Abstract
Background and Aims
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide. Mito-carboxy proxyl (Mito-CP), a lipophilic cationic nitroxide, accumulates in the mitochondria due to the large negative transmembrane potential. Studies have shown that these agents act by disrupting the energy producing mechanism, inducing mitochondrial-mediated apoptosis, and also enhancing the action of other chemotherapeutic agents in cancer cells. We hypothesized that the combination of Mito-CP and glycolysis inhibitor, 2-deoxyglucose (2-DG), would synergistically inhibit HCC in vitro.
Methods
HepG2 cells and primary hepatocytes were treated with various combinations of Mito-CP and 2-DG. Cell cytotoxicity was measured using methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay, and adenosine triphosphate (ATP) bioluminescence assay. In addition, caspase 3/7 enzymatic activity was examined after treatment.
Results
Mito-CP and 2-DG induced synergistic cytotoxicity in HepG2 cells in a dose- and time-dependent fashion, while primary cells remained viable and unaffected after treatment. The intracellular ATP levels of HepG2 cells were suppressed within 6 h of combination treatment, whereas primary cells maintained higher levels of ATP. Dose-dependent increases of caspase 3/7 activity occurred in HepG2 cells with time dependent manner, demonstrating the initiation of cell death via apoptotic pathway.
Conclusions
These findings indicate that a combination of Mito-CP and 2-DG effectively inhibit HCC growth in vitro. The increase in caspase 3/7 activity supports the occurrence of 2-DG and Mito-CP induced apoptotic death in HCC. Inability of the compounds to induce cytotoxicity or suppress the production of ATP in primary hepatocytes provides selective and synergistic approach for treatment of HCC.
Keywords: HCC; apoptosis; Warburg effect, mitochondrial target, glycolysis inhibitor; combination therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer related deaths worldwide. The major clinical risk factor for the development of HCC is cirrhosis of the liver. Chronic infections with Hepatitis B virus, Hepatitis C virus and increased alcohol consumption are major risk factors that can cause cirrhosis of the liver [1]. Early stage HCC is frequently asymptomatic; therefore the disease is detected primarily at an intermediate or advanced stage. At this stage, most patients are not eligible to undergo surgical interventions of liver resection or orthotopic liver transplantation [2]. Moreover, there has been a significant increase in the incidence of HCC in the past decade. These factors have contributed to the growing need for the development of innovative and effective treatment methodologies for the treatment of HCC [3].
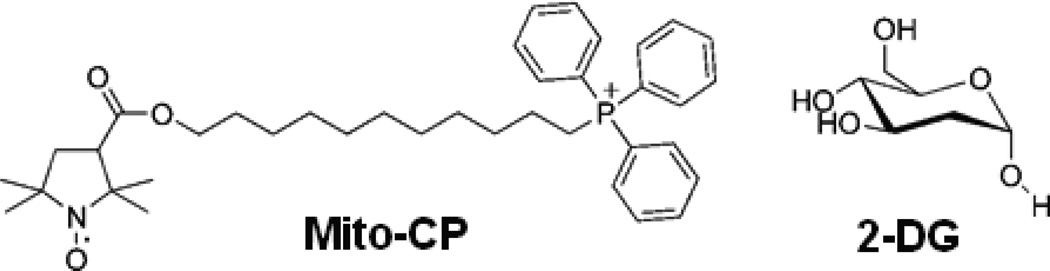
A significant difference in the metabolism of cancer cells and normal cells was discovered by Otto Warburg in the beginning of the twentieth century[4]. Warburg postulated that cancer cells unlike normal cells rely on glycolysis for most of their energy needs (ATP) to compensate for the loss that occurs due to defective oxidative phosphorylation in the mitochondria [5]. Agents that exploit this mechanism and selectively inhibit glycolytic metabolism have been tested for their anticancer potential in cellular systems and animal models [6, 7]. 2-deoxyglucose (2-DG) is one such agent (Figure 1) that is phosphorylated by hexokinase to 2-DG-p that cannot be further metabolized by the glycolytic pathway leading to depleting of cellular ATP [8]. However its efficacy remains limited due to the systemic toxicity induced by the high concentrations [9].
Figure 1.
Chemical structure of compounds used in the study.
Another prominent feature of cancer cells has been their prolonged resistance to mitochondrial apoptosis which renders them capable of indefinite growth [4, 10]. Mitochondria generate most of the cellular supply of chemical energy in the form of adenosine triphosphate (ATP). Apart from ATP synthesis, the mitochondrion is also involved in a wide array of cellular processes like cell metabolism, growth, cell death, differentiation and aging [11]. Being a central player in crucial cellular functions and the established role of mitochondrial defects in tumorigenesis has led to the mitochondria being a prime target for targeted anticancer treatment strategies [12].
This understanding of the molecular mechanisms and implications of both mitochondrial pathways and glycolytic metabolism in cancer has fuelled an increase in research focusing on drugs that target both pathways [13]. Mito-carboxy proxyl (Mito-CP) – a five membered nitroxide (Figure 1) that is covalently linked to a lipophilic triphenylphosphonium cation is a mitochondria-targeted antioxidant. Mito-CP accumulates in the mitochondria due to its lipophilic nature, chain length and negative mitochondrial membrane potential [14]. Previous studies have shown that Mito-CP and Mito-Q, another mitochondria- targeted antioxidant, effectively inhibited the proliferation of breast cancer cell lines MCF-7 and MDA-MB-231 and colon cancer cell line HCT-116 [15, 16]. Recently, Mito-CP has been used in combination with glycolysis inhibitor 2-deoxyglucose (2-DG) to eradicate breast cancer cells. This study has highlighted the ability of this combination treatment to target both mitochondrial and glycolytic pathways and effectively inhibit the growth of breast cancer both in vitro and in mouse models [17].
In this study, we evaluate the synergistic effects of mitochondrial targeted antioxidant, Mito-CP, and glycolysis inhibitor 2-DG in HepG2, a human hepatocellular carcinoma cell line. Primary hepatocytes were used as control cells to determine if combination treatment induced toxicity or potential side effects to the normal liver.
MATERIALS AND METHODS
Cell culture
HepG2, a hepatocarcinoma cell line (ATCC, Manassas, VA) was maintained in Eagles Minimum Essential Medium (ATCC) containing 10% FBS (Life Technologies, Carlsbad, CA). Cells were grown to about 70–80% confluence, trypsinized with TrypLE (Life Technologies) and seeded 12– 18 h prior to treatment in all the experiments.
Cryopreserved primary human hepatocytes (Life Technologies) was thawed immediately, washed with CHRM medium (Life Technologies), counted and adjusted to a density of 0.6 × 106 cells/ml with hepatocyte plating medium supplemented with plating supplements (Life Technologies). The cells were then seeded in collagen coated 96 well plates (Life Technologies) and placed in the incubator for 4–6 hr for cell attachment and form a monolayer. Then, the plate was shaken to loosen debris. The medium was aspirated and replaced with hepatocyte maintenance medium supplemented with maintenance supplements and placed in an incubator at 37°C and 5% CO2 overnight before treatment in all the experiments performed in this study.
A stock solution of 1M 2-DG (Sigma Aldrich, St Louis, MO) in PBS was prepared. A stock solution of 100 mM Mito-CP in ethanol was prepared. (Mito-CP was synthesized in the laboratory of Dr. Balaraman Kalyanaraman, Medical College of Wisconsin, Milwaukee, WI). Fresh dilutions of the Mito-CP and 2-DG in cell culture medium were made for each experiment.
Cell viability assay
HepG2 cells were seeded at density of 20,000 cells per well in 96 well plates and incubated for 12–18 hr under 5% CO2 at 37°C. Following incubation, cells were treated with increasing concentrations of Mito-CP (1 µM- 4 µM final concentration) and 2-DG (1 mM – 5 mM final concentration) both individually and in combination for a period of 24 hr and then MTT assay was carried out (Promega, Madison, WI). As a control, primary hepatocytes were also seeded in collagen coated 96-well plates and, treated with same concentrations of Mito-CP and 2-DG for a period of 24 hr and then the viability was determined by MTT assay. Experiments were performed in triplicates and data was plotted as an average ± standard error of the mean.
Cytotoxicity assay
HepG2 and primary hepatocytes cells were seeded at a density of 16,000 cells per well in 96-well plates and incubated overnight. The cells were then treated with Mito-CP and 2-DG as described above. For cytotoxicity assay, YOYO-1 (Life Technologies) was diluted in cell culture medium and added to a final concentration of 0.1 µM to both experimental and control wells. YOYO-1 is a cell impermeant cyanine dimer nucleic acid stain that can only enter cells with a compromised plasma membrane and fluorescently stain the nuclear DNA (18). By adding YOYO-1 to cells, we measured in real time the increase in YOYO-1 fluorescence, i.e., cell toxicity corresponding to treatment with Mito-CP and 2-DG. The treated plates were placed in an Incucyte FLR imaging system, and the YOYO-1 fluorescence was measured every 2 h for a period of 24 h. Following the 24 h incubation period, end point analysis was performed to measure the total number of DNA containing objects by treating cells with 0.0625% triton X-100 to permeabilize the cell membrane. The plate was further incubated for 1 – 2 h to allow staining of nuclear DNA by YOYO-1.
Object counting analysis was performed using the Incucyte FLR software to calculate the total number of YOYO- 1 fluorescence positive cells and total DNA containing objects (end point). The cytotoxicity index was calculated by dividing the number of YOYO-1 fluorescence positive objects by the total number of DNA containing objects for each treatment group.
ATP assay
HepG2 cells and primary hepatocytes were seeded at a density of 20,000 cells per well in 96 well plates and placed in the incubator for overnight as described earlier. The cells were then treated with increasing concentrations of Mito-CP (1 µM– 4 µM final concentration) and 2-DG (1 mM – 5 mM final concentration) both individually and in combination for a period of 6 h.
The ATP levels were measured using the ATP bioluminescent somatic cell assay kit (Sigma Aldrich) according to manufacturer’s instructions. Following the measurement of ATP, 20 L of the cell lysates was used to assay the protein concentration in all the treatment groups by the Bradford assay. The total ATP levels were normalized with respect to the total protein concentration in each well. Experiments were performed in triplicates and data was plotted as an average ± standard error of the mean.
Caspase 3/7 apoptosis assay
HepG2 and primary hepatocytes cells were seeded as described earlier. The next day cells were treated with Mito-CP and 2-DG dissolved to appropriate concentrations in cell culture medium. Caspase 3/7 apoptosis reagent (Essen Biosciences, Ann Arbor, Michigan) was diluted in cell culture medium and added to a final concentration of 5 µM to both experimental and control wells. Caspase 3/7 apoptosis reagent (Essen Biosciences) is an inert non fluorescent substrate that can enter cells by freely crossing the cell membrane. Once inside cells, this molecule gets cleaved by the activated caspase 3/7 in cells to release a fluorescent green DNA labeling dye. By treating cells with this reagent, we measured in real time the increases in caspase 3/7 apoptotic activity in response to increasing concentrations of combination treatment with Mito-CP and 2-DG.
The plate was placed within a micro plate tray in the Incucyte FLR Imaging system (Essen Biosciences) and scanned for fluorescence signal and Phase contrast images every 2 h for a period of 24 h. At the end of the 24 h time point, total DNA object count was performed by labeling all DNA containing objects using the Vibrant Dye Cycle Green DNA stain (Life Technologies). The dye was added to the wells at a final concentration of 1 µM and incubated for 1–2 h in the Incucyte FLR system before imaging. Object counting analysis was performed using the Incucyte FLR software to calculate the total number of caspase 3/7 positive cells and total DNA containing objects (end point). The apoptotic index was calculated by dividing the number of caspase 3/7 positive objects by the total number of DNA containing objects for each treatment group.
Western blot assay
HepG2 cells treated with indicated concentrations of control, 2-DG, Mito-CP, or in combinations were lysed in cell lysis buffer (Sigma) and the total protein concentration was quantified using bicinchoninic acid assay (BCA) kit (Pierce Biotechnology, Rockford, IL). Denatured cell lysates were resolved in a 4–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) gels, transferred onto nitrocellulose membranes (Bio-Rad Laboratories), blocked in 5% non-fat milk solution in PBS containing 0.1% tween (PBST) for 1 hr, and incubated with primary antibodies overnight at 4°C. The antibodies and dilutions were: PARP (1:2000), Procaspase-3, Procaspase-7, Mcl-1 (1:1000, Cell signaling Technology, Danvers, MA) survivin, Bax, BclXL, GAPDH (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA). Following incubation with primary antibody, the membranes were washed three times with PBST and incubated for 2 hr at room temperature with secondary antibodies (1:1000, Goat anti-mouse IgG-HRP or Goat anti-rabbit IgG-HRP, Santa Cruz Biotechnology). Then the membranes were washed with PBST three times for either 5 min or 10 min depending on the signal. ClarityTM western ECL substrate or Immun-Star substrate (Bio-Rad) was added to the membranes and the expression of proteins was detected using Molecular ImagerTM ChemiDoc XRS+ imager with image lab software (Bio-Rad).
RESULTS
Effect of Mito-CP and 2-DG on the cell viability of HepG2 cells and primary hepatocytes
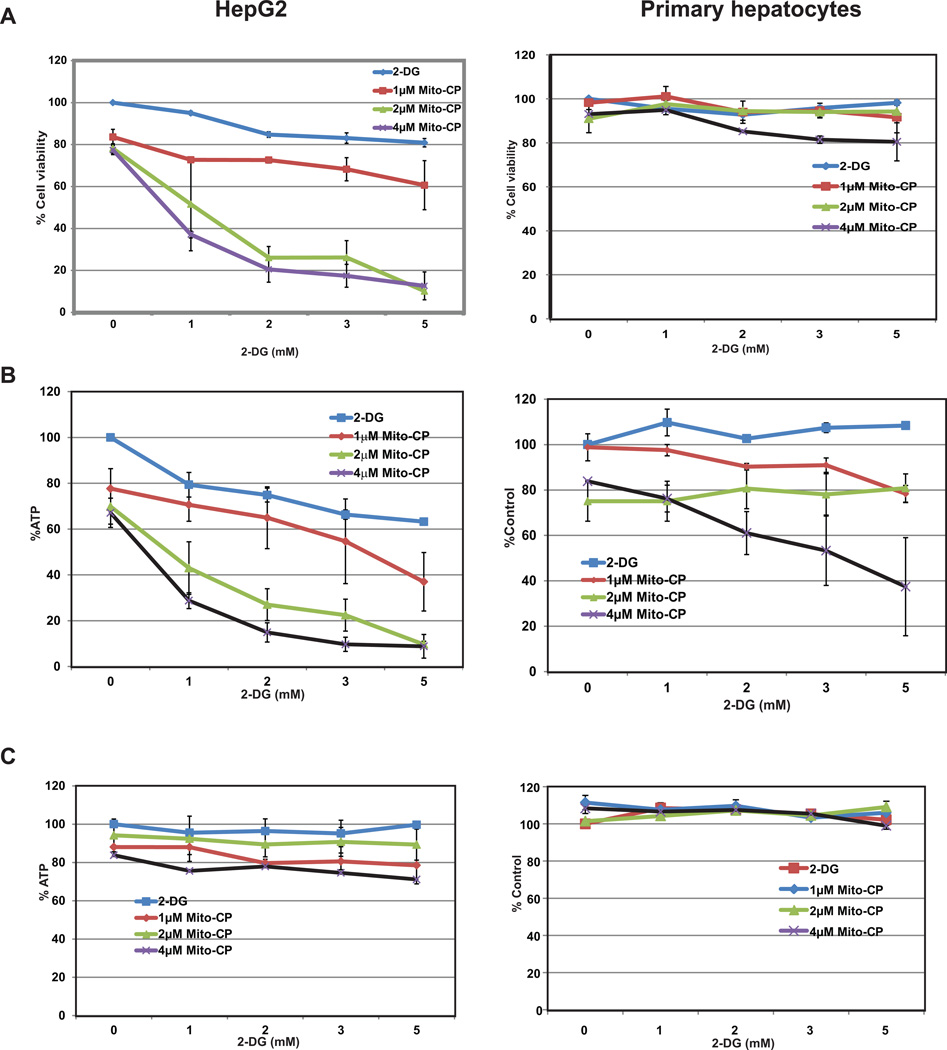
The results of the MTT assay post 24 h incubation with increasing concentrations of Mito-CP and 2-DG in HepG2 cells and primary hepatocytes are shown in Figure 2A. Both Mito-CP and 2-DG moderately decreased (approximately at higher dose 20%) the viability of HepG2 cells in a dose-dependent manner. Treatment with up to 5 mM 2-DG alone or up to 4 µM of Mito-CP alone did not visibly reduce the viability of HepG2 cells. Most importantly, treatment with 2 µM Mito-CP and 1 mM 2-DG in combination lead to an increased reduction in HepG2 cell viability by 50% (Figure 2A). On the other hand, primary cells remained unaffected at all individual and combination treatment doses maintaining a cell viability of >80% (Figure 2A).
Figure 2.
Effects of Mito-CP and 2-DG on proliferation of HepG2 and primary hepatocytes. A.HepG2 and primary hepatocytes cells were treated with increasing concentrations of Mito-CP, 2-DG, alone or in combinations for 24 hr. Proliferation results of average of two independent experiments are shown. HepG2 cell growth inhibition occurs significantly starting at a dose of 2 µM Mito-CP plus 2 mM 2-DG. Primary cells exhibit no reduction in cell viability. Individual treatment either Mito-CP or 2-DG did not affect the growth of HepG2 and primary hepatocytes. Analysis of ATP levels in HepG2 (B) and primary hepatocytes (C). Cells were treated with increasing concentrations of Mito-CP and 2-DG as indicated either alone or in combinations for 6 hr. and assayed for the levels of ATP. There is more than 50% depletion of ATP in HepG2 cells starting at treatment combinations of 2 µM Mito-CP and 2 mM 2-DG. However, the ATP levels in primary hepatocytes remain stable (>80%) at all treatment concentrations. Protein levels drop slightly at higher treatment concentrations in HepG2 cells but remain unaffected in primary hepatocytes.
Effect of Mito-CP and 2-DG on ATP levels of HepG2 cells and primary hepatocytes
To further confirm the growth inhibition by the combination treatment, we measured the intracellular ATP levels after treatment. Post treatment with Mito-CP and 2-DG (6 h), the ATP levels of HepG2 cells reduces significantly with increasing treatment concentrations (Figure 2B). While treatment with 2 µM Mito-CP reduced the ATP concentration to 70% of control cells ATP levels, a combination of 2 M CP and 1 mM 2-DG further brought it down to 40% of control cell ATP levels. The protein concentrations of HepG2 cells remained fairly stable except for a slight decrease at higher concentrations indicating that the cells remained viable at the time when the ATP levels were measured (Figure 2B). ATP levels and protein concentration remained unchanged in primary hepatocytes following 6 h treatment with both individual and combination doses of Mito-CP and 2-DG (Figure 2C).
Cytotoxic effects of Mito-CP and 2-DG on HepG2 cells and primary hepatocytes
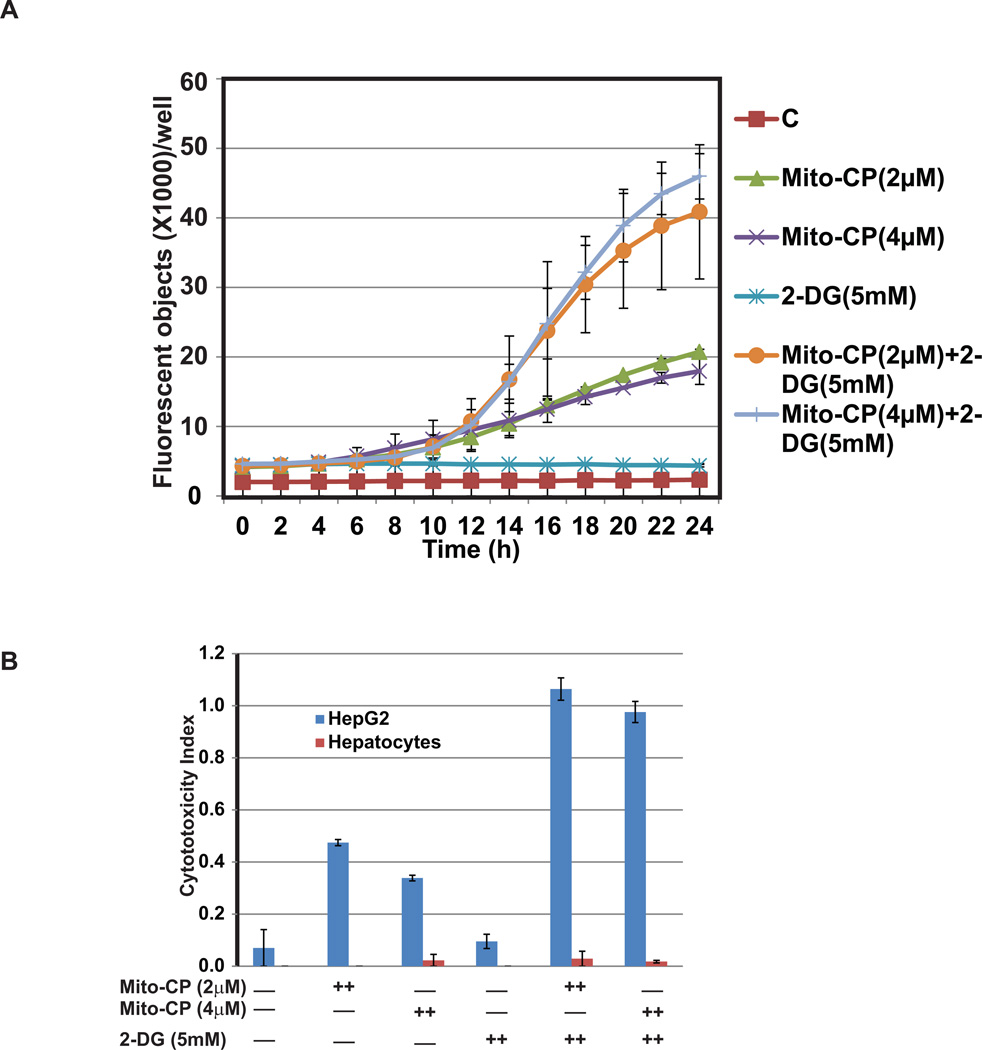
To determine the status of cytotoxic effect during the treatment, real time increases in cytotoxic effects of Mito-CP and 2-DG on HepG2 cells were monitored by using the YOYO-1 cell impermeant nucleic acid stain and Incucyte FLR imaging system (Figure 3A). We observed a steady increase in YOYO-1 fluorescence (caused by binding of the dye to nucleic acid) which initiated at about 10 h after treatment and peaked at 24 h on combination treatment with Mito-CP and 2-DG. Cells treated individually with Mito-CP or 2-DG did not induce an increase in YOYO-1 fluorescence signal proving the synergistic effects of the compounds in inhibiting HCC. The cytotoxicity index of HepG2 cells mirrors the real time data showing sharp increases with combination treatment (Figure 3B).The cytotoxic index of primary hepatocytes remained negligible in all treatment groups being consistent with cell viability assay results (Figure 3B). Based on the results from cell viability, cytotoxicity assay, and measurement of ATP levels, we confirmed that Mito-CP in combination with 2-DG treatment potentiate the reduction of HepG2 cellular growth compared to single agent treatment. Furthermore, the combination treatment appears to be synergic.
Figure 3.
Mito-CP and 2-DG combination induces cytotoxicity in HepG2 cells determined by YOYO-1 fluorescence assay (A) and cytotoxicity index (B). Measurement of cytotoxicity in real time using YOYO-1 fluorescence in HepG2 cells in response to treatment with 2, or 4 µM Mito-CP and 5 mM 2-DG both individually or in combination for 24 hr (A). YOYO-1 fluorescence increases significantly with combination treatment in HepG2 cells compared to control untreated group as well as treated individually with Mito-CP or 2-DG. Induction of cytotoxicity in HepG2 cells by combination treatment of Mito-CP and 2-DG determined by caspase 3/7 apoptosis assay (B). HepG2 and primary hepatocytes cells were treated with alone or in combination with Mito-CP and 2-DG for 24 hr. and scanned for the fluorescence signal and phase contrast images. Cytotoxicity index in HepG2 cells is highest in cells treated with a combination of 2, or 4 µM Mito-CP with 5 mM 2-DG compared to control treatment. Importantly treatment to primary hepatocytes showed very marginal cytotoxicity index compared to HepG2 cells under the same condition. Average of two independent experiments is shown.
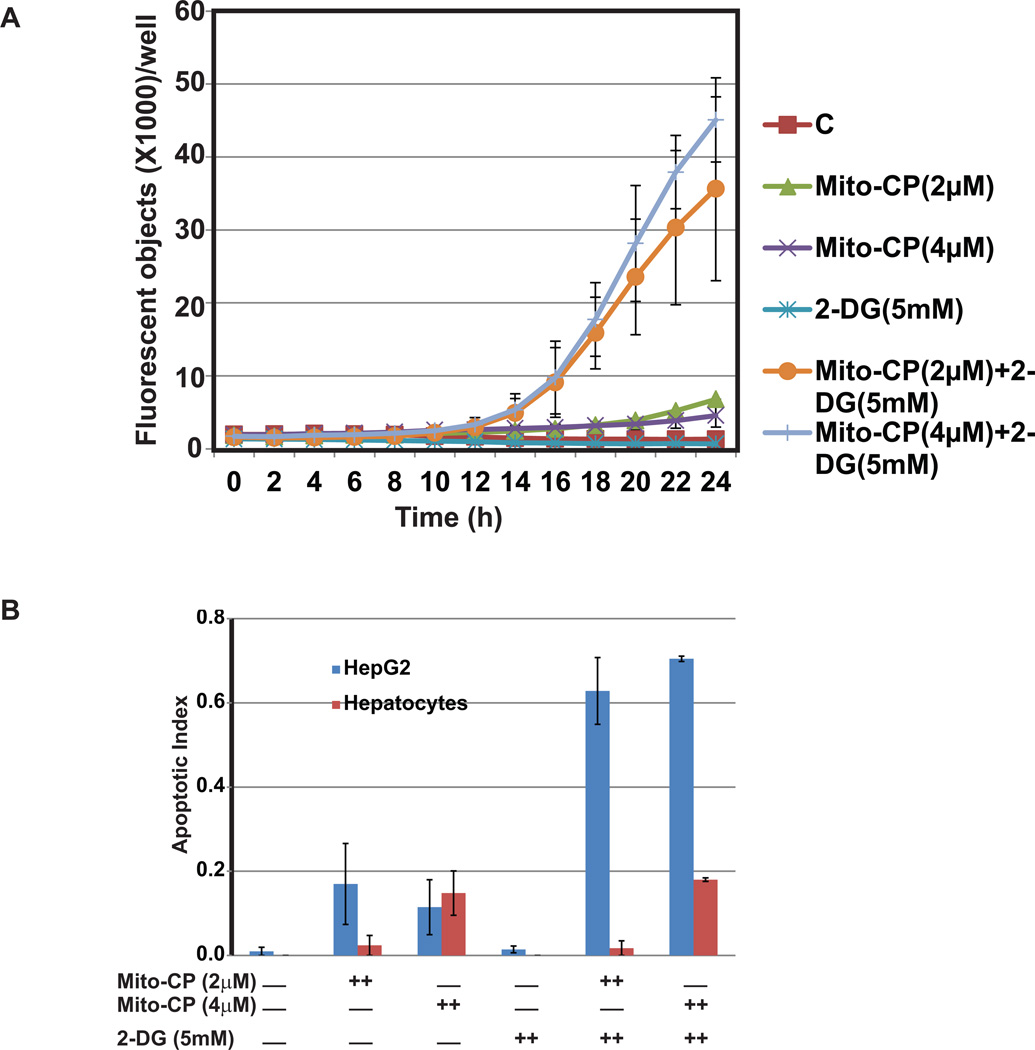
Combined treatment with Mito-CP and 2-DG induces apoptosis in HepG2 cells
To examine if Mito-CP and 2-DG inhibit the survival of HepG2 cells by triggering the apoptotic pathway, we measured the increase in caspase 3/7 activity following treatment. Real time increases in caspase 3/7 activity was monitored by using the Incucyte FLR imaging system. Caspase 3/7 activity increases consistently in both the combination treatment groups (2 µM Mito-CP+ 5mM 2-DG and 4 µM Mito-CP + 5 mM 2-DG) with time. The real time object counting analysis curve shows initiation of apoptotic activity at about 11 hr after treatment and peaks at 22 hr. Individual treatments with Mito-CP or 2-DG showed no increase in caspase 3/7 activity. The apoptotic index (as determined from the end point assay) is highest in the combination treatment groups (Figure 4A) and is thus consistent with the synergistic trend and real time caspase 3/7 activity for HepG2 cells. As predicted based on our MTT data, primary cells did not exhibit caspase 3/7 activity. The apoptotic index remained fairly low in all treatment groups with a minor increase in the groups treated with higher concentration of Mito-CP (4 µM) (Figure 4B).
Figure 4.
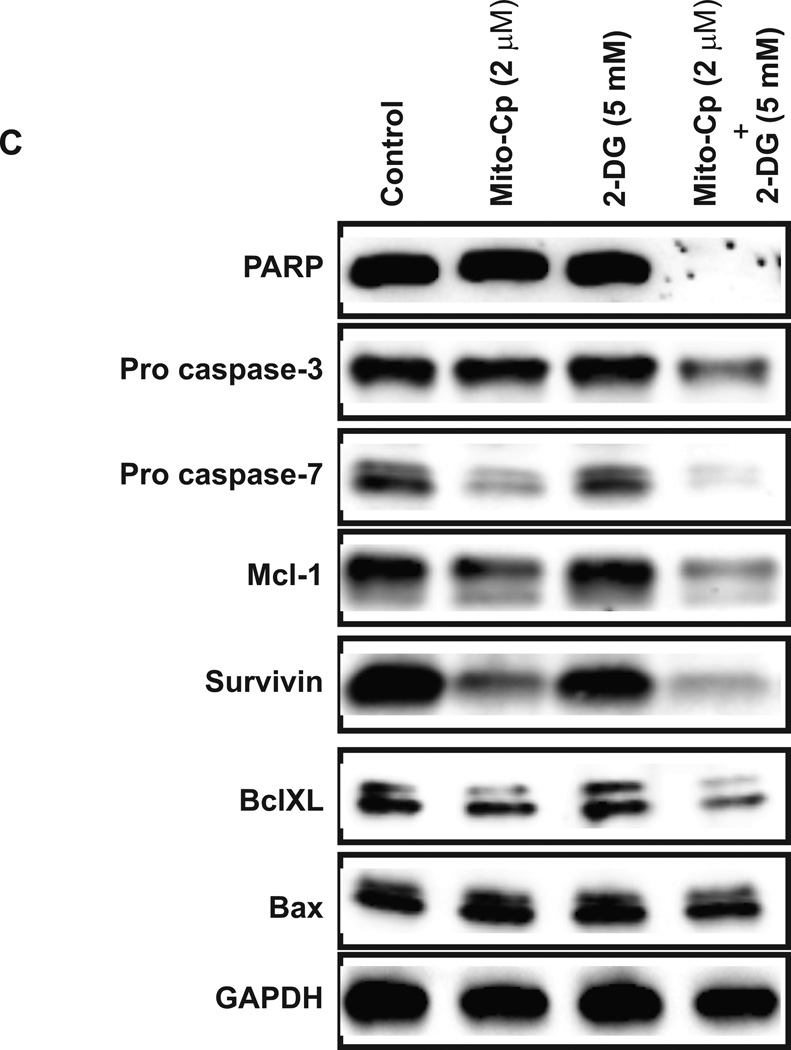
Induction of apoptosis by the combinations of Mito-CP and 2-DG. HepG2 cells and primary hepatocytes were treated with the indicated concentrations of Mito-CP, 2-DG, or in combinations for 24 hr. The DNA binding activity of the dye was visualized by fluorescence signal and phase contrast images for every 2 hr (A). Average of two independent experiments is shown here. Real time measurement of apoptotic activity as indicated by increase in caspase 3/7 positive HepG2 cells in response to treatment with 2, or 4 µM Mito-CP and 5 mM 2-DG both individually and in combination. Caspase 3/7 activity increases significantly with combination treatment compared to control untreated group and cells treated individually with Mito-CP and 2-DG. Apoptotic index in HepG2 cells is highest in groups treated with a combination of 2 or 4 µM Mito-CP with 5 mM 2-DG (B). Primary hepatocytes treatment did not induce apoptosis. (C). Effects of combination of Mito-CP and 2-DG on expression and activation of apoptosis markers. Lysates from control and treated groups of HepG2 cells were immunoblotted with antibodies against the indicated proteins.
To further confirm that HepG2 cell growth is inhibited by activation of apoptosis, we assessed the expression of pro and anti-apoptotic proteins in response to treatment with Mito-CP and 2-DG by western blot (Figure 4C). The combination of Mito-CP and 2-DG induced apoptosis by activating caspases-3 and 7 by reducing the procaspases more than either drug alone. To confirm the function of caspase activity, we looked for the expression level of total PARP. As shown in Figure 4C, the expression of PARP protein was completely reduced in combination treatment. Furthermore, the level of anti-apoptotic protein survivin is also reduced significantly compared to either treatment alone. A Bcl-2 family member, BclxL, and Mcl-1 localize to the mitochondria and inhibit apoptosis. In combination treatment, the levels of these proteins were reduced significantly. The level of Bax protein is not changed with treatment.
DISCUSSION
In the present study we show that Mito-CP – a mitochondrial targeted antioxidant in conjunction with a glycolysis inhibitor 2-DG caused a selective and synergistic cytotoxicity in hepatocellular carcinoma cells. Nitroxides have been used in cancer research as therapeutics, oximetric and imaging agents. In a previous study, the nitroxide 5-carboxy-1, 1, 3, 3 -tetramethylisoindolin-2-yloxyl (CTMIO) has been shown to ameliorate tumorigenic propensity in mice [18]. Another study highlights the potential use of tempol (nitroxide) as a therapeutic agent against malignant gliomas [19]. 2-DG on the other hand is a glycolysis inhibitor that has been extensively studied over the years for its cancer inhibiting properties. Recently, several studies have used 2-DG in innovative combination therapies and synergistic treatment strategies against a broad spectrum of cancers. Recently, 2-DG was used in conjunction with Bcl2 antagonists to study the therapeutic potential of this combination against prostate cancer [20]. Furthermore, 2-DG has also been used in combination with AMPK agonists for studying its ability to dual target energy pathways and induces growth inhibition in multiple types of cancer [21].
We hypothesized that using Mito-CP and 2-DG (whose anticancer properties have been established in a wide range of cancers) in conjunction might enable us to preferentially target and inhibit HCC cell proliferation at a much lower concentration than when used individually. Since this combination would directly target the site of energy generation in the cell and due to the previously established differences in the energy production mechanisms between cancer and normal cells, this combination might prove advantageous for specific targeted inhibition of HCC. Furthermore, a similar strategy using mitochondrial targeted nitroxides with cytotoxic chemotherapeutic compounds has resulted in enhanced anticancer effect against breast cancer cells while being nontoxic to normal cells [22].
Results of this preliminary study confirm that 2-DG and Mito-CP in conjunction induce cytotoxicity and inhibit proliferation of HepG2 cells in a possible synergistic manner while causing no measureable cytotoxicity or change in the viability of primary human hepatocytes (Figure 2A, Figure 3). Previous studies have shown the cytotoxic effects of 2-DG alone in various T cell and B cell lymphoma cell lines [23, 24]. Cytotoxic effects of 2-DG in combination with apoptosis inducing ligands in melanoma cells has been reported [23]. However, 2 –10 fold higher doses of 2-DG have been used in these studies compared to the present study [23, 24]. By using Mito-CP in combination with 2-DG, we are able to reduce the concentration of 2-DG by 2–10 folds compared to previous studies and still bring about a significant reduction in viability of HepG2 cells. Thus, this combination may provide reduced toxicity to normal liver as a valuable advantage.
The result of reduction in ATP level compared to either treatment alone or to treated primary hepatocyte showed no change in the total protein levels in all groups strongly suggests that the drop in ATP levels in HepG2 cells is solely the effect of treatment with Mito-CP and 2-DG. Based on this, we can anticipate the use of reduced doses in combination with decreased toxicity to the normal liver may be a potential treatment option for the HCC patient (Figure 2B, 2C).
Multi-cellular organisms maintain a delicate balance between the mechanisms of cell survival and death for normal tissue maintenance and development. Tumor development occurs when intrinsic deregulation in the apoptotic mechanism is triggered in certain cells, which enables continued growth [25]. Activation of a set of cysteine proteases termed caspases which further cleave cell substrates is a key step in the apoptotic process [26]. Caspase 3 and 7 are two such effector caspases whose activation indicates the cells irreversible commitment to apoptosis and therefore are reliable markers for apoptosis [27]. The synergistic increase in caspase 3/7 activity (Figure 4) is an indicative that Mito-CP and 2-DG in combination trigger apoptosis to induce HepG2 cell death in a time- and dose-dependent fashion. This was confirmed by western analysis for the expression of these proteins (Figure 4C). The increase in apoptotic activity is consistent and parallel in time to the observed cytotoxicity in HeG2 cells. However compared to HepG2 cells, the apoptotic activity in primary hepatocytes remained negligible (Figure 4B).
In summary, we have shown that Mito-CP and 2-DG increase cytotoxicity and decreases the viability of HepG2 cells compared to control as well as primary hepatocytes. This combination treatment invades the ATP producing mechanism and associated with induction of apoptosis in HepG2 cells while causing no measurable change in the viability or energy producing capabilities in primary hepatocytes. This provides evidence for further study into using this combination therapy against hepatocellular carcinoma.
Acknowledgments
Financial support: This research was supported by funding from “The Medical College of Wisconsin Dean’s Program Development Funding”.
Footnotes
Conflict of interest: The authors declared no conflict of interest with respect to this manuscript.
REFERENCES
- 1.Parikh S, Hyman D. Hepatocellular cancer: a guide for the internist. Am J Med. 2007;120:194–202. doi: 10.1016/j.amjmed.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Cahill BA, Braccia D. Current treatment for hepatocellular carcinoma. Clin J Oncol Nurs. 2004;8:393–399. doi: 10.1188/04.CJON.393-399. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Groof AJC, Lindert MMT, van Dommelen MMT, et al. Increased OXPHOS activity precedes rise in glycolytic rate in H-RasV12/E1A transformed fibroblasts that develop a Warburg phenotype. Molecular Cancer. 2009;8 doi: 10.1186/1476-4598-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Mjiyad N, Caro-Maldonado A, Ramirez-Peinado S, Munoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011;30:253–264. doi: 10.1038/onc.2010.466. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One. 2011;6:e17234. doi: 10.1371/journal.pone.0017234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwarakanath BS, Singh D, Banerji AK, et al. Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects. J Cancer Res Ther. 2009;5(Suppl 1):S21–S26. doi: 10.4103/0973-1482.55136. [DOI] [PubMed] [Google Scholar]
- 10.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 11.Pathania D, Millard M, Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev. 2009;61:1250–1275. doi: 10.1016/j.addr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen V, Staub RE, Fong S, et al. Bezielle selectively targets mitochondria of cancer cells to inhibit glycolysis and OXPHOS. PLoS One. 2012;7:e30300. doi: 10.1371/journal.pone.0030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtoglu M, Lampidis TJ. From delocalized lipophilic cations to hypoxia: blocking tumor cell mitochondrial function leads to therapeutic gain with glycolytic inhibitors. Mol Nutr Food Res. 2009;53:68–75. doi: 10.1002/mnfr.200700457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao VA, Klein SR, Bonar SJ, et al. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem. 2010;285:34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng G, Zielonka J, Dranka BP, et al. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012;72:2634–2644. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gueven N, Luff J, Peng C, et al. Dramatic extension of tumor latency and correction of neurobehavioral phenotype in Atm-mutant mice with a nitroxide antioxidant. Free Radical Biology and Medicine. 2006;41:992–1000. doi: 10.1016/j.freeradbiomed.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Gariboldi MB, Ravizza R, Petterino C, et al. Study of in vitro and in vivo effects of the piperidine nitroxide Tempol--a potential new therapeutic agent for gliomas. Eur J Cancer. 2003;39:829–837. doi: 10.1016/s0959-8049(02)00742-6. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi R, Janssen E, Perkins G, et al. Efficient elimination of cancer cells by deoxyglucose-ABT-263/737 combination therapy. PLoS One. 2011;6:e24102. doi: 10.1371/journal.pone.0024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheong JH, Park ES, Liang J, et al. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther. 2011;10:2350–2362. doi: 10.1158/1535-7163.MCT-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng G, Lopez M, Zielonka J, et al. Mitochondria-targeted nitroxides exacerbate fluvastatin-mediated cytostatic and cytotoxic effects in breast cancer cells. Cancer Biol Ther. 2011;12:707–717. doi: 10.4161/cbt.12.8.16441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin JZ, Xin H, Nickoloff BJ. 2-Deoxyglucose sensitizes melanoma cells to TRAIL-induced apoptosis which is reduced by mannose. Biochemical and Biophysical Research Communications. 2010;401:293–299. doi: 10.1016/j.bbrc.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 24.Zagorodna O, Martin SM, Rutkowski DT, et al. 2-Deoxyglucose-induced toxicity is regulated by Bcl-2 family members and is enhanced by antagonizing Bcl-2 in lymphoma cell lines. Oncogene. 2012;31:2738–2749. doi: 10.1038/onc.2011.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 26.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]