Summary
The border cells of Drosophila are a model system for coordinated cell migration. Ecdysone signaling has been shown to act as the timing signal to initiate the migration process. Here we find that mutations in phantom (phm), encoding an enzyme in the ecdysone biosynthesis pathway, block border cell migration when the entire follicular epithelium of an egg chamber is mutant, even when the associated germline cells (nurse cells and oocyte) are wildtype. Conversely, mutant germline cells survive and do not affect border cell migration, as long as the surrounding follicle cells are wildtype. Interestingly, even small patches of wildtype follicle cells in a mosaic epithelium are sufficient to allow the production of above-threshold levels of ecdysone to promote border cell migration. The same phenotype is observed with mutations in shade (shd), encoding the last enzyme in the pathway that converts ecdysone to the active 20-hydroxyecdysone. Administration of high 20-hydroxyecdysone titers in the medium can also rescue the border cell migration phenotype in cultured egg chambers with an entirely phm mutant follicular epithelium. These results indicate that in normal oogenesis, the follicle cell epithelium of each individual egg chamber must supply sufficient ecdysone precursors, leading ultimately to high enough levels of mature 20-hydroxyecdysone to the border cells to initiate their migration. Neither the germline, nor the neighboring egg chambers, nor the surrounding hemolymph appear to provide threshold amounts of 20-hydroxyecdysone to do so. This “egg chamber autonomous” ecdysone synthesis constitutes a useful way to regulate the individual maturation of the asynchronous egg chambers present in the Drosophila ovary.
Keywords: Phantom (phm), Shade (shd), ecdysone biosynthesis, border cell migration, oogenesis, Drosophila
Introduction
Guided cell migration is crucial for a range of events that take place in multicellular organisms during development, such as neural crest migration or germline migration, as well as during adulthood, for example during immune response and wound healing. In addition, cell motility contributes to pathological conditions such as metastatic cancer, when transformed epithelial cells acquire migratory properties and invade other tissues. Cell migration is a tightly regulated multi-step event. First of all, cells have to detach from their original location. Next they have to migrate over or through other tissues. As cells reach their target location, they have to attach to tissues and disengage from their migratory behavior in order to perform their functions. Consequently, cell migration processes are very complex and require several major changes in cellular properties.
Border cell (BC) migration in the developing Drosophila egg chamber provides a genetically tractable and relatively simple model system that is suitable to study the mechanisms of cell migration, chemotaxis and regulation of migration timing (see Montell, 2003; Naora and Montell, 2005; Rorth, 2002 for review). Each Drosophila egg chamber consists of fifteen nurse cells and one oocyte, which are germline derived, surrounded by a monolayer of somatic follicle cells (FCs) that form an epithelium of about 800–900 cells, after completion of several rounds of mitotic division (see Spradling, 1993 for review). BCs are specialized cells that derive from an initially non-motile anterior group of FCs. They are specified by the activation of the JAK-STAT pathway by its ligand Unpaired (Upd) that is produced and secreted by a pair of FCs at each end of the egg chamber, the so-called anterior and posterior polar cells (McGregor et al., 2002; Xi et al., 2003). Activation of JAK-STAT signaling from anterior polar cells is the earliest known step in BC specification, and was shown to be essential for the migration of BCs (Beccari et al., 2002; Silver and Montell, 2001). As a result of this specification at the beginning of stage 9, a group of 8–10 cells, including the pair of anterior polar cells, form a BC cluster consisting of the two non-motile polar cells in the center and 6–8 motile outer BCs. BCs extend protrusions in between the nurse cells, delaminate from the neighboring anterior non-motile follicular epithelium, and migrate in a tumor-like, invasive manner in between nurse cells towards the oocyte. The migration proceeds in a highly directional fashion, and by stage 10A of oogenesis, about 6 hours after the initiation of migration, the cluster reaches the border between the nurse cells and the oocyte (see Montell, 2003; Montell et al., 2012; Naora and Montell, 2005; Rorth, 2002 for review). BCs use chemotaxis to guide them through the tissue using oocyte-secreted growth factors, such as Pvf (the Drosophila PDGF / VEGF homolog) and Egfr ligands as attractive guidance signals (Duchek et al., 2001; McDonald et al., 2006).
The regulation of timing of cell migration during development is as crucial as the spatial control and requires additional signals that coordinate and integrate several events that occur at the same developmental time point. For example, BC migration has to be tightly concurrent with germline development and with the general rearrangements of the rest of the FC epithelium. The role of the steroid hormone ecdysone in the temporal control of the initiation of the BC migration was uncovered over the last years (Bai et al., 2000; Jang et al., 2009). Ecdysone functions through the Ecdysone receptor (EcR), consisting of the two subunits, EcR and Ultraspiracle (Usp), both of which are expressed throughout oogenesis including in the border cells at the time of migration. Ecdysone signaling also requires a receptor co-activator Taiman (Tai) (Bai et al., 2000). Ecdysone signaling reaches its highest activity level in the anterior group of FCs, including the BCs, at the beginning of stage 10 of oogenesis, the time point when the migration of the BC cluster is completed and serves as a temporal regulator of BC migration (Jang et al., 2009; Riddiford, 1993). Furthermore, the JAK-STAT pathway, a spatial regulator of BC specification, is integrated with the ecdysone signaling pathway via Abrupt protein. Abrupt is a repressor of ecdysone signaling and, hence, BC migration, and is normally lost from BCs during stage 9 in response to JAK-STAT pathway activation (Jang et al., 2009). While these results have shed some light on the integration between spatial pattering and the timing of migration, our understanding of the temporal control of BC migration of each egg chamber within the context of an ovary that usually contains many egg chambers at different stages of development is still incomplete.
In order to discover new genes involved in the control of the cell motility and developmental timing, we identified a set of mutants affecting the migration of BCs in a mosaic screen on the X-chromosome. Two mutants in particular, FL99 and FT59, specifically affected BC migration and did not show any other defects in FC differentiation. Here we demonstrate that FL99 and FT59 carry mutations in the gene phantom (phm), which is known to be required for ecdysone synthesis. We show that Drosophila Phm acts during oogenesis to initiate BC migration, and its function is required in the FCs and not in the germline cells. Ecdysone production seems autonomous to each egg chamber, such that wild-type FCs from adjacent egg chambers cannot rescue an egg chamber surrounded by mutant FCs. We further found that about 8–15% of FCs (50–100 cells), independent of their position within the egg chamber are sufficient to produce required levels of ecdysone to initiate BC migration. A similar phenotype was observed with mutations in the gene shade (shd), which catalyzes the last step in the ecdysone pathway converting ecdysone to active 20-hydroxyecdysone. Mutations in shd also lead to defects in border cell migration, and the function of Shd is required only in the follicular epithelium. In addition, we analyzed the migration of BCs in egg chambers cultured in vitro, and demonstrated that BC migration could be rescued in phm mutants by adding synthetically synthesized 20-hydroxyecdysone, the biologically active form of ecdysone, to the culture medium.
Results
Border cell migration is disrupted in egg chambers containing FL99 and FT59 whole epithelial homozygous mutant clones
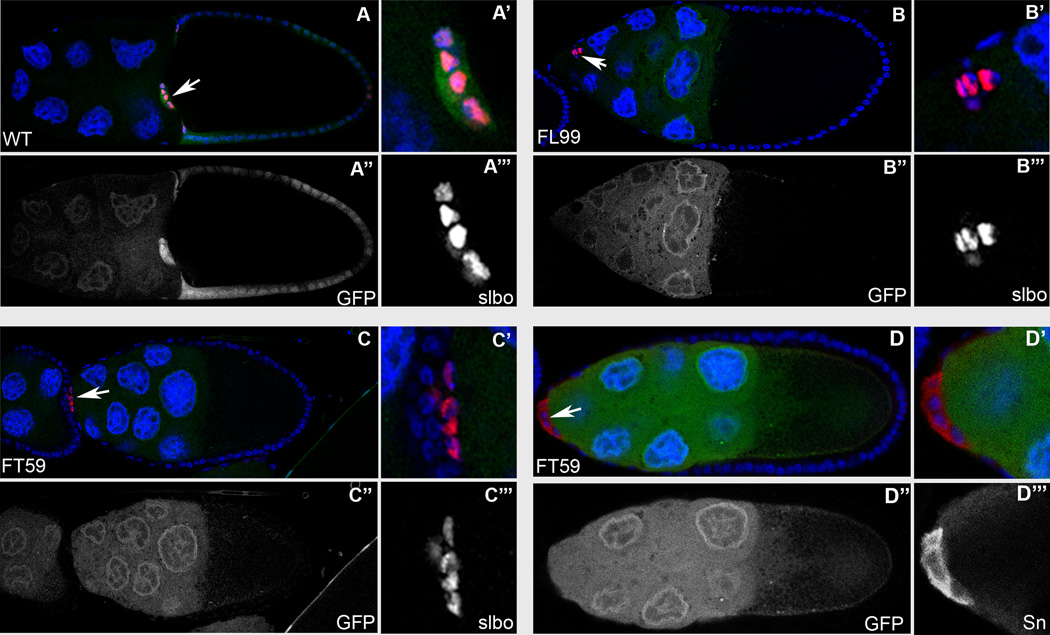
In a genetic mosaic screen designed to identify genes involved in FC patterning, differentiation and morphogenesis during Drosophila oogenesis (Denef et al., 2008; Yan et al., 2009; Yan et al., 2011; Yi et al., 2011; Domanitskaya et al., 2012), two homozygous lethal mutations, FL99 and FT59 were isolated, which fail to complement each other, indicating that they are alleles of the same gene. Using either Slow border cells (Slbo) which labels the border cells, or Cut, a marker for the polar cells, we found that in egg chambers containing FL99 or FT59 whole epithelial homozygous mutant clones, migration of the BCs was severely disrupted. In wild-type egg chambers BC clusters reach the border between the nurse cells and the oocyte at stage 10A of oogenesis (Fig. 1A). At the same developmental stage, the majority of BC clusters in egg chambers containing either FL99 or FT59 whole epithelial homozygous mutant clones did not initiate the migration, and retained their position at the most anterior end of the egg chambers (Fig. 1B, C, and D). To estimate the strength of the mutant phenotype, we quantified BC migration in stage 10A egg chambers containing FL99 or FT59 whole epithelial homozygous mutant clones. The penetrance of this phenotype was very similar for both mutant alleles. In 73% of the cases for FL99 (n=15) and 86% of cases for FT59 (n=21) mutant egg chambers, the BC clusters did not initiate the migration. Of the remaining clusters, in one out of 15 analyzed FL99 mutant egg chambers (6%) the BC cluster migrated only approximately 1/3 of the distance towards the oocyte, and in 3 egg chambers (20%) the BC clusters migrated over 2/3 of the distance. In the case of the FT59 mutant, we observed one out of 21 analyzed cases (5%) where the BC cluster migrated over 2/3 of the distance, and two out of 21 (10%) that completed migration.
Fig. 1. Border cell migration is disrupted in the phmFL99 and phmFT59 whole epithelial mutant egg chambers.
Stage 10A wild-type egg chambers (A) and egg chambers containing phmFL99 (B) or phmFT59 (C) whole epithelial homozygous mutant clones stained with Hoechst (blue) to label nuclei and anti-Slbo (A–C) (red), or anti-Sn (D) (red) to label border cells. Mutant cells are marked by the absence of GFP (green). BC clusters are marked by white arrows. Egg chambers are oriented with anterior to the left. (A, A’, A”, A”’) BC reached the border between the nurse cells and the oocyte in the wild-type egg chamber. (B-D) BC clusters failed to migrate in the egg chambers containing phmFL99 (B, B’, B”, B”’) or phmFT59 (C–D) whole epithelial homozygous mutant clones.
Homozygous mutant BC are specified normally, as they stain positive for border cell specific markers in the egg chambers containing phmFL99 or phmFT59 whole epithelial homozygous mutant clones.
Specification of border cells and cluster formation are unaffected in the FL99 and FT59 mutants
To determine if the phenotype observed in the FL99 and FT59 mutant egg chambers was due to defects either in the specification of the anterior cell fates, in cluster formation or in the migration process itself, we stained the whole epithelial homozygous mutant egg chambers for Slow Border Cells (Slbo), an early border cell marker (Montell et al., 1992), as well as for Singed (Sn) a marker for migrating BCs (Cant et al., 1994). We detected these markers in the BCs of wild-type as well as mutant egg chambers (Fig. 1), which indicates that border cell fate specification is unaffected in both of these mutants. In addition, the morphology of the BC clusters within whole epithelial mutant egg chambers seemed to be unaffected, and each cluster comprised 6–8 outer cells surrounding two non-motile polar cells (Fig. 1). Thus, the BC clusters in the FL99 and FT59 mutant egg chambers were formed normally, but instead of migrating towards the oocyte, in the majority of analyzed cases they remained at the anterior end of the egg chamber. Based on these data, we conclude that the FL99 and FT59 mutations affected the BC migration process and did not affect BC fate specification and cluster formation.
FL99 and FT59 are alleles of the gene phm
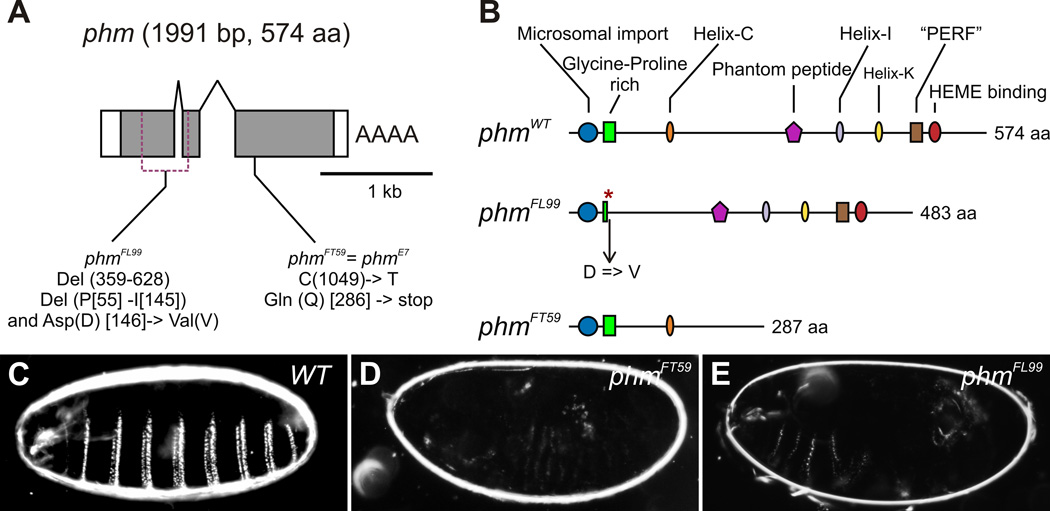
In order to identify the gene affected by the homozygous lethal FL99 and FT59 mutations, a combination of meiotic recombination with visible recessive markers, P-element insertions, duplications and SNP mapping approaches were used (see Experimental Procedures). This allowed us to map the FL99 and FT59 mutations to the gene phantom (phm, Cyp306a1). The Drosophila phm gene is located at the cytological interval 17C5-D2 of the X chromosome (Wieschaus et al., 1984), it comprises 3 exons and encodes for a 1991 bp cDNA product, or a protein consisting of 574 amino acids. (Fig. 2A) (Niwa et al., 2004; Warren et al., 2004). The phmFL99 allele carries an in-frame deletion removing amino acids 55–145, resulting in a Phm protein lacking the Glycine-Proline rich and Helix-C domains. In addition, the phmFL99 deletion results in a substitution of the aromatic Aspartic acid (D) at position 146 by the hydrophobic Valine (V) (Fig. 2A, B). The phmFT59 allele carries a cytosine to thymine point mutation at the nucleotide position 1049, leading to an in-frame stop codon at amino acid position 286 (Fig. 3A, B). This results in a truncated form of the Phm protein containing only 285 amno acids and lacking several functional domains, including a heme-binding site essential for the catalytical activity of all cytochrome P450 enzymes (Fig. 2B) (see Werck-Reichhart and Feyereisen, 2000 for review). Interestingly, the mutation in the phmFT59 allele is identical to the mutation carried in a previously isolated and characterized phmE7 allele (Niwa et al., 2004; Warren et al., 2004; Wieschaus et al., 1984), possibly indicating a mutagenesis hotspot.
Fig. 2. FL99 and FT59 are alleles of the gene phm.
(A) Schematic representation of the intron-exon structure of phm (adopted from Niwa et al., 2004). White rectangles correspond to 5’ and 3’ UTR, grey ones indicate translated regions. The gene phm encodes for 1991 bp cDNA product or 574 amino acids long protein. In the phmFL99 allele parts of the first and the second exons are deleted from 359 bp until 628 bp removing amino acids 55–145. The phmFL99 allele carries in addition a substitution of the aromatic Aspartic acid (Asp, D) at the position 146 by the hydrophobic Valine (Val, V). The phmFT59 allele carries the same mutation as phmE7 (Niwa et al., 2004; Warren et al., 2004), a C to T point mutation at the nucleotide position 1049, leading to a formation of an in-frame stop codon at amino acid position 286. (B) Schematic diagrams illustrating phantom proteins produced by phmWT (adopted from Warren et al., 2004), phmFL99 and phmFT59 alleles. The deletion of the part of the Glycine-Proline rich domain in the phmFL99 allele is depicted by a red asterisk. (C-E) Cuticle preparation. Wild-type cuticle (C), and lack of cuticle differentiation in phmFT59 (D) and phmFL99 (E) mutant embryos.
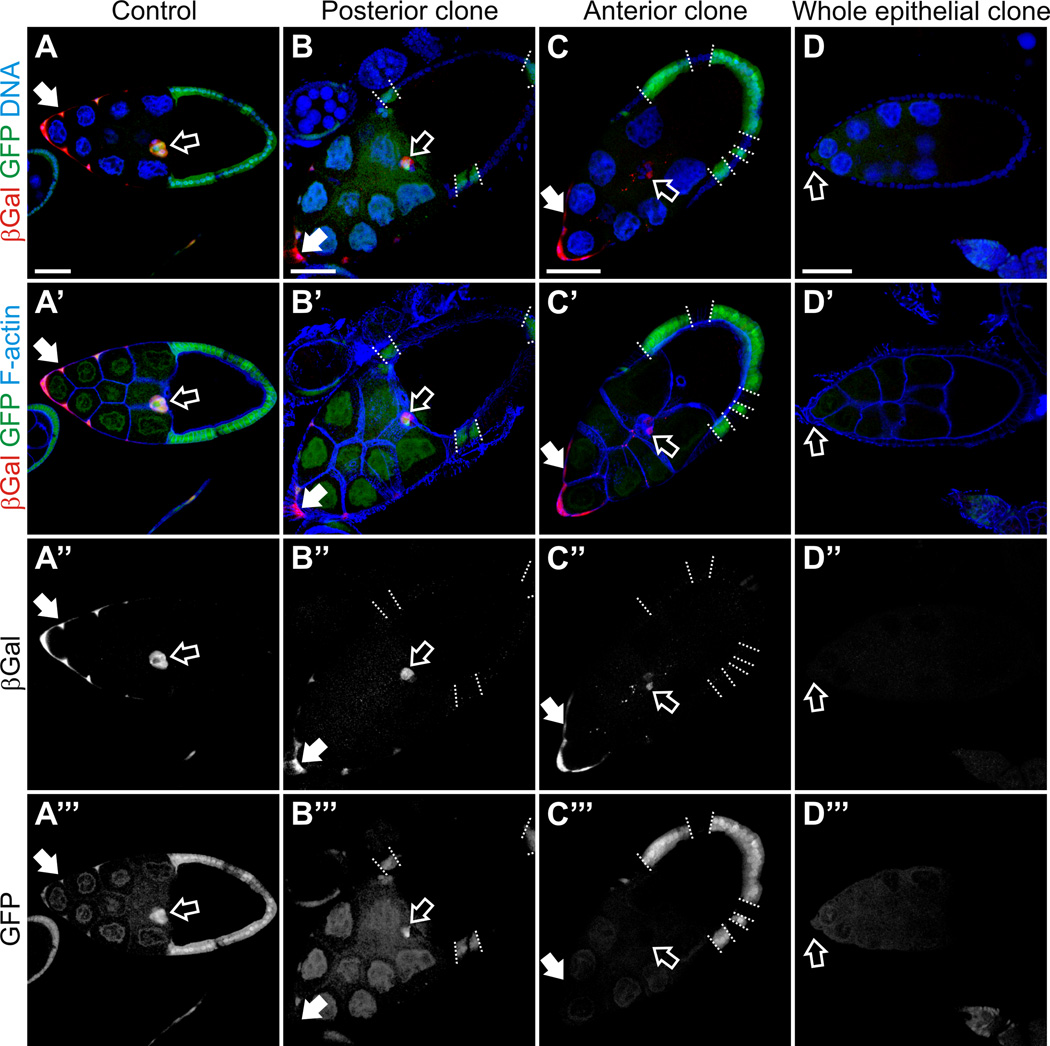
Fig. 3. Ecdysone signaling failed to be activated in the in the phmFL99 and phmFT59 whole epithelial homozygous mutant egg chambers.
Egg chambers from flies carrying EcR-lacZ reporter line in the genetic background stained with Hoechst to label nuclei, phalloidin to label F-actin, and with anti-βGal antibodies to detect activation of EcR. Egg chambers are oriented with the anterior to the left. Scale bars represent 40 µm. (A) Stage 10A wild-type egg chambers. The LacZ reporter expression was detected in the anterior FCs including the BCs (open arrow) and stretched cells overlying the nurse cells (filled arrow), which indicates that ecdysone signaling is activated in these cells types. (B, C) Mosaic phmFL99 and phmFT59 mutant egg chambers, stage 10A. phmFL99 and phmFT59 homozygous mutant clones are marked by the absence of GFP. The borders between mutant clones and neighboring wild-type cells are marked by dashed lines. Border cells are marked with open arrow, stretched cells by filled arrows. (D) We observed a lack of ecdysone signaling activation in the phmFL99 or phmFT59 homozygous mutant egg chambers containing whole epithelial mutant clones, which are marked by the absence of GFP (D’”). Border cells are marked by open arrow (D-D”’).
Phm is a member of the so-called Halloween group of genes, which were originally isolated due to their functions during embryogenesis. Mutations in Halloween group genes, disembodied (dib), shadow (sad), shade (shd), spook (spo) and spookier (spok), result in characteristic morphogenetic abnormalities, such as failure of cuticle formation and head involution, defects in dorsal closure and ecdysone deficiency, resulting in embryonic lethality (Chavez et al., 2000; Jurgens et al., 1984; Ono et al., 2006; Petryk et al., 2003; Warren et al., 2002; see Gilbert, 2004 and Gilbert et al., 2002 for review). As was previously shown, phmE7 mutant embryos display a range of the phenotypes characteristic for the members of the Halloween group, such as undifferentiated cuticle and abnormal morphogenetic movements (Niwa et al., 2004; Warren et al., 2004). We analyzed cuticle formation in phmFL99 and the phmFT59 homozygous mutant embryos, and observed a failure in the production of differentiated cuticle structures similar to the phmE7 allele (Fig. 2C–E).
Ecdysone signaling activity in the border cells is affected when follicle cells within the same egg chamber are mutant for phm
Phm encodes a cytochrome P450 ecdysteroidogenic enzyme, the microsomal 25-hydroxylase, which converts 2,22,25-trideoxyecdysone (3β,5β-ketodiol) to 2,22-dideoxyecdysone (3β,5β-ketotriol) (Niwa et al., 2004; Warren et al., 2004). Absence of Phm enzymatic function leads to a disruption of the biosynthetic production chain of ecdysone, and therefore an absence of its biologically active form, 20-hydroxyecdysone. The ecdysone biosynthesis and its temporal fluctuations play critical roles in temporal coordination of cell proliferation, differentiation, and apoptosis during embryonic and larval development (see Cranna and Quinn, 2009; Gilbert et al., 2002; Thummel, 2001 for review), and in adult reproduction, physiology and behavior (see Schwedes and Carney, 2012 for review). Activation of the ecdysone receptor in BCs regulates the timing of the migration and is necessary for the stimulation of the invasive cell behavior (Bai et al., 2000; Jang et al., 2009). We therefore hypothesized that in phm mutants, the ecdysone receptor in the BCs is not activated due to the block in ecdysone production, hence leading to the observed failure of BC migration.
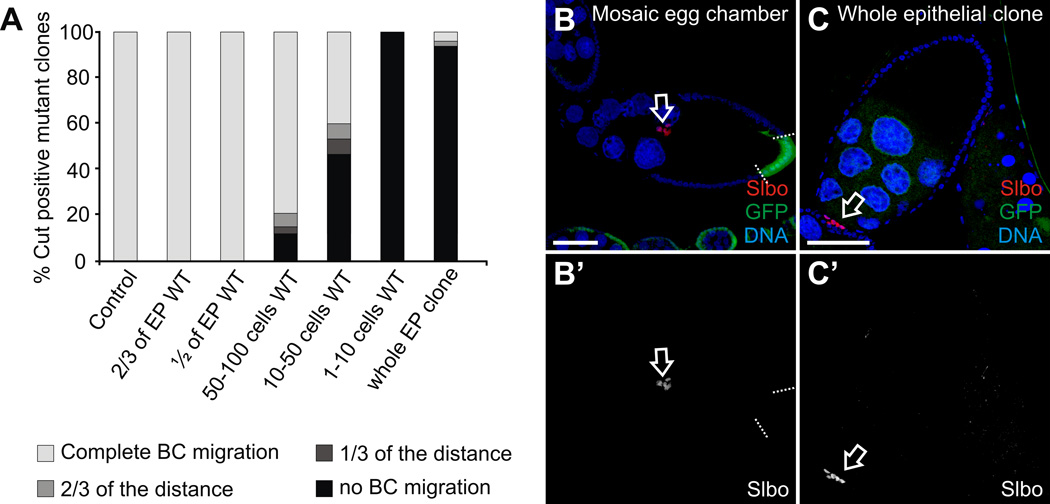
In order to assess the activation of ecdysone signaling, an EcR-lacZ transgenic reporter, containing seven copies of an EcR response element upstream of a minimal promoter and the E. coli lacZ gene, has been commonly used in Drosophila. The EcR-lacZ reporter is expressed only in cells that are exposed to ecdysone and competent to respond to it (Kozlova and Thummel, 2003b). To analyze the activity of ecdysone signaling in the phmFL99 and the phmFT59 homozygous mutant egg chambers, we crossed the EcR-lacZ reporter into the phmFL99 and phmFT59 genetic backgrounds and analyzed the expression of the reporter in the mosaic egg chambers (Fig. 3, open arrows). Consistent with previously reported data, no EcR-lacZ reporter expression was detected in the ovaries before stage 9 (Jang et al., 2009). During stage 9, we began to detect reporter expression in the anterior FCs including the BCs and stretched cells overlying the nurse cells, which indicates that ecdysone signaling is activated in these cells types (Jang et al., 2009) (Fig. 3A, closed arrows). We observed a lack of ecdysone signaling activation in the phmFL99 or phmFT59 homozygous mutant egg chambers containing whole epithelial mutant clones (Fig. 3D). Interestingly, in the presence of even a small patch of wild-type FCs in the otherwise homozygous mutant egg chambers, we detected the expression of the EcR-lacZ reporter, meaning that the ecdysone signaling was successfully activated in these egg chambers (Fig. 3B, C). Importantly, BC migration progressed normally in the egg chambers containing a small number of wild-type cells (Fig. 3B, C, open arrows). However, even though the migration of the BCs was normal, the intensity of the staining for the EcR-LacZ reporter line in these egg chambers with a few wild-type FCs was somewhat lower in comparison to egg chambers completely surrounded by wild-type FCs (Fig. 4). We performed a more detailed analysis of mosaic egg chambers, distinguishing different cases dependent on the position of the homozygous mutant clone affecting different cell types: polar cells, outer BCs, stretched cells, centripetal cells and posterior FCs. Interestingly, as long as the wild-type patch of FCs was of sufficient size (see detailed analysis below) we did not note any defects in the migration of the BC cluster, independent of the position of the mutant clone. We also noted that, in egg chambers with a mosaic follicular epithelium, independent of the clone position, the anterior stretched cells also all expressed EcR-LacZ reporter activity.
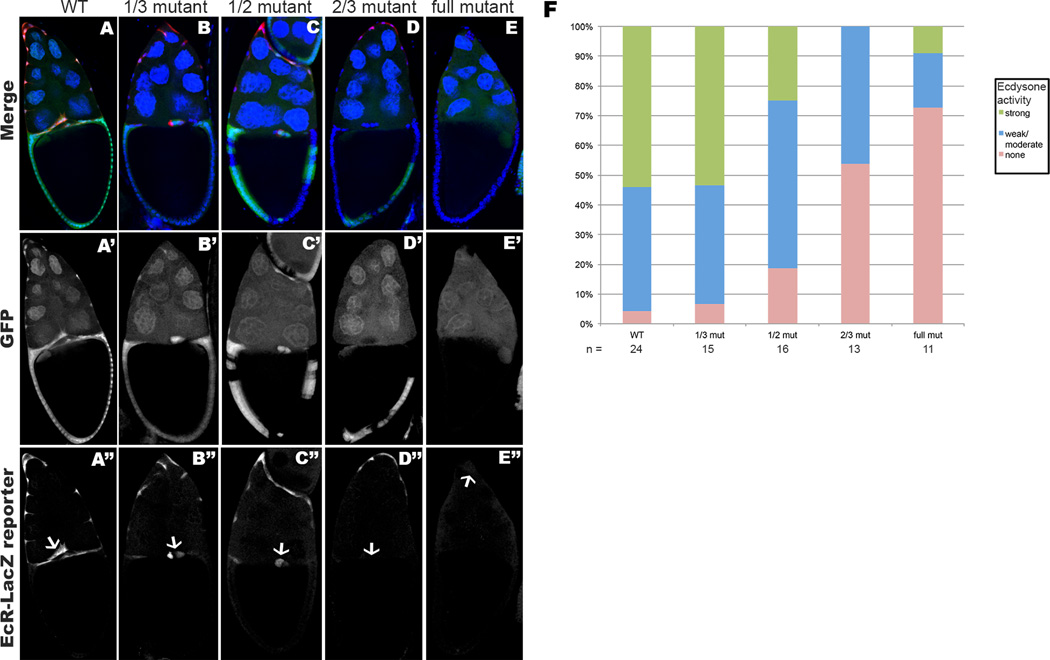
Fig. 4. Ecdysone reporter expression is reduced proportional to increasing numbers of mutant cells within the follicular epithelium.
(A–D) Stage 10 PhmFT59 mosaic egg chambers containing the EcR-LacZ reporter. Egg chambers are stained with Hoechst to label nuclei, and anti-β-galactosidase to detect ecdysone reporter expression. Homozygous mutant cells are marked by the absence of GFP. (A’–D’) show the GFP expression, and (A”–D”) show anti-β-galactosidase staining. Border cells are indicated with an arrow (A”–D”). Egg chambers with an entirely wild-type epithelium exhibit strong expression of the ecdysone activity reporter within the border cells (A”). When larger portions of the cells within the follicular epithelium are mutant, EcR-LacZ reporter expression in the border cells diminishes in direct relation to increasing numbers of mutant epithelial cells (B”–D”). Egg chambers with specific fractions of mutant cells within the follicular epithelium were scored for relative intensity of EcR-LacZ expression within the border cell cluster (F), re-affirming the trend demonstrated in (A”ȓD”).
In order to determine the minimal size of the patch of wild-type cells sufficient to produce the amount of ecdysone necessary to promote the migration of the BCs, we performed a detailed quantification counting numbers of wild-type cells in each of the mosaic egg chambers (Fig. 5). As a positive control we used wild-type stage 10A egg chambers, where we observed 100% of the analyzed BC clusters reaching the border between the nurse cells and oocyte by this stage (n=44). We used 10A stage egg chambers containing the phmFL99 or phmFT59 whole epithelial homozygous mutant clones as a negative control. In these whole epithelial mutant egg chambers, less than 10% of the BC clusters reached the oocyte and more than 90% of BC clusters did not initiate migration (n=46). In contrast, no defects in BC migration were observed in the egg chambers, where more than 30% of the FCs were wild-type (Fig. 5). In the presence of up to 10 wild-type FCs in the egg chambers (about 1–2% of all FCs) independent on their position, the BC clusters failed to migrate and the defect was as strong as in the egg chambers with the whole epithelial clones. Interestingly, in the egg chambers containing from 10 to 50 wild-type FCs (about 2–5% of FCs), we observed approximately 40% of BC clusters which completed their migration. In the egg chambers containing from 50 to 100 wild-type FCs (about 8–15% of FCs), approximately 80% of BC clusters completed their migration and reached the oocyte by stage 10A. Based on these data, we conclude that the presence of 50 to 100 wild-type FCs, which is about 8–15% of all FCs in the egg chamber, is sufficient to produce an essential level of ecdysone to induce and promote BC migration.
Fig. 5. Quantification of the border cell migration phenotype in the phmFL99 and phmFT59 homozygous mutant egg chambers.
(A) Percentage of the Cut positive mutant clones in the stage 10A egg chambers of the following classes: wild-type (control) egg chambers (n=44); egg chambers with 2/3 of the total FCs being wild-type (n=43); egg chambers with the half of the follicle epithelium being wild-type (n=32); mosaic egg chambers containing 50–100 wild-type FCs (n=33); 10–50 wild-type FCs (n=15); 1–10 wild-type FCs (n=8); and egg chambers containing whole epithelial phm mutant clones (n=46). BC migration in the egg chambers at stage 10A was scored distinguishing the following categories: “completed migration” when BCs reached the oocyte (white); BCs migrated over 2/3 of the distance between the most anterior end of the egg chamber and the border between the nurse cells and the oocyte (light grey); BCs migrated 1/3 of this specified distance (dark grey); and BC cluster failed to migrate and stayed at the most anterior end of the egg chamber, the so-called “no migration” phenotype (black). (B, C) Egg chambers stained with with Hoechst (B, C) to label DNA, and anti-Slbo (B) or anti-Cut (C) antibody to label border cell clusters. Egg chambers are oriented with the anterior to the left. Scale bars represent 40 µm. (B) Stage 10A phm mutant mosaic egg chamber. Mutant cells are marked by the absence of GFP (green), the borders between mutant clones and neighboring wild-type cells are marked by dashed lines. Border cells (open arrow) migrated normally. (C) Stage 10A egg chamber containing phm whole epithelial homozygous mutant clone. Border cells (open arrow) failed to migrate.
The GAL4 driver line that we used to induce the homozygous clones is only active in the FCs (Duffy et al., 1998). For this reason, the egg chambers that contained the homozygous whole epithelial mutant clones were surrounding a nurse cell-oocyte cluster of wild-type genotype. The fact that BC migration failed in the large majority of cases when only the FCs were mutant indicates that the germline derived nurse cells or the oocyte cannot contribute sufficient levels of ecdysone to allow BC migration.
Phantom activity is not required in the germline for survival and border cell migration
Germline clones homozygous for mutations in EcR or the E74 response gene, as well as germline clones homozygous for mutations in ecd degenerate at the onset of vitellogenesis (Buszczak et al., 1999; Gaziova et al., 2004) which indicates a strong requirement for ecdysone in the germline. In order to assess whether the germline requires Phantom activity to survive we induced germline clones of phmFL99 and phmFT59. We did not detect any increased rate of degeneration in the 69 phmFL99 ovaries, and 45 phmFT59 ovaries from females in which germline clones had been induced, compared to the 81 ovaries from FRT19A control females (6 degenerating phmFL99 egg chambers, 8 degenerating phmFT59 egg chambers, and 54 degenerating control egg chambers). In addition, we also induced germline clones by heatshock using the ovoD dominant female sterile technique (Perrimon, 1998) and observed that many of the females with germline clones mutant for phmFL99 or phmFT59 laid eggs that produced hatching larvae at a rate equal to FRT19A control females (90–95% for both mutant and control heatshocked females, see experimental procedures for genotypes). This indicates that the germline, even in the absence of endogenous Phm activity, will receive sufficient ecdysone levels for survival and developmental progression from the surrounding tissues. We next asked whether border cell migration was normal in the presence of phm mutant germline clones. We found that in all of the fully homozygous mutant germline clones the border cells migrated normally (in total 14/14; Supplementary Figure S1) indicating that phm expression is not required in the germline for BC migration.
In summary, these results show that phm expression in the follicle cells is both required and sufficient for border cell migration.
Shade, another component of the ecdysteroid synthesis pathway, is also required for border cell migration specifically in the follicle cells
Our findings that Phm activity is specifically required in the follicle epithelium to promote normal border cell migration led us to examine whether this indicates a more general requirement for the ecdysteroid synthesis pathway in the follicle cells of each egg chamber. We therefore repeated the analysis using mutations in shade (shd). Shd, which encodes 20-hydroxylase, is the P450 enzyme responsible for converting Ecdysone into 20-hydroxyecdysone, the active steroid that functions as the morphogenetic regulator in Drosophila (Petryk et al., 2003; Rewitz et al., 2006). We generated shd2 mosaic ovaries to determine whether the activation to 20-hydroxyecdysone also occurs within the follicle epithelium of each egg chamber, and is important for migration of the border cells.
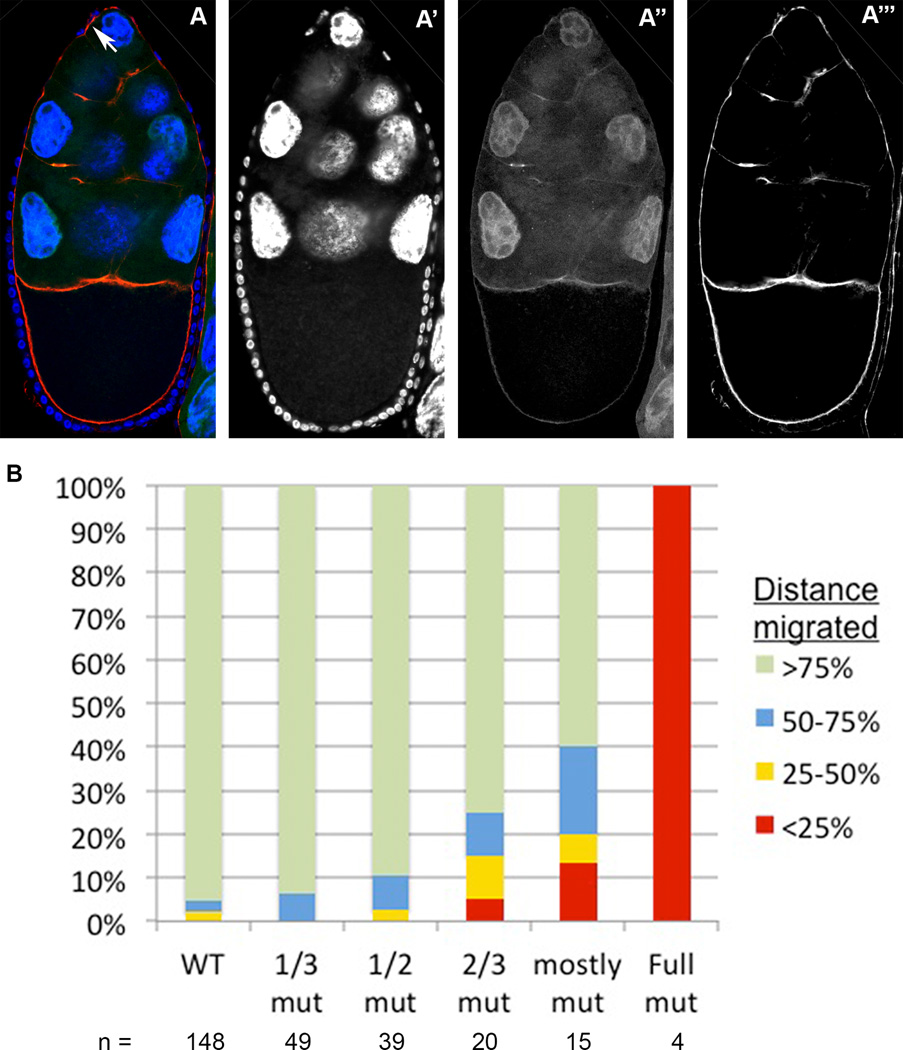
Similar to our observation for phm mutations, we found that border cell migration failed in egg chambers that contained follicle cell epithelia entirely mutant for shd. When we scored BC migration in mosaic egg chambers with shd2 mutant clones occupying increasingly larger portions of the follicle epithelium, without regards to the clonal position within the epithelium we found that BC migration defects were increasingly more severe as more shd2 mutant cells were present in the epithelium (Fig 6). This phenotype is also very similar to that observed for phm epithelial clones, in which larger clones result in more severe migration delays (Fig. 5). In addition, we determined that shd activity is not required in the germline. Upon scoring egg chambers with full germline clones only, we found no border cell migration defects in 13 out of 13 cases, as we saw with full germline clones mutant for phm (Supplementary Fig. S1).
Figure 6. The shd mutant border cell migration phenotype: increasingly larger mutant clones within the follicular epithelium result in more severe migration delays.
(A) A Stage 10 egg chamber with a follicular epithelium that is entirely mutant for shd (observe the lack of GFP in the follicular epithelium in A”), stained with Hoechst to label nuclei (A’), and phalloidin to label cell membranes (A”’). Anterior is oriented upward. The border cell cluster, indicated with a white arrow in A, fails to detach from the epithelium at the anterior of the egg chamber. (B) Quantification of border cell migration behaviors in egg chambers with epithelial clones of increasingly larger proportions. The size of each colored bar represents the percentage of egg chambers in which border cells have migrated through each quadrant of the wild type migration distance. Egg chambers with increasingly larger sized epithelial clones exhibit more severe delays in migration.
Therefore, the follicle epithelium provides all of the Shade activity necessary for border cell migration, just as in the case of Phantom. Thus, conversion of Ecdysone to the active 20-hydroxyecdysone is also spatially compartmentalized to the follicle cells within each individual egg chamber.
Medium supplemented with 20-hydroxyecdysone can rescue BC migration in phmFL99 or phmFT59 whole epithelial homozygous mutant egg chambers cultured in vitro
In the absence of Phm protein, ketodiol is not converted to ketotriol, and thus the production chain of the 20-hydroxyecdysone, a physiologically active ecdysteroid, is disrupted. The addition of exogenous 5×10−6 M 20-hydroxyecdysone to cultured larval organs and embryos at stage 9, when the ecdysone receptor is normally silent, leads to its activation (Kozlova and Thummel, 2002, 2003a). Feeding ecdysone to mutant animals with inadequate level of ecdysteroid titers due to a misregulation of the ecdysone synthetic pathway at different steps of the ecdysone production restored the molting and other mutant phenotypes, as for instance for Cyp307a2 (spookier, spok) (Ono et al., 2006), Cyp6t3 (Ou et al., 2011) and dnpc1a mutants (Huang et al., 2005). Furthermore, in adult females injected with 20-hydroxyecdysone, premature BC migration was detected in a few rare cases (Bai et al., 2000).
To address the question of whether BC migration can be rescued in the egg chambers containing phmFL99 or phmFT59 whole epithelial homozygous mutant clones by supplying these egg chambers with 20-hydroxyecdysone, we took advantage of the improved culture conditions supporting ex vivo development of stage 9 egg chambers in combination with live imaging (Dorman et al., 2004; He et al., 2011; Prasad et al., 2007; Prasad and Montell, 2007; Prasad et al., 2011). We introduced membrane-mCherry (Martin et al., 2010) to the phmFL99 or phmFT59 genetic background to visualize cell outlines and to analyze BC dynamics in the cultured egg chambers in the course of time-lapse live imaging. We cultured stage 9 egg chambers according to a previously established protocol (Prasad et al., 2007) and imaged the migration of the BC cluster for 6–8 hours. Normal migration of the BC clusters was observed in the wild-type egg chambers and in mosaic egg chambers containing both phmFL99 or phmFT59 homozygous mutant and wild-type clones, cultured in the control medium without 20-hydroxyecdysone supplement (Supplementary Movie S1). In the case of both stage 9 and stage 10A egg chambers containing the phmFL99 or phmFT59 whole epithelial homozygous mutant clones cultured in the control medium, we did not observe the migration of the BC clusters even after 6–8 hours of culturing (Supplementary Movie S2 and Supplementary Figure S2).
For the rescue experiments, we supplied the egg chamber culturing medium with 5×10−6 M 20-hydroxyecdysone, the physiological concentration of 20-hydroxyecdysone that leads to wide-spread activation of the ecdysone receptor in the cultured larval organs (Kozlova and Thummel, 2002). We cultured the stage 9 and stage 10A egg chambers containing the phmFL99 or phmFT59 whole epithelial homozygous mutant clones in the 20-hydroxyecdysone supplemented medium for approximately 6–8 hours. No migration of the BC clusters was observed within the first 3–4 hours of incubation, followed by the eventual detachment of the cluster from the neighboring non-motile epithelial cells and a relatively fast migration towards the oocyte (n=4, Supplementary Movie S3 and Supplementary Figure S2). Based on this observation, we propose that the first 3–4 hours are needed for the diffusion of sufficient 20-hydroxyecdysone into the egg chambers, and that after 3–4 hours the concentration of 20-hydroxyecdysone reached the essential level to initiate the migration of the BC clusters. Thus, the BC migration phenotype can be rescued in the phm homozygous mutant egg chambers by providing them with the synthetic 20-hydroxyecdysone.
Discussion
The ecdysteroids are steroid hormones that, in combination with juvenile hormones, program stage specific gene expression during insect development. Ecdysteroid levels show specific pulses during different stages of embryogenesis to initiate molting and metamorphosis. During the adult stage of many insects, ecdysteroids play important roles in reproduction, physiology and behavior (see Schwedes and Carney, 2012 for review). Ecdysone is the only known steroid hormone in Drosophila, and is produced by the prothoracic glands in immature insects (see Gilbert et al., 2002 for review). Little is known about tissue-specific production of ecdysone in adult animals due to its lower levels in comparison to the earlier developmental stages, and thus difficulties in its detection. However, ecdysone was detected in all 3 body segments of adult flies, in hemolymph, and in specific tissues such as testes, gut, Malpighian tubules and in ovaries (see Schwedes and Carney, 2012 for review). During the adult stage, the ovary is a major site of ecdysone synthesis, which peaks at stage 9 (Riddiford, 1993), when BC migration takes place. More recently, a novel function of ecdysone was demonstrated in the regulation of timing of BC migration in the developing egg chambers in the Drosophila females (Bai et al., 2000; Jang et al., 2009; McDonald et al., 2003). The precise source of the ecdysone that initiates border cell migration, was however not known. In the work reported here, we isolated two lethal alleles of Drosophila phm, a gene encoding for a cytochrome P450 ecdysteroidogenic enzyme, which converts ketodiol to ketotriol, the intermediate products in the ecdysone synthetic chain. We demonstrated that BC migration is severely disrupted in the phm mutants due to the lack of Phm activity in the follicle cells of the developing egg chambers, and we also found that the same egg chamber autonomous requirement applies to Shade, the last enzyme in the 20-hydroxyecdysone pathway.
Phm acts in follicle epithelial cells of developing egg chambers
It has previously been shown that ecdysone is synthesized within the egg chambers during oogenesis, although the precise cells that are crucial for ecdysone synthesis are not known (Riddiford, 1993; see Schwedes and Carney, 2012 for review). It is also not known whether the multi-step process of ovarian ecdysone synthesis takes place exclusively in one cell type in the egg chamber, or whether some steps of the synthesis are carried out in the germline derived cells, such as nurse cells or oocyte, while others are performed in the FCs. Expression of some enzymes in the biosynthetic pathway was detected in the germline cells, whereas others are found predominantly in FCs (Chavez et al., 2000; Namiki et al., 2005; Niwa et al., 2004; Ono et al., 2006; Petryk et al., 2003; Schwedes and Carney, 2012; Warren et al., 2002; Warren et al., 2004). It is therefore possible that certain intermediates in the pathway diffuse between the cell types. Phm RNA was detected in follicle cells at stage 8/9 and may be present later, possibly at low levels, in nurse cells (Niwa et al., 2004; Warren et al. 2004). We showed that this step of ecdysone synthesis takes place in the follicle epithelial cells and not sufficiently in the germline cells (neither in nurse cells nor in the oocyte) to promote border cell migration. Similarly, we demonstrated that the synthesis of 20-hydroxyecdysone from ecdysone via Shd also takes place within the follicular epithelium, and that the germline cannot rescue the absence of Shd activity in the follicle cells.
Interestingly, we observed a block in border cell migration in egg chambers where the follicle epithelium was fully mutant for phm or for shd, even when the adjacent egg chambers contained wild-type follicle cells. This further indicates that in terms of border cell migration, the egg chamber requires its own follicle cells to be actively engaged in the ecdysone pathway, and border cells cannot receive sufficient active ecdysone from the neighboring egg chambers to initiate the migration process. This is biologically justified, since each egg chamber has to time its own progression and therefore strict reliance on the egg chamber’s own follicle cells will assure coordination of the various developmental processes within each individual egg chamber.
A critical level of ecdysone is required for normal development
Threshold concentrations of ecdysone in the egg chambers are crucial for normal progression of oogenesis. Highly elevated levels of the ecdysteroid, exceeding the normal threshold, induce apoptosis at mid-oogenesis, in stage 8 and 9 egg chambers. This effect is observed, for example, under starvation conditions that cause ecdysone levels in the ovaries to increase, resulting in the degeneration of the egg chambers, and thereby controlling the rate of egg production in relation to food intake (Terashima et al., 2005). Insufficient levels of EcR activation, on the other hand, also cause similarly abnormal oogenesis and apoptosis. For example, there are no vitellogenic stages in the ovaries of ecd-1 females (Audit-Lamour and Busson, 1981), and the presence of EcR mutant germline clones in the developing egg chamber leads to developmental arrest and degeneration during mid-oogenesis (Buszczak et al., 1999). Therefore, certain intermediate levels of ecdysone seem to be crucial for wild-type egg chamber progression and ecdysone concentration significantly above or below this threshold affect oogenesis.
We took advantage of the ability to generate mutant clones of different size in the developing egg chambers to define amount of wild-type FCs necessary for the production of a sufficient level of ecdysone essential for normal BC migration. The major phenotype we observed in egg chambers in which all FCs were mutant for phm or shd, was the severe disruption of BC migration. Based on our detailed analysis of the phm phenotype, we demonstrated that in the presence of 50–100 wild-type FCs (about 8–15% of total amount of post-mitotic FCs in an egg chamber), BC migration is normal, indicating that a sufficient amount of ecdysone is produced in these egg chambers for this process. In addition, we used an EcR-LacZ reporter line to assay the level of EcR activation in the FCs of the mosaic chambers. We noticed that the intensity of staining for the EcR-lacZ reporter line varied depending on the amount of the wild-type FCs in the mosaic egg chamber. We observed a very strong staining in the wild-type egg chambers at mid-oogenesis, a lower intensity with a smaller amount of wild-type cells in the mosaic egg chambers, and no detectable EcR activation in whole epithelial mutant egg chambers. This data indicates that the resulting level of ecdysone, and thus EcR activation, depends on the amount of phm wild-type FCs in the egg chamber, and probably closely reflects the level of ketotriol production in the FCs. The fact that the BCs migrate normally even when only about 10% of the follicle cells are wild-type, irrespective of the location of the wild-type cells, indicates that certain intermediates and/or 20-hydroxyecdysone itself diffuse readily in the egg chamber, but again illustrates that neighboring egg chambers cannot supply these products to rescue the migration phenotype. Nevertheless, we were able to rescue the migration phenotype by supplying exogenous 20-hydroxyecdysone in the culture medium. The levels of ecdysone in the hemolymph that surrounds the ovaries in vivo must therefore be significantly lower than what we supplied in the culture medium. Maintaining a relatively low level of ecdysone in the hemolymph of the adult females is presumably also required to allow each egg chamber to individually regulate the timing of border cell migration and to coordinate this with the other developmental processes in the egg chambers.
In summary we have shown that the induction of phm expression at stage 8/9 specifically in the follicle cells is crucial for production of sufficiently high ecdysone levels in each individual egg chamber to initiate border cell migration. Within each egg chamber, however, only a minor fraction of the follicle cells have to carry a wildtype allele of phm to allow sufficient levels of ecdysone to be produced for the correct execution of border cell migration.
Experimental procedures
Fly stocks and Genetics
The phmFT59 and phmFL99 mutations were isolated in a mosaic screen for EMS-induced mutations in a y w FRT19A genetic background (Denef et al., 2008). The membrane-mCherry line was a gift from A. Martin (Martin et al., 2010). The phmE7 allele, EcR-LacZ line (Kozlova and Thummel, 2003b) used to assay EcR activity, and various duplication and P-element lines used for mapping were obtained from the Bloomington Drosophila Stock Center. The shd2 allele, (originally called shade7C94; Jurgens et al., 1984), was utilized to examine the effect of shd mutants on BC migration. phm mutant FC clones were generated using the FRT/UAS-Flp/GAL4 system (Duffy et al., 1998) using a Ubi-GFP FRT19A; e22c-Gal4, UAS-Flp line obtained from the Bloomington Drosophila Stock Center. shd2 FC clones were generated by first recombining the mutant allele with the FRT80B and then using an e22c-Gal4, UAS-Flp; Ubi-GFP FRT80B line. Germline clones for phm were generated by heat shocking y w phm FRT19A/Ubi-GFP FRT19A; TM3 hs-Gal4, UAS-Flp, Sb e larvae and pupae, or by heat shocking y w phm FRT19A/y w sn P{w+ OvoD1} FRT19A hsFLP122 larvae, or control larvae without a phm mutation, three times for 1 ¼ hours and testing the resulting adult females individually for egg production. Non-heat shocked females of either FRT19A/ovoD or phm FRT19A/ovoD did not lay any normal eggs, proving that P{ovoD1} is fully penetrant under our conditions. Germline clones for shd were produced using a similar heat-shock regime and a y w hs-Gal4, UAS-Flp; Ubi-GFP FRT80B line.
Mapping of the phmFT59 and phmFL99 mutations
We used recombination with visible recessive markers to map the lethal mutation in the phmFT59 and phmFL99 mutant alleles to the region proximal to f (proximal to 15F7). The lethal phenotype was rescued by duplications covering the genomic region 17A2–17A8; 18A7 (Dp(1;Y)BSC13) and 17A2–17B3; 18A7 (Dp(1;Y)BSC15). Subsequent SNP mapping (Berger et al., 2001; Chen et al., 2008; Chen et al., 2009; Hoskins et al., 2001) placed the lethal mutation between 17C6 and 17D1. We sequenced PCR products of at least 2 independent genomic isolations for each allele covering the region, and compared the sequences with those of the FRT19A control to map the mutations within the phm gene.
Immunofluorescence staining and microscopy
Ovaries were dissected in PBS, fixed for 20 min in 4% paraformaldehyde at room temperature, and stained according to standard procedures (Ashburner, 1989). Primary antibodies used were rabbit anti β-Gal (1:2000, Millipore), mouse anti-Cut (1:10, 2B10, DSHB), rat anti-Slbo (1:500, gift from P. Roth) (Borghese et al., 2006), and mouse anti-Sn (1:10, Sn-7c, DSHB). Secondary antibodies were AlexaFluor488, 568, 647 conjugated (Molecular probes) and used at 1:1000. Phalloidin conjugates and Hoechst were from Molecular Probes. Images were taken on a Zeiss LSM510 confocal microscope, a Nikon A1 confocal microscope, and a Nikon A1-RS spectral confocal microscope.
Cuticle preparations
Cuticle preparations were carried out as described previously (Wieschaus and Nüsslein-Volhard, 1986) and were visualized using dark field microscopy.
Egg chamber culturing and live imaging
Flies carrying phmFL99 or phmFT59 alleles and the fluorescent membrane-mCherry reporter were used to generate homozygous mutant follicle epithelial clones. Fluorescent membrane-mCherry (Martin et al., 2010) was used to visualize cell outlines in the cultured egg chambers during live imaging. Flies were put on yeast for 16–18 hours prior to dissection. The individual egg chambers were dissected out, separated from the neighboring egg chambers in the ovariole and cultured on the Greiner Lumox culture hydrophilic dishes (Sigma, Cat. No. Z376744) as it was previously described (Prasad et al., 2007). Briefly, medium containing Schneider’s Drosophila medium (Invitrogen, Cat. No 11720-034), 15% fetal bovine serum (Sigma, Cat. No. F2442) and 0.6X Streptomycin/penicillin (Invitrogen, Cat. No. 15140-122) was prepared in advance and stored at 4°C. This medium cocktail was supplemented with 10 mg/ml Insulin (Sigma, Cat. No. I5500) dissolved in acidified water on the day of use. For the rescue experiments, the medium was additionally supplemented with 5×10−6 M 20-hydroxyecdysone (Sigma, Cat. No. H5142), or with a vehicle control (1% ethanol). We cultured the stage 9 and stage 10A egg chambers containing whole epithelial homozygous phmFL99 or phmFT59 mutant clones or as a control, wild-type or mosaic egg chambers, in a drop of medium without covering the sample with a coverslip for approximately 6–8 hours. Live imaging was performed on a Perkin Elmer UltraView RS spinning disk confocal microscope. The egg chambers of interest were selected using the GFP channel. Live imaging was performed with 561 laser to detect mCherry signal. Generally, we defined an approximately 20–30 µm thick Z stack in order to capture the whole BC cluster. We defined a Z-step as 1 µm, and scanned the egg chamber every 3 minutes. For movie processing we used Volocity 3D Image Analysis Software.
Supplementary Material
Supplemental Figure S1. Neither Phantom, nor Shade are required in the germline for the formation and migration of the border cells.
(A–C) A stage 10 PhmFL99 mosaic egg chamber stained with Hoechst to label nuclei, and phalloidin to label F-actin. (B) All germline cells are homozygous mutant as shown by the absence of GFP staining. (C) The border cell cluster, indicated by an arrow, has formed and completed its migration. (D) Stage 10 egg chambers with fully mutant germline clones for phm, and also for shd were scored for formation of the border cell cluster, and for complete vs. defective migration of the cluster.
Supplementary Figure S2. GFP expression of egg chambers shown in Movies S2 and S3 to allow identification of mutant follicle cell epithelia.
(A) GFP expression in the egg chamber containing phmFT59 whole epithelial homozygous mutant clones, shown in the Supplementary Movie S2. (B) GFP expression in the egg chamber containing phmFL99 whole epithelial homozygous mutant clones, shown in the Supplementary Movie S3.
Supplementary Movie S1. Migration of the border cell clusters in wild-type egg chambers, expressing the membrane-mCherry reporter and cultured without 20-hydroxyecdysone supplement, at the onset of migration was recorded by time-lapse microscopy (as described in Methods).
Supplementary Movie S2. Absence of border cell migration in an egg chamber containing a phmFT59 whole epithelial homozygous mutant clone (see Supplementary Figure S2 for GFP expression) expressing a membrane-mCherry reporter and cultured in the control medium without 20-hydroxyecdysone supplement, recorded by time-lapse microscopy (as described in Methods). The egg chamber center in the lower half and to the right of the center is surrounded by mutant follicle cells and the border cells do not migrate, whereas the egg chamber on the left is surrounded by wildtype follicle cells and the border cells migrate.
Supplementary Movie S3. Migration of the border cell clusters in an egg chamber containing an phmFL99 whole epithelial homozygous mutant clone (see Supplementary Figure S2), expressing a membrane-mCherry reporter and cultured in the medium supplemented with 20-hydroxyecdysone, at the onset of migration was recorded by time-lapse microscopy (as described in Methods). In contrast to the control (non-mutant) egg chamber on the right, the border cell cluster in the mutant egg chamber in the middle stays stationary for a longer time period, but then migrates rapidly posteriorward.
HIGHLIGHTS.
Ecdysone biosynthesis enzymes are required only in follicle cells, not the germline, for border cell migration.
Ecdysone biosynthesis is required in an egg chamber autonomous fashion for border cell migration.
Regardless of position, a small number of wildtype follicle cells can rescue border cell migration.
Acknowledgements
We thank Adam Martin, Pernille Roth, the Developmental Studies Hybridoma Bank and the Bloomington stock center for sending Drosophila stocks and for providing antibodies. We are very grateful to Natalie Denef and Yan Yan for performing the initial mutagenesis screen, Gail Barcelo for technical help, Joe Goodhouse for support with confocal microscopy and members of the Schupbach and Wieschaus laboratories for feedback and suggestions. We also thank Julie Merkle, Olivier Devergne and Attilio Pane for critical reading and helpful comments on the manuscript. This work was supported by the Howard Hughes Medical Institute and by US Public Health Service Grant RO1 GM077620.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner M. Drosophila: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Audit-Lamour C, Busson D. Oogenesis defects in the ecd-1 mutant of Drosophila melanogaster, deficient in ecdysteroid at high temperature. J. Insect Physiol. 1981;27:829–837. [Google Scholar]
- Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Beccari S, Teixeira L, Rorth P. The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mechanisms of development. 2002;111:115–123. doi: 10.1016/s0925-4773(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Berger J, Suzuki T, Senti KA, Stubbs J, Schaffner G, Dickson BJ. Genetic mapping with SNP markers in Drosophila. Nat Genet. 2001;29:475–481. doi: 10.1038/ng773. [DOI] [PubMed] [Google Scholar]
- Borghese L, Fletcher G, Mathieu J, Atzberger A, Eades WC, Cagan RL, Rorth P. Systematic analysis of the transcriptional switch inducing migration of border cells. Developmental cell. 2006;10:497–508. doi: 10.1016/j.devcel.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126:4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- Cant K, Knowles BA, Mooseker MS, Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. The Journal of cell biology. 1994;125:369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez VM, Marques G, Delbecque JP, Kobayashi K, Hollingsworth M, Burr J, Natzle JE, O'Connor MB. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development. 2000;127:4115–4126. doi: 10.1242/dev.127.19.4115. [DOI] [PubMed] [Google Scholar]
- Chen D, Ahlford A, Schnorrer F, Kalchhauser I, Fellner M, Viragh E, Kiss I, Syvanen AC, Dickson BJ. High-resolution, high-throughput SNP mapping in Drosophila melanogaster. Nat Methods. 2008;5:323–329. doi: 10.1038/nmeth.1191. [DOI] [PubMed] [Google Scholar]
- Chen D, Berger J, Fellner M, Suzuki T. FLYSNPdb: a high-density SNP database of Drosophila melanogaster. Nucleic Acids Res. 2009;37:D567–D570. doi: 10.1093/nar/gkn583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranna N, Quinn L. Impact of steroid hormone signals on Drosophila cell cycle during development. Cell division. 2009;4:3. doi: 10.1186/1747-1028-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef N, Chen Y, Weeks SD, Barcelo G, Schupbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14:354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman JB, James KE, Fraser SE, Kiehart DP, Berg CA. bullwinkle is required for epithelial morphogenesis during Drosophila oogenesis. Dev Biol. 2004;267:320–341. doi: 10.1016/j.ydbio.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Duffy JB, Harrison DA, Perrimon N. Identifying loci required for follicular patterning using directed mosaics. Development. 1998;125:2263–2271. doi: 10.1242/dev.125.12.2263. [DOI] [PubMed] [Google Scholar]
- Gaziova I, Bonnette PC, Henrich VC, Jindra M. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development. 2004;131:2715–2725. doi: 10.1242/dev.01143. [DOI] [PubMed] [Google Scholar]
- Gilbert LI. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Molecular and cellular endocrinology. 2004;215:1–10. doi: 10.1016/j.mce.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annual review of entomology. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- He L, Wang X, Montell DJ. Shining light on Drosophila oogenesis: live imaging of egg development. Current opinion in genetics & development. 2011;21:612–619. doi: 10.1016/j.gde.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins RA, Phan AC, Naeemuddin M, Mapa FA, Ruddy DA, Ryan JJ, Young LM, Wells T, Kopczynski C, Ellis MC. Single nucleotide polymorphism markers for genetic mapping in Drosophila melanogaster. Genome Res. 2001;11:1100–1113. doi: 10.1101/gr.178001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Suyama K, Buchanan J, Zhu AJ, Scott MP. A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development. 2005;132:5115–5124. doi: 10.1242/dev.02079. [DOI] [PubMed] [Google Scholar]
- Jang AC, Chang YC, Bai J, Montell D. Border-cell migration requires integration of spatial and temporal signals by the BTB protein Abrupt. Nat Cell Biol. 2009;11:569–579. doi: 10.1038/ncb1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster II. Zygotic loci on the third chromosome. Wilhelm Roux’s Arch. Dev. Biol. 1984:283–295. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- Kozlova T, Thummel CS. Spatial patterns of ecdysteroid receptor activation during the onset of Drosophila metamorphosis. Development. 2002;129:1739–1750. doi: 10.1242/dev.129.7.1739. [DOI] [PubMed] [Google Scholar]
- Kozlova T, Thummel CS. Essential roles for ecdysone signaling during Drosophila mid-embryonic development. Science. 2003a;301:1911–1914. doi: 10.1126/science.1087419. [DOI] [PubMed] [Google Scholar]
- Kozlova T, Thummel CS. Methods to characterize Drosophila nuclear receptor activation and function in vivo. Methods in enzymology. 2003b;364:475–490. doi: 10.1016/s0076-6879(03)64027-9. [DOI] [PubMed] [Google Scholar]
- Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. The Journal of cell biology. 2010;188:735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA, Pinheiro EM, Kadlec L, Schupbach T, Montell DJ. Multiple EGFR ligands participate in guiding migrating border cells. Dev Biol. 2006;296:94–103. doi: 10.1016/j.ydbio.2006.04.438. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Pinheiro EM, Montell DJ. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development. 2003;130:3469–3478. doi: 10.1242/dev.00574. [DOI] [PubMed] [Google Scholar]
- McGregor JR, Xi R, Harrison DA. JAK signaling is somatically required for follicle cell differentiation in Drosophila. Development. 2002;129:705–717. doi: 10.1242/dev.129.3.705. [DOI] [PubMed] [Google Scholar]
- Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Rorth P, Spradling AC. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 1992;71:51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Yoon WH, Starz-Gaiano M. Group choreography: mechanisms orchestrating the collective movement of border cells. Nat Rev Mol Cell Biol. 2012;13:631–645. doi: 10.1038/nrm3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki T, Niwa R, Sakudoh T, Shirai K, Takeuchi H, Kataoka H. Cytochrome P450 CYP307A1/Spook: a regulator for ecdysone synthesis in insects. Biochemical and biophysical research communications. 2005;337:367–374. doi: 10.1016/j.bbrc.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nature reviews. Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- Niwa R, Matsuda T, Yoshiyama T, Namiki T, Mita K, Fujimoto Y, Kataoka H. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem. 2004;279:35942–35949. doi: 10.1074/jbc.M404514200. [DOI] [PubMed] [Google Scholar]
- Ono H, Rewitz KF, Shinoda T, Itoyama K, Petryk A, Rybczynski R, Jarcho M, Warren JT, Marques G, Shimell MJ, Gilbert LI, O'Connor MB. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev Biol. 2006;298:555–570. doi: 10.1016/j.ydbio.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Ou Q, Magico A, King-Jones K. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS biology. 2011;9:e1001160. doi: 10.1371/journal.pbio.1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N. Creating mosaics in Drosophila. Int J Dev Biol. 1998;42:243–247. [PubMed] [Google Scholar]
- Petryk A, Warren JT, Marques G, Jarcho MP, Gilbert LI, Kahler J, Parvy JP, Li Y, Dauphin-Villemant C, O'Connor MB. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A. 2003;100:13773–13778. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Jang AC, Starz-Gaiano M, Melani M, Montell DJ. A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nature protocols. 2007;2:2467–2473. doi: 10.1038/nprot.2007.363. [DOI] [PubMed] [Google Scholar]
- Prasad M, Montell DJ. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Developmental cell. 2007;12:997–1005. doi: 10.1016/j.devcel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Prasad M, Wang X, He L, Montell DJ. Border cell migration: a model system for live imaging and genetic analysis of collective cell movement. Methods in molecular biology. 2011;769:277–286. doi: 10.1007/978-1-61779-207-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochemical Society Transactions. 2006;34:1256–1260. doi: 10.1042/BST0341256. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormone receptors and the regulation of insect metamorphosis. Receptor. 1993;3:203–209. [PubMed] [Google Scholar]
- Rorth P. Initiating and guiding migration: lessons from border cells. Trends in cell biology. 2002;12:325–331. doi: 10.1016/s0962-8924(02)02311-5. [DOI] [PubMed] [Google Scholar]
- Schwedes CC, Carney GE. Ecdysone signaling in adult Drosophila melanogaster. Journal of insect physiology. 2012;58:293–302. doi: 10.1016/j.jinsphys.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Silver DL, Montell DJ. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell. 2001;107:831–841. doi: 10.1016/s0092-8674(01)00607-9. [DOI] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. New York: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- Terashima J, Takaki K, Sakurai S, Bownes M. Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. The Journal of endocrinology. 2005;187:69–79. doi: 10.1677/joe.1.06220. [DOI] [PubMed] [Google Scholar]
- Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Developmental cell. 2001;1:453–465. doi: 10.1016/s1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Warren JT, Petryk A, Marques G, Jarcho M, Parvy JP, Dauphin-Villemant C, O'Connor MB, Gilbert LI. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2002;99:11043–11048. doi: 10.1073/pnas.162375799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JT, Petryk A, Marques G, Parvy JP, Shinoda T, Itoyama K, Kobayashi J, Jarcho M, Li Y, O'Connor MB, Dauphin-Villemant C, Gilbert LI. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect biochemistry and molecular biology. 2004;34:991–1010. doi: 10.1016/j.ibmb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Werck-Reichhart D, Feyereisen R. Cytochromes P450: a success story. Genome biology. 2000;1 doi: 10.1186/gb-2000-1-6-reviews3003. REVIEWS3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E, Nüsslein-Volhard C. Looking at embryos. In: Roberts DB, editor. Drosophila: A practical approach. 1 ed. Oxford, Washington, DC: IRL Press; 1986. pp. 199–227. [Google Scholar]
- Wieschaus E, Nusslein-Volhard C, Jürgens G. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. III. Zygotic loci on the X-chromosome and fourth chromosome. Roux's Arch. Dev. Biol. 1984:296–307. doi: 10.1007/BF00848158. [DOI] [PubMed] [Google Scholar]
- Xi R, McGregor JR, Harrison DA. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Developmental cell. 2003;4:167–177. doi: 10.1016/s1534-5807(02)00412-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Neither Phantom, nor Shade are required in the germline for the formation and migration of the border cells.
(A–C) A stage 10 PhmFL99 mosaic egg chamber stained with Hoechst to label nuclei, and phalloidin to label F-actin. (B) All germline cells are homozygous mutant as shown by the absence of GFP staining. (C) The border cell cluster, indicated by an arrow, has formed and completed its migration. (D) Stage 10 egg chambers with fully mutant germline clones for phm, and also for shd were scored for formation of the border cell cluster, and for complete vs. defective migration of the cluster.
Supplementary Figure S2. GFP expression of egg chambers shown in Movies S2 and S3 to allow identification of mutant follicle cell epithelia.
(A) GFP expression in the egg chamber containing phmFT59 whole epithelial homozygous mutant clones, shown in the Supplementary Movie S2. (B) GFP expression in the egg chamber containing phmFL99 whole epithelial homozygous mutant clones, shown in the Supplementary Movie S3.
Supplementary Movie S1. Migration of the border cell clusters in wild-type egg chambers, expressing the membrane-mCherry reporter and cultured without 20-hydroxyecdysone supplement, at the onset of migration was recorded by time-lapse microscopy (as described in Methods).
Supplementary Movie S2. Absence of border cell migration in an egg chamber containing a phmFT59 whole epithelial homozygous mutant clone (see Supplementary Figure S2 for GFP expression) expressing a membrane-mCherry reporter and cultured in the control medium without 20-hydroxyecdysone supplement, recorded by time-lapse microscopy (as described in Methods). The egg chamber center in the lower half and to the right of the center is surrounded by mutant follicle cells and the border cells do not migrate, whereas the egg chamber on the left is surrounded by wildtype follicle cells and the border cells migrate.
Supplementary Movie S3. Migration of the border cell clusters in an egg chamber containing an phmFL99 whole epithelial homozygous mutant clone (see Supplementary Figure S2), expressing a membrane-mCherry reporter and cultured in the medium supplemented with 20-hydroxyecdysone, at the onset of migration was recorded by time-lapse microscopy (as described in Methods). In contrast to the control (non-mutant) egg chamber on the right, the border cell cluster in the mutant egg chamber in the middle stays stationary for a longer time period, but then migrates rapidly posteriorward.