 95% CI: 0.3-2.4; p = 0.02) and LV mass (B = 0.02%; 95% CI: 0.01-0.03; p = 0.03) remained significant.
Metabolic syndrome and LV longitudinal strain
Individuals with MetSyn had lower εLL as compared with those without MetSyn (-12.1% ± 2.5% versus -13.9% ± 2.3, p < 0.01) (Table 2). This result was consistent for men and women. The εLL was similar in women and men with MetSyn (p > 0.05).
On univariate analysis, εLL was significantly related to MetSyn, weight, BMI, WC, ethnicity, LV mass, LVEF, creatinine, hypertension status, diabetes status, and triglycerides (Table 4). The MLR analysis included presence of MetSyn, LV mass, LVEF, ethnicity, and creatinine, which were significant in the univariate analysis. Weight, BMI, WC, triglycerides, hypertension, and diabetes status were excluded from the model because they are directly or indirectly related to the MetSyn definition. Presence of MetSyn (B = 1.3%; 95% CI: 0.3-2.2; p < 0.01) and LV mass (B = 0.02%; 95% CI: 0.01-0.03; p = 0.02) were independently related to εLL in the model (Table 4). The results were similar when using LV mass indexed by height in meters: presence of MetSyn (B = 1.3%; 95% CI: 0.3 2.2; p < 0.01) and LV mass/height (B = 0.03%; 95% CI: 0.01 0.06; p = 0.04).
Table 4.
Univariate and multivariate analysis – Dependent variable: εLL on STE
| Independent variables | Univariate Analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| B coeff (%) | 95%CI | p value | B coeff (%) | 95%CI | Pp value | |
| Presence of MetSyn | 1.73 | (0.83;2.64) | < 0.01 | 1.25 | (0.32;2.19) | < 0.01 |
| Weight (Kg) | 0.04 | (0.02;0.07) | < 0.01 | - | - | - |
| Waist circumference (cm) | 0.04 | (0.01;0.07) | < 0.01 | - | - | - |
| BMI (Kg/m2) | 0.11 | (0.03;0.18) | < 0.01 | - | - | - |
| Age (years) | 0.02 | (-0.03;0.08) | 0.37 | - | - | - |
| Ethnicity (Caucasian) | 1.11 | (0.24;1.97) | 0.01 | 0.85 | (-0.0;1.71) | > 0.05 |
| LV mass (g) | 0.02 | (0.01;0.04) | < 0.01 | 0.02 | (0.01;0.03) | 0.02 |
| LVEF (%) | -0.04 | (-0.11;0.03) | 0.21 | -0.02 | (-0.08;0.05) | > 0.05 |
| Gender (female) | 0.59 | (-0.31;1.50) | 0.20 | - | - | - |
| Creatinine (mg/dL) | 2.10 | (0.23;3.97) | 0.03 | 0.29 | (-1.74;2.32) | > 0.05 |
| Smoking status (current) | 1.23 | (-0.35;2.82) | 0.13 | - | - | - |
| Hypertension (Yes) | 0.97 | (0.10;1.84) | 0.03 | - | - | - |
| Diabetes (Yes) | 1.55 | (0.35;2.75) | 0.01 | - | - | - |
| FPG (mg/dL) | 0.02 | (-0.01;0.04) | 0.12 | - | - | - |
| Total cholesterol (mg/dL) | -0.01 | (-0.02;0.01) | 0.33 | - | - | - |
| LDL-cholesterol (mg/dL) | -0.01 | (-0.02;0.01) | 0.19 | - | - | - |
| HDL-cholesterol (mg/dL) | -0.01 | (-0.04;0.01) | 0.26 | - | - | - |
| Triglycerides (mg/dL) | 0.01 | (0.01;0.02) | 0.02 | - | - | - |
BMI: body mass index; εCC: circumferential strain; FPG: fasting plasma glucose; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LV: left ventricle; LVEF: LV ejection fraction; MetSyn: Metabolic Syndrome; MRI:magnetic resonance imaging.
In another model of MLR analysis, we maintained LVEF, LV mass, ethnicity, and creatinine, and replaced the MetSyn variable for all five components of MetSyn. With this approach, LV mass (B = 0.02%, p < 0.01), ethnicity (B = 1.0%, p = 0.02), and FPG component (B = 1.2%, p = 0.03) remained as independent predictors of εLL (stepwise method). In a model using LV mass, ethnicity, the five MetSyn components, and all the possible interactions between terms (stepwise method), LV mass (B = 0.02%; 95% CI: 0.01-0.03; p < 0.01), ethnicity (B = 0.9%; 95% CI: 0.1-1.8; p = 0.03), and the interaction term WC*FPG (B = 1.5%; 95% CI: 0.3-2.7; p = 0.01) remained significant. The εLL changed from -14.2% in participants with ≤ one MetSyn component, to -13.4%, in participants with two, and to -12.1% in those with ≥ three MetSyn criteria (p < 0.01).
Discussion
In our data, MetSyn, as defined by NCEP ATP III, was associated with reduced myocardial function as indicated by an impaired εCC and εLL in a sample of MESA participants. This result becomes even more important considering that this study sample was constituted by asymptomatic individuals with no history of MI, HF, and/or LVEF<50%. These findings could indicate presence of subclinical CVD in that population. The strength of those relationships was maintained even after adjusting for age, ethnicity, creatinine, LVEF, and LV mass.
Subclinical CVD has been demonstrated in subjects with MetSyn3-8. Gong et al. have used tissue Doppler to analyze εLL in Chinese participants with MetSyn13. Consistent with our findings in African-American and Caucasian participants, they have demonstrated that mean systolic εLL was lower in participants with MetSyn than in controls. In our study, using STE analysis, longitudinal as well as circumferential myocardial functions were reduced, as indicated by lower εLL and εCC in participants with MetSyn as compared with those without MetSyn. As MetSyn is more prevalent in African-Americans and Caucasians than in the Chinese population, a more general conclusion can be drawn from our study. Furthermore, strain measured by using Doppler has some limitations such as poor reproducibility, angle dependency, and signal noise31.
Waist circumference has been cited as a marker of central obesity, carrying a stronger association with health risk indicators32. Our data support this concept, because when all five components of MetSyn and all possible interactions between terms were analyzed on MLR analysis, only WC remained significantly associated with εCC on STE.
The strain difference between participants with and without MetSyn was small. Differences in circumferential and longitudinal myocardial shortening are typically small, particularly in samples of asymptomatic individuals20,33-35. Our εCC values were higher than εLL in concordance with other results36. Participants with MetSyn had lower total and LDL-cholesterol than those without MetSyn, probably due to higher usage of lipid-lowering medication.
Our findings indicate that εCC was somewhat underestimated on MRI, when compared with εCC on STE, independently of the presence of MetSyn. The lower temporal resolution of tagged MRI could explain this difference14,15.
The use of STE could help to improve patient care while providing early identification of subclinical disease37. In agreement with other reports3-6,8,11-13, our results support the association between MetSyn and subclinical CVD. Thus, individuals with MetSyn should be encouraged to improve their quality of life and control all CV risk factors, particularly abdominal obesity.
Limitations
Because of the cross-sectional design of our study, a causal relationship between MetSyn and strain cannot be established accurately. More studies are necessary to evaluate the clinical relevance of our data. Although strain parameters are not disease-specific, strain has been introduced as a sensitive marker of LV systolic function18-21. The participants were asymptomatic for HF, and did not undergo exercise stress test to exclude disease at the time of the echocardiographic evaluation. Because reproducibility is better for two-dimensional strain, the strain rate was not analyzed in this study.
Conclusion
These results indicate that LV εLL and εCC, markers of subclinical CVD, are impaired in asymptomatic individuals with MetSyn and no history of MI, HF and/or LVEF<50%. Our findings support strict control of each risk component of MetSynd, mainly WC.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions.
Footnotes
Author contributions
Conception and design of the research: Almeida ALC, Teixido-Tura G, Lima JAC; Acquisition of data: Almeida ALC, Teixido-Tura G, Choi EY, Opdahl A, Fernandes VRS; Analysis and interpretation of the data: Almeida ALC, Teixido-Tura G, Choi EY, Opdahl A, Wu CO, Lima JAC; Statistical analysis: Almeida ALC, Teixido-Tura G, Choi EY, Opdahl A, Wu CO; Obtaining financing: Lima JAC; Writing of the manuscript: Almeida ALC, Choi EY; Critical revision of the manuscript for intellectual content: Almeida ALC, Teixido-Tura G, Choi EY, Opdahl A, Fernandes VRS, Wu CO, Bluemke DA, Lima JAC.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by National Heart, Lung and Blood Institute (Bethesda, Maryland, USA).
Study Association
This article is part of the thesis of post-doctoral submitted by André Luiz Cerqueira de Almeida, from Johns Hopkins
References
- 1.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 2.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Tzou WS, Douglas PS, Srinivasan SR, Bond MG, Tang R, Chen W, et al. Increased subclinical atherosclerosis in young adults with metabolic syndrome: the Bogalusa Heart Study. J Am Coll Cardiol. 2005;46(3):457–463. doi: 10.1016/j.jacc.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 4.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, et al. Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care. 2003;26(4):1251–1257. doi: 10.2337/diacare.26.4.1251. [DOI] [PubMed] [Google Scholar]
- 5.Hassinen M, Komulainen P, Lakka TA, Vaisanen SB, Haapala I, Gylling H, et al. Metabolic syndrome and the progression of carotid intima-media thickness in elderly women. Arch Intern Med. 2006;166(4):444–449. doi: 10.1001/archinte.166.4.444. [DOI] [PubMed] [Google Scholar]
- 6.Wong ND, Sciammarella MG, Polk D, Gallagher A, Miranda-Peats L, Whitcomb B, et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41(9):1547–1553. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 7.Aijaz B, Ammar KA, Lopez-Jimenez F, Redfield MM, Jacobsen SJ, Rodeheffer RJ. Abnormal cardiac structure and function in the metabolic syndrome: a population-based study. Mayo Clin Proc. 2008;83(12):1350–1357. doi: 10.4065/83.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voulgari C, Moyssakis I, Papazafiropoulou A, Perrea D, Kyriaki D, Katsilambros N, et al. The impact of metabolic syndrome on left ventricular myocardial performance. Diabetes Metab Res Rev. 2010;26(2):121–127. doi: 10.1002/dmrr.1063. [DOI] [PubMed] [Google Scholar]
- 9.Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, et al. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study) Am J Cardiol. 2004;93(1):40–44. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Cull CA, Jensen CC, Retnakaran R, Holman RR. Impact of the metabolic syndrome on macrovascular and microvascular outcomes in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study 78. Circulation. 2007;116(19):2119–2126. doi: 10.1161/CIRCULATIONAHA.107.733428. [DOI] [PubMed] [Google Scholar]
- 11.Ingelsson E, Sullivan LM, Murabito JM, Fox CS, Benjamin EJ, Polak JF, et al. Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes. 2007;56(6):1718–1726. doi: 10.2337/db07-0078. [DOI] [PubMed] [Google Scholar]
- 12.Kullo IJ, Cassidy AE, Peyser PA, Turner ST, Sheedy 2 PF. Association between metabolic syndrome and subclinical coronary atherosclerosis in asymptomatic adults. Am J Cardiol. 2004;94(12):1554–1558. doi: 10.1016/j.amjcard.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Gong HP, Tan HW, Fang NN, Song T, Li SH, Zhong M, et al. Impaired left ventricular systolic and diastolic function in patients with metabolic syndrome as assessed by strain and strain rate imaging. Diabetes Res Clin Pract. 2009;83(3):300–307. doi: 10.1016/j.diabres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Ishizu T, Seo Y, Enomoto Y, Sugimori H, Yamamoto M, Machino T, et al. Experimental validation of left ventricular transmural strain gradient with echocardiographic two-dimensional speckle tracking imaging. Eur J Echocardiogr. 2010;11(4):377–385. doi: 10.1093/ejechocard/jep221. [DOI] [PubMed] [Google Scholar]
- 15.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47(4):789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 16.Sivesgaard K, Christensen SD, Nygaard H, Hasenkam JM, Sloth E. Speckle tracking ultrasound is independent of insonation angle and gain: an in vitro investigation of agreement with sonomicrometry. J Am Soc Echocardiogr. 2009;22(7):852–858. doi: 10.1016/j.echo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12(3):167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 18.Dedobbeleer C, Rai M, Donal E, Pandolfo M, Unger P. Normal left ventricular ejection fraction and mass but subclinical myocardial dysfunction in patients with Friedreich's ataxia. Eur Heart J Cardiovasc Imaging. 2012;13(4):346–352. doi: 10.1093/ejechocard/jer267. [DOI] [PubMed] [Google Scholar]
- 19.Yagmur J, Sener S, Acikgoz N, Cansel M, Ermis N, Karincaoglu Y, et al. Subclinical left ventricular dysfunction in Behcet's disease assessed by two-dimensional speckle tracking echocardiography. Eur J Echocardiogr. 2011;12(7):536–541. doi: 10.1093/ejechocard/jer088. [DOI] [PubMed] [Google Scholar]
- 20.Nakai H, Takeuchi M, Nishikage T, Lang RM, Otsuji Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr. 2009;10(8):926–932. doi: 10.1093/ejechocard/jep097. [DOI] [PubMed] [Google Scholar]
- 21.Saha SK, Kiotsekoglou A, Toole RS, Moggridge JC, Nichols KJ, Govind S, et al. Value of two-dimensional speckle tracking and real time three-dimensional echocardiography for the identification of subclinical left ventricular dysfunction in patients referred for routine echocardiography. Echocardiography. 2012;29(5):588–597. doi: 10.1111/j.1540-8175.2011.01631.x. [DOI] [PubMed] [Google Scholar]
- 22.Ng AC, Delgado V, Bertini M, Antoni ML, van Bommel RJ, van Rijnsoever EP, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J. 2011;32(12):1542–1550. doi: 10.1093/eurheartj/ehr084. [DOI] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 25.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83(6):1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa K, Hozumi T, Sugioka K, Matsumura Y, Nishiura M, Kanda R, et al. Usefulness of automated quantitation of regional left ventricular wall motion by a novel method of two-dimensional echocardiographic tracking. Am J Cardiol. 2006;98(11):1531–1537. doi: 10.1016/j.amjcard.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 28.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 29.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52(25):2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, et al. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7(5):783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 31.Hanekom L, Cho GY, Leano R, Jeffriess L, Marwick TH. Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: an angiographic correlation. Eur Heart J. 2007;28(14):1765–1772. doi: 10.1093/eurheartj/ehm188. [DOI] [PubMed] [Google Scholar]
- 32.Shen W, Punyanitya M, Chen J, Gallagher D, Albu J, Pi-Sunyer X, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity. 2006;14(4):727–736. doi: 10.1038/oby.2006.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen BD, Lima JA, Nasir K, Edvardsen T, Folsom AR, Lai S, et al. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the multi-ethnic study of atherosclerosis. Circulation. 2006;114(4):289–297. doi: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes VR, Polak JF, Edvardsen T, Carvalho B, Gomes A, Bluemke DA, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA) . J Am Coll Cardiol. 2006;47(12):2420–2428. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 35.Rosen BD, Saad MF, Shea S, Nasir K, Edvardsen T, Burke G, et al. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47(6):1150–1158. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 36.Leitman M, Lysiansky M, Lysyansky P, Friedman Z, Tyomkin V, Fuchs T, et al. Circumferential and longitudinal strain in 3 myocardial layers in normal subjects and in patients with regional left ventricular dysfunction. J Am Soc Echocardiogr. 2010;23(1):64–70. doi: 10.1016/j.echo.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23(4):351–369. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]