Abstract
The CYP2D6 gene encodes for an enzyme that is involved in the metabolism of more than 25% of all medications, including many opioids and antiemetics. It may contribute to the risk of postoperative nausea and vomiting (PONV), a common surgical complication. However, little research has been conducted in this area. The purpose of this study was to explore the association of CYP2D6 genotypes with PONV in adult surgical trauma patients. Data from 112 patients (28% female) with single extremity fractures, aged 18–70 years, were analyzed. PONV was defined as present if patients reported nausea, were observed vomiting, or received medication for PONV. Saliva samples collected for DNA extraction and Taqman® allele discrimination and quantitative real time polymerase chain reaction (qRT-PCR) were used to collect genotype data that were then used to assign CYP2D6 phenotype classification. The incidence of PONV was 38% in the postanesthesia care unit and increased to 50% when assessed at 48 hr. CYP2D6 classification results were 7 (6%) poor metabolizers, 34 (30%) intermediate metabolizers, and 71 (63%) extensive metabolizers. No ultrarapid metabolizers were identified. Patients who were classified as poor metabolizers had less PONV and higher pain scores. Gender and history of PONV, but not smoking, were also significant risk factors. Findings suggest variability in CYP2D6 impacts susceptibility to PONV.
Keywords: CYP2D6, nausea, vomiting, postoperative symptoms, PONV
Postoperative nausea and vomiting (PONV) has long been a major concern for nurses caring for surgical patients. PONV is a strong predictor for prolonged hospital stay and the most frequent cause for unanticipated admission following outpatient procedures (Marla & Stallard, 2009). In addition to extreme discomfort, potential adverse effects include aspiration, wound dehiscence, bleeding, hematoma, dehydration, electrolyte imbalance, and delays in resuming oral medications, mobilization, and recovery (Jolley, 2001; Miaskowski, 2009). The use of opioids for postoperative pain is the primary predictor of PONV (Watcha & White, 1992). Three mechanisms involving opioids lead to stimulation of the emetic center in the medulla following surgery (Nielson & Olsen, 2008): First, reduced gastrointestinal (GI) mobility from postoperative opioids or manipulation of the GI tract during surgery generates a release of serotonin from the enterochromaffin cells in the GI tract (enterochromaffin cells produce 90% of the body’s serotonin). Serotonin then binds to serotonin receptors located peripherally in the vagal nerve terminals, stimulating vagal afferent neurons that in turn directly activate the vomiting center (Ho & Gan, 2006). The second mechanism occurs when opioids used for postoperative pain stimulate serotonin (5-HT3) receptors located in the chemoreceptor trigger zone which then send impulses to the vomiting center in the medulla oblongata. A third mechanism arises when opioids enhance the sensitivity of the middle ear to movement (Nelson, 2002). This action activates the emetic center through the release of histamine and acetylcholine from vestibular fibers. Other well-established predictors of PONV include female gender, a negative smoking history and previous history of PONV or motion sickness illness (Apfel et al., 2008). The use of serotonin antagonists has improved the management of PONV, but despite the evolution of new medications and risk protocols, 20–30% of postoperative patients continue to experience PONV (Kranke et al., 2009). It is not clear why some patients respond well to antiemetic protocols and others do not. Understanding genetic variability in the mechanisms underlying PONV may help to clarify this variability in PONV.
The gene CYP2D6, a member of the CYP450 super family, encodes for a hepatic enzyme involved in the metabolism of 25% of all medications, including many opioids and antiemetics (Owen, Sangkuhl, Klein, &Altman, 2009; Zhou, 2009). CYP2D6 is located on chromosome 22, where it forms part of the CYP2D6 gene cluster with two nonfunctional pseudogenes, CYP2D7P and CYP2D8P (Arneth, Shams, Hiemke, & Hartter, 2009). CYP2D6 is highly polymorphic, with over 100 known allelic variants (Ingelman-Sundberg, Daly, & Nebert, 2009). Some polymorphisms lead to a complete loss of CYP2D6 function and others to reduced activity. Researchers have developed a system to classify patients into one of four types based on allelic variation: poor metabolizers (PMs), intermediate metabolizers (IMs), extensive metabolizers (EMs) and ultrarapid metabolizers (UMs), (Daly et al., 1996). EMs have normal CYP2D6 metabolism, with CYP2D6*1 considered the “wild-type” or reference allele (Sweeney, 2003). Sachse, Brockmöller, Bauer, and Roots (1997) report the prevalence of the different metabolizers in the Caucasian population as EMs 70–80%, IMs 10–17%, PMs 5–10%, and UMs 2–5%. Due to potential overlap and clinical similarities, investigators sometimes report IM and EM types as one group labeled EM/IM (de Leon, Armstrong, & Cozza, 2006). Other research provides evidence that patients who are classified as IMs are unique, with decreased metabolic activity that may be closer to that of the PMs (Furman et al., 2004; Gaedigk et al., 2008).
The most common variant alleles found in Caucasians are CYP2D6*4 (23%), which causes a splicing defect resulting in a nonfunctioning allele, and CYP2D6*5 (4%), which results in the total deletion of CYP2D6 (Arneth et al., 2009). Patients who are homozygous for CYP2D6*5 and CYP2D6*4 are classified as PMs. PMs are actually at risk for two potential types of problems. First, because they metabolize CYP2D6 substrates (drugs for which CYP2D6 is necessary for their metabolism) more slowly, they have increased plasma exposure to those drugs, which can increase the risk of a drug-induced adverse reaction. PMs are also at risk of reduced efficacy from medications that require CYP2D6 for conversion to an active drug. For example, oxycodone, a medication frequently prescribed for postoperative pain, requires CYP2D6 for the creation of oxymorphone, the active metabolite of oxycodone (Holmquist, 2009). Ultrametabolizers (UMs) are the result of a copy number variant (extra genes), resulting in increased metabolic capacity (Arneth et al., 2009). UMs usually require less opioids in the postoperative period compared to the other groups due to enhanced metabolic activity (Yang, et al., 2012), yet they are also at risk of intense adverse events from this more rapid metabolism (Leppert, 2011).
Researchers first found that CYP2D6 effected drug metabolism in a pharmacokinetic study of the antiarrhythmic drug sparteine when they serendipitously observed that some subjects experienced symptoms of nausea and diplopia, which are indicative of toxic doses of sparteine (Eichelbaum, Spannbrucker, Steincke, & Dengler, 1979). Only recently have researchers demonstrated that the defective metabolism first localized by Eichenbaum and colleagues 30 years ago is inherited as an autosomal recessive trait affecting the CYP2D6 gene (Sweeney, 2003). Several studies have examined the association of CYP2D6 and nausea and vomiting. Candiotti and colleagues (2005) compared 250 female patients in terms of their CYP2D6 metabolic status and incidence of PONV. When they analyzed the 88 subjects who experienced PONV by genotype, they found that 45% of the patients classified as UM (5 of the 11 patients) experienced PONV as compared to 8% in the PM group, 17% in the IM group, and 15% in the EM group. Janicki, Schuler, Jarzembowski, and Rossi (2006) compared treatment of PONV with granisetron and dolasetron in relation to the CYP2D6 genotype in 150 subjects considered to have moderate-to-high risk of PONV. Among the subjects treated with dolasetron (a drug that is metabolized by CYP2D6), the greatest incidence of nausea and vomiting occurred in subjects who were classified as UM. Kirchheiner and colleagues (2008) studied the response to opioids in healthy subjects preclassified as either UM or EM. They found that subjects classified as UM were more sensitive to opioids than those who were classified as EM. In a different group of patients, Kaiser and colleagues (2002) also found significantly more nausea and vomiting in their oncology patients who were defined as UMs.
The current antiemetic drug of choice is ondansetron, a serotonin receptor antagonist that is metabolized in part by CYP2D6. Stamer, Rauers, Eun-Hae, Mubhoff, and Stüber (2009) found that surgical subjects that possessed three or more active CYP2D6 alleles (and were thus classified as UM) had reduced ondansetron plasma concentration compared to those subjects that had zero-to-two active alleles. Candiotti and colleagues (2005) also observed a higher incidence of ondansetron failure for vomiting in their subjects with three or more copies of the CYP2D6 gene. There is strong evidence that subjects who are genotyped as UMs will experience rapid drug metabolism and be at an increased risk of PONV, though based on findings of other population-level studies of Caucasians, we would expect as few as 3% of patients to be categorized as UMs (Zhou, 2010). Because of the low incidence of UMs, other researchers have looked more closely at the larger number of patients classified as IM or PM. Zwisler and colleagues (2009) compared only PMs and EMs in their study of the hypoalgesic effects of oxycodone in 33 healthy volunteers. Though working with a small sample, they found statistically significant differences in the analgesic effects of oxycodone based on genotype, but they did not find differences in the opioid side effects of nausea and vomiting. A published case study about a CYP2D6 poor metabolizer demonstrates the potential of personalized medicine. The report describes an 85-year-old woman recovering from hip surgery. The patient had a long-standing intolerance to codeine, and during her first admission clinicians ordered treatment with oxycodone combined with tramadol. The initial oxycodone dose was 5 mg combined with 500 mg of acetaminophen every 12 hr. The dosage was increased to 10 mg every 12 hr and then 7.5 mg every 6 hr. Analgesia was still not achieved, and the patient suffered severe nausea and vomiting. When she was readmitted for further surgery, clinicians made the decision to analyze her CYP2D6 status and classified her as PM (Susce, Murray-Carmichael, & de Leon, 2006).
Increasing our understanding of both known risk factors for PONV and possible genetic risk factors has the potential to decrease or eliminate this common and distressing postoperative complication. The purpose of the present study was to explore the association between PONV and CYP2D6 genotypes in trauma patients admitted for surgical repair of a single extremity fracture. We selected this patient population to minimize the possibility of potential confounding variables related to comorbidities and surgical procedure.
Methods
Design and Sample
With Institutional Review Board approval, we enrolled 143 patients. Participants were between 18 and 70 years old, received general anesthesia or general and regional anesthesia for surgery that was expected to be ≤ 4 hr, had an isolated orthopedic injury, and had an American Society of Anesthesiologists physical status of I, II, or III. We excluded potential participants for reported opioid use during the 6 months prior to surgery, reported alcohol use in the 24 hr preceding surgery, recreational drug use in the preceding 6 months, or any documented history of alcohol abuse, mental illness, hepatic disease, renal disease, neurologic conditions such as stroke, head injury, spinal cord injury, or intracerebral hemorrhage, or previous history of arthritis or bone disease to eliminate the potential confounding role of these variables. To control for population stratification, we limited our analyses to self-reported Caucasians, which produced a sample size of 112 participants.
Data we collected included demographic characteristics, pain scores, presence of PONV as measured by the need for rescue antiemetics, amount of opioids given during surgery and amount of opioids given while in the postanesthesia care unit (PACU), current smoking status and history of previous PONV or motion sickness. We recorded amount and type of anesthesia agents from the anesthesia record. We assessed pain via self-report 15 min after the participant was admitted to the PACU and again at 45 min after admission to the PACU. We operationalized pain on an 11-point verbal pain score (VPS), with 0 representing no pain and 10 representing the worst pain ever imagined. Investigators have shown the VPS to be equivalent in the measure of pain as the frequently used visual analog scale (Cork et al., 2004). We assessed PONV as a binary variable. If the participant received antiemetic medication postoperatively for PONV, we considered them to be positive for PONV. Because it is standard practice in our institution to medicate all surgical patients with 4 mg of IV ondansetron before they leave the operating room (OR), we did not include this dose in our data collection. We obtained PONV measures at two points: that which occurred in the PACU and that which occurred within 48 hr of surgery. In addition, we recorded the total amount of opioids administered during the procedure and in the PACU, converting all administered opioids to morphine equivalents for comparison (McCaffery & Pasero, 1999) and then adjusting by weight for the score used in this study.
Genetic Data
We used Oragene DNA self-collection kits (DNA Genotek Corporation, Ottawa, Canada) to collect saliva samples after surgery. We extracted DNA from the saliva/buffer combination utilizing the protocol and reagents for extraction supplied with the Oragene kit. Using the prepared DNA, we determined CYP2D6 genotypes by the TaqMan Allelic Discrimination Assay (Applied Biosystems, Foster City, CA) using the ABI Prism7000 sequence detection system. We used quantitative real-time PCR based-Copy Number assays to determine the number of CYP2D6 gene copies (Applied Biosystems, Foster City, CA). The Allelic Discrimination assay includes the following alleles: rs16497 (which defines allele CYP2D6*2), rs3572686 (CYP2D6*3), rs3892097 (CYP2D6*4), rs5030656 (CYP2D6*9), rs1065852 (CYP2D6*10), rs28371706 (CYP2D6*17), rs1135840 (CYP2D6*39), and CYP2D6*5, which is defined by whole gene deletion. Based on genotyping results, we classified participants as EMs, IMs, or PMs, as shown in Table 1. There were no participants in this study with multiple CYP2D6 genes who would have been classified as UM. We classified participants who had two fully functional alleles as EMs, those with one fully functional allele and one decreased function or a nonfunctioning allele as IMs, and those with two nonfunctioning alleles as PMs.
Table 1.
CYP2D6 Alleles Analyzed in This Study.
| Allele | SNP | Change | Metabolic activity | Classification |
|---|---|---|---|---|
| *1 | Reference allele | Normal | Extensive metabolizer | |
| *2 | rs16497 | 1661G>C; 4180G>C | Normal | Extensive metabolizer |
| *3 | rs3574268 | 2549delA | None | Poor metabolizer |
| *4 | rs3892097 | 1846G>A | None | Poor metabolizer |
| *5 | Whole gene deletion | None | Poor metabolizer | |
| *9 | rs5030656 | 2615-2617del AAG | Decreased | Intermediate metabolizer |
| *10 | rs1065852 | 100C>T and the absence of 1846G>A | Decreased | Intermediate metabolizer |
| *17 | rs28371706 | 1023C>T | Decreased | Intermediate metabolizer |
| *39 | rs1135840 | 1661G>C | Normal | Extensive metabolizer |
Note. SNP = single-nucleotide polymorphism.
We stored all DNA samples in 1X TE buffer at 4 °C. All analyses were performed in the genetics laboratory at the University of Pittsburgh, School of Nursing.
Data Analysis
We performed statistical analyses using SPSS 17.0 (Statistical Package for the Social Sciences [SPSS], 2009). We computed means and standard deviations for each variable. To assess for the risk of multicollinearity, we computed correlations for all predictor variables. We computed the associations between suspected covariates and all independent variables of interest and the dependent variable to determine that the possible covariates were not confounders. Suspected covariates were the known risk factors for nonsmoking, history of PONV or motion sickness, and gender. Because all patients received opioids during surgery or in the PACU, we did not consider opioids to be a covariate in this study. We screened data for outliers and missing data. We computed unadjusted odds ratios with confidence intervals using univariate logistic regression. We used hierarchical binary logistic regression to determine the relationship of PONV with the CYP2D6 classifications, controlling for the known risk factors of smoking, history of PONV, and gender by their inclusion in the first block. Because the study model suggested that CYP2D6 could be a moderator in the relationship between the amount of opioids administered and PONV, we investigated an interaction between total opioids by weight and CYP2D6 classification. We employed the Hosmer Leme-show test to evaluate the goodness of fit of the binary logistic regression model and the omnibus test of model coefficients (model chi-square) to ensure that there was no evidence to suggest lack of fit. We used analysis of variance (ANOVA) to compare the means of the three CYP2D6 classifications on amounts of ondansetron given in 48 hr and total amount of opioids administered. We set the level of significance at p < .05 for two-sided hypothesis testing.
Results
Subject characteristics are reported in Table 2. The sample of 112 patients was predominately male nonsmokers (n = 66, 59%) with a mean age of 39.4 ± 12.7 years. Few subjects reported PONV with previous surgery. In the present study, the incidence of PONV (subjects who required antiemetic medication) was 38% in the PACU but increased to 50% during the first 48 hr after surgery. Mean postoperative pain scores were 6.83 ± 3.08 (15 min) and 6.20 ± 2.82 (45 min) after admission to the PACU. The type of anesthetic agents used were isoflurane (n = 7), desflurane (n = 57), and sevoflurane (n = 49). There were no differences in PONV across the three agents.
Table 2.
Patient Characteristics (N = 112).
| Variable | n (%) or M ± SD |
|---|---|
| Female sex | 31 (28) |
| Age (years) | 39.1 ± 12.7 |
| Nonsmoker | 66 (59) |
| History of PONV | 24 (21) |
| PONV in the PACU | 38 (34) |
| PONV 48 hr postsurgery | 56 (50) |
| CYP2D6—extensive metabolizer | 71 (63) |
| CYP2D6—intermediate metabolizer | 34 (30) |
| CYP2D6—poor metabolizer | 7 (6.3) |
| Total opioidsa (mg) | 0.49 ± 0.26 |
| Total ondansetronb (mg) | 3.39 ± 4.26 |
| PACU pain score at 15 min | 6.83 ± 3.08 |
| PACU pain score at 45 min | 6.20 ± 2.82 |
Note. PACU = postanesthesia care unit; PONV = postoperative nausea and vomiting.
Adjusted by weight.
Does not include ondansetron given in the operating room.
There were considerably more EMs than IMs or PMs. The most frequently occurring allele was CYP2D6*2, with 86 alleles, followed by CYP2D6*1, with 75 alleles. More than one third (n = 32 [34%]) of the subjects had at least one CYP2D6*4 allele, with six (5%) subjects being homozygous *4/*4 for that allele (Table 3). In addition, one subject had two decreased-functioning alleles (*9/*9) and, based on phenotype, we assigned that subject, along with the six homozygous for *4, to the PM category.
Table 3.
CYP2D6 Classification Assignment Based on Allele Combinations.
| Allele combination | n (%) | Classification |
|---|---|---|
| *1/*1 | 15 (13.5) | EM |
| *1/*2 | 5 (4.5) | EM |
| *2/*2 | 18 (16) | EM |
| *2/*39 | 25 (22) | EM |
| *39/*39 | 2 (1.8) | EM |
| *1/*3 | 1 (.09) | IM |
| *1/*9 | 3 (2.7) | IM |
| *1/*10 | 4 (3.6) | IM |
| *1/*4 | 32 (28.5) | IM |
| *9/*9 | 1 (.09) | PM |
| *4/*4 | 6 (5.3) | PM |
Note. EM = extensive metabolizer; IM = intermediate metabolizer; PM = poor metabolizer.
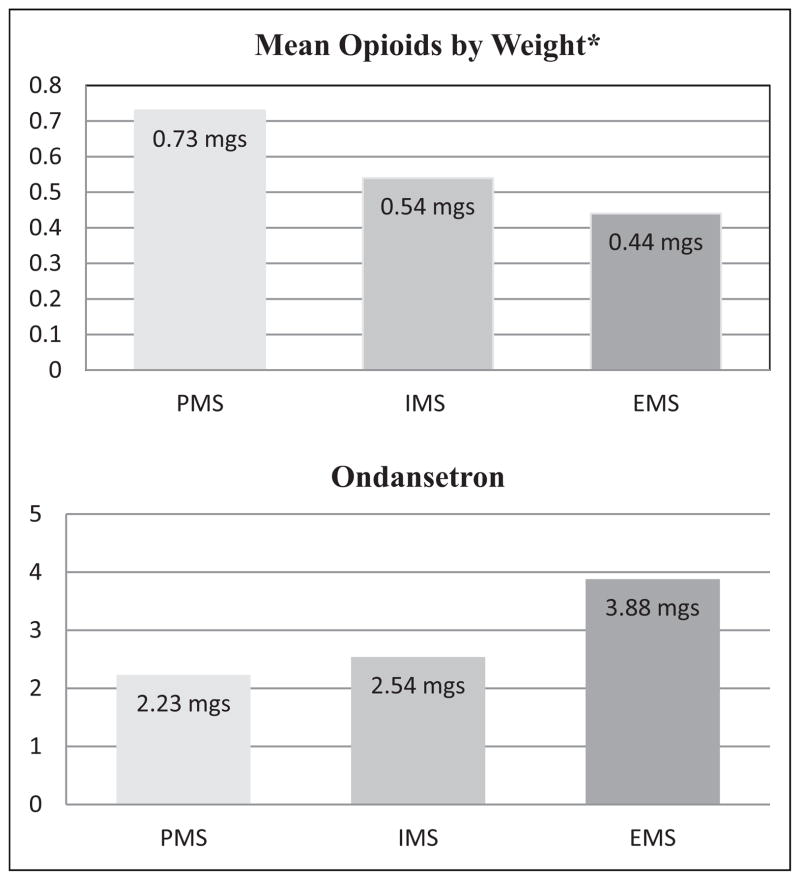
Female gender and history of PONV, but not smoking status, were significant risk factors for PONV in the present study. The omnibus test of model coefficient χ2 for the model was 31.839 (p < .001). The Hosmer Lemeshow goodness-of-fit test for the model was χ2 = 13.261 (p = .130), meaning this model shows no evidence of lack of fit. Using the EM group as the reference for binary hierarchical logistic regression (Table 4), we found a significant difference with the PM group for presence of PONV (p = .003) but not with the IM group (p > .563). We observed a significant interaction between total opioids adjusted by weight and a classification of PM (p = .010) but not the other CYP2D6 classifications. Subjects classified as PM (who received higher doses of opioids) had a decreased risk of experiencing PONV. We noted a significant difference in the amount of total opioids received (p = .007)) when we compared total ondansetron and total opioids adjusted by weight across the three CYP2D6 classifications (Figure 1). We did not find significant differences in the amount of ondansetron received across the same three groups (p = .270). However, the PM group required a smaller amount (2.28 ± 3.15 mg) of ondansetron for PONV compared to the EM group (3.88 ± 4.38 mg; Figure 1). The range of total ondansetron dosage for the PM group was 0–8 mg, while the range for the EM group was 0–20 mg. Because of the small sample size for the PM group, we combined the PM and IM groups and compared the combined group to the EM group for incidence of PONV. The odds ratio for this comparison was 2.016 (95% CI = [.92, 4.413]).
Table 4.
Multivariate Binary Logistic Regression for Postoperative Nausea and Vomiting (PONV).
| Variable | Odds ratio (OR) | 95% Confidence intervals for OR
|
p value | |
|---|---|---|---|---|
| Lower | Upper | |||
| History of PONV | 5.379 | 1.574 | 18.387 | .007 |
| Gender | 0.236 | .084 | .662 | .006 |
| Smoking status | 1.359 | .552 | 3.343 | .505 |
| Total opioidsa | 0.073 | .005 | 1.046 | .054 |
| CYP2D6 phenotype | ||||
| EM (REF) | 1.00 | |||
| IM | 0.492 | .010 | 24.171 | .721 |
| PM | 0.026 | .002 | .288 | .003 |
| Age | 0.966 | .931 | 1.003 | .071 |
| CYP2D6 phenotype by total opioids | ||||
| EM | 1.00 | .036 | ||
| IM | 4.616 | .026 | 822.837 | .563 |
| PM | 207.726 | 3.525 | 12240.88 | .010 |
Note. EM = extensive metabolizers; IM = intermediate metabolizers; PM = poor metabolizers.
Adjusted by weight.
Figure 1.
Means of total opioids and total ondansetron by CYP2D6 classification.
Note. Mean total mg of opioids adjusted by weight compared to mean total mg ondansetron for the three CYP2D6 classification groups. The three groups were significantly different in amount of opioids received (*p = .007) when analyzed by analysis of variance (ANOVA). The groups were not statistically different for total doses of ondansetron (p = .27), yet those subjects who required more opioids required less antiemetic medication. PMs = poor metabolizers; IMs = intermediate metabolizers; EMs = extensive metabolizers.
Discussion
The major findings of this study were (1) PMs experienced less PONV compared to the EM group but received higher doses of total opioids adjusted by weight and (2) 50% of all patients experienced some level of PONV within 48 hr of surgery.
The distribution of CYP2D6 classification groups for this study was consistent with previous studies. There were 65 (58%) and 40 (35%) patients classified as EMs and IMs, respectively. The 7 subjects (6.9%) classified as PMs fall within the 5–10% range usually reported in the literature. Most studies have reported that 2–3% of the population are UMs, so it was disappointing but not unexpected that we did not identify a UM (Owen et al., 2009; Zhou, 2009).
PMs in this study were less likely to experience PONV compared to the EM group, yet they received higher doses of total opioids adjusted by weight. Most researchers have reported no significant difference in incidence of PONV between EMs and PMs (Candiotti et al., 2005; Janicki, Schuler, Jarzembowski, & Rossi, 2006). In one recent study, however, Yang and colleagues (2012) reported the occurrence of severe postoperative pain was significantly higher in PMs as compared to the other metabolizer groups, requiring higher doses of opioids for pain relief.
Many researchers combine the EMs and IMs into one group when reporting results. But in a recent study by Arneth and colleagues (2009) in which they examined serum levels of patients treated with the antidepressant venlafaxine, the ratio of venlafaxine to the active metabolite O-desmethylvenlafaxine (ODV) [major metabolite from venlafaxine produced by CYP2D6] in serum was higher for patients with one CYP2D6*4 allele than for patients with two *4 alleles but significantly lower than for patients who were EMs; thus the investigators classified them as IMs. We chose to attempt to differentiate between the EMs and IMs, given this evidence that there may be detectable differences. In fact, the mean total opioid dose adjusted by weight was significantly different across the three groups, with a modest “intermediate” step between the EM and PM group, providing support for an autonomous intermediate group.
The *4 allele is present in high frequency and accounts for >75% of allelic variants in Caucasians (Stamer, Bayerer, Wolf, Hoeft, & Stüber, 2002). Prior findings are consistent with results of this study, in which CYP2D6*4 was the variant allele we detected most frequently (n = 38). The two other variant alleles we detected were CYP2D6*9 (n = 4) and CYP2D6*3 (n = 2). The one subject with two decreased-function alleles (*9/*9) by definition should probably be considered an IM (Owen et al., 2009), but based on phenotype we assigned this subject PM status. This subject required the highest amount of postoperative opioids of all subjects and reported pain levels of 10 at both 15 and 45 min postoperatively.
In our study, 56 (50%) patients were treated with ondansetron during their stay in the PACU and during the 48-hr post-surgery compared to 38 (34%) patients in the PACU alone. This is consistent with many studies focused on postoperative complications (Janicki et al., 2006; Meng & Quinlan, 2006). It is standard practice in our institution to medicate all surgical patients with 4 mg of IV ondansetron prior to their leaving the OR. Yet even with the administration of this prophylactic dose of ondansetron in the OR, a third of our subjects experienced PONV in the PACU. The American Society of PeriAnesthesia Nursing (ASPAN, 2006) describes problems associated with postdischarge nausea and vomiting; yet, there is limited research describing outcomes after the first 24-hr postoperative period. The present study’s finding of a 16% increase in PONV during the 48 hr after discharge from the PACU is highly significant, particularly in light of the number of ambulatory surgical procedures being performed. Discharge education in regard to PONV is, thus, of critical importance.
When we examined ondansetron dosage for the three CYP2D6 classifications, we found that mean ondansetron dosage for the PM group was less than 2.5 mg for the 48-hr postoperative period. No subject in this group required more than two doses of ondansetron (ondansetron is given in 4 mg doses usually every 6 hr). The range was broader for the IM and EM groups, with some patients in both groups requiring up to 20 mg of ondansetron 48 hr postoperatively. The results for total opioids are paradoxical compared to those for ondansetron. Those patients who required the most opioids (PMs) required the least amount of ondansetron, and those who required the most ondansetron required the lowest amount of opioids for pain (Figure 1). These findings are consistent with the recent findings of Kim, Choi, Kang, and Bae (2010), who reported that subjects with knee osteoarthritis taking tramadol for less than 14 days and classified as EM had significantly more nausea and vomiting than similar subjects taking tramadol and classified as IM. Investigators in two other studies reported results for postoperative patients who required more opioids for pain but experienced less PONV. In both studies, researchers believed that the difference was due to variability in the mu-opioid receptor gene; subjects who had the wild type allele required less opioids yet experienced more PONV (Chou et al., 2006; Sia et al., 2008).
It is not surprising that two of the major risk factors for PONV, a positive history of PONV and gender, were significant predictors of PONV, as this finding has been well documented in previous research (Apfel et al., 2008). Recent studies have also advanced the knowledge of risk factors (Apfel et al., 2008; Murphy, Hooper, Sullivan, Clifford, & Apfel, 2006; White, O’Hara, Robertson, Wender, & Candiotti, 2008). Risk factors that researchers identified most frequently include gender, smoking status, history of PONV or motion sickness, and use of opioids for postoperative pain. Apfel and colleagues developed a data-based assessment tool to predict risk of PONV (Apfel, Laara, Koivuranta, Greim, & Roewer, 1999). The tool assigns one point for each known risk factor. For example, a nonsmoking female with a previous history of PONV that was treated with opioids for postoperative pain would be assigned a score of 4. In a study of 1,137 postsurgical patients, Apfel and colleagues reported that the presence of one, two, three, and four of these risk factors increased the incidence of PONV by 21%, 39%, 61%, and 79%, respectively. The PONV risk factor assessment tool provides guidance to physicians and nurses regarding the identification of high-risk patients and need for prophylactic antiemetic therapy (Apfel et al., 2008).
Smoking, which is usually a strong predictor of decreased PONV, did not have a significant influence in the present study. This finding may be due to the greater-than-average number of smokers in the sample, or it may be because the smokers were primarily male and female gender is a stronger predictor than smoking (Apfel et al., 2008). The American Heart Association (Roger et al., 2011) reports that 18–21% of Americans are current smokers, while 43% of the present group of orthopedic trauma patients were current smokers. Brattwall and colleagues (2009), who reported that 32% of subjects in their study were current smokers or snuff users, found that PONV was reduced by 50% in tobacco users when compared to the nontobacco use group in both genders on the day of surgery and the first postoperative day. Interestingly, they reported that smoking and snuff use equally reduced PONV. We were unable to test this in our sample because we did not record data on snuff usage.
Though it is not included as one of the four major risk factors in most adult PONV scoring systems (Apfel et al., 1999), researchers have identified age as a moderate predictor for PONV (Junger et al., 2001; Sinclair, Chung, & Mezei, 2000). The relatively young mean age of the present sample (39 years) might have contributed to the influence of age in our study.
We controlled for two additional factors that researchers have described as possible predictors for PONV by study inclusion criteria: type and length of surgery (Junger et al., 2001; Sinclair et al., 2000). All study participants were admitted for an orthopedic procedure for single isolated extremity fracture, and we did not includes any subjects with surgery time greater than 4 hours.
Limitations
We conducted this study in a single center, and practices in regard to administration of pre- and postoperative medications may have influenced the occurrence of PONV. We gathered several of our variables through retrospective data collection (history of PONV and smoking); this retrospective data collection may increase the risk of missing data, mistakes during interpretation of data, or incorrect documentation. In addition, we only analyzed Caucasians in this study due to the small number of non-Caucasians enrolled to date. Thus, our findings cannot be generalized to other racial/ethnic groups. Finally, our study’s sample size was relatively small. A larger fully powered prospective, controlled study that follows patients postoperatively and measures both the episodes of vomiting and the severity of nausea directly would allow for a more robust analysis, especially if it were large enough to identify patients that fall into the UM classification.
Conclusion and Implications for Clinical Practice
The goal of personalized medicine is to provide physicians and nurses with the knowledge to utilize the most appropriate treatment and dosing scheme for each individual patient. To prepare for that eventuality, it is necessary for clinical genetic research to continue to identify risk factors that may predispose patients to a negative outcome. Sweeney (2003) noted that health care providers should consider interindividual variability in response to medications as a major clinical problem. An enhanced ability to recognize when patients are not responding to treatment due to genetic variants or other factors will make nurses more understanding when a postoperative patient requires more opioids or antiemetics than usual and more likely to know when to implement alternative strategies for successful postoperative recovery.
The finding that PONV increased after discharge from the PACU also has important implications for postoperative treatment. Appropriate treatment of PONV is typically available for hospitalized patients but may not be as available for patients who undergo outpatient surgery. Nurses should provide patient education on the risk of PONV after discharge and suggest appropriate strategies for patients to use at home for treatment.
Future research should also explore the variability in the neurotransmitter genes related to the emetic pathway that may help explain the 20–30% of postoperative patients who do not respond to antiemetics.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Eta Chapter of Sigma Theta Tau International; American Association of Nurse Anesthetist Foundation; grant UL1RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research, and a special grant from the Office of the Senior Vice Chancellor for the Health Sciences, University of Pittsburgh.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Reprints and permission: sagepub.com/journalsPermissions.nav
References
- American Society of PeriAnesthesia Nurses. ASPAN’s evidence-based clinical practice guideline for the prevention and/ or management of PONV/PDNV. Journal of PeriAnesthesia Nursing. 2006;71:230–250. doi: 10.1016/j.jopan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Apfel C, Greim C, Habuitz I, Goepfert C, Usadel J, Sefrin P, Roewer N. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiologica Scandinavica. 2008;42:495–501. doi: 10.1111/j.1399-6576.1998.tb05157.x. [DOI] [PubMed] [Google Scholar]
- Apfel C, Laara E, Koivuranta M, Greim C, Roewer N. A simplified risk score for predicting PONV: Conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- Arneth B, Shams M, Hiemke C, Hartter S. Rapid and reliable genotyping procedure for detection of alleles with mutations, deletion, and/or duplication of the CYP2D6 gene. Clinical Biochemistry. 2009;42:1282–1290. doi: 10.1016/j.clinbiochem.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Brattwall M, Stomberg M, Rawal N, Segerdahl, Houltz E, Jakobbson J. Postoperative impact of regular tobacco use, smoking or snuffing, a prospective multi-center study. Acta Anaesthesiologica Scandinavica. 2009;10:1–7. doi: 10.1111/j.1399-6576.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- Candiotti KA, Birnbach DJ, Lubarsky DA, Nhuch F, Kamat A, Koch, Andrews D. The impact of pharmacogenomics on postoperative nausea and vomiting: Do CYP2D6 allele copy number and polymorphisms affect the success or failure of ondansetron prophylaxis? Anesthesiology. 2005;102:543–549. doi: 10.1097/00000542-200503000-00011. [DOI] [PubMed] [Google Scholar]
- Chou W, Wang C, Liu P, Liu C, Tseng C, Jawan B. Human opioid receptor A188G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105:334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- Cork R, Isaac I, Elsharydah A, Saleemi S, Zavisca F, Alexander L. A comparison of the verbal rating scale and the visual analog scale for pain assessment. Internet Journal of Anesthesiology. 2004;8 Retrieved March 21, 2010, from http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ija/vol8n1/vrs.xml. [Google Scholar]
- Daly A, Brockmöller J, Broly F, Eichelbaum M, Evans W, Gonzalez F, Zanger UM. Nomenclature for human CYP2D6 alleles. Pharmacogenetics. 1996;6:193–201. doi: 10.1097/00008571-199606000-00001. [DOI] [PubMed] [Google Scholar]
- de Leon J, Armstrong SC, Cozza K. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psyschosomatics. 2006;47:75–85. doi: 10.1176/appi.psy.47.1.75. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M, Spannbrucker N, Steincke B, Dengler H. Defective N-oxidation of sparteine in man: A new pharmacogenetic defect. European Journal of Clinical Pharmacology. 1979;16:183–187. doi: 10.1007/BF00562059. [DOI] [PubMed] [Google Scholar]
- Furman KD, Grimm DR, Mueller T, Hollery-Shanks RR, Bertz RJ, Williams LA, Katz DA. Impact of CYP2D6 intermediate metabolizer alleles on single-dose desipramine pharmacokinetics. Pharmacogenetics. 2004;14:279–284. doi: 10.1097/00008571-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Simon S, Pearce R, Bradford L, Kennedy M, Leeder J. The CYP2D6 activity score: Translating genotype information into a qualitative measure of phenotype. Clinical Pharmacology and Therapeutics. 2008;83:234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- Ho K, Gan T. Pharmacology, pharmacogenetics and clinical efficacy of 5HT3 receptor antagonists for postoperative nausea and vomiting. Current Opinions in Anaesthesiology. 2006;19:606–611. doi: 10.1097/01.aco.0000247340.61815.38. [DOI] [PubMed] [Google Scholar]
- Holmquist G. Opioid metabolism and effects of cytochrome P450. Pain Medicine. 2009;10:S20–S29. [Google Scholar]
- Ingelman-Sundberg M, Daly A, Nebert D. Home page of Human Cytochrome P450(CYP) Allele Nomenclature Committee. 2009 Retrieved from NCBI website at http://www.cypalleles.ki.se/cyp2d6.htm.
- Janicki P, Schuler H, Jarzembowski T, Rossi M. Prevention of postoperative nausea and vomiting with granisetron and dolasetron in relation to CYP2D6 genotype. Anesthesia & Analgesia. 2006;102:1127–1133. doi: 10.1213/01.ane.0000200364.55798.3f. [DOI] [PubMed] [Google Scholar]
- Jolley S. Managing post-operative nausea and vomiting. Nursing Standards. 2001;15:47–52. doi: 10.7748/ns2001.06.15.40.47.c3044. [DOI] [PubMed] [Google Scholar]
- Junger A, Hartmann B, Benson M, Schindler E, Dietrich G, Jost A, Hempelmannn G. The use of an anesthesia information management system for prediction of antiemetic rescue treatment at the postanesthesia care unit. Anesthesia & Analgesia. 2001;92:1203–1209. doi: 10.1097/00000539-200105000-00023. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Sezer O, Papies A, Bauer S, Schelenz C, Tremblay P, Brockmöller J. Patient-tailored anti-emetic treatment with 5HT3 receptor antagonists according to cytochrome P-450 2D6 genotypes. Journal of Clinical Oncology. 2002;20:2805–2811. doi: 10.1200/JCO.2002.09.064. [DOI] [PubMed] [Google Scholar]
- Kim E, Choi C, Kang C, Bae S. Adverse events in analgesic treatment with tramadol associated with CYP2D6 extensive-metabolizer and OPRM1 high-expression variants. Annals of Rheumatoid Arthritis. 2010;69:1888–1891. doi: 10.1136/ard.2009.124347. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Jan-Tobias H, Keulen M, Bauer S, Roots I, Brockmöller J. Effects of the CYP2D6 gene duplication on the pharmacokinetics and pharmacodynamics of tramadol. Journal of Clinical Psychopharmacology. 2008;28:78–83. doi: 10.1097/JCP.0b013e318160f827. [DOI] [PubMed] [Google Scholar]
- Kranke P, Roewer N, Smith A, Piper S, Wallenborn J, Eberhart L. Postoperative nausea and vomiting: What are we waiting for? Anesthesia Analgesia. 2009;108:706–712. doi: 10.1213/ane.0b013e318194c755. [DOI] [PubMed] [Google Scholar]
- Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87:274–285. doi: 10.1159/000326085. [DOI] [PubMed] [Google Scholar]
- Marla S, Stallard S. Systematic review of day surgery for breast cancer. International Journal of Surgery. 2009;7:318–323. doi: 10.1016/j.ijsu.2009.04.015. [DOI] [PubMed] [Google Scholar]
- McCaffery M, Pasero C. Pain: Clinical manual. 2. St. Louis, MO: Mosby; 1999. [Google Scholar]
- Meng L, Quinlan J. Assessing risk factors for postoperative nausea and vomiting: A retrospective study in patients undergoing retromastoid craniectomy with microvascular decompression of cranial nerves. Journal of Neurosurgical Anesthesiology. 2006;18:235–239. doi: 10.1097/00008506-200610000-00003. [DOI] [PubMed] [Google Scholar]
- Miaskowski C. A review of the incidence, causes, consequences and management of gastrointestinal effects associated with postoperative opioid administration. Journal of PeriAnesthesia Nursing. 2009;24:222–228. doi: 10.1016/j.jopan.2009.05.095. [DOI] [PubMed] [Google Scholar]
- Murphy M, Hooper V, Sullivan E, Clifford T, Apfel C. Identification of risk factors for postoperative nausea and vomiting in the perianesthesia adult patient. Journal of PeriAnesthesia Nursing. 2006;21:377–384. doi: 10.1016/j.jopan.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Nelson T. Postoperative nausea and vomiting: Understanding the enigma. Journal of PeriAnesthesia Nursing. 2002;17:178–189. doi: 10.1053/jpan.2002.33207. [DOI] [PubMed] [Google Scholar]
- Nielson M, Olsen N. Genetic polymorphisms in the cytochrome P450 and efficacy of 5-HT type 3 receptor antagonists for postoperative nausea and vomiting. (2008) British Journal of Anaesthesia. 2008;101:441–445. doi: 10.1093/bja/aen246. [DOI] [PubMed] [Google Scholar]
- Owen R, Sangkuhl K, Klein T, Altman R. Cytochrome P450 2D6. Pharmacogenetics and Genomics. 2009;19:559–562. doi: 10.1097/FPC.0b013e32832e0e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger V, Go S, Lloyd-Jones M, Benjamin J, Berry D, Borden B, Turner M on behalf of the American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: Allele frequencies and phenotypic consequences. American Journal of Human Genetics. 1997;60:284–295. [PMC free article] [PubMed] [Google Scholar]
- Sia A, Lim Y, Lim E, Goh R, Law H, Landau R, Tan E. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 2000;91:109–118. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- Stamer U, Rauers N, Eun-Hae L, Mubhoff F, Stuber F. Ondansetron for the treatment of opioid induced nausea and vomiting: Impact of cytochrome polymorphisms. European Journal of Pain. 2009;13:S193. [Google Scholar]
- Stamer UM, Bayerer B, Wolf S, Hoeft A, Stüber F. Rapid and reliable method for cytochrome P450 2D6 genotyping. Clinical Chemistry. 2002;48:1412–1417. [PubMed] [Google Scholar]
- Susce M, Murray-Carmichael E, de Leon J. Response to hydrocodone, codeine and oxycodone in a CYP2D6 poor metabolizer. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30:1356–1358. doi: 10.1016/j.pnpbp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Sweeney B. Do genes influence outcome from anaesthesia? British Journal of Anaesthesia. 2003;103:725–727. doi: 10.1093/bja/aeg103. [DOI] [PubMed] [Google Scholar]
- Watcha M, White P. Postoperative nausea and vomiting. Its etiology, treatment and prevention. Anesthesiology. 1992;77:162–184. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- White P, O’Hara J, Roberson C, Wender R, Candiotti K. The impact of current anti-emetic practices on patient outcomes: A prospective study on high-risk patients. Anesthesia & Analgesia. 2008;107:452–458. doi: 10.1213/ane.0b013e31817b842c. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yang Z, Ahreart K, Morris R, Zhang Y, Rodriguez Y, Candiotti K. CYP2D6 poor metabolizer genotype and smoking predict severe postoperative pain in female patients on arrival to the recovery room. Pain Medicine. 2012;13:604–609. doi: 10.1111/j.1526-4637.2012.01296.x. [DOI] [PubMed] [Google Scholar]
- Zhou S. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clinical Pharmacokinetics. 2009;48:689–723. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Zhou S. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part II. Clinical Pharmacokinetics. 2010;48:761–804. doi: 10.2165/11318070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Zwisler S, Enggaard T, Noehr-Jensen L, Pederson R, Mikkelsen S, Nielsen F, Sindrup S. The hypoalgesic effect of oxycodone in human experimental pain models in relation to the CYP2D6 oxidation polymorphism. Basic and Clinical Pharmacology. 2009;104:335–344. doi: 10.1111/j.1742-7843.2009.00378.x. [DOI] [PubMed] [Google Scholar]