Abstract
We report three families presenting with hypertrophic cardiomyopathy, lactic acidosis, and multiple defects of mitochondrial respiratory chain (MRC) activities. By direct sequencing of the candidate gene MTO1, encoding the mitochondrial-tRNA modifier 1, or whole exome sequencing analysis, we identified novel missense mutations. All MTO1 mutations were predicted to be deleterious on MTO1 function. Their pathogenic role was experimentally validated in a recombinant yeast model, by assessing oxidative growth, respiratory activity, mitochondrial protein synthesis, and complex IV activity. In one case, we also demonstrated that expression of wt MTO1 could rescue the respiratory defect in mutant fibroblasts. The severity of the yeast respiratory phenotypes partly correlated with the different clinical presentations observed in MTO1 mutant patients, although the clinical outcome was highly variable in patients with the same mutation and seemed also to depend on timely start of pharmacological treatment, centered on the control of lactic acidosis by dichloroacetate. Our results indicate that MTO1 mutations are commonly associated with a presentation of hypertrophic cardiomyopathy, lactic acidosis, and MRC deficiency, and that ad hoc recombinant yeast models represent a useful system to test the pathogenic potential of uncommon variants, and provide insight into their effects on the expression of a biochemical phenotype.
Keywords: MTO1, hypertrophic cardiomyopathy, lactic acidosis, mitochondrial disorder, yeast
Introduction
Mitochondrial disorders are a group of syndromes associated with severe dysfunction of oxidative phosphorylation (OXPHOS), the main energy bioreactor of cells. Cardiomyocytes, with their extremely high request of energy, are one of the major targets of OXPHOS impairment, and infantile hypertrophic cardiomyopathy is a key clinical feature in many mitochondrial disorders. We have recently reported the first patients affected by hypertrophic cardiomyopathy and lactic acidosis carrying mutations in MTO1 (MIM #614702) [Ghezzi et al., 2012]. Two were siblings, compound heterozygous for c.1858dup (p.Arg620Lysfs*8) and c.1282G>A (p.Ala428Thr) mutations, who died in their first days of life due to sudden bradycardia. Muscle and fibroblasts showed decreased activities of mitochondrial respiratory chain (MRC) complex I (CI) and CIV. The third patient, homozygous for the c.1282G>A (p.Ala428Thr) mutation, had also early-onset cardiac hypertrophy with severe lactic acidosis, and defective CI + CIV activities in muscle; however, he dramatically improved on a permanent treatment with dichloroacetate (DCA) and cofactors, being now 20 years old with compensated, stable hypertrophic cardiomyopathy.
MTO1 (MIM #614667), a gene conserved in all eukaryotes, encodes one of the two subunits of the enzyme that catalyzes the 5-carboxymethylaminomethylation (mnm5s2U34) of the wobble uridine base in the mitochondrial tRNAs specific to Gln, Glu, Lys, Leu(UUR), and possibly Trp [Suzuki et al., 2011; Wang et al., 2010]. The other subunit is encoded by MSS1 in yeast and GTPBP3 in humans (MIM #608536). For mt-tRNAs for Gln, Glu, Lys, this modification is usually coupled to the 2-thiolation of the same uridine moiety, a reaction catalyzed by 2-thiouridylase, encoded by yeast MTO2 or human TRMU (MIM #610230). Both these posttranscriptional modifications increase accuracy and efficiency of mitochondrial DNA (mtDNA) translation by influencing tRNA structure, binding to the ribosome, stabilization of the correct codon-anticodon pairing [Kurata et al., 2008; Murphy et al., 2004; Takai, 2005; Umeda et al., 2005; Urbonavicius et al., 2001; Wang et al., 2010; Yarian et al., 2000; Yasukawa et al., 2001], and tRNA recognition by the cognate aminoacyltransferase [Krüger and Sørensen, 1998; Sylvers et al., 1993].
In our previous work, we investigated the functional consequences of the MTO1 mutations in a simple eukaryotic model system, Saccharomyces cerevisiae [Ghezzi et al., 2012]. The analysis was performed mainly in a mutant yeast strain harboring a C>G transversion at nucleotide 1,477 of the 15S rRNA mtDNA gene [Colby et al., 1998], which results in a synthetic phenotype with MTO1 disruption. The mutation disrupts the C1477–G1583 base pairing in a functionally relevant hairpin structure, which is part of the decoding site (site A) of the ribosome, where codon–anticodon recognition takes place [Yan et al., 2005]. This mutation confers resistance to the antibiotic paromomycin by destabilizing the hairpin. We chose this strain because the human mitochondrial 12S rRNA contains a hairpin structure that corresponds to the paromomycin-resistant variant in yeast. We showed that the yeast Ala431Thr change, corresponding to human Ala428Thr, reduced mitochondrial respiratory activity, whereas the mutation equivalent to human Arg620Lysfs*8 behaved as a null allele.
We present here the identification of five additional MTO1 mutant subjects (two couples of siblings, and a sporadic case) who also present with hypertrophic cardiomyopathy and lactic acidosis, thus, strengthening a consistent genotype/phenotype correlation. We confirm the pathogenic role of the two novel mutations in the yeast model and, for the milder variant, by complementation studies in mutant fibroblasts.
Materials and Methods
Patients
Informed consent for participation in this study was obtained from the parents of all patients, in agreement with the Declaration of Helsinki and approved by the Ethical Committees of the Institutes participating in this study, where biological samples were obtained.
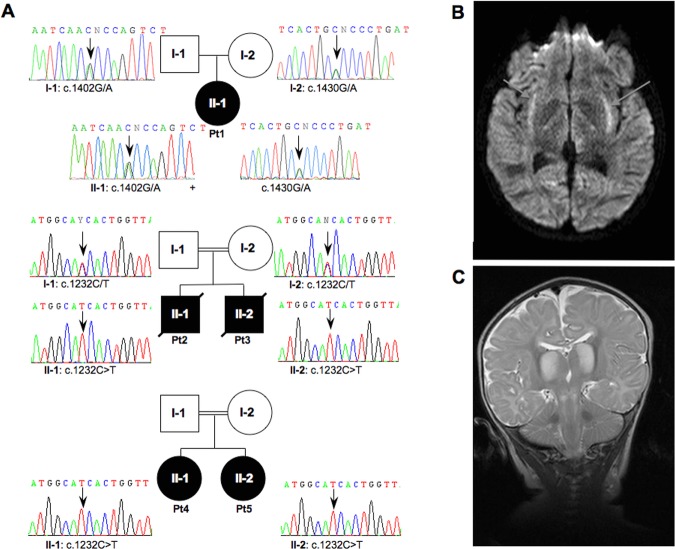
We studied a first cohort of 30 patients with cardiomyopathy and a biochemical defect of the MRC, affecting either CI alone or multiple complexes, and a second small group of four cases with isolated CIV deficiency and at least one affected sibling, irrespective of their clinical presentations (ranging from cardiomyopathy to encephalopathy). Table 1 summarizes the main clinical, laboratory and biochemical features of five patients from three families (Fig. 1A) with MTO1 mutations. All these subjects showed early-onset, progressive hypertrophic cardiomyopathy, and lactic acidosis. Some did also display neurological features affecting the peripheral or the central nervous system, or both, associated with neuropathological abnormalities documented by MRI (Fig. 1B and C). Detailed case reports are described in the Supporting Information.
Table 1.
Clinical Synopsis and Biochemical Features of MTO1 patients
| Patient | Familiarity | Gender | Age of onset | Relevant clinical features | Actual age/Outcome | Cause of death | Metabolic findings | Biochemical MRC defects | Mutations in MTO1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Pt1, present study | No | F | 2 days | Psychomotor delay, hypotonia, dystonia. Later, hypertrophic cardiomyopathy. | 14 yrs | – | Lactic acidemia, hyperalaninemia | Ms: ↓ CI and CIVFbs: ↓ MRR | p.[Ala428Thr]; [Arg477His] |
| #2 | Pt2, present study | Brother of #3; consanguineous parents | M | Birth | Poor feeding due to swallowing difficulties.Failure to thrive. Later, hypertrophic cardiomyopathy. Aspiration pneumonia. Hypotonia. | +12 mo | Cardio-respiratory arrest. | Hypoglycaemia, lactic acidemia | Ms: ↓ CI and CIVFbs: ↓ MRR | p.[Thr411Ile];[Thr411Ile] |
| #3 | Pt3, present study | Brother of #2; consanguineous parents | M | Birth | Poor feeding due to swallowing difficulties. Failure to thrive. Early-onset hypertrophic cardiomyopathy. Hypotonia. | +3 mo | n.d. | Lactic acidemia | n.d. | p.[Thr411Ile];[Thr411Ile] |
| #4 | Pt4, present study | Sister of #5; consanguineous parents | F | 3 mo | Early-onset hypertrophic cardiomyopathy. Bronchiolitis-like illness. Encephalopathy and seizures. | 19 yrs | – | Lactic acidemia | Ms: ↓ CIV | p.[Thr411Ile];[Thr411Ile] |
| #5 | Pt5, present study | Sister of #4; consanguineous parents | F | 5 mo | Upper respiratory illness. Hypertrophic cardiomyopathy and WPW. Psychomotor delay. | 12 yrs | – | Lactic acidemia, hyperalaninemia, ketonuria | Ms: ↓ CIV | p.[Thr411Ile];[Thr411Ile] |
| #6 | Pt1, Ghezzi et al., (2012) | Brother of #7 | M | Birth | Hypertrophic cardiomyopathy. | +19 days | Sudden bradycardia | Lactic acidemia, hyperalaninemia | Ms: ↓ CI and CIVFbs: ↓CIII and CIV; ↓ MRR | p.[Ala428Thr];[Arg620Lysfs*8] |
| #7 | Pt2, Ghezzi et al., (2012) | Sister of #6 | F | Birth | Hypertrophic cardiomyopathy with tachycardia. Hypotonia. | +40 days | Sudden bradycardia | Lactic acidemia | Ms: ↓ CI and CIVFbs: ↓ CI; ↓ MRR | p.[Ala428Thr];[Arg620Lysfs*8] |
| #8 | Pt3, Ghezzi et al., (2012) | No | M | 1 mo | Weakness, lack of ocular fixation. Hypertrophic cardiomyopathy with sinus bradycardia. Moderate bilateral optic atrophy. | 20 yrs | – | Lactic acidemia | Ms: ↓ CI and CIV | p.[Ala428Thr];[Ala428Thr] |
Ms, muscle biopsy; Fbs, fibroblasts; MRC, mitochondrial respiratory chain; CI–CIV, complexes I–IV; WPW, Wolff–Parkinson–White syndrome.
Figure 1.
Pedigrees and radiological features. A: Pedigrees and electropherograms of the MTO1 genomic region encompassing the nucleotide substitutions in patients and available parents. Black symbols designate affected subjects. B: Brain MRI of Pt1. Transverse FLAIR image showing abnormal hyperintensity in the region of the claustrum and surrounding capsulae (arrows). C: Brain MRI of Pt2. Coronal T2-weighted sequence showing abnormal hyperintense signals of the thalami and diffusely abnormal signal in the subcortical white matter. Lesions are also present in the brainstem. The cerebellar folia are normal.
Molecular Analysis
Genomic DNA was extracted by standard methods. Exons and exon–intron boundaries of human MTO1 (NM_012123.3; NP_036255) were amplified using primers listed in Supp. Table S1, and analyzed by Sanger sequencing. Whole-exome next-generation sequencing (WES) and variant filtering were performed as described [Ghezzi et al., 2012]. Nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence, according to journal guidelines (www.hgvs.org/mutnomen). The initiation codon is codon 1. All variants reported have been submitted to LSDB (http://www.lovd.nl/MTO1).
Biochemical Assays
The activities of MRC complexes and citrate synthase in muscle homogenates were measured as described [Bugiani et al., 2004]. Microoxygraphy was used to measure maximal respiration rate (MRR), spare respiratory capacity (SRC), respiratory control ratio, and oxygen consumption rate (OCR)/extracellular acidification rate (ECAR) in fibroblasts, using SeaHorse FX-24 or FX-96 [Invernizzi et al., 2012]. For transduced cells, F14 medium (Euroclone), supplemented with EGF, FGF, insulin, and uridine, was used instead of DMEM.
In Silico Analysis
The pathogenicity of the human mutations was predicted by using five bioinformatic tools based on heuristic methods: PANTHER (http://www.pantherdb.org), SIFT (http://sift.jcvi.org), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2), SNPs&GO (http://snps-and-go.biocomp.unibo.it/snps-and-go), and MutPred (http://mutpred.mutdb.org). Structural analysis was performed using the structure of Chlorobium tepidum GidA (PDB ID 3CP8 at http://www.rcsb.org/pdb/home/home.do). Models of mutant proteins were constructed using SwissModel (http://swissmodel.expasy.org/) and superimposed with Swiss-Pdb Viewer Magic fit tool. Protein regions were visualized by the RasMol software package.
Analysis in Yeast
We used the yeast strain W303 PR mto1 (MATα trp1-1 mto1::URA3) [Colby et al., 1998]. MTO1 was cloned in the centromeric vector pFL39 [Bonneaud et al., 1991] through PCR amplification of MTO1 and digestion with SalI and SacI. The mto1 mutant alleles were obtained by site-directed mutagenesis of a MTO1 fragment [Ho et al., 1989], using suitable primers (Supp. Table S1). Mutant fragments were cloned in the AvaI and SacI cloning sites of pFL39-MTO1. The mto1 strain was transformed with pFL39 harboring wt or mutant MTO1 alleles by lithium-acetate based methods [Gietz and Woods, 2002]. Respiratory activity and in vitro mt-DNA protein synthesis were performed as previously described [Barrientos et al., 2002; Goffrini et al., 2009]. Cytochrome c oxidase activity was measured according to Fontanesi et al., (2008) and Barrientos et al., (2009) on a mitochondrial-enriched fraction prepared according to Soto et al., (2009).
Lentiviral Transduction
The wt MTO1 cDNA was cloned into the pLenti6.3/V5-TOPO Vector (Invitrogen, Carlsbad, CA, USA), and virions were obtained as previously described [Zhang et al., 2009]. Mutant and wt fibroblasts were infected with viral supernatant and selected upon exposure to 2 µg/ml Blasticidin (Invitrogen).
Results
Molecular and Biochemical Analyses in Human Samples
By Sanger sequencing we screened MTO1 in a cohort of mitochondrial defective patients with cardiomyopathy, and found that Pt1 was compound heterozygous for the previously described c.1402G>A/p.Ala428Thr mutation and for a novel missense substitution (c.1430G>A/p.Arg477His), whereas siblings Pt2 and Pt3 harbored a homozygous c.1232C>T/p.Thr411Ile change (Fig. 1A). By WES analysis on a second group of familial cases with CIV deficiency (see Materials and Methods) and no known genetic defect, we identified an additional case, Pt4, with the same homozygous c. 1232C>T/p.Thr411Ile change. Her clinically affected sister (Pt5) was shown to harbor the identical homozygous variant (Fig. 1A).
Spectrophotometric biochemical assays of the respiratory chain complexes activities revealed defects in CI, CIV, or both (Table 1). No clear evidence of CIII deficiency was obtained in any tissue sample of the present study, in contrast with a previous study [Ghezzi et al., 2012; #6 in Table 1]. A partial but significant reduction in MRR, SRC, and OCR/ECAR was measured by SeaHorse microscale oxygraphy in fibroblast cell lines of Pt1 and Pt2 (Supp. Table S2).
In Silico Analysis
To test the potential deleterious effects of the p.Thr411Ile and p.Arg477His, we first used bioinformatic tools based on heuristic methods for predicting pathogenic variants. The prediction of a putative pathogenic change is based on evolutionary conservation, plus other features, such as predicted structural effects (Polyphen-2, MutPred), predicted functions (MutPred), and local sequence and gene ontology score (SNPs&GO). Both mutations scored a “probably pathological” prediction by each method (Supp. Table S3).
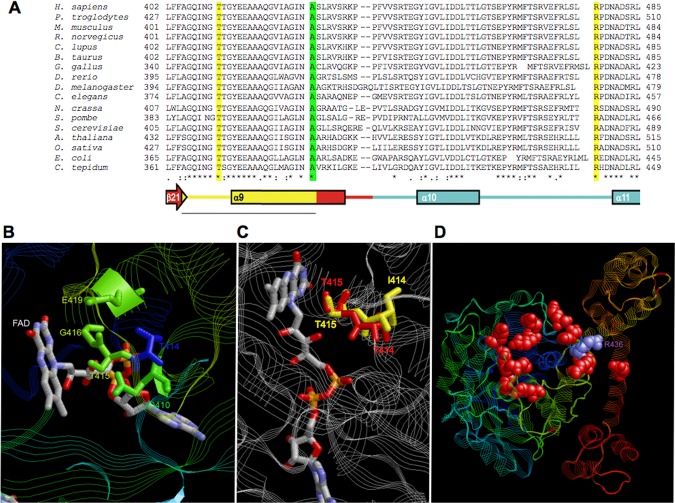
We further tested the pathogenicity of these mutations on the basis of the structure of GidA, the eubacterial ortholog of Mto1. Its structure has been resolved in Escherichia coli, C. tepidum, and Aquifex aeolicus [Meyer et al., 2008; Osawa et al., 2009; Shi et al., 2009]. GidA contains four domains: an α/β FAD-binding domain; a small N-terminal insertion-domain 1; a large α/β insertion-domain 2, which binds NADH; a large α C-terminal domain, which contributes to the binding of the tRNA and promotes the dimerization with MnmE, the ortholog of Mss1/Gtpbp3. Human threonine 411 (hThr411) is part of motif 2, which is highly conserved from bacteria to eukaryotes (Fig. 2A) and is contained in the α/β FAD-binding domain. Motif 2 includes residues from the C-terminus of sheet β21, the loop between β21 and the residues from the N-terminal of helix α9. In C. tepidum GidA, this motif includes three conserved residues: Gln366 (hGln407, yeast Gln410), Gly372 (hGly413, yGly416), and Glu375 (hGlu 416, yGlu 419), plus Ser371, which is either conserved or substituted conservatively by threonine (e.g., hThr412, yThr415). The residues are all involved in FAD binding through interaction with the FAD ribitol and pyrophosphate moieties (Gln366 and Gly372) or with the isoalloxazine ring-containing active site (Ser371 and Glu375) (Fig. 2B). Moreover, the side chain of serine 371 is oriented to the central ring of isoalloxazine, suggesting a functional role in the catalytic process and/or binding/stabilization of FAD [Meyer et al., 2008]. As a matter of fact, substitution of Thr382 in A. aeolicus GidA, corresponding to Ser371 in C. tepidum GidA, results in inability of complementing the methylaminomethyl modification at position 5 of uridine (mnm5) during exponential growth of GidA-deficient E. coli [Osawa et al., 2009]. We hypothesized that the substitution of hydrophilic hThr411/yThr414 (corresponding to threonine at position 370 of GidA) with a hydrophobic, bulky isoleucine changes the position of the adjacent amino-acid residue (hThr412/yThr415 or bacterial Ser371). To support this hypothesis, we constructed a structural model in which the threonine at position 370 (yThr414) was changed to isoleucine. This change altered the orientation of the side chain of the adjacent amino acid and increased the distance relative to the isoalloxazine ring (Fig. 2C).
Figure 2.
In silico structural analysis. A: Alignment of Mto1 proteins from animals, yeasts, plants, and eubacteria around mutated amino acids. In yellow the amino acids corresponding to mutations hThr411Ile and hArg477His, in green the amino acid corresponding to mutation Ala428Thr [Ghezzi et al., 2012]. The corresponding secondary structure elements for the C. tepidum GidA structure are indicated and colored according to Meyer et al. [2008]. B: Structure of the C. tepidum GidA region around the FAD moiety. Amino acids of the motif 2 of GidA which bind the FAD group are indicated. For simplicity, the numbers refer to the equivalent position in yeast Mto1. C: Structure of the C. tepidum GidA region around the FAD moiety superimposed to the model structure of GidA. The wt structure of the amino acids equivalent to threonines 414 (T414) and 415 (T415) in yeast Mto1 is in red. The predicted structure of the amino acids equivalent to yeast mutant isoleucine 414 (I414, corresponding to the human mutation Thr411Ile) and adiacent threonine 415 (T415) is in yellow. For simplicity, the numbers refer to the position in yeast Mto1. D: Overall structure of the C. tepidum GidA with basic amino acids (in red), which form a pocket who is predicted to bind the D-stem of the incoming tRNA. The bacterial Arg436 residue (R436), equivalent to hArg477 and yArg481, is in magenta.
We identified the hArg477 residue in humans as equivalent to C. tepidum Arg436, which is located in a highly conserved loop between helices α10 and α11 in the C-terminal domain (Fig. 2A). Arg436 takes part in a cluster of several basic amino acids (Lys, Arg, and His), conserved in bacteria and eukaryotes, and predicted to form a positively charged pocket, which binds the phosphates of the D-stem backbone of the incoming tRNA (Fig. 2D) [Meyer et al., 2008; Osawa et al., 2009]. The substitution of the equivalent arginine with alanine in GidA of E. coli is known to decrease the efficiency of mnm5 modification [Shi et al., 2009]. Therefore, we hypothesized that substitution of the fully charged human Arg477 with the partially charged His477 could also decrease the affinity for the incoming tRNA.
Analysis in Yeast
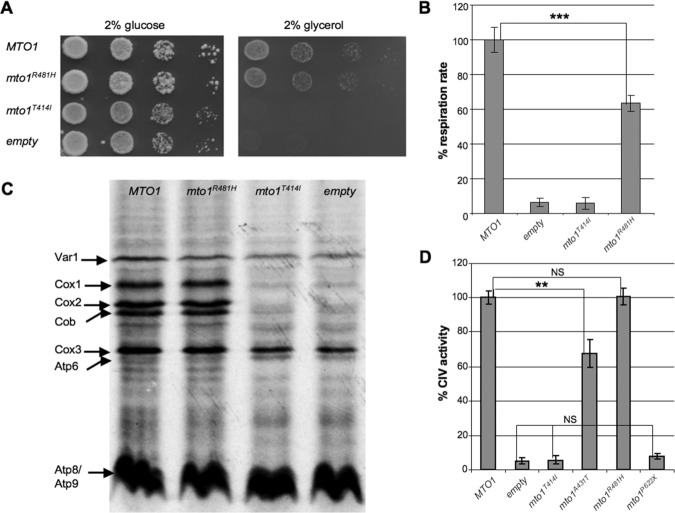
To confirm the pathogenic role of p.Thr411Ile and p.Arg477His mutations predicted by in silico analysis, we introduced the corresponding mutant alleles (mto1T414I and mto1R481H) in the paromomycin-resistant yeast strain disrupted in MTO1 (Δmto1 PR strain). The parental Δmto1 PR strain is unable to grow on oxidative carbon sources (Fig. 3A) [Colby et al., 1998]; the expression of mto1T414I mutant allele failed to correct this phenotype, whereas the expression of mto1R481H was able to restore oxidative growth, although to a lesser extent than wt MTO1 (Fig. 3A). Accordingly, mitochondrial respiration was abolished in both Δmto1 and mto1T414I strains, whereas it was restored by the mto1R481H strain to approximately 60% of the wt strain (Fig. 3B).
Figure 3.
Yeast studies. A: Growth of Δmto1 strain transformed with MTO1 wt allele, mto1R481H, and mto1T414I mutant alleles or empty plasmid on YP medium supplemented with 2% glucose (left panel) or 2% glycerol (left panel). Cells were pregrown on YP+glucose and plated after serial dilutions to obtain spots of 5 × 104, 5 × 103, 5 × 102, and 5 × 101 cells/spot. Pictures were taken after 2 days of growth. B: Respiratory activity of Δmto1 strains transformed with MTO1 wt allele, mto1R481H, and mto1T414I mutant alleles or empty plasmid. Respiratory rates were normalized to the strain transformed with wt MTO1, for which the respiratory rate was 34.7 nmol min−1 mg−1. Values are the mean of three independent experiments, each with an independent clone. Two-tail, paired t-test was applied for statistical significance. ***P < 0.001. C: In vivo mitochondrial translation of Δmto1 strain transformed with MTO1 wt allele, mto1R481H, and mto1T414I mutant alleles or empty plasmid. Mitochondrial gene products were labeled with [35S]-methionine in whole cells in the presence of cycloheximide for 10 min at 28°C. Cox: cytochrome c oxidase; Cob: cytochrome b; Atp: ATP synthase; Var1: small mitochondrial ribosome subunit. D: Cytochrome c oxidase (CIV) activity of Δmto1 strain transformed with MTO1 wt allele, mto1T414I, mto1A431T, mto1R481H, and mto1P622X mutant alleles or empty plasmid. Cytochrome c oxidase activities were normalized to the strain transformed with wt MTO1, for which the activity was 368.8 units per mg of mitochondrial proteins. Values are the mean of three independent experiments, each with an independent clone. Two-tail, unpaired t-test was applied for statistical significance. **P < 0.01.
Since the p.Thr414Ile mutation is predicted to alter the position of the adjacent Ser371, which participates in the catalytic process involving FAD and/or in its binding/stabilization, we carried out a second set of experiments in the presence of increasing concentration of riboflavin (from 1 to 25 μM); however, neither defective oxidative growth nor reduced respiratory activity of the mto1T414I strain were rescued by riboflavin, suggesting that the catalytic activity of the mutant Mto1T414I is fully impaired (data not show). An alternative explanation is that the mutant Mto1T414I is unstable and quickly degraded; the absence of an antibody against the yeast Mto1 prevented us from evaluating the levels of Mto1 in different mutant strains.
To analyze the molecular consequences of the p.Thr414Ilr and p.Arg481His MTO1 mutations, we performed in vivo mitochondrial protein synthesis (Fig. 3C). As previously observed, we detected radiolabeled bands in the Δmto1 strain corresponding to ribosomal protein Var1, cytochrome c oxidase subunit 3 (Cox3), and Atp subunits (Atp6, Atp8, Atp9), whose levels were similar to those of the MTO1 strain. However, cytochrome c oxidase subunits 1 and 2 (Cox1 and Cox2) and cytochrome b (Cob) were absent. The mto1T414I strain behaved like the Δmto1 strain, whereas mto1R481H was indistinguishable from wt MTO1, as previously observed for mto1A431T. Accordingly, CIV activity of mto1T414I and mto1P622X strains was indistinguishable from that of Δmto1 strain (4%–7% relative to MTO1 strain), it was 70% in mto1A431T relative to the wt, and identical to the wt in mto1R481H strains (Fig. 3D) (Supp. Table S4). In mto1T411H mutant strain, we measured the CI–CIII activity (following the reduction of cytochrome c in presence of NADH as electron donor and KCN as inhibitor of cytochrome c oxidase), to identify a possible explanation for the respiratory phenotype but no reduction was observed (data not shown).
Complementation in Fibroblasts
To further confirm the pathogenic role of the milder mutation p.Arg477His, we analyzed the respiration in fibroblasts from Pt1, compound heterozygous for p.Arg477His and p.Ala428Thr, after transduction with a recombinant lentiviral construct expressing the wt MTO1 cDNA. Infected cells were cultured in F14 medium, enriched in growth factors, to facilitate the recovery after infection and speed up cell growth. In our experience, these culturing conditions increase the values for SRC, an indicator of the bioenergetic reserve, in both control and mutant cells. Infected Pt1 fibroblasts showed marked increase of MRR (+146%) up to normal values. A mild MRR increase (+34%) was also observed in MTO1wt cells (Supp. Fig. S1). These results support a causative role for both p.Arg477His and p.Ala428Thr MTO1 variants in defective mitochondrial respiration of Pt1.
Discussion
A quite broad phenotypic spectrum was observed in MTO1 mutant patients: from severe, rapidly progressive, ultimately fatal presentation in two compound heterozygous children for Arg620Lysfs*8 and Ala428Thr mutations [Ghezzi et al., 2012], to fulminant postnatal phenotype, or severe, but long-lasting, encephalo-cardiomyopathy in the two families with a homozygous p.Thr411Ile mutation (this work), to benign, compensated hypertrophic cardiomyopathy with modest neurological abnormalities in patients [Pt1 in this work; Pt3 in Ghezzi at al., 2012], bearing two missense mutations.
In silico analysis suggested potential pathogenic role for the missense MTO1 mutations identified in our patients, but the yeast model allowed us to experimentally confirm their deleterious effects, dissecting the contribution of single allelic variants and giving an idea of the severity of each mutation. As summarized in Supp. Table S4, the severity of the yeast phenotype associated with mto1 mutations is: yArg481His (hArg477His) < yAla431Thr (hAla428Thr) ≪ yThr414Ile (hThr411Ile) = yPro622* (hArg620Lysfs*8) ≈ mto1Δ. In particular, the behavior of mto1R481H mutant is intermediate between that of the mto1A431T mutant, and that of the MTO1 wt allele as far as oxidative growth, respiratory activity [Ghezzi et al., 2012], and CIV activity are concerned. A moderate effect of the yArg481His substitution is in agreement with the observation that the Arg versus His change is electrostatically conservative, the equivalent Arg in GidA from C. tepidum being predicted to participate in a positively charged pocket, formed by several Arg, Lys, and His residues, that binds the phosphates of the D-stem backbone of the incoming tRNA. This was confirmed by the partial, but significant, reduction of oxygen consumption but virtually normal CIV and CI–CIII activities detected in the mto1R481H mutant strain. A defect of CV in the mto1R481H mutant is unlikely, owing to the presence of normal amount of Atp6, Atp8, Atp9, and the previous observation that yeast strains carrying mutations in ATP6, 8, or 9 display defective oxidative growth but normal respiratory activity [Dujon, 1981] or reduced respiratory activity due to an indirect decrease of CIV [Kucharczyk et al., 2009].
Both mto1T414I and mto1P622X alleles behave as the null allele as for oxidative growth, respiratory activity, mitochondrial protein synthesis [Ghezzi et al., 2012], and CIV activity, albeit it is unclear if this is due to instability or loss of function of the mutant protein.
In some patients with MTO1 mutations, the clinical presentations seemed to depend on the genotype and partly to comply with the phenotypic observations in yeast. For instance, the presence of one allele expressing the p.Ala428Thr variant, which, in yeast, is of intermediate severity, is probably not sufficient to complement the defects caused by the variant Arg620Lysfs*8, which is functionally null. Contrariwise, patients homozygous for the p.Ala428Thr mutation [Ghezzi et al., 2012] or heterozygous with the less severe p.Arg477His mutation (Pt1 in this report), have milder symptoms, and are alive and relatively well at 20 and 14 years of age respectively, although both with compensated hypertrophic cardiomyopathy.
However, in spite of carrying the same mutant genotype (Thr411Ile), the disease course was very different for the patients of the two families presented in this article. Although Pt2 and Pt3 both had perinatal onset and died very early, Pt4 and Pt5 presented with the first symptoms after only a few months of life and yet have reached adolescence, being now 19 and 12 years old, respectively. This observation highlights the importance of genetics and environmental variations in modulating the phenotype in humans. It is tempting to speculate that, in addition to protection/risk genetic factors differentially expressed in the two families, the different outcome could be due to the different pharmacological intervention, which was merely supportive in the first family, whereas included timely correction of lactic acidosis in the second, following DCA administration. Although the number of reported MTO1 mutant patients is very low, as a matter of fact all patients that survived beyond infancy and are still alive had chronic DCA treatment starting immediately after the clinical onset. DCA was remarkably effective on metabolic acidosis, suggesting that vigorous treatment of this life-threatening condition allows compensatory mechanisms to take place, which can mitigate the effects of hypertrophic cardiomyopathy. DCA administration should therefore be considered in MTO1 mutant patients. In spite of these encouraging effects on survival, DCA treatment could not prevent the development of neurological symptoms associated with highly deleterious mutations such as the Thr411Ile in Pt4 and 5, suggesting that neurodegeneration can progress independently from the correction of the metabolic status if MTO1 function is severely impaired.
Given the role of MTO1 as an optimizer of mtDNA translation, MTO1 mutations can be associated with any combination of MRC deficiency, from isolated CIV deficiency (Family 2 in this article) to combined CI + CIV deficiency, the most common biochemical signature observed in MTO1 mutant cases, to combined CIV + CIII deficiency, as previously reported [Ghezzi et al., 2012].
A rather specific genotype/phenotype correlation has been reported for several mutant factors involved in mtDNA translation [Rotig, 2011], an observation that still requires a finer dissection of the pathomechanism. Hypertrophic cardiomyopathy seems to be the clinical hallmark of MTO1 mutations, although in the present study most of the patients were preselected on the basis of cardiac symptoms. In addition to the heart, clinical/radiological signs of brain involvement were clearly present in several MTO1 mutant patients. Interestingly, a recently reported patient, carrying p.Gly59Ala and p.Thr308Ala MTO1 compound heterozygous changes, showed refractory infantile spasms and CIV deficiency, but no cardiac involvement [Vasta et al., 2012]. However, the pathogenic role of these very variants remains unproven and the c.922A>G/p.Thr308Ala is reported as a SNP (dbSNP: rs145043138) with a minor allele frequency of 0.3%.
Yeast strains harboring mto1A431T or mto1R481H mutant alleles did show no evident defects in mitochondrial proteins synthesis; this observation is concordant with the lack of obvious impairment in mtDNA translation found in Ala428Thr and Arg620Lysfs*8 mutant fibroblasts [Ghezzi et al., 2012], suggesting that the pathogenic effects of MTO1 mutations are not due to reduced levels of mtDNA-encoded subunits of the respiratory chain. Likewise, mitochondrial protein synthesis was not reduced in cells carrying deleterious mutations of, or having been knocked down for, MTO2/TRMU [Sasarman et al., 2011]. It is possible that amino-acid substitutions, that is, qualitative alterations of the primary structure of mtDNA proteins, rather than quantitative decrease of global protein synthesis, may play a major pathogenic role in both MTO1 and MTO2 mutant cells. The 5-carboxymethylaminomethylation and the 2-thiolation of the wobble uridine increase the accuracy of translation when guanidine is the third base of Gln, Glu or Lys codons, and prevent codon-anticodon pairing when the third base is a pyrimidine [Kurata et al., 2008; Murphy et al., 2004; Yarian et al., 2002]. Accordingly, in Ala428Thr and Arg620Lysfs*8 compound heterozygous fibroblasts, mtDNA-dependent CI, CIII, and/or CIV showed reduced activity, in spite of quantitatively normal mitochondrial protein synthesis, suggesting that errors in translation can determine the synthesis of qualitatively altered CI, CIII, and CIV mtDNA-encoded subunits [Ghezzi et al., 2012]. Likewise, CIV activity was reduced in the mto1A431T yeast strain, although the total levels of Cox1, Cox2, and Cox3 were similar to those of MTO1 wt. This hypothesis is testable, by systematic investigation of human or yeast mutant cells, through mass spectrometry and other proteomics approaches. Another possibility is that MTO1 may play a second role in mitochondria besides 5-carboxymethylaminomethylation of the wobble uridine, as previously reported for other enzymes, which modify tRNA in bacteria [Nicholson, 1999; Roovers et al., 2008] and, potentially, for MTO2 [Sasarman et al., 2011]. Alternatively, the 5-carboxymethylaminomethylation of the tRNA can have additional functions besides the optimization of mitochondrial translation, as hypothesized for the thiolation of the wobble position catalyzed by MTO2 [Sasarman et al., 2011].
This study confirmed that MTO1 mutations are associated with a mitochondrial disorder, characterized by hypertrophic cardiomyopathy, lactic acidosis, and MRC deficiency, albeit with a broad range of severity and frequent involvement of brain, possibly depending on the treatment. Moreover, we showed that the use of a suitable recombinant yeast model can validate the pathogenic role of variants found in human patients.
Acknowledgments
The Cell lines and DNA bank of Paediatric Movement Disorders and Neurodegenerative Diseases, member of the Telethon Network of Genetic Biobanks (project no. GTB12001), funded by Telethon Italy, provided us with specimens.
Disclosure statement: The authors declare no conflict of interest.
Additional Supporting Information may be found in the online version of this article.
References
- Barrientos A, Korr D, Tzagoloff A. Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh's syndrome. EMBO J. 2002;21:43–52. doi: 10.1093/emboj/21.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A, Fontanesi F, Díaz F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr Protoc Hum Genet. 2009;19:19.3. doi: 10.1002/0471142905.hg1903s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud N, Ozier-Kalogeropoulos O, Li GY, Labouesse M, Minvielle-Sebastia L, Lacroute F. A family of low and high copy replicative, integrative and single-stranded S. cerevisiaeE. coli shuttle vectors. Yeast. 1991;7:609–615. doi: 10.1002/yea.320070609. [DOI] [PubMed] [Google Scholar]
- Bugiani M, Invernizzi F, Alberio S, Briem E, Lamantea E, Carrara F, Moroni I, Farina L, Spada M, Donati MA, Uziel G, Zeviani M. Clinical and molecular findings in children with complex I deficiency. Biochim Biophys Acta. 2004;1659:136–147. doi: 10.1016/j.bbabio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Colby G, Wu M, Tzagoloff A. MTO1 codes for a mitochondrial protein required for respiration in paromomycin-resistant mutants of Saccharomyces cerevisiae. J Biol Chem. 1998;273:27945–27952. doi: 10.1074/jbc.273.43.27945. [DOI] [PubMed] [Google Scholar]
- Dujon B. The molecular biology of the yeast saccharomyces: life cycle and inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1981. Mitochondrial genetics and functions; pp. 505–635. [Google Scholar]
- Fontanesi F, Jin C, Tzagoloff A, Barrientos A. Transcriptional activators HAPNFY rescue a cytochrome c oxidase defect in yeast and human cells. Hum Mol Genet. 2008;17:775–788. doi: 10.1093/hmg/ddm349. [DOI] [PubMed] [Google Scholar]
- Ghezzi D, Baruffini E, Haack TB, Invernizzi F, Melchionda L, Dallabona C, Strom TM, Parini R, Burlina AB, Meitinger T, Prokisch H, Ferrero I, Zeviani M. Mutations of the mitochondrial-tRNA modifier MTO1 cause hypertrophic cardiomyopathy and lactic acidosis. Am J Hum Genet. 2012;90:1079–1087. doi: 10.1016/j.ajhg.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Goffrini P, Ercolino T, Panizza E, Giachè V, Cavone L, Chiarugi A, Dima V, Ferrero I, Mannelli M. Functional study in a yeast model of a novel succinate dehydrogenase subunit B gene germline missense mutation (C191Y) diagnosed in a patient affected by a glomus tumor. Hum Mol Genet. 2009;18:1860–1868. doi: 10.1093/hmg/ddp102. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Invernizzi F, D'Amato I, Jensen PB, Ravaglia S, Zeviani M, Tiranti V. Microscale oxygraphy reveals OXPHOS impairment in MRC mutant cells. Mitochondrion. 2012;12:328–335. doi: 10.1016/j.mito.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger MK, Sørensen MA. Aminoacylation of hypomodified tRNAGlu in vivo. J Mol Biol. 1998;284:609–620. doi: 10.1006/jmbi.1998.2197. [DOI] [PubMed] [Google Scholar]
- Kucharczyk R, Rak M, Di Rago JP. Biochemical consequences in yeast of the human mitochondrial DNA 8993T>C mutation in the ATPase6 gene found in NARPMILS patients. Biochim Biophys Acta. 2009;1793:817–824. doi: 10.1016/j.bbamcr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Kurata S, Weixlbaumer A, Ohtsuki T, Shimazaki T, Wada T, Kirino Y, Takai K, Watanabe K, Ramakrishnan V, Suzuki T. Modified uridines with C5-methylene substituents at the first position of the tRNA anticodon stabilize U.G wobble pairing during decoding. J Biol Chem. 2008;283:18801–18811. doi: 10.1074/jbc.M800233200. [DOI] [PubMed] [Google Scholar]
- Meyer S, Scrima A, Versées W, Wittinghofer A. Crystal structures of the conserved tRNA-modifying enzyme GidA: implications for its interaction with MnmE and substrate. J Mol Biol. 2008;380:532–547. doi: 10.1016/j.jmb.2008.04.072. [DOI] [PubMed] [Google Scholar]
- Murphy FV, 4th, Ramakrishnan V, Malkiewicz A, Agris PF. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat Struct Mol Biol. 2004;11:1186–1191. doi: 10.1038/nsmb861. [DOI] [PubMed] [Google Scholar]
- Nicholson AW. Function, mechanism and regulation of bacterial ribonucleases. FEMS Microbiol Rev. 1999;23:371–390. doi: 10.1111/j.1574-6976.1999.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Osawa T, Ito K, Inanaga H, Nureki O, Tomita K, Numata T. Conserved cysteine residues of GidA are essential for biogenesis of 5-carboxymethylaminomethyluridine at tRNA anticodon. Structure. 2009;17:713–724. doi: 10.1016/j.str.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Roovers M, Oudjama Y, Kaminska KH, Purta E, Caillet J, Droogmans L, Bujnicki JM. Sequence–structure–function analysis of the bifunctional enzyme MnmC that catalyses the last two steps in the biosynthesis of hypermodified nucleoside mnm5s2U in tRNA. Proteins. 2008;71:2076–2085. doi: 10.1002/prot.21918. [DOI] [PubMed] [Google Scholar]
- Rötig A. Human diseases with impaired mitochondrial protein synthesis. Biochim Biophys Acta. 2011;1807:1198–1205. doi: 10.1016/j.bbabio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Sasarman F, Antonicka H, Horvath R, Shoubridge EA. The 2-thiouridylase function of the human MTU1 (TRMU) enzyme is dispensable for mitochondrial translation. Hum Mol Genet. 2011;20:4634–4643. doi: 10.1093/hmg/ddr397. [DOI] [PubMed] [Google Scholar]
- Shi R, Villarroya M, Ruiz-Partida R, Li Y, Proteau A, Prado S, Moukadiri I, Benítez-Páez A, Lomas R, Wagner J, Matte A, Velázquez-Campoy A, et al. Structure–function analysis of Escherichia coli MnmG (GidA), a highly conserved tRNA-modifying enzyme. J Bacteriol. 2009;191:7614–7619. doi: 10.1128/JB.00650-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto IC, Fontanesi F, Valledor M, Horn D, Singh R, Barrientos A. Synthesis of cytochrome c oxidase subunit 1 is translationally downregulated in the absence of functional F1F0-ATP synthase. Biochim Biophys Acta. 2009;1793:1776–1786. doi: 10.1016/j.bbamcr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Nagao A, Suzuki T. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu Rev Genet. 2011;45:299–329. doi: 10.1146/annurev-genet-110410-132531. [DOI] [PubMed] [Google Scholar]
- Sylvers LA, Rogers KC, Shimizu M, Ohtsuka E, Söll D. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry. 1993;32:3836–3841. doi: 10.1021/bi00066a002. [DOI] [PubMed] [Google Scholar]
- Takai K. Possible conformations of 5-aminomethyluridine derivatives recognizing a G at the third position of the codon. Nucleic Acids Symp Ser (Oxf) 2005;49:317–318. doi: 10.1093/nass/49.1.317. [DOI] [PubMed] [Google Scholar]
- Umeda N, Suzuki T, Yukawa M, Ohya Y, Shindo H, Watanabe K, Suzuki T. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J Biol Chem. 2005;280:1613–1624. doi: 10.1074/jbc.M409306200. [DOI] [PubMed] [Google Scholar]
- Urbonavicius J, Qian Q, Durand JM, Hagervall TG, Björk GR. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001;20:4863–4873. doi: 10.1093/emboj/20.17.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta V, Merritt JL, 2nd, Saneto RP, Hahn SH. Next-generation sequencing for mitochondrial diseases: a wide diagnostic spectrum. Pediatr Int. 2012;54:585–601. doi: 10.1111/j.1442-200X.2012.03644.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Yan Q, Guan MX. Combination of the loss of cmnm5U34 with the lack of s2U34 modifications of tRNALys, tRNAGlu, and tRNAGln altered mitochondrial biogenesis and respiration. J Mol Biol. 2010;395:1038–1048. doi: 10.1016/j.jmb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Li X, Faye G, Guan MX. Mutations in MTO2 related to tRNA modification impair mitochondrial gene expression and protein synthesis in the presence of a paromomycin resistance mutation in mitochondrial 15 S rRNA. J Biol Chem. 2005;280:29151–29157. doi: 10.1074/jbc.M504247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarian C, Marszalek M, Sochacka E, Malkiewicz A, Guenther R, Miskiewicz A, Agris PF. Modified nucleoside dependent Watson-Crick and wobble codon binding by tRNALysUUU species. Biochemistry. 2000;39:13390–13395. doi: 10.1021/bi001302g. [DOI] [PubMed] [Google Scholar]
- Yarian C, Townsend H, Czestkowski W, Sochacka E, Malkiewicz AJ, Guenther R, Miskiewicz A, Agris PF. Accurate translation of the genetic code depends on tRNA modified nucleosides. J Biol Chem. 2002;277:16391–16395. doi: 10.1074/jbc.M200253200. [DOI] [PubMed] [Google Scholar]
- Yasukawa T, Suzuki T, Ishii N, Ohta S, Watanabe K. Wobble modification defect in tRNA disturbs codon–anticodon interaction in a mitochondrial disease. EMBO J. 2001;20:4794–4802. doi: 10.1093/emboj/20.17.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Sun L, Nie QH, Huang CX, Jia ZS, Wang JP, Lian JQ, Li XH, Wang PZ, Zhang Y, Zhuang Y, Sun YT, Bai X. Down-regulation of CXCR4 expression by SDFKDEL in CD34(+) hematopoietic stem cells: an antihuman immunodeficiency virus strategy. J Virol Methods. 2009;16:30–37. doi: 10.1016/j.jviromet.2009.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.