Abstract
Identification of appropriate donor cell types is important for lung cell therapy and for lung regeneration. Previous studies have indicated that mesenchymal stromal cells derived from human bone marrow (hBM-MSCs) and from human adipose tissue (hAT-MSCs) may have the ability to trans-differentiate into lung epithelial cells. However, these data remain controversial. Herein, the ability of hBM-MSCs and hAT-MSCs to repopulate acellular rodent lung tissue was evaluated. hBM-MSCs and hAT-MSCs were isolated from bone marrow aspirate and lipoaspirate, respectively. Rat lungs were decellularized with CHAPS detergent, followed by seeding the matrix with hBM-MSCs and hAT-MSCs. Under appropriate culture conditions, both human MSC populations attached to and proliferated within the lung tissue scaffold. In addition, cells were capable of type 2 pneumocyte differentiation, as assessed by marker expression of surfactant protein C (pro-SPC) at the protein and the RNA level, and by the presence of lamellar bodies by transmission electron microscopy. Additionally, hAT-MSCs contributed to Clara-like cells that lined the airways in the lung scaffolds, whereas the hBM-MSCs did not. We also tested the differentiation potential of MSCs on different extracellular matrix components in vitro, and found that protein substrate influences MSC epithelial differentiation. Together our data show the capacity for human MSCs to differentiate toward lung epithelial phenotypes, and the possibility of using these cells for lung cell therapies and tissue engineering.
Introduction
One of the roadblocks to success in the nascent tissue engineering field is the identification of cells that can be used to repopulate bioengineered organs. For lung tissue engineering, good candidate cells are most likely to be derived from patient-specific samples. Cells should be easily expandable, and be capable of lung epithelial cell differentiation or of lung epithelial cell support.1–4 Various groups have described the capacity for bone-marrow-derived cells to contribute to lung repair and regeneration.5–8 Interestingly, these reports show contribution to lung epithelium by the hematopoietic stem cell component of the bone marrow, as well as the mesenchymal stromal cell (MSC) fraction. In many of these studies, the contribution by bone-marrow-derived cells to lung epithelium appears to require lung injury, along with possible concomitant exposure of the lung basement membrane to repopulating cells.9
Another potential source of mesenchymal cells can be found in the human-adipose-tissue-derived MSCs (hAT-MSCs).10–13 These cells have previously been shown to have the capacity for differentiation into pulmonary-like cells in vitro when cultured in specialized media or when cocultured. No study to date has used hAT-MSCs to recellularize acellular lung tissue.14
Further, a subpopulation of human and rodent bone marrow MSC-like cells may express Clara cell secretory protein (CCSP), a marker that is associated in the lung with Clara cells.6 Previous studies have shown that tail vein administration of murine CCSP+ bone marrow cells into CCSP-knockout mice resulted in the incorporation of CCSP+ cells in the host lung following lung injury. Taken together, these studies and others may imply that MSCs and other bone-marrow-derived cells have the potential to contribute functional epithelial cells to the lung following injury. However, controversies surrounding the differentiation of MSCs to epithelial phenotypes largely appear to derive from variations in experimental methods used between investigators, particularly the use of eGFP as a means to lineage trace the cells of interest, and the resultant inability of investigators to definitively tell donor from recipient cells.9
Bone-marrow- and adipose-tissue-derived MSCs have also been shown to have immunomodulatory roles.15,16 These include the lack of activation of T cells, as well as a reduction of activated lymphocytes, when MSCs are delivered in animal models in vivo.16 Additionally, MSCs produce paracrine signals that have been demonstrated to have anti-inflammatory roles in lung.17,18 From a therapeutic standpoint, the evidence favoring the use of MSCs as a potential treatment of lung disease, either via a direct contribution to lung epithelium or through an indirect paracrine immunomodulatory mechanism, is of high interest.
Past work from our laboratory has utilized neonatal rodent lung epithelial cells for the repopulation of bioengineered rat lungs.19 These experiments demonstrated the feasibility of using decellularized lung matrices as a means to direct mixed populations of epithelial cells to anatomically correct locations within the lung matrix. In this report, we have tested the ability of both hAT-MSCs and human-bone-marrow-derived MSCs (hBM-MSCs) to directly contribute to lung epithelium on decellularized rodent lung lobes. We find that hBM-MSCs and hAT-MSCs are capable of attachment and growth in the lung scaffold. Following 7 days of culture in small airway growth medium (SAGM), the hBM-MSCs express the type 2 pneumocyte marker pro-surfactant protein C (SPC) at the RNA and protein level. hBM-MSCs also secrete surfactant into the culture medium, and contain lamellar bodies as shown by transmission electron microscopy (TEM). Additionally, hBM-MSCs give rise to cells that are positive for the proximal airway marker cytokeratin-5 after culture in the rat lung scaffold within a bioreactor. In contrast, the hAT-MSCs give rise to pro-SPC-positive cells as well as Clara-like cells that line the small airways. However, the hAT-MSCs do not give rise to cytokeratin-5-positive cells following culture in the lung bioreactor, unlike hBM-MSCs.
We further explored the influence of substrate matrix composition on the differentiation of the MSCs by seeding hBM-MSCs and hAT-MSCs onto tissue culture dishes coated with a mixed composition of human extracellular matrix (ECM), Matrigel, laminin, collagen I, collagen IV, and fibronectin. We find, and quantify by fluorescence-activated cell sorting (FACS) and reverse-transcription polymerase chain reaction (RT-PCR), that matrix surface coatings influence the percentage of MSCs that express lung epithelial markers when grown in standard tissue culture flasks. Additionally, we cultured hBM-MSCs on decellularized liver matrix, and show that MSCs seeded onto this matrix do not maintain the same suite of epithelial markers present when cultured on the lung matrix. These data indicate that the decellularized lung scaffolds retain specific cues to direct MSC differentiation into epithelial phenotypes, and that the anatomic source of the MSCs influences potential cell fates. To the best of our knowledge, this is the first indication of human-derived hBM-MSCs and hAT-MSCs giving rise to multiple lung epithelial cell types when placed onto a decellularized lung.
Materials and Methods
Isolation and characterization of human bone marrow and adipose tissue MSCs
Fresh, unprocessed human bone marrow samples were purchased from Lonza (Cat. No. 1M-125). Three total donor samples were acquired, two women and one man, ages ranging between 22 and 29. The bone marrow cells were plated on tissue culture plastic at a density of 5×105 cells per cm2 in high-glucose DMEM containing 10% FBS. The medium was changed every 2 to 3 days. Only low-passage (≤5) cells were used in experiments. Cells were passaged every 7–10 days at a 1:3 ratio. hBM-MSCs were characterized by flow cytometry (Becton Dickenson LSR II) for expression of CD90, CD105, CD73, and CD45 (all antibodies were acquired from eBiosciences).
hAT-MSCs were acquired from three donors ranging in age from 44 to 63. These samples were discarded and anonymous and do not fall under human subject research. Lipoaspirates were washed 2×with DPBS, followed by digestion with 0.15% collagenase type 1 (Gibco; Cat No. 17100-017) in DMEM for 60 min at 37°C. The digest was stopped by adding 10% FBS/DMEM, followed by centrifugation and resuspension in 10% FBS/DMEM and filtration through a 100-μm filter. Cells were fed every 2–3 days.
Fluorescence-activated cell sorting
Single cells were fixed with a 2% paraformaldehyde solution for 10 min, and washed two times in PBS for 5 min each. The cells were incubated with 10% FBS and 0.2% Triton X-100 containing the diluted antibody of interest. The cells were incubated on ice with the following antibodies (all from eBiosciences) for 25 min in the dark: CD45-PE (12-9459-41), CD90-FITC (11-0909-41), CD73-PE (12-0739-41), and CD105-APC (17-1057-41). Additional antibodies used were as follows: pro-SPC 1/100 (Millipore; ab 3786) and CCSP 1/100 (Millipore; 07-623). Secondaries for these antibodies were all species appropriate Invitrogen Alexa Fluors diluted at 1/500. Isotype controls used included anti-mouse IgG-PE (eBiosciences; 12-4714), anti-mouse IgG-FITC (ebiosciences; 11-4724), anti-mouse IgG APC (ebiosciences; 17-4015-80), rabbit IgG-FITC (ebiosciences; 11-4614-80), and purified rabbit IgG (Invitrogen 02-6102). In addition to isotype controls, secondary-only antibody controls were run in parallel.
Decellularization and reseeding of rat lung samples
Yale IACUC approved all procedures utilizing animals. Adult Sprague Dawley rat lungs (between 3 and 5 months old) were decellularized as previously described.19 Briefly, rats were euthanized with an IP injection of sodium pentobarbital (Euthasol). The rat lungs were excised and the trachea and pulmonary artery were cannulated. A 10-mL mixture of heparin (50 U/mL) and sodium nitroprusside (1 μg/mL) was infused by gravity through the pulmonary artery. The pulmonary artery was instilled with 500 mL of pH-12 decellularization solution composed of 8 mM CHAPS, 25 mM EDTA, and 1 M NaCl in PBS at 37°C at a constant pressure of 20 mmHg. The lungs were then infused via the airways with 10 mL of benzonase and incubated for 1 h at 37°C. The cellular remnants were washed away with 2.5 L of PBS. The lungs were incubated in a mixture of antibiotics and antimycotics (1% gentamicin, 4 mg/mL amphotericin, and 10% penicillin/streptomycin) for at least 16 h prior to seeding with cells. During this incubation, the solution was perfused through the pulmonary artery at 1 mL/min. Prior to seeding cells, the antibiotic/antimycotic solution was removed from the lungs and replaced with PBS. The lungs were perfused with PBS for an additional 30 min before cellular seeding. Cells were seeded as a bolus of between 2.5×106 and 10×106 cells through the trachea into the single decellularized upper right rat lung lobe. The cultures were maintained for 7 days in small-airway growth medium (SAGM; Lonza; Cat. No. CC-3118) with perfusion of the medium via the pulmonary artery at 1 mL/min. Medium was changed every 2–3 days.
Immunohistochemistry
Lungs were instilled with a 10% formalin solution through the airways, and placed in 10% formalin for 4 h at room temperature with constant rocking. Lungs were embedded in paraffin, and sectioned at 5 μm. The sections were deparaffinized following a standard rehydration alcohol/xylene series. Antigen retrieval was performed by incubation of the rehydrated tissue sections with a Tris-EDTA buffer (10 mM Tris Base, 1 mM EDTA, and 0.05% Tween-20 at pH 9.0) at 75°C for 20 min. The tissue sections were then allowed to cool at room temperature for an additional 20 min. The sections were washed once in PBS prior to immunostaining. The sections were incubated in blocking reagent (10% NGS or FBS, and 0.2% Triton X-100 in PBS) for 45 min. The primary antibodies (CCSP 1/50, Millipore, Cat. No. 07-623; pro-SPC 1/100, Millipore, Cat. No. ab 3786; caveolin-1 1/100, Abcam, Cat. No. 39541; and alpha smooth muscle actin 1/100, Dako, Cat. No. M0851) were incubated for 2 h at room temperature, or overnight at 4°C. The sections were washed in PBS 3 times for 3 min per wash, followed by incubation with secondary antibodies (all species-specific secondary antibodies from Invitrogen Alexa Fluor series diluted at 1/500) at room temperature for 45 min. Tissue sections were mounted in Vector labs Vectashield mounting medium containing DAPI (Vector Science; Cat. No. H1200).
Processed sections were imaged with a Zeiss fluorescence microscope and images were acquired with Volocity software. Confocal microscope images were acquired with a Leica TCS SP5.
Real-time quantitative RT-PCR
Total RNA was extracted from cells using the RNeasy Mini Kit from Qiagen following the manufacturer's instructions. First-strand complementary DNA (cDNA) was synthesized with random hexamers as primers, using the SuperScript First-Strand Synthesis System according to manufacturer's protocol (Invitrogen). An equal volume mixture of the products was used as templates for PCR amplification. Reactions were performed in a 25 μL volume with iQ™ SYBR Green Supermix (Bio-Rad) and 200 nM each of forward and reverse primers shown using iCyler and iQ software (Bio-Rad). Each sample was run in triplicate. PCR conditions included an initial denaturation step of 4 min at 95°C, followed by 40 cycles of PCR consisting of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C. Average threshold cycle (Ct) values from the triplicate PCR for a gene of interest (GOI) were normalized against the average Ct values for GAPDH from the same cDNA sample. Fold change of GOI transcript levels between sample A and sample B equals 2−ΔΔCt, where ΔCt=Ct(GOI)−Ct(GAPDH), and ΔΔCt=ΔCt(A)−ΔCt(B). Table 1 lists the primers used for the genes of interest.
Table 1.
Primers Used for Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction
| Gene | Length (bp) | Primer sequences |
|---|---|---|
| hSPC | 94 | Forward: CCTTCTTATCGTGGTGGTGGT |
| Reverse: TCTCCGTGTGTTTCTGGCTCAT | ||
| hAQ5 | 79 | Forward: ACTGGGTTTTCTGGGTAGGG |
| Reverse: ATGGTCTTCTTCCGCTCTTC | ||
| hCaveolin-1 | 122 | Forward: CTACAAGCCCAACAACAAGG |
| Reverse: CATCGTTGAGGTGTTTAGGGT | ||
| hGAPDH | 122 | Forward: GACAACAGCCTCAAGATCATCAG |
| Reverse: ATGGCATGGACTGTGGTCATGAG |
Transmission electron microscopy
A modified protocol from Schmiedl et al. was followed.23 Native rat lungs and recellularized lungs were inflation fixed at 37°C with 2.5% glutaraldehyde/2.0% paraformaldehyde in 0.2 M sodium cacodylate for 30 min, followed by a 2-h incubation at 4°C. The fixed tissue was rinsed with 0.1 M sodium cacodylate. The tissues were postfixed in 1% OsO4 for 2 h, followed by en block uranyl acetate staining. The tissues were dehydrated in a standard ethanol series and embedded in EPON. Sections of 70 nm were obtained and poststained with uranyl acetate and lead citrate. Images were obtained with a Philips Tecnai transmission electron microscope.
Coating of matrix proteins for cell culture
hBM-MSCs and hAT-MSCs were cultured on different extracellular proteins, including fibronectin (50 μg/mL), collagen I (100 μg/mL), collagen IV (50 μg/mL), Matrigel (1:80), and a mixture of human ECM proteins (1:100) (consisting of collagens, laminin, fibronectin, tenascin, elastin, and a number of proteoglycans and glycosaminoglycans; Sigma Aldrich) for 7 days (all ECM components purchased from Sigma Aldrich). Fibronectin, collagen I, collagen IV, and laminin are principal components of lung matrix.
ELISA analysis for SPC
ELISA was performed on cell culture media collected from the supernatant of hBM-MSCs and hAT-MSCs cultured on rat acellular lung scaffolds to quantify secreted SPC according to the manufacturer's instructions (Life Science Advanced Technology). SPC values were normalized to the total number of cells, and values for experimental samples were subtracted from fresh SAGM medium alone.
Statistical analyses
All statistical analyses were performed with the Origin software (OriginLab). The data were expressed as mean±SEM (standard error of measurement). T-tests were performed to evaluate whether two groups were significantly different from each other and p≤0.05 was considered statistically significant.
Results
Characterization of MSCs isolated from bone marrow and adipose tissue
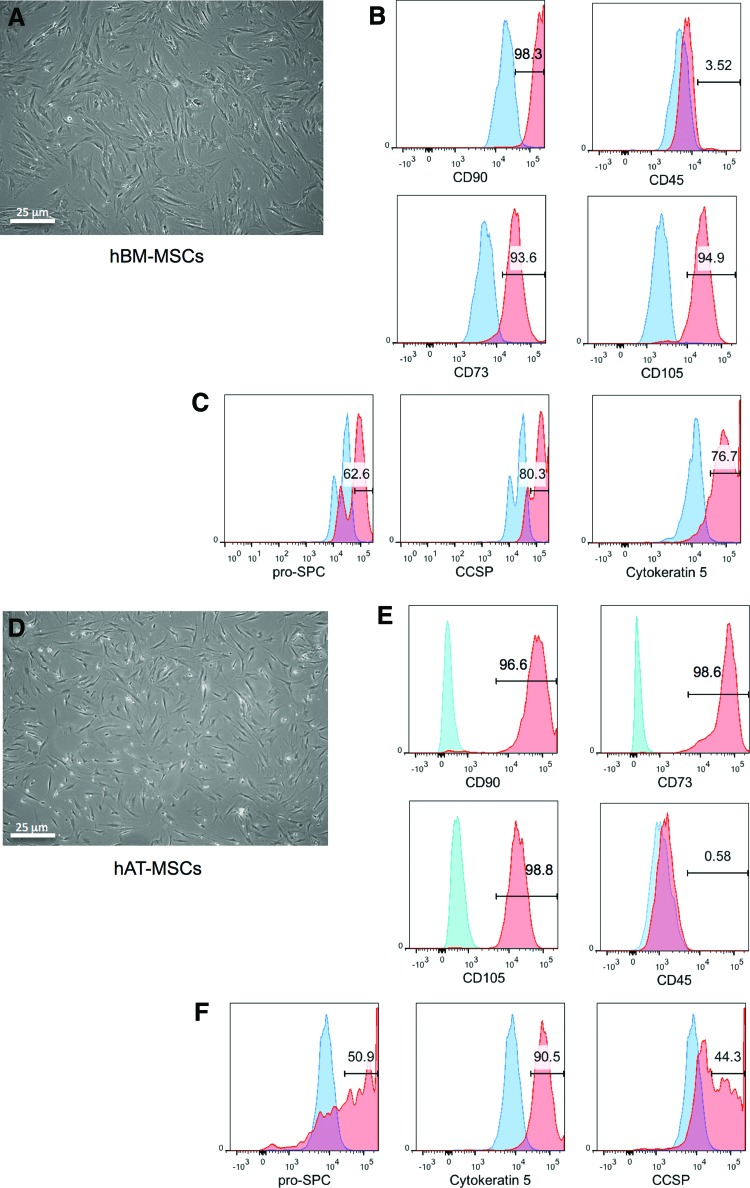
hBM-MSCs were obtained from freshly isolated bone marrow samples (Fig. 1A–C). The unfractionated marrow was placed on tissue culture flasks and the resultant adherent cells were immunophenotyped for expression of canonical MSC cluster of differentiation markers. Early passage hBM-MSCs, cultured in 10% FBS/DMEM between two and four passages, were >93% positive for CD90, CD105, and CD73, while being predominantly negative for CD45 (Fig. 1B). We also assayed for the expression of epithelial markers, including CCSP, pro-SPC, and cytokeratin 5, in cells cultured on tissue culture plastic and maintained in DMEM/10% FBS (Fig. 1C), since previous work has shown that a subfraction of cultured MSC-like cells can express epithelial markers by FACS.20 Our FACS analysis indicated that hBM-MSCs are positive for CCSP (80%), pro-SPC (63%), and cytokeratin-5 (77%) (Fig. 1C). As a negative control for these experiments, whole unfractionated bone marrow mononuclear cells were analyzed by FACS and these were uniformly negative for the lung epithelial markers assessed (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
FIG. 1.
Fluorescence-activated cell sorting (FACS) analysis of adherent, passage 2, human bone marrow mesenchymal stromal cell (hBM-MSC) and human adipose tissue mesenchymal stromal cell (hAT-MSC) samples indicates that cells express mesenchymal stromal cell markers and markers of epithelium. (A) Morphology of passage-2 hBM-MSCs. (B) hBM-MSCs are positive for MSC markers, including CD90, CD105, and CD73, and they are CD45 negative. (C) A subpopulation of hBM-MSCs express the epithelial markers pro-SPC, Clara cell secretory protein (CCSP), and cytokeratin-5. (D) Morphology of passage-2 hAT-MSCs. (E) FACS analysis of hAT-MSCs confirms that these cells are also positive for MSC markers, including CD90, CD105, and CD73, and they are CD45 negative. (F) hAT-MSCs also contain a population of cells that are positive for pro-SPC, CCSP, and cytokeratin 5. Isotype controls are shown in blue, and experimental samples are shown in red. SPC, surfactant protein C. Color images available online at www.liebertpub.com/tea
hAT-MSCs were isolated from freshly harvested lipoaspirates (Fig. 1D–F). The cells were isolated via collagenase 1 tissue digest and placed onto tissue culture flasks. These cells were maintained in 10% FBS/DMEM medium for expansion. Immunophenotyping of hAT-MSCs confirmed their identity as CD90+/CD105+/CD73+ and CD45− (Fig. 1E). Similar to hBM-MSCs, hAT-MSCs were also immunopositive by FACS for various epithelial markers (Fig. 1F). Interestingly, there were differences in the fractions of cells that express epithelial markers between the hAT-MSCs and the hBM-MSCs. In contrast to the hBM-MSC population, approximately half of the population of hAT-MSCs were positive for CCSP (44%); hAT-MSCs also were positive for pro-SPC (51%), and cytokeratin 5 (91%) (Fig. 1F).
Repopulation of rat acellular matrix with MSCs in lung bioreactor cultures
hBM-MSCs reseeded onto rat lung acellular matrix and cultured in lung bioreactor
In an effort to understand whether adipose- or bone-marrow-derived MSCs can repopulate lung extracellular matrices, and whether these cells can take on an epithelial phenotype, MSCs were cultured in a previously described biomimetic rat lung bioreactor system.19 H&E histology and DAPI staining of the native and the decellularized lung matrix confirmed that decellularized lungs are devoid of remnant native lung cells prior to seeding with MSCs (Supplementary Fig. S2A, B). To date our laboratory has decellularized hundreds of rodent lungs and not one has shown traces of viable cells following the procedure.19
hBM-MSCs that were cultured from between two and four passages were seeded via the airway at a density between 2.5 and 10×106 cells into the single right upper lobe of a decellularized rat lung. These cells were injected as a bolus into the trachea and cultured in the lung bioreactor for 7 days in SAGM. The lung bioreactor culture allows for perfusion of medium at a constant rate via the pulmonary artery, and allows for the cells to be injected into the trachea in sterile fashion.19 SAGM was chosen as a potentially suitable medium for the culture of the recellularized lung after pilot experiments with 10% FBS/DMEM resulted in cells that were uniformly fibroblastic in morphology, with nearly all cells expressing α-smooth muscle actin (α-sma), a marker of myofibroblasts, following 7 days of culture (Supplementary Fig. S3).
Moreover, SAGM was selected as a candidate to promote lung epithelial differentiation, because of the retinoic acid and human epidermal growth factor it contains have been shown to promote proliferation and epithelial differentiation of pluripotent cells.21,22 In vitro pilot experiments were performed in which MSCs were grown in tissue culture flasks with SAGM medium or in 10% FBS/DMEM. MSCs grown in SAGM did not express α-sma, while the cells maintained CCSP expression to a similar level as was present at early passages (Supplementary Fig. S3C–F). As a result of the pilot in vitro experiments, we used SAGM in an effort to curb the amount of cells that express α-sma after seeding into the lung matrix, and in turn to promote lung epithelial differentiation. However, prior to seeding the hBM-MSCs into the acellular lung, the cells were maintained in 10% FBS/DMEM medium on tissue culture plastic to promote robust growth.
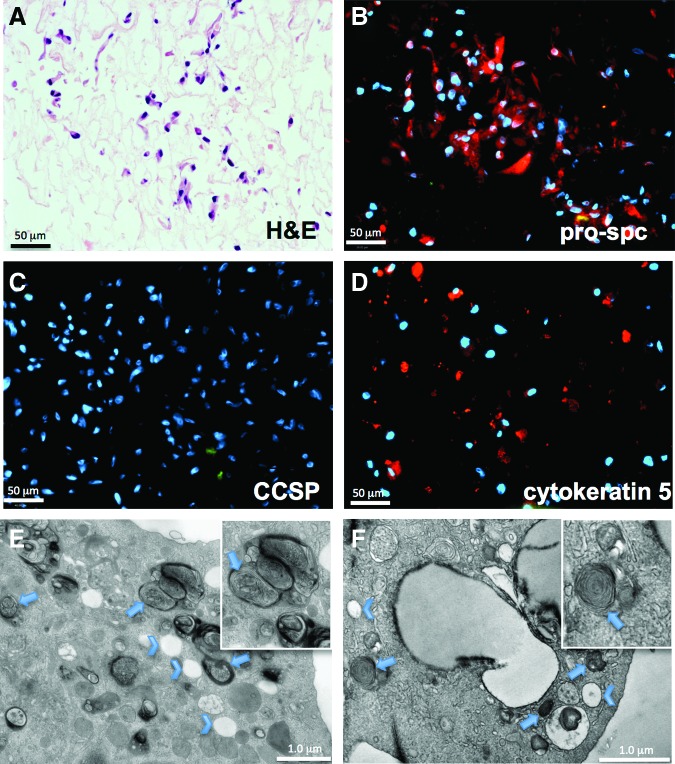
H&E staining of hBM-MSC-seeded lungs that were cultured for 7 days in SAGM demonstrated a cuboidal appearance of the attached cells, when compared with cells that were grown in 10% FBS/DMEM in the lung bioreactor (Fig. 2A and Supplementary Fig. S3). In agreement with the in vitro pilot cultures, immunostaining for α-sma was almost entirely absent in the hBM-MSC-recellularized rodent lungs (data not shown). Most hBM-MSCs attached to lung alveolar matrix, with few to no cells adhering to proximal airway structures. Approximately 65–70% of the attached cells expressed pro-SPC, a type 2 pneumocyte marker (Fig. 2B). Cytokeratin-5, a marker expressed by basal epithelial cells of the airways, was also present in a subset of the attached cells (Fig. 2D). Interestingly, there were no cells that were positive for CCSP after culture in the lung bioreactor, even though the starting population of hBM-MSCs expressed CCSP by immunostaining and FACS (Figs. 1C and 2C). The attached cells were also negative for P63, a marker of basal cells, and for caveolin-1, a marker of type 1 pneumocytes (data not shown). These data indicate that hBM-MSCs are capable of predominately repopulating alveolar structures, and take on a phenotype akin to type 2 pneuomcytes based on location of attachment as well as marker expression.
FIG. 2.
Lung bioreactor cultures seeded with hBM-MSCs. (A) H&E histological section of lungs seeded with hBM-MSCs and cultured for 7 days in small airway growth medium (SAGM). (B) Immunostaining for pro-SPC shows numerous cells that are positive for the type 2 pneumocyte marker. (C, D) There were no cells that were positive by immunostaining for the Clara cell marker CCSP (C), while there were sparse cells that were cytokeratin-5 positive (D). (E, F) As an additional method to positively identify the hBM-MSC-derived cells as type 2 pneumocytes, transmission electron microscopy (TEM) analysis was performed. TEM analysis of a native type 2 cell (E) (arrows indicate lamellar bodies; chevrons indicate secretory vesicles). (F) Lungs reseeded with hBM-MSCs contain cells that have both lamellar bodies (arrows) and secretory vesicles (chevrons); both are characteristics of type 2 pneumocytes. Color images available online at www.liebertpub.com/tea
The identification of lamellar bodies via TEM is a method of definitive identification of type 2 cells.23,24 Lamellar bodies serve as a repository of secretory surfactants and lipids contained within the type 2 cells, and both human and rat type 2 pneumocytes are characterized by the presence of lamellar bodies. By TEM, lamellar bodies in native type 2 cells are ∼500-nm long and are characterized by electron-dense deposits in structures that are formed of concentric whorls. Additionally, another characteristic of native type 2 pneumocytes is the presence of secretory vesicles (Fig. 2E, chevrons). hBM-MSC-derived cells cultured on the lung scaffold for 7 days had abundant electron-dense lamellar bodies, as well as many secretory vesicles (arrows and chevrons, respectively, Fig. 2F). Morphologically, the native rat type 2 pneumocytes were very similar to the hBM-MSCs that had been cultured within the bioreactor on lung matrix and in the presence of SAGM for 7 days. Hence, this is strong evidence that hBM-MSCs can take on the phenotype of type 2 alveolar epithelial cells, when expanded and cultured under suitable conditions in lung ECM.
hAT-MSCs reseeded onto rat lung acellular matrix and cultured in lung bioreactor
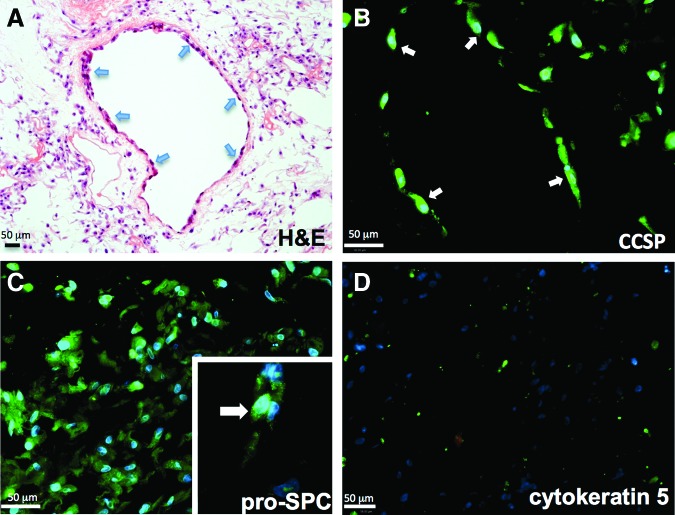
We seeded 2.5–10×106 hAT-MSCs as a bolus into the upper right decellularized rodent lung lobe and cultured these in SAGM medium for 7 days under the same conditions as the hBM-MSCs. H&E histological preparations of seeded lungs demonstrated that the hAT-MSCs attached throughout the matrix, but had a particular affinity to attach to and repopulate the proximal and small airways, unlike the hBM-MSCs that did not attach in the airways (Fig. 3A, B). Immunostaining revealed that the cells attached to the airways were positive for CCSP. This is in contrast to hBM-MSCs, which do not maintain expression of CCSP following culture on the lung matrix, nor do these cells adhere to the proximal and small airways.
FIG. 3.
hAT-MSCs give rise to type 2 pneumocyte-like cells, and to Clara-like cells that line the airways when cultured in the lung bioreactor. (A) hAT-MSCs robustly repopulate the lung matrix following 7 days of culture in SAGM in the acellular rat lung bioreactor. H&E staining reveals the particular affinity for the cells to inhabit the lining of the airways (arrows). (B) The cells that line the airways (arrows) are positive for the Clara cell marker CCSP. (C) The cells that are growing on the matrix are positive for pro-SPC. The inset shows the granular, cytoplasmic staining of the cells positive for pro-SPC. (D) There is no indication that the hAT-MSCs maintain cytokeratin-5 expression when grown on the decellularized matrix. Color images available online at www.liebertpub.com/tea
hAT-MSCs cultured in the lung bioreactor were also positive for the type 2 pneumocyte marker pro-SPC. Immunofluorescence for pro-SPC revealed cells with clear, punctate cytoplasmic staining for this marker (inset, Fig. 3C). Another difference between hAT-MSCs and hBM-MSCs is that the hAT-MSCs do not give rise to cells that are cytokeratin-5 positive by immunofluorescence, whereas the hBM-MSCs do (Figs. 2D and 3D). Aside from tissue of origin for the MSCs, another independent difference that may factor in these data is the age ranges of the donor cell types. The bone marrow donors were all in the mid 20s, whereas the youngest adipose MSC donor was 44. Overall, these data indicate that there are intrinsic differences between MSCs derived from different tissue sources with regard to their ability to repopulate the lung acellular matrix, but these differences may also be due to differences in the donor ages for the two cell types.
Alveolar epithelial gene expression following lung bioreactor culture
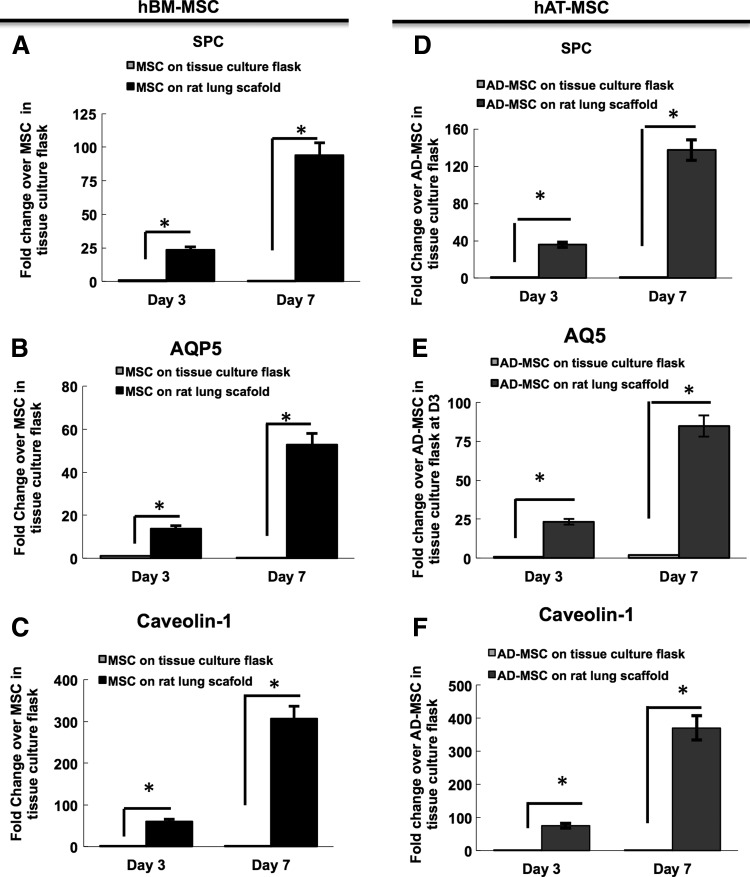
We evaluated gene expression for various distal lung epithelial markers in the hBM-MSC- and hAT-MSC-recellularized lungs in SAGM. Real-time quantitative RT-PCR (qRT-PCR) was used to assay for expression of surfactant protein C (SPC; type 2 alveolar cells), and type 1 alveolar cell markers caveolin-1 and aquaporin 5 (AQP5) (Fig. 4).
FIG. 4.
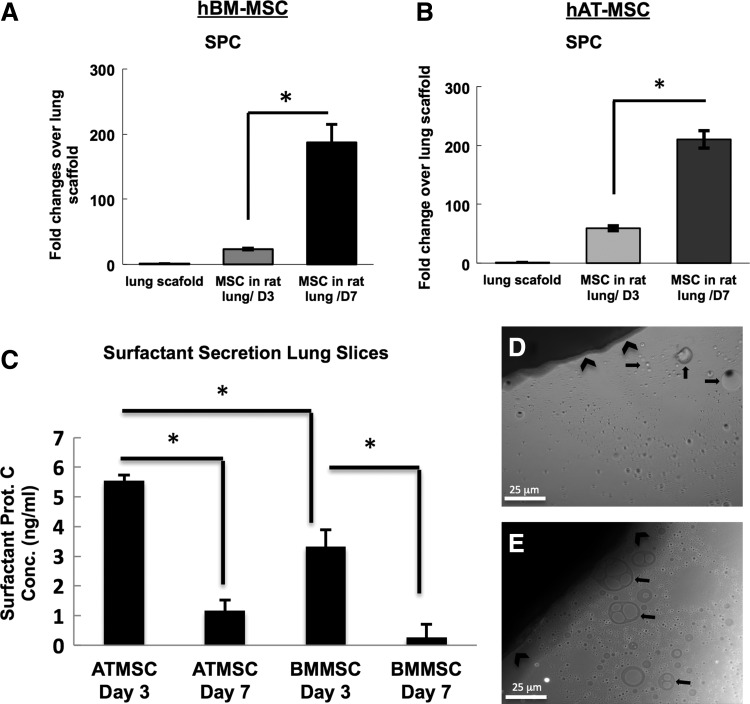
Reverse-transcription polymerase chain reaction (RT-PCR) of day-3 and −7 lung bioreactor cultures seeded with either hBM-MSCs or with hAT-MSCs. (A–C) hBM-MSCs have increased gene expression levels of distal epithelial genes, including SPC, aquaporin-1, and caveolin-1, with increased time in the lung bioreactor culture. (D–F) Similarly, hAT-MSCs increase the expression of distal genes with longer culture periods. The fold change of the cells in the lung bioreactors is compared with MSCs grown in tissue culture flasks.
SPC is widely used as a marker of type 2 pneumocytes as well as early lung progenitor cells. RT-PCR revealed that SPC expression in hBM-MSC-recellularized lungs increased from day 3 to 7, and was higher than for MSCs grown in a flask in DMEM/10% FBS (Fig. 4A). These results show that there is a progressive increase in the amounts of SPC transcripts present at day 3 and 7, by 23- and 93-fold, respectively (Fig. 4A). A similar pattern of SPC expression was observed in the hAT-MSC-seeded lung scaffolds. The expression of SPC increased from day 3 (36×) to day 7 (137×) when compared with hAT-MSCs grown in flasks. The increase in SPC gene expression was greater for the hAT-MSC-recellularized lungs when compared with the hBM-MSC-recellularized lungs (p<0.05).
Additionally, we evaluated the expression of two specific alveolar type 1 markers, including caveolin-1 and AQP5. The expression of both caveolin-1 and AQP5 increased over time on days 3 and 7, when compared with MSCs grown on tissue culture flasks (Fig. 4B–F; p<0.05). While the gene expression for these two type 1 cell markers increased with time in culture, we were unable to detect protein expression based on immunostaining (data not shown).
Overall, qRT-PCR revealed that the levels of gene expression for SPC, caveolin-1, and AQP5 increased with time in the bioreactor with both hBM-MSC- and hAT-MSC-seeded lung scaffolds. However, protein expression as shown by immunostaining indicates that of these genes, only pro-SPC is detectable in hBM-MSCs and hAT-MSCs after 7 days on the lung scaffold, while there is no detectable protein expression for the type 1 markers. This apparent discrepancy is likely explained by protein levels that are not robust enough for detectable signal with immunostaining, whereas the sensitivity of qRT-PCR allows us the capacity to detect small amounts of mRNA expression from the cells seeded on the lung scaffold.
Functionality of type 2 pneumocytes derived from hBM-MSCs and hAT-MSCs
To determine whether the MSCs seeded onto the decellularized lung scaffolds exerted a functional impact on the organ, we assessed whether there was active surfactant secretion into the medium by the cells growing on the decellularized scaffold (Fig. 5). hBM-MSCs were seeded at a density of 2×106 cells into the upper right decellularized lung lobe, and allowed to attach to the matrix for 2 h, followed by cutting the lobe in half, and placing the pieces in a six-well culture plate containing SAGM. The lung sections, thus seeded, were cultured for either 3 or 7 days and the culture medium was collected at these time points. RT-PCR of cell-seeded scaffolds indicated an increase in SPC gene expression between days 3 and 7 when compared with the decellularized scaffold alone (Fig. 5A, B). Brightfield microscopy of active cultures revealed a visible layer of oil-like droplets that were mostly concentrated near the lung section (Fig. 5D, E). As a control for these experiments, scaffolds that were not seeded with MSCs were placed in the same culture conditions. These controls did not contain oil-like droplets (Supplementary Fig. S4). Because of the likelihood that the visible droplets were surfactant that was actively being produced by the seeded cells, enzyme-linked immunosorbent assay (ELISA) was performed to test for the presence of SPC (Fig. 5C). SPC was present in hBM-MSC slice cultures both at days 3 and 7, at 3.3 ng/mL and 0.26/mL, respectively (Fig. 5C; p<0.05). The lung cultures that were seeded with hAT-MSCs had a larger amount of surfactant protein present in the culture medium (p<0.05). The conditioned SAGM from these cultures contained 5.55 ng/mL and 1.16 ng/mL, respectively (Fig. 5C; p<0.05). The decrease in protein levels between days 3 and 7 is likely due to time-dependent changes in the differentiation state of the hAT-MSCs and the hBM-MSCs when cultured in the slice culture system used for the ELISA studies. In particular, RT-PCR analysis of recellularized lungs indicated that there was large increase in RNA levels of type 1-associated genes, specifically AQP5 and caveolin-1 between days 3 and 7 of culture in recellularized lungs (Fig. 4). While RT-PCR results also indicate an increase in SPC RNA between days 3 and 7, it can be that the MSCs are in a transitional state wherein mature SPC protein is not made to the same extent as in the earlier, day-3 culture time point. Previous work also supports the possibility of a similar transition from a type 2 cell phenotype to a type 1 cell phenotype in a span of only 4 days.25,26 These previous studies indicated that lung recellularized with IPS-derived type 2 pneumocytes can undergo a rapid phenotypic change wherein up to 32% of the cells with an initial type 2 cell phenotype undergo a transition to cells with a type 1 cell phenotype. These data indicate that the seeded MSCs take a function akin to type 2 pneumocytes, in that the MSCs actively produce and secrete surfactant when cultured on decellularized lung scaffolds.
FIG. 5.
hAT-MSCs and hBM-MSCs grown in SAGM actively produce surfactant. (A) RT-PCR analysis of hBM-MSCs (A) and hAT-MSCs (B) seeded on an acellular rat lung, then cultured in slices, indicates a progressive increase in SPC expression with time in culture. hBM-MSCs (D) and hAT-MSCs (E) cultured in SAGM for 7 days in a lung slice have visible surfactant droplets in the culture medium adjacent to the lung slice. (C) Enzyme-linked immunosorbent assay (ELISA) of the media samples indicates that at day 3, 5.5 ng/mL of SPC is present in the hAT-MSC lung slice cultures, and 3.3 ng/mL is present in the hBM-MSC cultures. At day 7, the concentration of SPC is 1.1 ng/mL for hAT-MSCs and 0.26 ng/mL for the hBM-MSCs. All ELISA values were normalized to SAGM medium alone (*p<0.05). Bars represent SEM.
Influence of substrate matrix on the differentiation of MSCs
In an effort to further understand the influence of the culture substrate on the differentiation of MSCs, either hAT-MSCs or hBM-MSCs were cultured on tissue culture flasks that had been coated with various ECM proteins, including collagen I, collagen IV, laminin, fibronectin, human ECM, and Matrigel (Fig. 6). Collagen I, collagen IV, laminin, and fibronectin were chosen because each is a represented component of lung ECM27,28; the human ECM and Matrigel coatings were chosen in an effort to provide the cells with a mixed ECM composition. After a 7-day culture period in SAGM, the population of cells was characterized for epithelial marker expression by FACS and by RT-PCR.
FIG. 6.
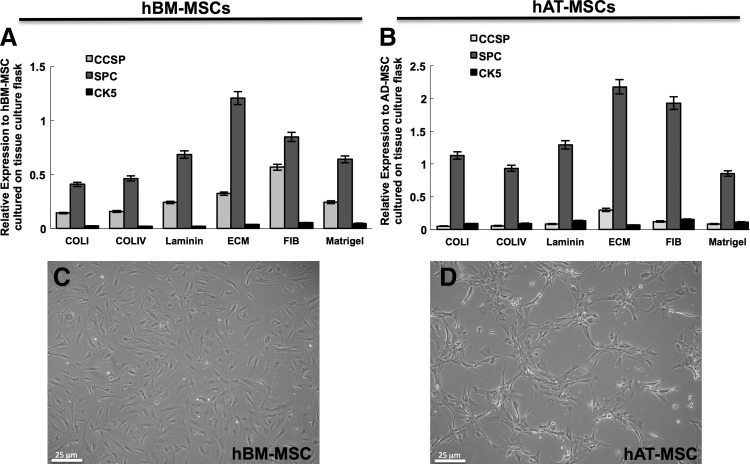
Substrate coatings influence epithelial marker expression in hBM-MSCs and hAT-MSCs. hBM-MSCs and hAT-MSCs were cultured on various extracellular matrix (ECM) coatings (human ECM, laminin, fibronectin, collagen IV, collagen I, and Matrigel) in SAGM media. After 7 days of culture, the hBM-MSCs (A) and hAT-MSCs (B) were analyzed by RT-PCR for expression of CCSP, pro-SPC, and cytokeratin 5. Both sources of MSCs varied in terms of epithelial marker expression when cultured on different surface coatings. These data indicate that the highest expression of SPC is found in MSCs grown in human ECM compared with MSCs grown on other surface coatings. Also evident from these experiments were differences in cell morphology between hBM-MSCs (C) and hAT-MSCs (D) grown on human ECM.
Data obtained from FACS analysis experiments from hBM-MSCs cultured on the various substrates revealed large differences in the population of cells that expressed cytokeratin 5, CCSP, and pro-SPC depending on the ECM component on which the cells were cultured (Supplementary Figs. S5 and S6). Cells grown on collagen I substrate had the lowest fraction of cells that were cytokeratin 5 positive; ∼3.0% of the population was positive, a reduction of approximately threefold when compared to cells that were grown on fibronectin-coated plastic flasks. The fraction of CCSP-positive cells in the hBM-MSC populations also varied drastically depending on the substrate on which these were grown. The largest population of CCSP-positive cells was in the fibronectin-coated flasks (44%), whereas the least amount of CCSP-positive cells was found in hBM-MSCs grown on collagen IV (27%). Likewise, the population of hBM-MSCs that express pro-SPC varied between surface coatings where the highest amount of cells positive was present in the human-ECM-coated flasks (54%), while the lowest was in cells grown on Matrigel (34%).
The behavior of hAT-MSCs grown on different ECM matrices also showed differences in the populations that express CCSP, pro-SPC, and cytokeratin-5. For example, the population of pro-SPC-positive cells ranged from a high of 81% in the human ECM condition to a low of 32% in the collagen I condition. The population of CCSP-positive cells also was greatest in the human-ECM-coated flasks (10%), when compared with the other surface coatings. While there existed differences in the fractions of cells that expressed epithelial markers between the hAT-MSCs and the hBM-MSCs, also evident were phenotypic differences between the populations especially between those that were cultured on human ECM (Fig. 6C, D). The hAT-MSCs cultured on human ECM formed a lattice-like network throughout the culture dish, whereas the hBM-MSCs maintained canonical MSC morphology. These data indicate that particular ECM components allow for MSCs to modulate epithelial marker expression. This is particularly striking in the condition in which the MSCs were grown on a mixed human ECM substrate. These data also indicate that retained ECM components within the decellularized lung can play a role in the differentiation of cultured MSCs.
RT-PCR was performed as an additional means to quantify the impact of different ECM substrates. A similar pattern of CCSP, cytokeratin-5, and SPC gene expression was observed in the hBM-MSCs and hAT-MSCs cultured on different ECM proteins by qPCR as was seen by flow cytometry (Fig. 6A, B and Supplementary Figs. S5 and S6). Specifically, qPCR data showed that cells cultured on mixed human ECM protein substrate resulted in the highest expression of SPC in both hBM-MSCs and hAT-MSCs on day 7 when compared with cells cultured on other ECM proteins.
The observed differences in the ability of hAT-MSCs and hBM-MSCs to modulate phenotype based on the substrate on which they are cultured may indicate an intrinsic difference between these two MSC populations in their ability to react to interactions with ECM proteins. Specifically, hAT-MSCs have a nearly twofold greater expression of SPC relative to hBM-MSCs when these are cultured on human ECM substrate that consists of a mixture of collagens, laminins, fibronectin, tenascin, and elastin. Further, these and other differences between the MSC populations may play a role in the ability of these cells to interact with remnant ECM proteins of the decellularized lung matrix. This in turn may partly explain the propensity of hAT-MSCs to repopulate decellularized lung airways, whereas hBM-MSCs do not.
Finally, to evaluate whether the phenotypic plasticity of the MSCs cultured on lung ECM was organ specific, we analyzed the phenotype of hBM-MSCs that were seeded onto decellularized liver matrix (Supplementary Fig. S7). hBM-MSCs were seeded onto sections of decellularized liver matrix and cultured for 3 days, followed by histological analysis. These data show that hBM-MSCs cultured in either 10% FBS/DMEM or in SAGM on sections of liver matrix do not express cytokeratin 5 or CCSP, and only rarely express pro-SPC by immunostaining (Supplementary Fig. S7). We restricted the culture period to only 3 days because longer periods resulted in lower MSC viability, as indicated by disrupted nuclei and uniform caspase expression (data not shown). These data further show that the lung ECM environment in concert with soluble factors play a critical role on the capability of MSCs to adhere, survive, and differentiate, and to maintain expression of lung epithelial markers.
Discussion
For purposes of lung tissue regeneration either in vivo or in vitro, characteristics that are essential for the donor cell type include (1) the ability to differentiate into lung epithelial or other cell types, (2) high proliferative potential (must be able to expand these cells to high numbers prior to seeding onto the decellularized lung scaffolds), (3) be easily accessible from a patient (i.e., an autologous cell source, either differentiated or stem), and (4) lack of immunogenicity. In toto, these characteristics point to the possibility of using MSCs as a source for reseeding decellularized lungs or as in vivo lung cell therapy.
hBM-MSCs have previously been shown to give rise to various epithelial cell types including lung epithelium, though some of these findings have remained controversial.5,6,18,29,30 Additionally, MSCs are less immunogenic than most cell types, do not express MHC class II markers, and do not elicit a strong immune response as evidenced by lack of activation of T cells.16 Our study of human marrow- and adipose-derived MSCs in rat lung matrix points to clear and indisputable evidence of epithelial cell differentiation for these two cell types.
Herein we demonstrate that human BM-MSCs, when placed onto decellularized rat lung matrices and cultured in SAGM, are capable of expressing the type 2 pneumocyte marker pro-SPC as well as the proximal airway marker cytokeratin-5. These results contrast with previous work that failed to show any substantial contribution to lung epithelium from hBM-MSCs that were seeded onto decellularized lung scaffolds.31,32 This discrepancy may partly be explained by differences in the lung decellularization process, and our use of continuous perfusion of medium via the pulmonary artery in our bioreactor cultures. In contrast, the work of these other groups used a lung slice culture system, and used a lung scaffold that likely differed in retained ECM components. Our decellularization method makes use of 8 mM CHAPS, whereas the other groups used 0.1% Triton-X in the decellularization protocol. A previous report that compared CHAPS and SDS detergents for decellularization found differences in retention of collagen and elastin following lung decellularization.27 It is likely that different matrix components remain following CHAPS lung decellularization as compared with Triton-X decellularization, which may influence the differentiation of the seeded MSCs. Another, although more remote, possibility for the discrepancy between reports is that we have used human-derived MSCs seeded onto rat lungs, whereas Daly and colleagues used mouse lungs and cells, and Bonvillain et al. used both macaque lungs and cells. Another major difference between our study and those preceding is that we are the only report that uses hAT-MSCs in repopulating decellularized lung tissue.
We also show that hAT-MSCs give rise to Clara-like cells that line the airways and express CCSP protein, a characteristic that is not found in any of the three human bone marrow donor samples we assayed, regardless of donor age. Additionally, similar to hBM-MSCs, hAT-MSCs also give rise to type 2-like cells, but do not give rise to cells that are positive for cytokeratin-5 after recellularizing the lung. These differences are particularly interesting given that both MSC sources start out with common CD marker expression. However, the initial MSC populations vary with regard to expression of the epithelial markers CCSP, pro-SPC, and cytokeratin-5. These differences in initial epithelial marker expression may result in the downstream variation between the MSC sources after culture in the lung bioreactor.
Various reports have documented a difference with regard to differentiation potential of MSCs depending on the tissue of origin.10,33–36 Gene expression comparisons between MSCs derived from bone marrow, adipose, and skin have shown a marked difference in the ability to express genes associated with osteogenic and adipogenic lineages.34 Given our results comparing hAT-MSC and hBM-MSC sources, it is likely that additional MSC sources may have distinct differentiation potential when cultured under appropriate lung biomimetic conditions.
A particularly interesting candidate to assess are cells isolated from bronchoalveolar lavage fluid, so-called “lung MSCs” that attach to plastic and express canonical markers associated with bone marrow MSCs.35,37,38 The presence of lung-resident MSCs (L-MSCs) has been reported in mice and humans. Coculture of L-MSCs with type 2 cells induced the expression of CK18, CK19, occludin, and SPC in L-MSCs, suggesting that these lung-resident stromal cells have the ability to differentiate into alveolar-like cells in vitro.4 Other studies have shown that L-MSCs derived from human lungs are capable of generating Clara, ATI, and ATII cells in vitro.39,40 Hence, L-MSCs are an interesting population having lung epithelial differentiation capability under the appropriate culture conditions. Other mesenchymal cells from distinct origins may also have differences with regard to differentiation potential; these may include fetal-associated mesenchymal cells from the amniotic fluid, cord blood, Wharton's jelly, placenta, and amnion-derived MSCs.12,13,41
While other groups have reported conflicting results regarding the ability of MSCs to undergo lung epithelial differentiation based on differences of methodology, the results presented here solidify the differentiation capability of MSCs to produce cells with a lung epithelial phenotype. Our results are particularly conclusive because our starting materials are decellularized lung and purified hBM-MSCs or hAT-MSCs. Since the decellularized lung is completely devoid of cells (Supplementary Fig. S2), this eliminates the possibility of contaminating cell types or cell fusion confounding the experimental observations.
One of the main functions of alveolar type 2 cells is the production of surfactant protein. Both hAT-MSC and hBM-MSC populations expressed SPC by immunofluorescence, and RT-PCR. MSCs also actively produce SPC as assayed by ELISA, which resulted in visible surfactant droplets in the culture medium adjacent to the lung slices. These data are further evidence that MSCs are capable of taking on a functional type 2 cell phenotype. Interestingly, there was no visual evidence of surfactant secretion in cultures maintained in 10% FBS from either MSC populations, which lends further support regarding the importance of the culture medium in cellular differentiation (Supplementary Fig. S4).
We also cultured MSCs in SAGM on different protein substrates and assessed several epithelial markers. These data show that the MSC populations markedly vary in terms of marker expression when grown on different ECM substrates. In a related experiment, hBM-MSCs were cultured on decellularized liver in either SAGM or in DMEM/10% FBS for 3 days (Supplementary Fig. S7). These experiments were aimed at determining whether there is an organ-specific ECM effect on the differentiation of cells. We found that, on liver matrix, hBM-MSCs do not express cytokeratin-5 and are mostly negative for pro-SPC. Hence, lung epithelial differentiation appears to depend critically upon both culture medium and matrix substrate.
These data highlight intrinsic differences between MSCs from bone marrow and adipose tissue and the resultant ability for each of these cell populations to recellularize tissue-engineered lungs. In future lung tissue engineering efforts, the tissue of origin of MSCs should be taken into account, as MSCs from different sources may have variant differentiation potential. With regard to hAT-MSCs and hBM-MSCs, it appears that the former may be more beneficial for lung tissue engineering since these are capable of a broader range of differentiation and attachment to various locations on the decellularized lung, unlike the hBM-MSCs, which appear mostly restricted to repopulate the distal lung, alveolar regions. We are currently investigating the potential of these MSC populations, as well as others, in conjunction with primary and IPS-derived lung epithelial cells in an effort to better understand the optimal recellularization cell cocktail to use in lung tissue engineering efforts.
Collectively, these experiments demonstrate that MSCs from both human adipose and human bone marrow are capable of taking on lung epithelial phenotypes and surfactant secretion function following culture in a decellularized rat lung bioreactor system, while only hAT-MSCs are capable of colonizing the airways with CCSP-positive cells. These data are of importance because they provide a springboard for dissecting the pathways and determinants for epithelial differentiation from human mesenchymal cells. These findings, in turn, will support both in vivo lung cell therapy efforts, as well as in vitro efforts in whole-lung regeneration.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the Yale School of Medicine TEM core director Xinran Liu for technical support with TEM data acquisition. We are also grateful to Diane Krause for discussions regarding the project and to Elizabeth Calle for critical reading of the article and with technical support. This work was financially supported by a grant from United Therapeutics, Inc. United Therapeutics did not have any influence on the data interpretation nor presentation. J.J.M is supported by NIH T32 GM086287; LEN is also supported by NIH R01 HL098220.
Author's Contributions
L.E.N and J.J.M conceptualized and wrote the article. J.J.M isolated MSCs, decellularized lungs and liver, and performed FACS, TEM, immunostaining, and ELISA. M.G. performed PCR on MCSs pre- and postlung bioreactor culture.
Disclosure Statement
L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Badylak S.F., Weiss D.J., Caplan A., and Macchiarini P.Engineered whole organs and complex tissues. Lancet 379,943, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Soto-Gutierrez A., Werner S., Ott H.C., and GILBERT T.Perspectives on whole-organ assembly:moving toward transplantation on demand. J Clin Invest 122,3817, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song J.J., and Ott H.C.Organ engineering based on decellularized matrix scaffolds. Trends Mol Med 17,424, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Wagner D., et al. . Can stem cells be used to generate new lungs? Ex vivo bioengineering with decellularized whole lung scaffolds. Respirology 18,895, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause D.S., et al. . Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105,1, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Wong A.P., Keating A., and Waddell T.K.Airway regeneration: the role of the Clara cell secretory protein and the cells that express it. Cytotherapy 11,676, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Kotton D.N., et al. . Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128,5181, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Rojas M., et al. . Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 33,145, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause D.S.Bone marrow-derived cells and stem cells in lung repair. Proc Am Thoracic Soc 5,323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baer P.C., and Geiger H.Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012,1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sng J., and Lufkin T.Emerging stem cell therapies: treatment, safety, and biology. Stem Cells Int 2012,1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chistiakov D.A.Endogenous and exogenous stem cells: a role in lung repair and use in airway tissue engineering and transplantation. J Biomed Sci 17,92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolios G., and Moodley Y.Introduction to stem cells and regenerative medicine. Respiration 85,3, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Li M., and Ikehara S.Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int 2013,1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DelaRosa O., et al. . Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev 21,1333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmusson I., Ringdén O., Sundberg B., and Le Blanc K.Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76,1208, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lee J.W., Fang X., Krasnodembskaya A., Howard J.P., and Matthay M.A.Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells 29,913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz L.A., et al. . Mesenchymal stem cell engraftment in lung isenhanced in response to bleomycin exposureand ameliorates its fibrotic effects. PNAS 100,8408, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen T.H., et al. . Tissue-engineered lungs for in vivo implantation. Science 329,538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong A.P., et al. . Identification of a bone marrow–derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest 119,336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rackley C.R., and Stripp B.R.Building and maintaining the epithelium of the lung. J Clin Invest 122,2724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenssen J., and Stolk J.Pulmonary stem cells and the induction of tissue regeneration in the treatment of emphysema. Int J COPD 2,131, 2007 [PMC free article] [PubMed] [Google Scholar]
- 23.Schmiedl A., Ochs M., Muhlfeld C., Johnen G., and Brasch F.Distribution of surfactant proteins in type II pneumocytes of newborn, 14-day old, and adult rats: an immunoelectron microscopicand stereological study. Histochem Cell Biol 124,465, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kassmer S.H., and Krause D.S.Detection of bone marrow-derived lung epithelial cells. Exp Hematol 38,564, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghaedi M., et al. . Human iPS cell–derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest 123,4950, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghaedi M., et al. . Alveolar epithelial differentiation of human induced pluripotent stem cells in a rotating bioreactor. Biomaterials 35,699, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen T.H., Calle E.A., Colehour M.B., and Niklason L.E.Matrix Composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs 195,222, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortiella J., et al. . Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 16,2565, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Wong A.P., et al. . Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol 293,L740, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Wang X., et al. . Characterization of mesenchymal stem cells isolated from mouse fetal bone marrow. Stem Cells 24,482, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Daly A.B., et al. . Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A 18,1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonvillain R.W., et al. . A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A 18,2437, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VIDAL M.A., et al. . Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg 37,713, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Nbaheen M., et al. . Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev 9,32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman A.M., et al. . Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung. Stem Cells Dev 20,1779, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pevsner-Fischer M., Levin S., and Zipori D.The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev and Rep 7,560, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Lama V.N., et al. . Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117,989, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarvinen L., et al. . Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 181,4389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubo H.Tissue engineering for pulmonary diseases: insights from the laboratory. Respirology 17,445, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Weiss D.J.Stem Cells, cell therapies and bioengineering in lung biology and diseases: comprehensive review of the recent literature 2010–2012. Ann Am Thorac Soc 10,S45, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes M., Curley G., Ansari B., and Laffey J.Clinical review: stem cell therapies for acute lung injury/acute respiratory distress syndrome-hope or hype? Crit Care 16,1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.