Abstract
Rising atmospheric CO2 concentrations are placing spatially divergent stresses on the world's tropical coral reefs through increasing ocean surface temperatures and ocean acidification. We show how these two stressors combine to alter the global habitat suitability for shallow coral reef ecosystems, using statistical Bioclimatic Envelope Models rather than basing projections on any a priori assumptions of physiological tolerances or fixed thresholds. We apply two different modeling approaches (Maximum Entropy and Boosted Regression Trees) with two levels of complexity (one a simplified and reduced environmental variable version of the other). Our models project a marked temperature-driven decline in habitat suitability for many of the most significant and bio-diverse tropical coral regions, particularly in the central Indo-Pacific. This is accompanied by a temperature-driven poleward range expansion of favorable conditions accelerating up to 40–70 km per decade by 2070. We find that ocean acidification is less influential for determining future habitat suitability than warming, and its deleterious effects are centered evenly in both hemispheres between 5° and 20° latitude. Contrary to expectations, the combined impact of ocean surface temperature rise and acidification leads to little, if any, degradation in future habitat suitability across much of the Atlantic and areas currently considered ‘marginal’ for tropical corals, such as the eastern Equatorial Pacific. These results are consistent with fossil evidence of range expansions during past warm periods. In addition, the simplified models are particularly sensitive to short-term temperature variations and their projections correlate well with reported locations of bleaching events. Our approach offers new insights into the relative impact of two global environmental pressures associated with rising atmospheric CO2 on potential future habitats, but greater understanding of past and current controls on coral reef ecosystems is essential to their conservation and management under a changing climate.
Keywords: Bioclimatic Envelope Modeling, boosted regression trees, coral reef ecosystems, global warming, MaxEnt, maximum entropy, ocean acidification
Introduction
Anthropogenic climate change has emerged as a serious global-scale threat to the future viability of coral reef ecosystems (e.g., Hoegh-Guldberg et al., 2007; Riegl et al., 2009; Veron et al., 2009; Glynn, 2012), with studies linking widespread bleaching events to increasing sea surface temperatures (SSTs; Hoegh-Guldberg, 1999). There is concern that the growing frequency and severity of mass bleaching episodes may lead to species composition shifts and functional collapse in coral reefs in the near future (Hughes et al., 2003; Baker et al., 2008; Glynn, 2012; van Hooidonk et al., 2013), although this is likely to be strongly influenced by the extent of other anthropogenic stresses and the corals’ capacity for thermal adaptation (e.g., Baskett et al., 2009; Kennedy et al., 2013). In contrast, global warming also has the potential to improve currently marginal environmental conditions and extend the range of tropical coral reefs into higher latitudes (e.g., Precht & Aronson, 2004; Yamano et al., 2011), as demonstrated in the fossil record in response to warmer geological periods (e.g., Lighty et al., 1978; Veron, 1992; Precht & Aronson, 2004; Greenstein & Pandolfi, 2008; Woodroffe et al., 2010; Kiessling et al., 2012). If expansion is not limited by additional factors, higher latitude reefs could provide ‘refuges’ for many coral reef species as increasing temperatures cause ecosystem failure at low-latitude reef sites (e.g., Zamagni et al., 2012; Halfar et al., 2005; Vargas-Ángel et al., 2003; Glynn, 1996; though see, e.g., Lybolt et al., 2011).
A second global environmental pressure associated with rising atmospheric CO2 – ‘ocean acidification’ – complicates the temperature-only picture of a shift of suitable habitats to high latitudes and contraction in the tropics. Ocean acidification is the chemical consequence of the excess dissolution of CO2 derived from fossil fuel combustion in surface seawater (Turley et al., 2010). In addition to lower seawater pH, anthropogenic ocean acidification is also characterized by a reduction in the saturation state of calcium carbonate (Hönisch et al., 2012). Corals precipitate skeletons of the CaCO3 polymorph aragonite, and declining aragonite saturation (ΩArag) increases the metabolic cost of skeletogenesis. Ocean acidification may thus lead to weaker reef structures that are more susceptible to erosion and competitive advantages for bioeroding organisms (Kleypas et al., 1999b; Smith & Buddemeier, 1992; e.g., Manzello et al., 2008). On the basis of this parameter (aragonite saturation) alone, it has been noted that for atmospheric CO2 concentrations beyond ca. 550 ppm, the expected open ocean surface ΩArag value for most reef locations will be below that associated with the limits of coral distributions today (ca. 3.25; Cao & Caldeira, 2008; Hoegh-Guldberg et al., 2007). This has raised concerns over the corals’ ability to maintain reef structures, pushing the view that declining aragonite saturation represents a pressing threat (e.g., Cao & Caldeira, 2008; Silverman et al., 2009; Ricke et al., 2013). However, the actual effect of increased acidification on calcification rates is complex and species dependent (Chan & Connolly, 2013; Kroeker et al., 2013), with many scleractinian corals being capable of buffering their internal pH for instance (e.g., Krief et al., 2010; McCulloch et al., 2012). The present-day relationship between coral reef locations and aragonite saturation is hence very likely modulated by other environmental factors such as temperature and nutrient availability (e.g., Cohen & Holcomb, 2009; Chauvin et al., 2011), meaning that habitat suitability implications cannot be drawn from future aragonite saturation changes in isolation.
With continuing fossil fuel CO2 emissions, these two main global marine environmental changes – surface warming and acidification, create a biogeographical tension, with warming tending to drive the zone of suitable coral reef habitat polewards, and decreasing saturation forcing it to contract toward the equator (Meissner et al., 2012; Yara et al., 2012). The net outcome is far from trivial, as the physiological responses of corals vary between regions, species, and even experimental manipulations, with interactions between stressors that are complex and nonlinear (e.g., Lough & Barnes, 1997; Silverman et al., 2007; Anthony et al., 2008; Chauvin et al., 2011; Edmunds et al., 2012). Despite a pressing need for a better understanding of the ongoing impact of environmental change on coral reef ecosystems (Pandolfi et al., 2011), few quantitative modeling studies have taken into account changes in SST and ΩArag simultaneously. Buddemeier et al. (2011) modeled the response to warming and acidification of eastern Caribbean reefs and projected that 95% coral cover would be lost by 2035, or delayed until 2065 if thermal tolerance of corals increases by 1–1.5 °C. Hoeke et al. (2011) found similar results for the Hawaiian region, with a precipitous decline in coral cover expected sometime between 2030 and 2050 unless corals are able to adapt to higher temperatures. They also predicted a temperature-driven increase in coral growth rates, particularly toward higher latitudes and despite the lower aragonite saturation levels there. Yara et al. (2012) explored changes in SST and ΩArag along the Japanese coastline. On the basis of a minimum ΩArag cutoff value of 3 (2.3) for tropical (temperate) corals, they found that a possible SST-driven range expansion into higher latitudes would be strongly limited by ocean acidification, and projected that all potential habitats in Japan might become unsuitable for tropical/subtropical coral reefs by 2040.
In this study, we assess global patterns of short-term range shifts in potential coral reef habitat under the spatially divergent stresses of ocean warming and acidification at the spatial scale of 1° × 1°. We employ a suite of statistical models based on the environmental factors thought to be limiting to the present equilibrium distribution of shallow-water coral reefs (Fig. 1, see Couce et al., 2012), perturbing them with Earth System Model projected future SST and aragonite saturation changes (the simulations used in Turley et al., 2010). We considered a range of potential future CO2 emissions scenarios, but focus here on the consequences of the ‘A2’ scenario (characterized by regionally oriented economic development and high population growth, expecting ca. 850 atmospheric CO2 by 2100). Significantly, the coral suitability models are not based on specific predetermined cutoff values for either ΩArag or SST, but instead seek to encapsulate the multidimensional climate envelope where the synergies between all relevant drivers favor the presence of coral reefs. Based on equilibrium relationships, these models do not incorporate information on coral responses or potential adaptation to dynamic environmental drivers, such as warming-induced bleaching and sea-level rise. Instead, the models identify where environmental conditions become adverse for coral reef presence based on the range of conditions in which they are currently found. The purpose of the study is thus not to predict the fate of existing coral reefs, but to identify the broad net impacts of warming and acidification on the potential future habitat suitability of these emblematic ecosystems.
Figure 1.
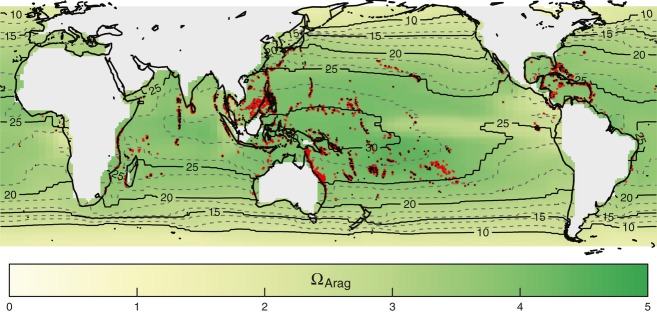
Current global distribution of coral reefs (red) according to ReefBase v2000 data set. The 1990 UVic-predicted fields for aragonite saturation (yellow–green color bar) and mean annual sea surface temperature (gray/black contour lines) used to train the Bioclimatic Envelope Models are also shown.
Materials and methods
Habitat suitability models
Bioclimatic Envelope Modeling establishes relationships between environmental factors and the distribution of a species or ecosystem through statistical analysis. It defines an n-dimensional volume (or ‘envelope’) in the space of all relevant environmental factors where conditions are adequate for a species or ecosystem, identifying the relative significance of the different factors and the changes in environmental suitability across climatic gradients. We employed two different techniques: Maximum Entropy (MaxEnt; Phillips & Dudík, 2008; Phillips et al., 2006), and Boosted Regression Trees (BRT; De'ath, 2007; Friedman, 2001). Further details on these modeling approaches are provided in Table 1. Both MaxEnt and BRT are machine-learning techniques, able to handle nonlinear relationships and to take into account synergistic effects between the different factors affecting a species’ distribution. This flexible approach is advantageous for studying ecosystems because the tolerance limits of an ecosystem are influenced by contributions from many species and would be expected to give rise to more complicated relationships with climatic variables. MaxEnt is widely used in ecological studies, including the prediction of climate change impacts on a species or ecosystem's potential distribution (e.g., tree sparrows within North America, Graham et al., 2011a; sagebrush ecosystems in Nevada, Bradley, 2010; or invasive weeds species in Australia, Wilson et al., 2009). To date, BRT is less widely used, despite having comparable predictive capabilities (e.g., Elith et al., 2006; De'ath, 2007).
Table 1.
Modelling techniques used in the study, model names used in article, and the list of environmental variables considered. See Materials and methods, Data S1 and Couce et al. (2012) for more details
| Model Description | Model name | Variables |
|---|---|---|
| Boosted regression trees (BRT; Friedman, 2001) is a decision-tree-based statistical technique. A single tree is built by repeatedly splitting the data finding a cutoff value for one of the environmental variables so that the homogeneity of the resulting two groups is maximized. For BRT models, a sequence of trees (typically >1000) is produced, each grown on reweighted versions of the data, assigning ever-increasing weight to the cases misclassified by previous trees. The final prediction is obtained by the weighted average of predicted values across the sequence of trees. | ||
| BRTOPT | 27 environmental variables:
|
|
| BRT models were fitted in R, with version 1.6–3.1 of the gbm library (Ridgeway, 2007). Different values for the Tree Complexity (TC), Learning Rate (LR) and Bagging Fraction (BF) were considered, and the combination that minimized the predictive deviance was chosen in each case (for BRTOPT: TC = 10, LR = 0.01 and BF = 75; for BRTSIM: TC = 7, LR = 0.01 and BF = 50). See Couce et al. (2012) for more details. | ||
| BRTSIM | Five variables containing the most relevant information according to BRTOPT's output:
|
|
| Maximum entropy modeling (MaxEnt; Phillips et al., 2006) is a presence-only technique for the prediction of species geographic distributions, based on the environmental conditions of sites of known occurrence. Assumes environmental factors act as constraints on the distribution of a species and that within those constraints, the species will occupy the available habitat in a way that maximizes entropy. | ||
| MaxEntOPT | Same variable set as BRTOPT | |
| Our models were developed with Maxent version 3.3.3e, with default values for the convergence threshold (10−5) and maximum number of iterations (500). | MaxEntSIM | Same variable set as BRTSIM |
All models were trained with coral location data from ‘ReefBase’ (version 2000; http://www.reefbase.org; Vergara et al., 2000). This database was chosen for consistency and ease of comparison with previous studies: Kleypas et al. (1999a), Couce et al. (2012, 2013). At the 1° × 1° spatial resolution of our study, previous testing has shown the results are insensitive to the reef location data set used. For example, BRT and MaxEnt models developed using the UNEP-WCMC compilation (http://data.unep-wcmc.org/datasets/13; UNEP-WCMC, WorldFish Centre, WRI, TNC, 2010; IMaRS-USF, IRD, 2005; IMaRS-USF, 2005) replicated model performance and environmental variable relevance with insignificant differences to the equivalent ReefBase-trained models (E. Couce, unpublished data).
The optimal ‘OPT’ models were trained with a complete suite of relevant environmental fields identified in Couce et al., 2012 (a total of 27 parameters; these are the MaxEntOPT and BRTOPT models), whereas the simple ‘SIM’ models were trained with a reduced subset of 6 of the most significant variables (the MaxEntSIM and BRTSIM models) – see Table 1 for complete variable list. Annual average SST and ΩArag values were obtained from climate and marine carbon cycle projections made using the University of Victoria (UVic) Earth System Model (Weaver et al., 2001), while most of the remaining variables were observationally based. The UVic mean annual data were interpolated to the 1° × 1° resolution of the reef suitability models using the Inverse Distance Weighted method (2nd degree). Maximum and minimum SST values were computed by adding observed present-day anomalies to UVic 1990 mean SST. Additional information and analysis of the model development, data sources, and variables’ contribution to predictions are available in the Supporting Data S1 and is described in further detail in Couce et al. (2012).
Grid and mask
All fields, including coral reef presence data, were mapped onto a 1° × 1° global grid between −60° and 60° latitude. The suitability models were trained on a shallow water mask established as the grid cells with regions shallow enough for adequate light penetration. The shallowest depth of a grid cell was determined from 30″ resolution SRTM30 Plus bathymetry data (Becker et al., 2009). The mask was defined as the grid cells for which the shallowest point fell within twice the mean annual depth of penetration of light (Z), estimated after Kleypas et al. (1999a):
where PARcell was the monthly mean Photosynthetically Active Radiation (PAR) using data from ISCCP (Bishop & Rossow, 1991; Bishop et al., 1997), PARmin was 125 dWm−2 (to encapsulate mesophotic reefs; Gattuso et al., 2006) and K490cell was the monthly mean diffuse attenuation coefficient of light (wavelength 490 nm) obtained from Globcolour (2008) satellite measurements. In addition, the study region was further constrained to the open-ocean areas covered by UVic projections (i.e., excluding the Red Sea and Arabian Gulf; Fig. S1.1 in Data S1). The environmental fields were extrapolated 1° shoreward by linear average of nearest neighbors to cover near-shore coral reef locations. This process resulted in a total of 32 582 oceanic grid cells, 4002 of which were located within the shallow-water mask. A total of 1124 cells contained at least one entry from the ReefBase v2000 data set (Fig. 1) and were designated ‘presence’ cells.
Future projections
Future habitat suitability was computed by applying the Bioclimatic Envelope Models to UVic-generated projections for annual average SST and aragonite saturation under the IPCC TAR scenarios A2, B1, and A1B. Future maximum and minimum SST values were computed by adding observed present anomalies to UVic mean SST data (i.e., assuming future variability will remain the same). All other environmental fields were kept fixed at present values. Projections were then generated at 10-year intervals from 2010 to 2070.
To isolate the impacts of warming and ocean acidification, changes in SST variables and ΩArag were considered first separately and then in combination, while all other environmental variables were kept constant at modern observed values. Future projections can involve novel environmental conditions, exceeding the values of the models’ training range. Understanding the way in which the model deals with these data is critical for the correct interpretation of the results. The four models used in this study (Table 1) extrapolate by maintaining a constant response outside the training range (i.e., treating variables exceeding the values present in the training range as if they remained at the limit of the training range; see Data S2 for a detailed discussion on extrapolating to novel conditions and the impact on the predictions). In the results presented here, we explicitly indicate regions with novel SST or ΩArag conditions where the method of extrapolation significantly affects the projections and statistical relationships observed in training may no longer hold. These regions were defined as areas where MaxEntOPT's projected suitability was found to vary by 0.1 or more when extrapolation was carried out using a different method (i.e., by simple linear extrapolation of the model's response curves to each climatic variable, instead of maintaining a constant response). The 2070 cutoff for future projections was chosen because over 10% of coral reef cells are out of training range by this date.
Model evaluation
Model performance on 1990 training data was tested via Receiver Operating Characteristic curves (ROC curves; Peterson et al., 1954) and Area-Under-the-Curve scores (AUC; Swets, 1988; Fig. S1.2 in Data S1). To assess the models’ predictive ability we considered published reports of fossil evidence from past warm geological periods and reports of present-day range expansion. We also explored whether rapid changes in equilibrium habitat suitability were correlated with areas where conditions have already been identified as challenging for existing coral reefs as evidenced by recent bleaching episodes. The data set of bleaching events was obtained from ReefBase (http://www.reefbase.org); it was originally developed by the WCMC and the WorldFish Center and subsequently extended to include published records and reports of personal observations. The changes in suitability between 2010 and training (1990) conditions on cells where bleaching episodes had been reported (between 2008 and 2012) were compared with the changes predicted in all reef presence cells; distributions and p-values of the two populations were compared via a Welsh's t-test, after testing the data for colinearity.
Results
Impact of future surface warming
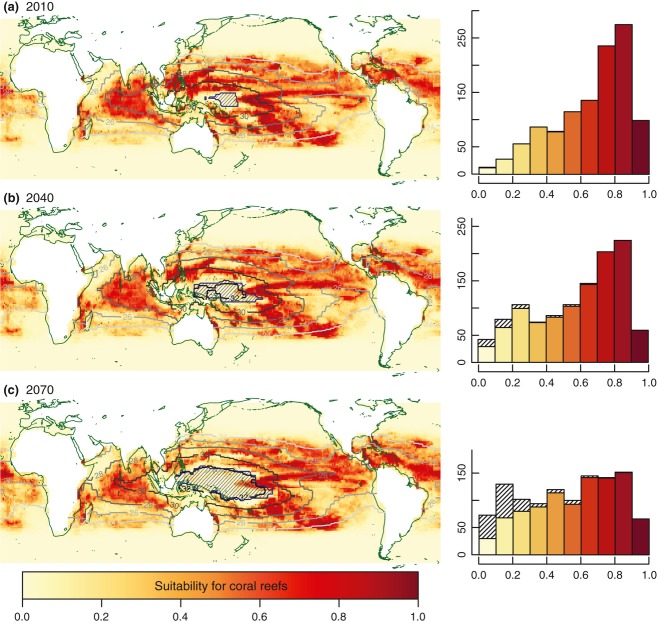
Considering changes in SST variables only, sea surface warming has a marked impact on habitat suitability for coral reef ecosystems in currently favorable areas (defined as suitability ≥0.5), especially in the central Indo-Pacific region (Fig. 2). The percentage of presence cells (those currently with reefs or nonreef coral communities) for which the model is predicting suitability above a value of 0.5 falls from 77% in 2010 to 66% in 2040 and 54% by 2070 (BRTOPT projections). This reduction is particularly pronounced in the Western Pacific Warm Pool (WPWP) region, affecting the Coral Triangle and some of the most biodiverse coral reef regions in the world. Suitability elsewhere either declines slightly (e.g., most of the Indian Ocean), or slightly increases (e.g., some areas of the Atlantic, including the Caribbean, and high latitudes). By 2070, SST conditions move out of training range for a large fraction of the Indo-Pacific region, with the area of less reliable model projection (as defined in ‘Materials and methods’) affecting up to ca. 13% of coral reef cells under the A2 scenario (hashed areas in Fig. 2 maps and histograms). If areas requiring extrapolation into novel conditions are excluded from the analysis, the observed patterns of change to 2070 remains robust (e.g., the decrease in habitat suitability among presence cells in the histograms in Fig. 2 is still evident).
Figure 2.
Predicted changes in suitability for coral reef ecosystems under the A2 scenario for future change in sea surface temperature (SST) variables only; 2010 (a), 2040 (b) and 2070 (c). Histograms show predicted suitability values for the 1124 presence sites of the study grid (i.e., cells currently with reefs according to the ReefBase v2000 data). All environmental fields apart from SST variables were kept constant at their present values (used in model training). Areas where projections are less reliable (i.e., SST out of training range and MaxEnt clamping value >0.1; see Data S2) are indicated by the hatched pattern, in both maps and histograms. The figure shows BRTOPT model's results (see figure S3.1 in Data S3 for MaxEntOPT's projections).
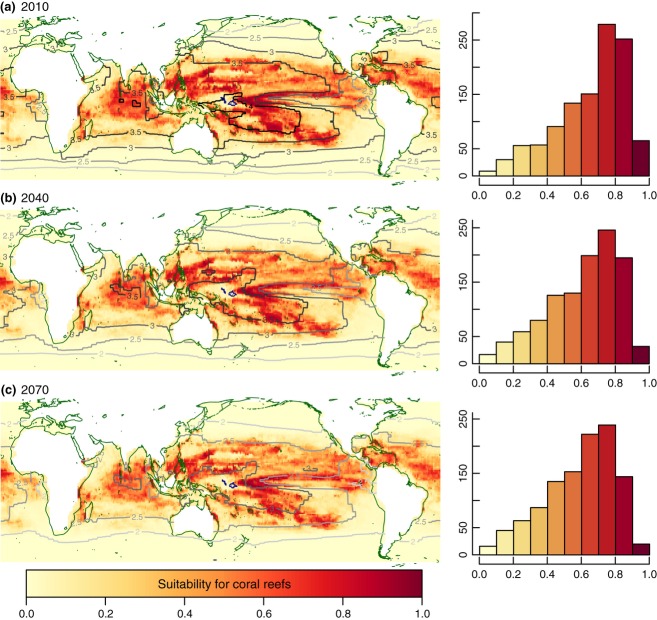
Impact of future ocean acidification
A reduction of surface ΩArag – while maintaining all other variables fixed at present values – leads to a decline in suitability for all areas currently favorable to coral reef formation (Fig. 3). However, the reduction is more subdued than that due to future SSTs (Fig. 2). For instance, whereas the projected SST rise caused the average suitability of presence cells to fall by 17%, from 0.69 in training (1990) conditions to 0.52 in 2070, the projected change in ΩArag over the same period caused a decrease of 10%, to an average of 0.59 (BRTOPT). The projected decline is also more uniformly distributed globally (Fig. 3) in contrast to the more extreme pattern of warming-induced impact. However, some geographical differences are still apparent, with regions most affected by increased acidification corresponding to off-equatorial zones in the Pacific, adjacent to the WPWP zone most affected by warming, and to a lesser degree in localized regions of the central Indian Ocean and the Caribbean. Unlike SST, changing ΩArag alone does not lead to extrapolation uncertainties (Fig. 3), as the areas with novel ΩArag conditions are confined to high latitudes, well beyond the present-day latitudinal range of scleractinian coral reefs.
Figure 3.
Predicted changes in suitability for coral reef ecosystems under the A2 scenario for future change in aragonite saturation only; 2010 (a), 2040 (b) and 2070 (c). Histograms show predicted suitability values for the 1124 presence sites of the study grid (i.e., cells currently with reefs according to the ReefBase v2000 data). All environmental fields apart from ΩArag were kept constant at their present values (used in model training). Unlike the SST results (Fig. 2), changes in ΩArag do not give rise to extrapolation uncertainties by producing novel conditions. The figure shows BRTOPT results (see figure S3.2 in Data S3 for MaxEntOPT projections).
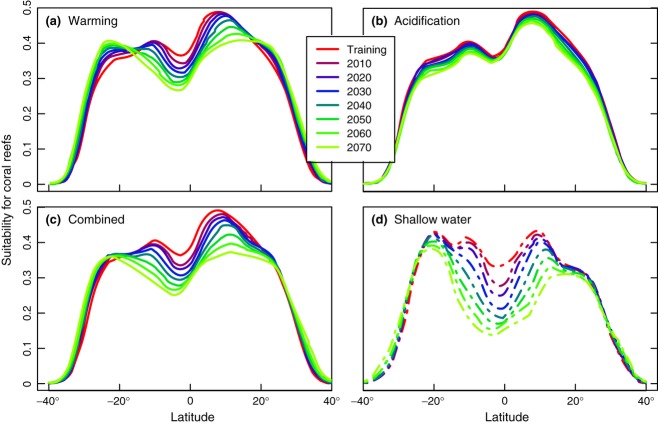
Synergistic impacts of warming and acidification
On a zonally averaged basis, ocean acidification reduces the environmental suitability for reef presence across all latitudes, but maximal influence is in the off-equatorial zone (ca. 5° to 20° in both hemispheres, Fig. 4a). The projected rise in SST significantly reduces coral reef suitability for all latitudes between 15°S and 15°N, while driving slight increases at latitudes above ca. 20° (Fig. 4b). With simultaneous changes in SST and ΩArag, the suitability for coral reef presence drops across all latitudes between 20°S and 20°N (Fig. 4c). This reduction is more extreme when the analysis is restricted to the grid cells with substrate within the euphotic zone adequate for reef formation (Fig. 4d). The SST-driven expansion at latitudes above 20° still persists, although it is restricted by the countereffect of acidification.
Figure 4.
Predicted suitability of coral reef presence as a function of latitude, when changing sea surface temperature variables only (a), aragonite saturation only (b) and both factors simultaneously, for the entire ocean (c) and restricted to shallow waters (d). Different colors show variations over time under the A2 scenario. All figures have been made averaging the modeled suitability values in 5° latitudinal bands, and show MaxEntOPT model's results (see figure S3.3 in Data S3 for the equivalent BRTOPT projections).
The zonal averaged changes (Fig. 4) conceal a more heterogeneous geographical response, with the 2070 projections forecasting decreased suitability for coral reef ecosystems in nearly all reef areas (including the Caribbean, GBR, and Coral Triangle region) and with the greatest decline in suitability taking place in the western Pacific (Fig. 5). All four models project a 27–30% decline within shallow waters over the next 60 years (Fig. 6). The three CO2 emission scenarios considered (A2, A1B, B1) produce very similar projected changes in suitability, corresponding to the consistent levels of warming to 2070 expected across all scenarios (IPCC, 2007).
Figure 5.
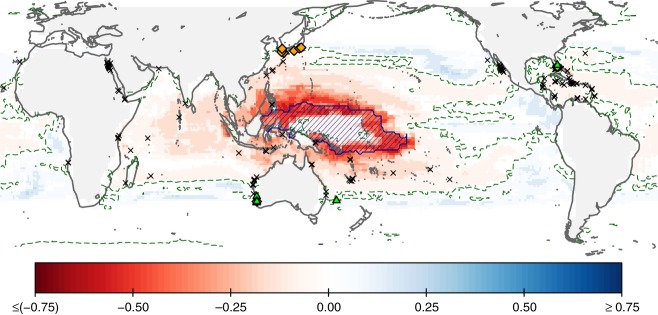
Projected change in suitability for coral reef ecosystems between 1990 and 2070 under the A2 scenario, considering simultaneous changes in aragonite saturation and surface ocean temperature. The projected response has been averaged across both ‘OPT’ models and the MaxEntSIM model (models defined in Table 1; BRTSIM's results were excluded from the average, as discussed in the text and Data S3). Green dashed line indicates null projected change, whereas the hatched pattern identifies areas with novel conditions where projections are less reliable, due to a significant impact of the chosen extrapolation method (as explained in Methods). The sites of past (green triangles) and present (orange diamonds) range expansion of coral are also indicated, based on data from Lighty et al. (1978), Veron (1992), Marsh (1993), Vargas-Ángel et al. (2003), Greenstein & Pandolfi (2008), Woodroffe et al. (2010), and Yamano et al. (2011). Black crosses indicate coral reef distribution during the last interglacial period (data from Kiessling et al., 2012).
Figure 6.
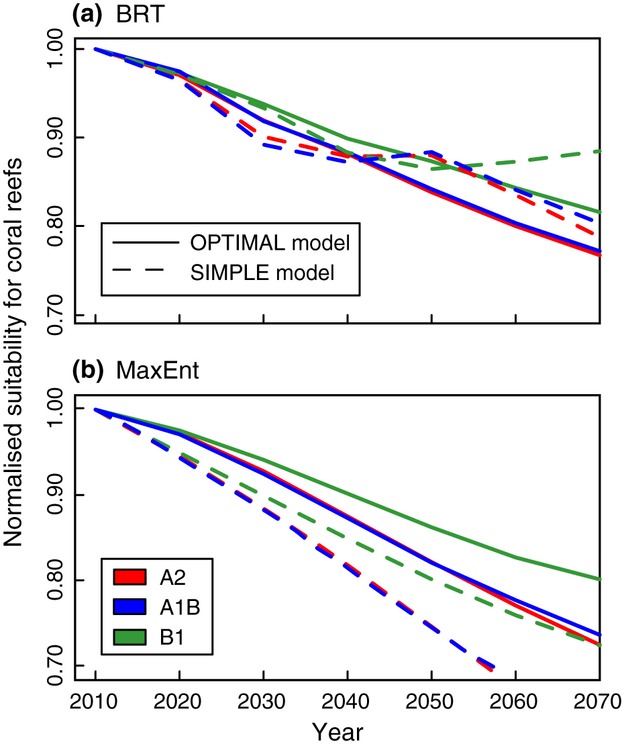
Average global suitability for coral reef presence in shallow water areas over time for boosted regression trees (a) and MaxEnt (b) models. Color of the line refers to the CO2 emission scenario, with red, blue, and green for A2, A1B, and B1, respectively, while the pattern of the line represents the model, with continuous lines for ‘OPT’, and dashed for ‘SIM’ models. Suitability values have been normalized to 2010 average.
Model performance, comparison with fossil data and recent bleaching episodes
All models performed adequately on training data, with average AUC scores close to 0.9 when evaluated on a random 25% subset data that had been excluded for model training (Fig. S1.2 in Supporting Data S1). The simple MaxEntSIM model, based on a reduced set of climatic variables, showed the poorest performance in ROC space, with an AUC score of 0.84 ± 0. 01. There is a clear correspondence between the changes in suitability predicted for present-day (2010) by the ‘SIM’ versions of the models and the locations of over 3500 reported bleaching episodes from 2008 to 2012 (Fig. 7). However, this correlation was not present in the forecasts obtained with the full ‘OPT’ models. In general, there was good agreement between forecasts obtained with the different models (Data S3), with the exception of BRTSIM.
Figure 7.
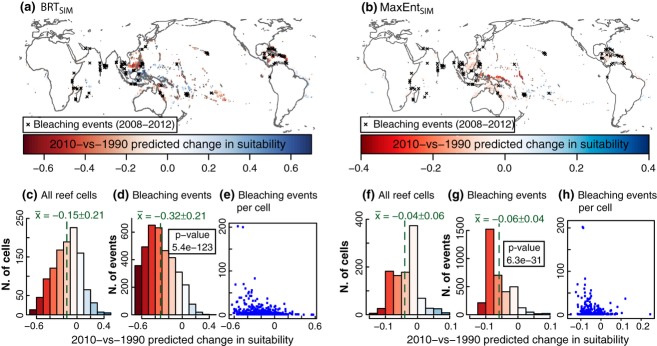
Changes in suitability for coral reef ecosystems between 1990 and 2010 predicted by BRTSIM (left) and MaxEntSIM (right) and the point location of over 3500 bleaching events taking place between 2008 and 2012 according to the ReefBase database. The changes in suitability assigned to grid cells currently with reefs (c, f) provide the baseline to which those corresponding to the bleaching events’ locations (d, g) should be compared (in figures c, d, f and g the mean and standard deviation are indicated). The p-values from a Welsh's t-test comparing the suitability distribution of bleaching events and that of all reef cells is also shown in d and g. Figures e and h illustrate the number of bleaching events within a cell as a function of the change in suitability assigned to that cell.
Sites of observed range expansion and fossil evidence from warmer periods in the past (based on data from Kiessling et al., 2012; Woodroffe et al., 2010; Greenstein & Pandolfi, 2008; Veron, 1992; Lighty et al., 1978) are consistent with the areas showing improved environmental suitability in our future projections (Fig. 5). In addition, the SST-driven decline projected at low latitudes is consistent with the equatorial decline described by Kiessling et al. (2012) from analysis of locations of coral fossil records from the last interglacial period.
Discussion
Isolating the impacts of warming and acidification
Areas where projected coral reef habitat suitability is most critically degraded by ocean surface warming (Fig. 2) correspond to areas with the highest mean annual SSTs today (Fig. 1), and also map onto regions identified as being particularly susceptible to future coral bleaching (e.g., Guinotte et al., 2003; Donner et al., 2005; van Hooidonk et al., 2013). We find that the projected decrease in suitability is most strongly associated with the weekly maximum – and to a lesser degree, minimum – SST variable, and very high values (>32 °C) are linked to marked declines (see Figs. S1.7–S1.8 in Data S1 and Fig. S2.5 in Data S2). Both BRT and MaxEnt statistical modeling approaches assign a relatively high significance to maximum weekly SST; in 10 randomly generated versions of MaxEntOPT/BRTOPT, this parameter ranked 5th/8th of a total of 27 parameters in average relative contribution to model output (however, correlations between variables do make it difficult to clearly establish hierarchy; Couce et al., 2012). The importance placed by the Bioclimatic Envelope Models on SST variables in general, and the negative contribution to modeled suitability at the higher end of the SST range, indicates that the global data set used for model training contains sites where present-day coral reef distribution is already limited by an upper thermal threshold. Although equivalent maximum SSTs are tolerated by Red Sea and Arabian Gulf coral reefs, these sites could not be included in the training data set and analysis because it was not possible to simulate conditions using the UVic model in these enclosed seas. It has been suggested that Red Sea and Arabian Gulf coral are potentially conditioned to extreme SSTs by very high SST variability (Ateweberhan & McClanahan, 2010), however, this is not a characteristic shared with the equatorial regions projected to decline in habitat suitability (Lough, 2012). Adding support to our model projections of an equatorial retraction, in addition to a poleward expansion, is the matching distribution range shift identified in fossil records of coral distribution during the last interglacial period, when the global average temperature exceeded that of today (Kiessling et al., 2012).
The areas most affected by reductions in ΩArag levels correspond to off-equatorial latitudes, particularly in the western Pacific (Fig. 3 and 4b). Generally highly stratified, therefore with little buffering by mixing with subsurface waters, these regions will tend to experience the strongest degree of saturation decline. In addition, these are slightly cooler waters, where other environmental factors, including ΩArag, are likely to out-weigh temperature variables in terms of importance. For instance, Cooper et al. (2012) argued that, at present, SST was far more significant than ΩArag to explain long-term changes in calcification rates for massive Porites colonies off Western Australia, whereas the GBR has been identified, in modeling studies (McNeil et al., 2004) and skeletal measurements in massive Porites colonies (e.g., Cooper et al., 2008; De'ath et al., 2009), as a region already exceeding the thermal optima for coral calcification and sensitive to reduced ΩArag.
In contrast, our results suggest higher latitude sites (e.g., southern GBR, southern Japan) and upwelling regions (e.g., East Pacific) will be relatively little affected by the acidification resulting from the projected increases in atmospheric CO2. The suitability for reef presence at 1990 levels is maintained in these locations up until 2070 across the majority of the models (Fig. 3). These areas experience the lowest ΩArag levels at which reefs are found today and are considered marginal for reef formation (e.g., Smith, 1992; Kleypas et al., 1999a; Glynn et al., 2007; Manzello et al., 2008) due to low calcification and cementation rates, which are finely balanced with erosion processes (Glynn, 1976; Cortes, 1997). Previous studies (e.g., Hoegh-Guldberg et al., 2007; Cao & Caldeira, 2008; Manzello, 2010) have identified coral reefs from higher latitudes and upwelling regions as the most sensitive to future reductions in ΩArag. However, statistical modeling has indicated that SST variables and light availability may be stronger determinants of coral reef presence at these marginal sites than ΩArag (Couce et al., 2012). Our future projections reflect this balance of controls. In addition, upwelling of deeper waters, isolated from the atmosphere, leads to a substantial degree of pCO2 disequilibrium between ocean surface and atmosphere and may hence provide some buffering against ocean acidification in regions such as the Eastern Pacific. Fabricius et al. (2011) observed changes in coral reef communities along a natural pH gradient on volcanic seeps at Papua New Guinea, and found that despite significant losses in species composition and biodiversity, coral cover remained constant for acidification levels comparable to what is expected by 2070 under the A2 scenario. This was achieved through a transition from branching species such as Acropora sp. toward predominance of more massive Porites sp. corals, whose calcification rates were less affected. Such shifts in species composition could have significant implications for ecosystem services; however, this study models coral reef ecosystems as a single entity, and is not sensitive to changes in species composition or biodiversity losses.
Synergistic impacts of warming and acidification
Under the combined influences of ocean surface warming and acidification, habitat suitability for coral reef ecosystems declines across the latitudinal band between 20°N and 20°S, affecting significant reef areas such as the Caribbean, GBR, and Coral Triangle region (Fig. 4c and 5). Areas where shallow water substrate is available are particularly susceptible to the impacts of warming and acidification (e.g., in Fig. 4d, modeled suitability in shallow equatorial regions more than halves by 2070). Although a marginal improvement at higher latitudes is projected by 2070, range expansion is constrained by availability of shallow waters where benthic substrate is present within light penetration depths (Fig. 5). Range expansion is also projected onto areas at the extreme end of oceanographic circulation pathways along the western boundaries, where the most depauperate reefs are found today (e.g., Glynn et al., 2007; Macintyre, 2003; Veron & Minchin, 1992; but see Thomson & Frisch, 2010). Coral reef expansion to higher latitude sites with improved conditions will, therefore, depend on larval influx, introducing a likely limitation for sites situated away from current transport such as the south-eastern Pacific (Wood et al., 2013). The rate of projected range expansion of suitable environmental conditions for coral reef presence varies between models. The MaxEntOPT model predicts a moderate (ca. 5 km per decade) expansion at present, accelerating to 30–45 km per decade by 2070 (Fig. 4d; calculated as rate at which curve edges intersects a horizontal line at 0.2). In contrast, BRTOPT projects an initial global shrinkage under the effects of acidification (at ca. 25 km per decade), with increasingly rapid expansion from 2020–2030 as the effect of rising SST becomes dominant (up to 70–80 km per decade by 2070). For comparison, Burrows et al. (2011) found the average poleward speed of surface isotherm movement in the oceans (50°S to 80°N) over the last 50 years was 27.5 km per decade. As already stated, our coral reef range expansion rate estimates do not take into account connectivity limitations related to the dispersal of coral larvae by ocean currents, and thus follow the thermal shift tempered by ocean acidification.
The combined impacts of increasing SST and acidification have little impact on environmental suitability for coral reef presence in the eastern Pacific, south east Atlantic, and the north Brazilian coast over the coming decades. These are recognized as marginal reef sites (e.g., Smith, 1992; Kleypas et al., 1999a; Glynn et al., 2007; Manzello et al., 2008); however, the decline of ΩArag is relatively slow in these predominantly upwelling regions, average SST is within tolerance levels, and further increases in SST might actually be advantageous for reef formation. The low ΩArag values in the eastern Pacific are not a limiting factor according to any of our statistical models, which instead assign constant, or at times increasing, suitability values to areas with ΩArag values falling below 2.2–2.3. While these levels of ΩArag will obviously impact reef structure and the balance of carbonate accretion and dissolution (e.g., Kleypas et al., 1999b; Manzello et al., 2008), and is likely to affect species composition and biodiversity (e.g., Fabricius et al., 2011), the region remains suitable for reef presence in our models, potentially assisted by warming where temperatures were previously suboptimal. That conditions remain suitable does not necessarily imply that existing reefs will be able to cope with the changes anticipated by 2070. We have not considered future changes in SST variability in our analysis, and this factor could play a key role for coral reef survival in these marginal areas (Glynn & Colgan, 1992; Toth et al., 2012), which are ranked globally among the slowest in terms of recovering from disturbances (Graham et al., 2011b).
Model evaluation and limitations
Empirical evidence is important to increasing our confidence in Bioclimate Envelope Models and projected distribution changes (Araújo et al., 2005) and the fossil coral record can provide a test for the models’ predictive ability. If SST is the stronger limiting factor for the presence of shallow coral reefs at high latitudes, we would expect fossil evidence of such an expansion into higher latitudes during warmer geological periods. In Fig. 5, we compare our 2070 predictions with relevant fossil records, including reports of extensive relic reef formations in high-latitude sites in Japan (Veron, 1992), Florida (Lighty et al., 1978), and Lord Howe Island (Woodroffe et al., 2010) dating from the Holocene, and along the Western Australian coast dating back to the Late Pleistocene (Greenstein & Pandolfi, 2008). Kiessling et al. (2012) documented both a range expansion and an equatorial retraction of coral reef distribution during the last interglacial period of the Pleistocene (ca. 125 000 years ago; Fig. 5), when average SST might have been about 1 °C higher than today (McKay et al., 2011). In addition, there is evidence supporting present-day range expansion. Yamano et al. (2011) report several tropical coral species expanding their range into higher latitudes along the coast of Japan, among them two Acropora sp. key for reef formation in the Indo-Pacific region. Veron (1992) also describes a high latitude fossil reef in Japan being re-colonized as a response to increasing temperature and Marsh (1993) reported Acropora sp. growing at a high-latitude site off Perth, Western Australia. In the Atlantic region, Vargas-Ángel et al. (2003) describe thickets of Acropora cervicorvis growing off the coast of Florida at higher latitudes than found previously. All these reports of past and present range expansion are consistent with our projections under current environmental change (Fig. 5).
Bioclimatic Envelope Models trained with equilibrium data are not suitable tools for the prediction of transient coral bleaching episodes. In particular, the models employed here have not been trained with the most relevant environmental variables (e.g., Degrees Heating Weeks or other short-term measures of thermal stress), or the location, date, and severity of bleaching episodes. Our projections correspond, instead, to the expected equilibrium response if conditions were maintained constant for sufficient time. Within these limitations, we wanted to establish a comparison of our predictions for the present-day with current observations. Coral bleaching reflects a stress response (Glynn, 1996), and degrading environmental conditions may lead to an increased number of bleaching episodes. To test this hypothesis, we compared the distribution of over 3500 recent bleaching events (observed between 2008 and 2012) with projected changes in suitability between model training conditions (1990) and the present-day (2010). We found bleaching episodes were more frequent in areas to which both MaxEntSIM and BRTSIM's 2010 projections assign higher decreases in suitability, when compared to the average on presence cells (Fig. 7). This is particularly significant for the BRT ‘SIM’ model; however, no such correlation is present with projections by the ‘OPT’ models, developed from the full suite of 27 predictive variables. Models relying on a limited number of variables are more sensitive to any change in those variables, and thus the responses of the ‘SIM’ models generally amplify that of the ‘OPT’ models, which can help explain why the ‘SIM’ models correlate better to short-term variations. MaxEntSIM and BRTSIM do however differ substantially in their predictions, especially in the WPWP region and surrounding areas, where the highest decrease in suitability is to be found according to MaxEntSIM, but where BRTSIM actually forecasts improved conditions. Few bleaching events are reported for the WPWP, but as it contains some of the least monitored coral sites in the world, this may be due to underreporting. BRT methods tend to overfit to training data, despite careful model development designed to minimize this (e.g., Elith et al., 2008), and models relying on a limited number of variables are more affected. Despite acceptable performance by BRTSIM within training conditions (similar AUC scores to that of MaxEntSIM; Fig. S1.2 in Data S1), overfitting makes its projections unstable, creating an oscillatory response to rising temperatures (e.g., Fig. S1.7) and leading to unreliable projections for anything but the smallest changes in environmental variables, including out-of-range conditions (e.g., the WPWP and surrounding areas in Fig. 7). For this reason, we chose to exclude BRTSIM projections from the average 2070 change in suitability (Fig. 5). Interestingly, the same qualities that make BRTSIM a poor long-term predictor may determine its strength in identifying areas that are currently experiencing thermal stress (Fig. 7). The MaxEnt technique is not as prone to overfitting, and we find strong agreement between projections by the ‘SIM’ and ‘OPT’ model versions. In addition, this issue is not present with the full ‘OPT’ version of the BRT model, which has a similar performance to both MaxEnt models (Data S3).
Caveats in the use of Bioclimatic Envelope Models to predict the effect of climate change on the distribution of a species include uncertainties associated with the modelling technique used (e.g., Thuiller, 2004; Lawler et al., 2006), migration limitations (reviewed in Thuiller et al., 2008), the method employed for out-of-range extrapolation (e.g., Webber et al., 2011), the availability of absence data or background selection (e.g., Elith et al., 2010), the spatial scale of the analysis (e.g., Pearson & Dawson, 2003), and the equilibrium hypothesis (e.g., Hirzel et al., 2001). Some of these caveats can be addressed by understanding the limitations of the results, which represent changes in equilibrium habitat suitability. Projections of the fate of existing coral reef ecosystems require the consideration of additional factors, such as local anthropogenic pressures and management, stress responses and recovery, bleaching, mortality, rates of sea-level rise, potential for adaptation, and migration capabilities among others. Instead, the Bioclimatic Envelope Models used here simply identify areas with future conditions similar to those where present-day coral reefs are found. In addition, the 1° × 1° spatial scale of the study may be too coarse to resolve many of the subtleties of the changing environmental conditions; however, this scale is typical of global projections from current climate models. Within those limitations, our results are informative, representing an initial estimation of the magnitude of the impact and providing a basis for future modelling work.
We employed two Bioclimatic Envelope Model approaches that make different use of absence data. MaxEnt is a presence-only technique, meaning it does not assume absence in areas where presence has not been established, unlike BRT. These two approaches have divergent responses to some of the limitations listed above, including performance in out-of-equilibrium situations (Hirzel et al., 2001) and dealing with incompleteness of the training database and/or absences due to factors other than climatic unsuitability (e.g., Pulliam, 2000). In addition, the development of models with two different levels of complexity – the ‘OPT’ and ‘SIM’ versions – helps establish the impact of variable selection in the model output and illustrate model-related performance issues, such as BRTSIM's poor performance for long-term and out-of-equilibrium predictions. Overall, we find relatively high model agreement between presence-only MaxEnt and presence/absence BRT (for the ‘OPT’ versions) and between the ‘OPT’ and ‘SIM’ model versions in the case of MaxEnt, particularly in areas projected to experience worsening conditions (Fig. 5 and Data S3). This, together with the congruence with the distribution of fossils from warmer geological times, increases our confidence in the predictions.
Despite their limitations, we note that the use of Bioclimatic Envelope techniques together with climate models output remains among the best tools in the study of species or ecosystem responses to changing conditions (e.g., Pearson & Dawson, 2003; Wiens et al., 2009). This study represents a significant advance over previous studies discussing the conflicting effects of warming and acidification because our models do not rely on specified thresholds for SST and ΩArag variables, but instead make simultaneous use of all relevant variables in the definition of an optimal climatic ‘envelope’ based only on the statistical analysis of the current coral reef distribution. This ‘envelope’ allows for the synergistic or antagonistic responses between variables that complicate physiological experimental results of thermal tolerance and ocean acidification studies.
Our main findings for future environmental suitability of coral reef ecosystems in all three CO2 emission scenarios considered are as follows: (i) range expansion at the high-latitude boundaries; (ii) no decreased suitability in currently marginal eastern Equatorial Pacific locations as well as in the Atlantic generally; and (iii) severe temperature-driven impacts in the WPWP and surrounding regions. The potential range expansion at high latitudes, however, may in many places be severely constrained by a lack of suitable benthic environment available for colonization and could additionally be affected by dispersal limitations. Currently, reefs in the Eastern Tropical Pacific will remain marginal, with increased warming offsetting the negative impacts of ocean acidification, while impacts in the suitability of the Atlantic basin as a whole may be minor. However, our models also forecast a significant overall decline in coral reef habitat suitability, with a decrease in suitability for coral reef ecosystems by 2070 of up to 30% in shallow water areas. We find that the decline, driven mainly by short-term SST maxima, is greatest around the WPWP region, and therefore would affect some of the most biodiverse coral reef regions. These results present important implications for future coral reef management, as they suggest that more emphasis should be placed in conservation efforts on marginal reefs as they are not necessarily a ‘lost cause’. They also suggest that coral reef presence is more likely to be preserved throughout much of the central and western Indian Ocean as well as the Atlantic, assuming other anthropogenic stresses are minimized.
Acknowledgments
This study was supported by University of Bristol alumnus A. Wilmot-Sitwell and a University of Bristol postgraduate scholarship to E. C., a Royal Society Advanced Fellowship and UK Ocean Acidification Research Program grant (NE/H017453/1) to A. R., and a RCUK Academic Fellowship to E. J. H. We thank Sally Wood and Nancy Jones for stimulating ideas and discussions, and the handling Editor and two anonymous Reviewers for their insightful comments.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Further details on the Bioclimatic Envelope Models. Details on model training, shallow water mask used in the study, variable contributions, and model performance on training data.
Data S2. Extrapolating to novel climates. Details on the method used to extrapolate for data outside training range, and discussion on its impacts on projections and the regions affected.
Data S3. Analysis of model agreement. Alternative model results for all figures presented in the main document, and discussion on agreement between 2070 projections by all four models.
References
- Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Science of the United States of America. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo MB, Whittaker RJ, Ladle RJ, Erhard M. Reducing uncertainty in projections of extinction risk from climate change. Global Ecology and Biogeography. 2005;14:529–538. [Google Scholar]
- Ateweberhan M, McClanahan TR. Relationship between historical sea-surface temperature variability and climate change-induced coral mortality in the western Indian Ocean. Marine Pollution Bulletin. 2010;60:964–970. doi: 10.1016/j.marpolbul.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuarine Coastal and Shelf Science. 2008;80:435–471. [Google Scholar]
- Baskett ML, Gaines SD, Nisbet RM. Symbiont diversity may help coral reefs survive moderate climate change. Ecological Applications. 2009;19:3–17. doi: 10.1890/08-0139.1. [DOI] [PubMed] [Google Scholar]
- Becker JJ, Sandwell DT, Smith WHF, et al. Global bathymetry and elevation data at 30 arc seconds resolution: SRTM30_PLUS. Marine Geodesy. 2009;32:355–371. [Google Scholar]
- Bishop JKB, Rossow WB. Spatial and temporal variability of global surface solar irradiance. Journal of Geophysical Research-Oceans. 1991;96:16839–16858. [Google Scholar]
- Bishop JKB, Rossow WB, Dutton EG. Surface solar irradiance from the International Satellite Cloud Climatology Project 1983–1991. Journal of Geophysical Research-Atmospheres. 1997;102:6883–6910. [Google Scholar]
- Bradley BA. Assessing ecosystem threats from global and regional change: hierarchical modeling of risk to sagebrush ecosystems from climate change, land use and invasive species in Nevada, USA. Ecography. 2010;33:198–208. [Google Scholar]
- Buddemeier R, Lane D, Martinich J. Modeling regional coral reef responses to global warming and changes in ocean chemistry: Caribbean case study. Climatic Change. 2011;109:375–397. [Google Scholar]
- Burrows MT, Schoeman DS, Buckley LB, et al. The pace of shifting climate in marine and terrestrial ecosystems. Science. 2011;334:652–655. doi: 10.1126/science.1210288. [DOI] [PubMed] [Google Scholar]
- Cao L, Caldeira K. Atmospheric CO2 stabilization and ocean acidification. Geophysical Research Letters. 2008;35:L19609. [Google Scholar]
- Chan NCS, Connolly SR. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Global Change Biology. 2013;19:282–290. doi: 10.1111/gcb.12011. [DOI] [PubMed] [Google Scholar]
- Chauvin A, Denis V, Cuet F. Is the response of coral calcification to seawater acidification related to nutrient loading? Coral Reefs. 2011;30:911–923. [Google Scholar]
- Cohen AL, Holcomb M. Why corals care about ocean acidification: uncovering the mechanism. Oceanography. 2009;22:118–127. [Google Scholar]
- Cooper TF, De'ath G, Fabricius KE, Lough JM. Declining coral calcification in massive Porites in two nearshore regions of the northern Great Barrier Reef. Global Change Biology. 2008;14:529–538. [Google Scholar]
- Cooper TF, O'leary RA, Lough JM. Growth of western Australian corals in the anthropocene. Science. 2012;335:593–596. doi: 10.1126/science.1214570. [DOI] [PubMed] [Google Scholar]
- Cortes J. Biology and geology of eastern Pacific coral reefs. Coral Reefs. 1997;16:S39–S46. [Google Scholar]
- Couce E, Ridgwell A, Hendy EJ. Environmental controls on the global distribution of shallow-water coral reefs. Journal of Biogeography. 2012;39:1508–1523. [Google Scholar]
- Couce E, Irvine PJ, Gregoire L, Ridgwell AJ, Hendy EJ. Tropical coral reef habitat in a geoengineered, high-CO2 world. Geophysical Research Letters. 2013;40:1799–1804. [Google Scholar]
- De'ath G. Boosted trees for ecological modeling and prediction. Ecology. 2007;88:243–251. doi: 10.1890/0012-9658(2007)88[243:btfema]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- De'ath G, Lough JM, Fabricius KE. Declining coral calcification on the Great Barrier Reef. Science. 2009;323:116–119. doi: 10.1126/science.1165283. [DOI] [PubMed] [Google Scholar]
- Donner SD, Skirving WJ, Little CM, Oppenheimer M, Hoegh-Guldberg O. Global assessment of coral bleaching and required rates of adaptation under climate change. Global Change Biology. 2005;11:2251–2265. doi: 10.1111/j.1365-2486.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- Edmunds PJ, Brown D, Moriarty V. Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Global Change Biology. 2012;18:2173–2183. [Google Scholar]
- Elith J, Graham CH, Anderson RP, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. Journal of Animal Ecology. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Methods in Ecology and Evolution. 2010;1:330–342. [Google Scholar]
- Fabricius KE, Langdon C, Uthicke S, et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Climate Change. 2011;1:165–169. [Google Scholar]
- Friedman JH. Greedy function approximation: a gradient boosting machine. Annals of Statistics. 2001;29:1189–1232. [Google Scholar]
- Gattuso JP, Gentili B, Duarte CM, Kleypas JA, Middelburg JJ, Antoine D. Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences. 2006;3:489–513. [Google Scholar]
- Globcolour. Diffuse Attenuation Coefficient at 490 nm (KD490) Sophia Antipolis: Observation de la Terre – Environnement (ACRI-ST); 2008. [Google Scholar]
- Glynn PW. Some Physical and Biological Determinants of Coral Community Structure in Eastern Pacific. Ecological Monographs. 1976;46:431–456. [Google Scholar]
- Glynn PW. Coral reef bleaching: facts, hypotheses and implications. Global Change Biology. 1996;2:495–509. [Google Scholar]
- Glynn PW. Global warming and widespread coral mortality: evidence of first coral reef extinctions. In: Hannah L, editor. Saving a Million Species: Extinction Risk from Climate Change. Washington, DC: Island Press; 2012. pp. 103–119. [Google Scholar]
- Glynn PW, Colgan MW. Sporadic disturbances in fluctuating coral reef environments: El Niño and coral reef development in the Eastern Pacific. American Zoologist. 1992;32:707–718. [Google Scholar]
- Glynn PW, Wellington GM, Riegl B, Olson DB, Borneman E, Wieters EA. Diversity and biogeography of the scleractinian coral fauna of Easter Island (Rapa Nui) Pacific Science. 2007;61:67–90. [Google Scholar]
- Graham J, Jarnevich C, Young N, Newman G, Stohlgren T. How will climate change affect the potential distribution of Eurasian tree sparrows Passer montanus in North America? Current Zoology. 2011a;57:648–654. [Google Scholar]
- Graham NaJ, Nash KL, Kool JT. Coral reef recovery dynamics in a changing world. Coral Reefs. 2011b;30:283–294. [Google Scholar]
- Greenstein BJ, Pandolfi JM. Escaping the heat: range shifts of reef coral taxa in coastal Western Australia. Global Change Biology. 2008;14:513–528. [Google Scholar]
- Guinotte JM, Buddemeier RW, Kleypas JA. Future coral reef habitat marginality: temporal and spatial effects of climate change in the Pacific basin. Coral Reefs. 2003;22:551–558. [Google Scholar]
- Halfar J, Godinez-Orta L, Riegl B, Valdez-Holguin JE, Borges JM. Living on the edge: high-latitude Porites carbonate production under temperate eutrophic conditions. Coral Reefs. 2005;24:582–592. [Google Scholar]
- Hirzel AH, Helfer V, Metral F. Assessing habitat-suitability models with a virtual species. Ecological Modelling. 2001;145:111–121. [Google Scholar]
- Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Marine and Freshwater Research. 1999;50:839–866. [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Hoeke RK, Jokiel PL, Buddemeier RW, Brainard RE. Projected changes to growth and mortality of Hawaiian corals over the next 100 years. PLoS ONE. 2011;6:1–13. doi: 10.1371/journal.pone.0018038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönisch B, Ridgwell A, Schmidt DN, et al. The geological record of ocean acidification. Science. 2012;335:1058–1063. doi: 10.1126/science.1208277. [DOI] [PubMed] [Google Scholar]
- van Hooidonk R, Maynard JA, Planes S. Temporary refugia for coral reefs in a warming world. Nature Climate Change. 2013;3:508–511. [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- IMaRS-USF. 2005. Millennium Coral Reef Mapping Project (unvalidated maps are unendorsed by IRD, and were further interpreted by UNEP-WCMC), UNEP World Conservation Monitoring Centre, Cambridge (UK). Availabe at: http://data.unep-wcmc.org/datasets/13 (accessed 15 June 2013)
- IMaRS-USF, IRD. 2005. Millennium Coral Reef Mapping Project (validated maps), UNEP World Conservation Monitoring Centre, Cambridge (UK). Available at: http://data.unep-wcmc.org/datasets/13 (accessed 15 June 2013)
- IPCC. Global Climate Projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Contributions of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change: “The Physical Science Basis”. Cambridge: Cambridge University Press; 2007. pp. 747–843. [Google Scholar]
- Kennedy EV, Perry CT, Halloran PR, et al. Avoiding coral reef functional collapse requires local and global action. Current Biology. 2013;23:912–918. doi: 10.1016/j.cub.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Kiessling W, Simpson C, Beck B, Mewis H, Pandolfi JM. Equatorial decline of reef corals during the last Pleistocene interglacial. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21378–21383. doi: 10.1073/pnas.1214037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleypas JA, McManus JW, Menez LAB. Environmental limits to coral reef development: where do we draw the line? American Zoologist. 1999a;39:146–159. [Google Scholar]
- Kleypas JA, Buddemeier RW, Archer D, Gattuso JP, Langdon C, Opdyke BN. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science. 1999b;284:118–120. doi: 10.1126/science.284.5411.118. [DOI] [PubMed] [Google Scholar]
- Krief S, Hendy EJ, Fine M, Yam R, Meibom A, Foster GL, Shemesh A. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochimica Et Cosmochimica Acta. 2010;74:4988–5001. [Google Scholar]
- Kroeker KJ, Kordas RL, Crim R, et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology. 2013;19:1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JJ, White D, Neilson RP, Blaustein AR. Predicting climate-induced range shifts: model differences and model reliability. Global Change Biology. 2006;12:1568–1584. [Google Scholar]
- Lighty RG, Macintyre IG, Stuckenrath R. Submerged early Holocene barrier reef south-east Florida shelf. Nature. 1978;276:59–60. [Google Scholar]
- Lough JM, Barnes DJ. Several centuries of variation in skeletal extension, density and calcification in massive Porites colonies from the Great Barrier Reef: a proxy for seawater temperature and a background of variability against which to identify unnatural change. Journal of Experimental Marine Biology and Ecology. 1997;211:29–67. [Google Scholar]
- Lough JM. Small change, big difference: sea surface temperature distributions for tropical coral reef ecosystems, 1950-2011. Journal of Geophysical Research-Oceans. 2012;117:C09018. [Google Scholar]
- Lybolt M, Neil D, Zhao J, Feng Y, Yu KF, Pandolfi J. Instability in a marginal coral reef: the shift from natural variability to a human-dominated seascape. Frontiers in Ecology and the Environment. 2011;9:154–160. [Google Scholar]
- Macintyre IG. A classic marginal coral environment: tropical coral patches off North Carolina, USA. Coral Reefs. 2003;22:474. [Google Scholar]
- Manzello DP. Ocean acidification hot spots: spatiotemporal dynamics of the seawater CO2 system of eastern Pacific coral reefs. Limnology and Oceanography. 2010;55:239–248. [Google Scholar]
- Manzello DP, Kleypas JA, Budd DA, Eakin CM, Glynn PW, Langdon C. Poorly cemented coral reefs of the eastern tropical Pacific: possible insights into reef development in a high-CO2 world. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10450–10455. doi: 10.1073/pnas.0712167105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh LM. The occurrence and growth of Acropora in extra-tropical waters off Perth, western Australia. In: Richmond RH, editor. Proceedings of the Seventh International Coral Reef Symposium Guam 22–26 June 1992, Volume 2. Mangilao, Guam: University of Guam Press; 1993. pp. 1233–1238. [Google Scholar]
- McCulloch M, Falter J, Trotter J, Montagna P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nature Climate Change. 2012;2:623–633. [Google Scholar]
- McKay NP, Overpeck JT, Otto-Bliesner BL. The role of ocean thermal expansion in Last Interglacial sea level rise. Geophysical Research Letters. 2011;38:L14605. [Google Scholar]
- McNeil BI, Matear RJ, Barnes DJ. Coral reef calcification and climate change: the effect of ocean warming. Geophysical Research Letters. 2004;31:L22309. [Google Scholar]
- Meissner KJ, McNeil BI, Eby M, Wiebe EC. The importance of the terrestrial weathering feedback for multimillennial coral reef habitat recovery. Global Biogeochemical Cycles. 2012;26:GB3017. [Google Scholar]
- Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. Projecting coral reef futures under global warming and ocean acidification. Science. 2011;333:418–422. doi: 10.1126/science.1204794. [DOI] [PubMed] [Google Scholar]
- Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography. 2003;12:361–371. [Google Scholar]
- Peterson W, Birdsall T, Fox W. The theory of signal detectability. Information Theory. 1954;4:171–212. [Google Scholar]
- Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. [Google Scholar]
- Precht WF, Aronson RB. Climate flickers and range shifts of reef corals. Frontiers in Ecology and the Environment. 2004;2:307–314. [Google Scholar]
- Pulliam HR. On the relationship between niche and distribution. Ecological Letters. 2000;3:349–361. [Google Scholar]
- Ricke KL, Orr JC, Schneider K, Caldeira K. Risks to coral reefs from ocean carbonate chemistry changes in recent earth system model projections. Environmental Research Letters. 2013;8(3) [Google Scholar]
- Ridgeway G. gbm: Generalized Boosted Regression Models. R Package Version 1.6-3.1. 2007. . Available at: http://CRAN.R-project.org/package=gbm (accessed 22 March 2012) [Google Scholar]
- Riegl B, Bruckner A, Coles SL, Renaud P, Dodge RE. Coral reefs: threats and conservation in an era of global change. Annals of the New York Academy of Sciences. 2009;1162:136–186. doi: 10.1111/j.1749-6632.2009.04493.x. [DOI] [PubMed] [Google Scholar]
- Silverman J, Lazar B, Erez J. Effect of aragonite saturation, temperature, and nutrients on the community calcification rate of a coral reef. Journal of Geophysical Research-Oceans. 2007;112:C05004. [Google Scholar]
- Silverman J, Lazar B, Cao L, Caldeira K, Erez J. Coral reefs may start dissolving when atmospheric CO2 doubles. Geophysical Research Letters. 2009;36:L05606. [Google Scholar]
- Smith SR. Patterns of coral recruitment and postsettlement mortality on Bermudas reefs – comparisons to Caribbean and Pacific reefs. American Zoologist. 1992;32:663–673. [Google Scholar]
- Smith SV, Buddemeier RW. Global change and coral reef ecosystems. Annual Review of Ecology and Systematics. 1992;23:89–118. [Google Scholar]
- Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Thomson DP, Frisch AJ. Extraordinarily high coral cover on a nearshore, high-latitude reef in south-west Australia. Coral Reefs. 2010;29:923–927. [Google Scholar]
- Thuiller W. Patterns and uncertainties of species’ range shifts under climate change. Global Change Biology. 2004;10:2020–2027. doi: 10.1111/gcb.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Albert C, Araújo MB, et al. Predicting global change impacts on plant species’ distributions: future challenges. Perspectives in Plant Ecology Evolution and Systematics. 2008;9:137–152. [Google Scholar]
- Toth LT, Aronson RB, Vollmer SV, et al. ENSO drove 2500-year collapse of eastern Pacific coral reefs. Science. 2012;337:81–84. doi: 10.1126/science.1221168. [DOI] [PubMed] [Google Scholar]
- Turley C, Eby M, Ridgwell AJ, et al. The societal challenge of ocean acidification. Marine Pollution Bulletin. 2010;60:787–792. doi: 10.1016/j.marpolbul.2010.05.006. [DOI] [PubMed] [Google Scholar]
- UNEP-WCMC, WorldFish Centre, WRI, TNC. Cambridge, UK: UNEP World Conservation Monitoring Centre; 2010. Global distribution of warm-water coral reefs, compiled from multiple sources, including the Millennium Coral Reef Mapping Project. See attribute table for details. Available at: http://data.unep-wcmc.org/datasets/13 (accessed 15 June 2013) [Google Scholar]
- Vargas-Ángel B, Thomas JD, Hoke SM. High-latitude Acropora cervicornis thickets off Fort Lauderdale, Florida, USA. Coral Reefs. 2003;22:465–473. [Google Scholar]
- Vergara SG, McManus JW, Kesner-Reyes KN, et al. ReefBase 2000: Improving Policies for Sustainable Management of Coral Reefs, ver. 2000. Manila, Philippines: International Centre for Living Aquatic Resources Management; 2000. [Google Scholar]
- Veron J. Environmental control of Holocene changes to the world's most northern hermatypic coral outcrop. Pacific Science. 1992;46:402–425. [Google Scholar]
- Veron JEN, Minchin PR. Correlations between sea-surface temperature, circulation patterns and the distribution of hermatypic corals of Japan. Continental Shelf Research. 1992;12:835–857. [Google Scholar]
- Veron JEN, Hoegh-Guldberg O, Lenton TM, et al. The coral reef crisis: the critical importance of <350 ppm CO2. Marine Pollution Bulletin. 2009;58:1428–1436. doi: 10.1016/j.marpolbul.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Weaver AJ, Eby M, Wiebe EC, et al. The UVic Earth System Climate Model: model description, climatology, and applications to past, present and future climates. Atmosphere-Ocean. 2001;39:361–428. [Google Scholar]
- Webber BL, Yates CJ, Le Maitre DC, et al. Modelling horses for novel climate courses: insights from projecting potential distributions of native and alien Australian acacias with correlative and mechanistic models. Diversity and Distributions. 2011;17:978–1000. [Google Scholar]
- Wiens JA, Stralberg D, Jongsomjit D, Howell CA, Snyder MA. Niches, models, and climate change: assessing the assumptions and uncertainties. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19729–19736. doi: 10.1073/pnas.0901639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PD, Downey PO, Leishman M, Gallagher R, Hughes L, O'donnell J. Weeds in a warmer world: predicting the impact of climate change on Australia's alien plant species using MaxEnt. Plant Protection Quarterly. 2009;24:84–87. [Google Scholar]
- Wood S, Paris CB, Ridgwell A, Hendy EJ. Modeling dispersal and connectivity of broadcast spawning corals at the global scale. Global Ecology and Biogeography. 2013 , doi: 10.1111/geb.12101. [Google Scholar]
- Woodroffe CD, Brooke BP, Linklater M, et al. Response of coral reefs to climate change: expansion and demise of the southernmost Pacific coral reef. Geophysical Research Letters. 2010;37:L15602. [Google Scholar]
- Yamano H, Sugihara K, Nomura K. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophysical Research Letters. 2011;38:L04601. [Google Scholar]
- Yara Y, Vogt M, Fujii M, et al. Ocean acidification limits temperature-induced poleward expansion of coral habitats around Japan. Biogeosciences. 2012;9:4955–4968. [Google Scholar]
- Zamagni J, Mutti M, Kosir A. The evolution of mid Paleocene-early Eocene coral communities: how to survive during rapid global warming. Palaeogeography Palaeoclimatology Palaeoecology. 2012;317:48–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Further details on the Bioclimatic Envelope Models. Details on model training, shallow water mask used in the study, variable contributions, and model performance on training data.
Data S2. Extrapolating to novel climates. Details on the method used to extrapolate for data outside training range, and discussion on its impacts on projections and the regions affected.
Data S3. Analysis of model agreement. Alternative model results for all figures presented in the main document, and discussion on agreement between 2070 projections by all four models.