Abstract
The human gut microbiota is inextricably linked to health and disease. One important function of the commensal organisms living in the intestine is to provide colonization resistance against invading enteric pathogens. Because of the complex nature of the interaction between the microbiota and its host, multiple mechanisms likely contribute to resistance. In this review, we dissect the biological role of short-chain fatty acids (SCFA), which are fermentation end products of the intestinal microbiota, in host–pathogen interactions. SCFA exert an extensive influence on host physiology through nutritional, regulatory, and immunomodulatory functions and can also affect bacterial fitness as a form of acid stress. Moreover, SCFA act as a signal for virulence gene regulation in common enteric pathogens. Taken together, these studies highlight the importance of the chemical environment where the biology of the host, the microbiota, and the pathogen intersects, which provides a basis for designing effective infection prevention and control.
1. INTRODUCTION
1.1. Microbiota and colonization resistance
The human intestine is populated by a diverse collection of microorganisms, the composition of which is a key determinant in human health and disease. However, the complex nature of the interactions between microbial cells and their host presents challenges in elucidating the contribution of the microbiota to health or the causal relationship between the microbiota and disease. Evidence supports a role for “healthy” microbiota in protecting individuals from colonization and infection by enteric pathogens, a phenomenon commonly referred to as “colonization resistance” (Lawley & Walker, 2013). This is best illustrated with the observation that oral antibiotic usage, which disrupts the intestinal microbiota, often increases the risk of Clostridium difficile infection, a common hospital-acquired nosocomial infection with severe sequelae. There are likely multiple mechanisms that contribute to colonization resistance. One major resistance mechanism derives from the gut microbiota closely interacting with the host mucosal surface, the epithelium, and the immune system to modulate host responses against colonization of pathogens (Duerkop, Vaishnava, & Hooper, 2009; Hooper, Midtvedt, & Gordon, 2002; Kau, Ahern, Griffin, Goodman, & Gordon, 2011; Littman & Pamer, 2011).
The microbiota itself poses a significant barrier to foreign bacterial pathogens through niche and nutrient competition and bacteriocin production—two examples of resistance mechanisms. The colonizing microorganisms in the gut are well adapted to host physical and nutritional constraints and therefore can outcompete invading pathogens. This mechanism has been clearly demonstrated for infection by Escherichia coli or C. difficile, where colonization of nonpathogenic strains can successfully prevent subsequent challenge of pathogenic strains (Chang et al., 2004; Leatham et al., 2009; Merrigan, Sambol, Johnson, & Gerding, 2003; Sambol, Merrigan, Tang, Johnson, & Gerding, 2002). In addition, many bacteria also produce peptides with anti-microbial functions or “bacteriocins,” that can target and kill invading pathogens. Numerous reports have confirmed the antimicrobial activity of purified bacteriocins in vitro, and evidence for successful prevention of pathogen colonization in vivo is increasing (Corr et al., 2007; Cursino et al., 2006; Millette et al., 2008; Schamberger & Diez-Gonzalez, 2004). These studies support the feasibility of using live bacteriocin-producing organisms as probiotics for consumption to protect individuals against infection by enteric pathogens and to promote overall intestinal health (Corr, Hill, & Gahan, 2009; Dobson, Cotter, Ross, & Hill, 2012; Ross, Mills, Hill, Fitzgerald, & Stanton, 2010).
1.2. Intestinal SCFA production
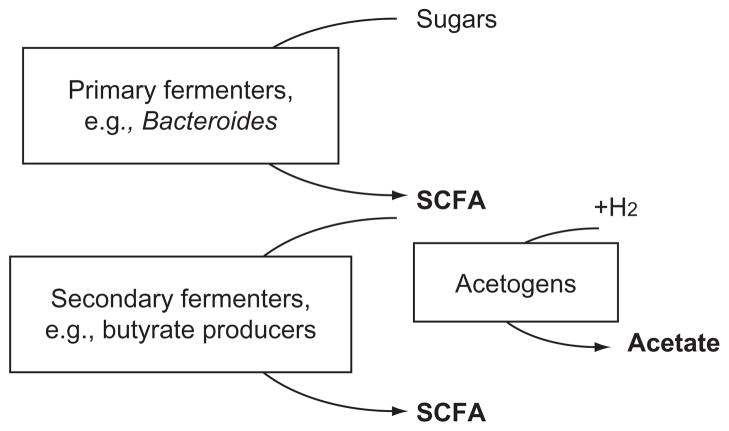
The metabolic activity of the human gut microbiota defines the chemical environment in the intestinal lumen (Hooper et al., 2002). Nondigestible carbohydrates are broken down and oxidized incompletely in the anaerobic lumen by the intestinal microbiota releasing short-chain fatty acids (SCFA) as fermentation byproducts. SCFA can be formed through multiple pathways by the concerted effort of different members of the microbiota as depicted in the simplified schematic shown in Fig. 3.1. In general, Bacteroidetes represent the primary fermenters that will transform simple sugars derived from breakdown of complex carbohydrates to organic acids including SCFA and hydrogen. Secondary fermenters such as Clostridium species and butyrate-producing bacteria further utilize the organic acids to generate additional SCFA. Moreover, acetogens (Rey et al., 2010) can deplete the hydrogen as an energy source and contribute to the pool of acetate, the dominant component of intestinal SCFA.
Figure 3.1.
An overview of short-chain fatty acid (SCFA) production in the intestines. Primary fermenters such as Bacteroides species oxidize mono- and oligosaccharides and release SCFA that can be subsequently utilized by secondary fermenters to generate additional SCFA. Acetogens also utilize hydrogen released from fermentation reactions along with carbon dioxide to form acetate, thereby contributing to intestinal SCFA content.
The other two major constituents of intestinal SCFA are butyrate and propionate. After the formation of butyryl-CoA from condensation of acetyl-CoA, two different pathways have been proposed for the final step of butyrate production. In the first scenario exemplified by Clostridium acetobutylicum (Hartmanis & Gatenbeck, 1984), butyryl-CoA is converted to butyrate through the intermediate butyryl-phosphate by two separate enzymes, butyrate kinase and phosphotransbutyrylase. An alternative butyrate-producing pathway involves the butyryl-CoA:acetate-CoA transferase, which catalyzes the transfer of coenzyme A between acetate and butyrate (Duncan, Barcenilla, Stewart, Pryde, & Flint, 2002). An in vitro survey of 38 butyrate-producing intestinal isolates using degenerate PCR and enzymatic assays suggests the latter pathway as the major source of butyrate in the intestines (Louis et al., 2004). Finally, propionate can be formed through carbon fixation reactions from succinyl-CoA (Miller & Wolin, 1996) as demonstrated by in vitro analysis of a Bacteroides fragilis pure culture (Macy, Ljungdahl, & Gottschalk, 1978). Understanding the metabolic pathways for butyrate and propionate productions has enabled the development of molecular markers based on genes coding for metabolic enzymes to study the functional aspects of microbial ecology in the intestines (Hosseini, Grootaert, Verstraete, & Van de Wiele, 2011).
The chemical structures of available complex carbohydrates play a critical role in determining the kinds of fermentation products produced by the microbiota. Therefore, the level and composition of intestinal SCFA are heavily influenced by diet and the endogenous microbial community structure (Campbell, Fahey, & Wolf, 1997; Cummings, 1981; Cummings & Macfarlane, 1991; Rechkemmer, Rönnau, & Engelhardt, 1988; Roy, Kien, Bouthillier, & Levy, 2006; Topping & Clifton, 2001). There isa distinct spatial organization of the intestinal microbiota (Nava, Friedrichsen, & Stappenbeck, 2011; Pedron et al., 2012) that influences the distribution of SCFA. In general, different regions of the small and large intestines exhibit distinct levels of SCFA, which result in environments with different pH values (Cummings & Macfarlane, 1991; Macfarlane, Gibson, & Cummings, 1992; Walter & Ley, 2011). The small intestine also contains a lower microbial burden with a different composition than the large intestine (Walter & Ley, 2011). This heterogeneous distribution of microorganisms in the intestines leads to spatial variation in the relative proportions of acetate, butyrate, and propionate (Cummings, 1981). Collectively, knowledge derived from many studies suggests that an invading enteric pathogen encounters changing levels and composition of SCFA and commensal microbes as it traverses the intestines. Understanding how enteric pathogens respond to the changing intestinal environment is important in providing a framework for identifying new ways to prevent and treat enteric infections. This review will focus on how common enteric pathogens respond to intestinal SCFA by regulating virulence functions.
2. BIOLOGICAL ACTIVITIES OF SCFA
2.1. Biological activities of SCFA in the host organism
The chemical environment established through metabolic activity of the microbiota plays critical nutritional roles in the host organism. The SCFA produced by the microbiota, especially butyrate, have profound effects on energy homeostasis. Butyrate is taken up by colonocytes and used as their primary energy source (Wong, de Souza, Kendall, Emam, & Jenkins, 2006). Colonocytes from germ-free (GF) mice that are deficient in intestinal SCFA exhibit decreased intermediary metabolism that results in activation of the nutrient and energy sensor, AMPK, which eventually leads to autophagy (Donohoe et al., 2011). Butyrate, when provided exogenously, rescues the GF colonocytes from AMPK activation-directed autophagy, indicating that microbiota-derived butyrate is essential for normal host colonocyte metabolism. The authors used chemical inhibitors to further show that the requirement for butyrate to prevent autophagy was based on its contribution to energy generation, not to the known property of butyrate as a histone deacetylase (HDAC) inhibitor (Donohoe et al., 2011). In fact, the ability of normal versus transformed colonocytes to use butyrate as an energy source could be shown to alter cellular responses to butyrate. In contrast to normal cells where butyrate is the primary energy source, transformed cells rely on glycolysis as the primary source of energy generation, leading to the accumulation of butyrate which functions in these cancerous cells predominantly as a HDAC inhibitor (Donohoe et al., 2012).
In addition to serving as metabolic substrates, SCFA also modulate host immune functions. Butyrate or propionate is taken up into immune cells through the SLC5A8 transporter, where the HDAC activity of these SCFA exerts immunomodulatory effects by blockade of dendritic cell development and by inducing Fas upregulation followed by Fas-mediated T cell apoptosis (Singh et al., 2010; Zimmerman et al., 2012). Butyrate also decreases IL-12 expression, but increases IL-23 production, by activated dendritic cells, emphasizing the importance of this microbiota-derived SCFA in gut immune homeostasis (Berndt et al., 2012).
SCFA are recognized by a family of G-protein-coupled receptors (FFAR) and can trigger signaling at both the gut epithelium and systemic sites. Several reports suggest that binding of acetate and propionate to FFAR2 (GPR43) or propionate and butyrate to FFAR3 (GPR41) regulates gut hormone production, obesity, and inflammation (Layden, Angueira, Brodsky, Durai, & Lowe, 2013; Xiong et al., 2004). Mice lacking FFAR2 or FFAR3 exhibited decreased glucagon-like peptide-1 levels in vivo and impaired glucose tolerance (Tolhurst et al., 2012), implicating a role for intestinal SCFA in diabetes. Furthermore, SCFA treatment appears to stimulate adipogenesis in mice by FFAR-dependent (Hong et al., 2005) and FFAR-independent mechanisms (Lin et al., 2012). FFAR2 binding of SCFA also suppressed intestinal inflammation; FFAR2-deficient mice did not resolve disease in mouse models of colitis and arthritis (Maslowski et al., 2009). Thus, accumulating evidence provides a compelling picture that implicates microbiota-produced SCFA as key regulators of energy homeostasis, gut hormone production, and inflammation. Further elucidation of the diverse mechanisms by which SCFA and their host receptors may protect against long-term development of chronic diseases, such as colitis and diabetes, will provide an evidence-based platform to examine the effects of probiotics or prebiotics on human health.
The endogenous microbiota aid the gut epithelium in defense against attachment and invasion of enteric pathogens by stimulating production of antimicrobial peptides (AMPs) (Gallo & Hooper, 2012). One mechanism by which the microbiota may contribute to AMP production in the healthy intestine is through SCFA-dependent induction of LL-37 production demonstrated in a human colonic epithelial cell line (Termén et al.,). Sim- 2008 ilarly in chickens, SCFA enhanced the expression of host defense peptide gene expression, and including exogenous SCFA in feed resulted in lower Salmonella colonization in the cecum (Sunkara et al., 2011; Sunkara, Jiang, & Zhang, 2012). Thus, augmentation of animal feed with SCFA or with pre-biotics that promote SCFA production by the indigenous microbiota may be a viable alternative to antibiotic usage for reducing livestock colonization by potential human pathogens.
2.2. Biological activities of SCFA in bacteria
SCFA not only affect host functions but also serve as a carbon source for the endogenous microbiota (Fischbach & Sonnenburg, 2011) and at high concentration can exhibit toxic effects on bacteria. Numerous in vitro studies have demonstrated that the toxicity was attributable to the nonionized forms of these acids, which exist more prominently at low pH (Baskett & Hentges, 1973; Bergeim, 1940; Hentges, 1967; Weiner & Draskoczy, 1961). These early studies also established the pleiotropic effects of weak organic acids ranging from inhibiting oxidative metabolism (Weiner & Draskoczy, 1961) to eliciting chemotactic responses (Repaske & Adler, 1981). Currently, the general mechanism for SCFA-dependent toxicity involves the entry of nonionized acids into the bacterial cytoplasm (Fig. 3.2). The non-ionized acids are small and uncharged and therefore are thought to freely diffuse across the bacterial membrane. Once inside the bacterial cytoplasm, which generally has a circumneutral pH, these nonionized acids dissociate, leading to an accumulation of protons and SCFA anions (Lambert & Stratford, 1999; Repaske & Adler, 1981; Russell, 1992; Salmond, Kroll, & Booth, 1984). On one hand, the influx of protons acidifies the intracellular compartment and dissipates proton motive force (Axe & Bailey, 1995) that can ultimately compromise metabolic reactions (Roe, O’Byrne, McLaggan, & Booth, 2002) and energy conservation. On the other hand, the accumulation of SCFA anions in the cytoplasm also significantly impacts cellular physiology, such as alterations in osmotic balance (Roe, McLaggan, Davidson, O’Byrne, & Booth, 1998).
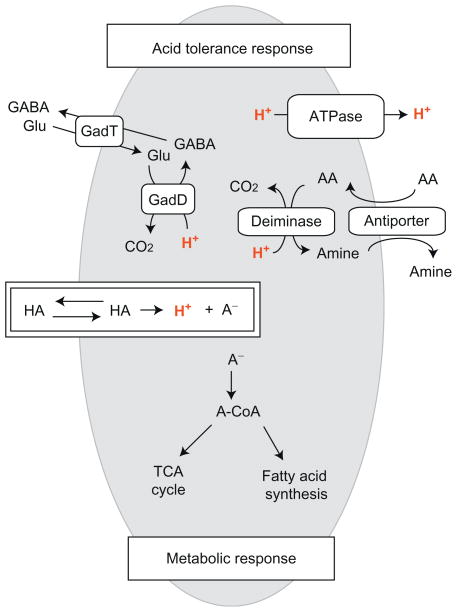
Figure 3.2.
A representative schematic of bacterial responses to weak organic acids. Nonionized organic acids, symbolized as “HA,” can diffuse across bacterial membrane and dissociate into protons (H+) and anions (A−) in the circumneutral cytoplasm. This influx of proton will induce the acid tolerance response (ATR) that functions to maintain intracellular pH homeostasis by removing cytoplasmic protons. ATR, in general, includes a glutamate decarboxylase system (GadD, glutamate decarboxylase; GadT, glutamate-GABA antiporter), an F0F1-ATPase, and a deamination system (e.g., AA, arginine; amine, ornithine). The organic anions accumulated in the cytoplasm can feed into metabolic pathways such as TCA cycle or membrane fatty acid synthesis after addition of coenzyme A.
SCFA diffusion process and the consequent toxicity are strongly influenced by external pH, which predicts the relative amount of non-ionized SCFA. Thus, SCFA toxicity is often more prevalent under acidic conditions where the pKa value of SCFA (4.76 for acetate, 4.82 for butyrate, and 4.87 for propionate) is closer to or higher than the external pH. Furthermore, SCFA-mediated toxicity is also influenced by internal pH, which affects the transmembrane pH gradient that drives the influx of acid. Although bacterial cytoplasm is relatively resistant to pH perturbation because of the intrinsic impermeability of the membrane to protons (Raven & Beardall, 1981) and the buffering capacity established by ionizable moieties such as amino acids side chains (Booth, 1985; Slonczewski, Fujisawa, Dopson, & Krulwich, 2009), there are still various adaptive mechanisms, such as proton transporters, that are involved in active maintenance of intracellular pH (Booth, 1985). When external pH is low, organisms that are more stringent with maintaining pH at around neutral levels will face a higher transmembrane pH gradient that will enhance acid influx and thereby will be more susceptible to SCFA toxicity than those that can tolerate lower intracellular pH values (Diez-Gonzalez & Russell, 1997; Russell, 1991).
SCFA-induced toxicity often results in growth inhibition attributable to pleiotropic defects in cellular processes (Cherrington, Hinton, Mead, & Chopra, 1991) that are likely to vary by pathway, organism, and environmental condition. For example, DNA synthesis is more sensitive to propionate than synthesis of proteins, RNA, lipids, or cell walls in E. coli (Cherrington, Hinton, & Chopra, 1990). Similarly, amino acid uptake was inhibited in Bacillus subtilis after exposure to acetate and propionate (Freese, Sheu, & Galliers, 1973). However, more recent proteomic analysis showed an increased level of some amino acid transporters in E. coli after acetate treatment (Kirkpatrick et al., 2001), suggesting that metabolic responses to SCFA might vary by organism. The same study also demonstrated an alternative proteomic response to acetate in a defined minimal medium compared to rich medium, indicating the importance of environmental context in bacterial responses to SCFA.
3. VIRULENCE REGULATION OF ENTERIC PATHOGENS BY SCFA
3.1. Salmonella spp
According to Centers for Disease Control and Prevention, Salmonella infection is one of the most common foodborne illnesses with more than 1 million cases estimated per year in the United States. Among thousands of known serotypes that can cause human disease, Salmonella enterica serotypes Enteriditis, Typhimurium, and Newport are responsible for more than 60% of all laboratory confirmed incidences in 2011. A critical component of Salmonella pathogenesis after adherence to the host cells involves the delivery of bacterial effector proteins into host cytosol through two Type III Secretion Systems (T3SS) (Galan, 2001). During the gastrointestinal phase of the infection, Salmonella must navigate within the luminal environment rich in SCFA before gaining access to the host epithelium. Therefore, understanding how Salmonella responds to SCFA will reveal key aspects of pathogenesis that can ultimately provide useful insight into designing prevention and treatment strategies.
Molecular responses to SCFA have been extensively studied in Salmonella species. In general, Salmonella can assimilate SCFA, such as propionate (Horswill & Escalante-Semerena, 1999), as a carbon source when provided at low concentrations. At higher levels and low pH, SCFA strongly inhibit the growth of Salmonella (Goepfert & Hicks, 1969; McHan & Shotts, 1993; Van Immerseel et al., 2003), an activity that has been the basis for using SCFA in food preservatives or poultry feed to minimize Salmonella contamination (Wales, Allen, & Davies, 2010). As a foodborne pathogen that encounters several host environments with low pH and high SCFA levels in the gastrointestinal tract, Salmonella adopts a variety of active mechanisms to survive the acid stress by eliminating proton accumulation in the cytosol (Álvarez-Ordóñez et al., 2011).
In addition to serving as metabolic precursors and agents of acid stress, SCFA also regulate Salmonella virulence gene expression in vitro in a pH-and species-specific manner (Boyen et al., 2008; Cardenal-Muñoz & Ramos-Morales, 2011; Durant, Corrier, & Ricke, 2000; Gantois et al., 2006; Gong et al., 2009; Huang, Suyemoto, Garner, Cicconi, & Altier, 2008; Zabala Díaz & Ricke, 2004). In Salmonella dublin, all SCFA with two to six carbons induce genes spvABCD, which are important for virulence (El-Gedaily, Paesold, & Krause, 1997). In contrast, single supplementation of butyrate (four carbons) or propionate (three carbons), but not acetate (two carbons), reduces expression of invasion genes in WT S. enterica Typhimurium. Mixtures representing colonic SCFA concentrations, which contain higher total SCFA as well as relative proportions of butyrate and propionate, exhibit a greater inhibitory effect than ileal SCFA concentrations, suggesting spatial orientation for S. enterica Typhimurium colonization in the host intestines (Lawhon, Maurer, Suyemoto, & Altier, 2002). Detailed analyses to study the molecular mechanisms of inhibition have highlighted the importance of SCFA metabolism, for example, formation of acetyl-phosphate and propionyl-CoA from acetate and propionate, respectively, in regulation of virulence gene expression (Hung et al., 2013; Lawhon et al., 2002).
The effect of SCFA on virulence gene expression in vitro has been tested during Salmonella interactions with the host using both tissue culture and animal infection models. As observed in gene expression analyses in vitro (Durant et al., 2000), the effect of SCFA on S. enterica Typhimurium association and invasion into HEp-2 cells depends heavily on the medium pH. All three SCFA tested, acetate, butyrate, and propionate reduced cell association more efficiently at pH 6 than at pH 7 (Durant et al., 1999). Pre-treatment of S. enterica Enteriditis with butyrate reduces invasion of the avian intestinal cell line DIV-1 (Van Immerseel et al., 2003) and primary chicken cecal epithelial cells (Van Immerseel et al., 2004). While these studies collectively suggest a protective role of SCFA during Salmonella infections, they overlook the host response to SCFA that may affect the infection outcome. In animal models of infection where SCFA exposure is shared by host epithelium and invading Salmonella, supplementing SCFA in feed reduced the Salmonella number in ceca of chicks (McHan & Shotts, 1992) and pigs (Boyen et al., 2008), agreeing with the protective effects of SCFA against Salmonella colonization demonstrated in tissue culture models of infection. Furthermore, antibiotic-treated mice that have an altered microbiota composition and decreased levels of SCFA are more susceptible to Salmonella infection (Garner et al., 2009). Taken together, these studies suggest that individuals with sufficient levels of intestinal SCFA, specifically butyrate and propionate, are less likely to be susceptible to Salmonella infections.
3.2. Escherichia coli
Enterohemorrhagic E. coli (EHEC) is one of the leading foodborne pathogens that causes attaching and effacing lesions of the intestinal epithelium through delivery of effector proteins into host cells by the T3SS (Wong et al., 2011). Key virulence determinants including the T3SS for EHEC are encoded on a chromosomal locus for enterocyte effacement (LEE). Based on protein and transcriptomic analyses, expression of LEE genes in EHEC strain Sakai is strongly induced by sodium butyrate but not by sodium acetate or sodium propionate (Nakanishi et al., 2009). This particular response to butyrate relies on the transcriptional regulator Lrp or leucine-responsive regulatory protein (Nakanishi et al., 2009), which belongs to a group of related proteins that are widely distributed among bacteria and Archaea and are often involved in metabolic responses to nutrient availability (Brinkman, 2003; Calvo & Matthews, 1994; Newman & Lin, 1995; Yokoyama et al., 2006). Based on analyses of site-directed Lrp mutants, butyrate may interact with the Lrp ligand-binding domain and thereby affect Lrp activity (Nakanishi et al., 2009).
In contrast to butyrate promoting bacterial adherence, all three major SCFA induce production of flagella in EHEC through both Lrp-dependent and -independent mechanisms (Tobe, Nakanishi, & Sugimoto, 2011). As adherence and flagellar motility exert opposing effects on bacterial cells, the authors postulate an in vivo scenario in which EHEC expresses flagella inside the intestinal lumen and only initiates adherence as butyrate levels increase in the large intestine leading to colonization and delivery of T3SS effector proteins (Tobe et al., 2011). This hypothesis is consistent with the observation that EHEC has the ability to inhibit butyrate uptake in the human colonic Caco-2 cell line (Borthakur et al., 2006), thereby increasing local butyrate level near the epithelium for optimal induction of the T3SS. Moreover, a recent study (Herold, Paton, Srimanote, & Paton, 2009) demonstrated in three different EHEC strains that colonic but not ileal levels of SCFA induce expression of iha, which encodes an outer membrane protein involved in adherence, supporting the ability of EHEC to navigate within different intestinal environments by responding to SCFA levels. However, these studies do not agree with the observation that bovine colonic tissues incubated with SCFA support a reduced load of EHEC (Cobbold & Desmarchelier, 2004). Therefore, additional in vivo studies will be necessary to better elucidate the complex functions of SCFA in EHEC pathogenesis.
3.3. Listeria monocytogenes
Listeria monocytogenes is a prevalent contaminant in food products that are slightly acidic in nature such as dairy products or food with organic acid preservatives because of its ability to survive and grow under acid conditions. After ingestion, the bacterium must survive acid stress in the stomach and the SCFA challenge in the lower intestines for colonization and pathogenesis to occur. Therefore, understanding L. monocytogenes acid response is of particular importance from the perspective of food safety as well as bacterial pathogenesis.
Prior acid exposure enhances L. monocytogenes survival of subsequent acid stress (Davis, Coote, & O’Byrne, 1996; Kroll & Patchett, 1992; O’Driscoll, Gahan, & Hill, 1996). This adaptive behavior, termed acid tolerance response (ATR) (Cotter & Hill, 2003; Ryan, Hill, & Gahan, 2008), encompasses three major cellular adaptations in response to the decreased intracellular pH (Shabala et al., 2002) as shown in Fig. 3.2. The glutamate decarboxylase system (Cotter, Gahan, & Hill, 2001; Cotter, O’Reilly, & Hill, 2001; Cotter, Ryan, Gahan, & Hill, 2005; Wiedmann, Arvik, Hurley, & Boor, 1998), the F1F0 ATPase (Bowman, Hages, Nilsson, Kocharunchitt, & Ross, 2012; Bowman, Lee Chang, Pinfold, & Ross, 2010; Cotter, Gahan, & Hill, 2000; Datta & Benjamin, 1997; Phan-Thanh & Mahouin, 1999), and the arginine and agmatine deiminase system (Ryan, Begley, Gahan, & Hill, 2009) all function to reduce the intracellular level of protons. In addition to survival in acid stress, ATR plays a critical role in promoting L. monocytogenes virulence (Conte et al., 2000; Conte et al., 2002; Marron, Emerson, Gahan, & Hill, 1997).
ATR studies using in vitro survival assays (Ferreira, 2003) or proteomics approaches (O’Driscoll et al., 1997) all reported that organic acids eliciting a distinct response from inorganic acids. This can be explained by the intracellular accumulation of organic acid anions, which are carbon metabolites, interfering with metabolic reactions. For example, exposure to butyrate significantly alters membrane fatty acid composition (Julotok, Singh, Gatto, & Wilkinson, 2010; Sun, Wilkinson, Standiford, Akinbi, & O’Riordan, 2012) because of butyrate assimilation into straight chain fatty acids, which normally represent a minor component of membrane fatty acids. This response is notably different from changes in membrane fatty acid composition caused by exposure to HCl, acetic acid, or lactic acid (Mastronicolis et al., 2010). Moreover, high levels of butyrate strongly inhibit virulence factor production in L. monocytogenes at the transcriptional level (Sun et al., 2012), suggesting a protective effect of intestinal SCFA against L. monocytogenes infection.
Work published as early as 1979 revealed that GF animals show increased susceptibility to Lm colonization and that the intestinal microbiota, introduced either individually or as a community, is capable of decreasing Lm colonization of GF mice (Archambaud et al., 2012; Bambirra et al., 2007; dos Santos et al., 2011; Nakamura et al., 2012; Vieira et al., 2008; Zachar & Savage, 1979) and rats (Czuprynski & Balish, 1981). Although these studies do not provide clear mechanisms for colonization resistance, they nevertheless demonstrate a functional requirement for the gut microbiota in protection against L. monocytogenes infection. Thus, mechanistic understanding of how intestinal SCFA affect L. monocytogenes virulence gene regulation and pathogenesis in vivo remains to be determined.
3.4. Campylobacter jejuni
Campylobacter jejuni is the most common bacterial foodborne pathogen causing diarrheal disease in humans with more than 2 million cases per year according to reports available at the Centers for Disease Control and Prevention. As contaminated chickens are considered the main source of exposure, numerous studies are conducted to establish proper housing regimens to minimize the spread of C. jejuni (Hermans et al., 2011), including those specifically testing the effect of SCFA in animal feed on C. jejuni colonization (Heres, Engel, Urlings, Wagenaar, & van Knapen, 2004; Heres, Engel, Van Knapen, Wagenaar, & Urlings, 2003; Van Deun, Haesebrouck, Van Immerseel, Ducatelle, & Pasmans, 2008). These studies have not reported a consistent protective effect by SCFA. Studies that evaluated C. jejuni virulence responses to SCFA using a tissue culture infection model showed that pretreating C. jejuni with SCFA did not compromise its invasion into human colonic epithelium-derived Caco-2 cell, but pretreating Caco-2 cells significantly reduced subsequent C. jejuni invasion (Van Deun, Pasmans, Van Immerseel, Ducatelle, & Haesebrouck, 2008). Therefore, it is possible that SCFA are not involved in bacterial virulence gene regulation in C. jejuni but provide a protective value to the host against C. jejuni infection.
3.5. Shigella spp
Shigella represents another model enteric pathogen that is widely studied to probe host–pathogen interactions. Islam et al. (2001) has demonstrated that Shigella infection causes a downregulation in the production of cathelicidin, an AMP that is part of the innate defense repertoire, in both human rectal mucosal biopsies and in a tissue culture model of infection (Van Deun, Pasmans, Van Immerseel, Ducatelle, & Haesebrouck, 2008). This bacterial modulation of host immune defense is thought to be important for colonization and pathogenesis but can be overcome by oral administration of butyrate or bolus infusion of SCFA into the colon, both of which significantly improve clinical manifestations in an adult rabbit infection model (Rabbani et al., 1999; Raqib et al., 2006). The potential health benefit of SCFA proposed by these studies is mainly based on upregulation of rabbit cathelicidin, which efficiently eliminates Shigella. This was subsequently tested in a human clinical trial where patients with Shigella infections receiving butyrate-containing enemas showed improved pathology and higher expression of cathelicidin compared to patients receiving the placebo control (Raqib et al., 2012). Although there may be multiple effects of SCFA on Shigella virulence regulation that remain to be defined, they likely include indirect effects on Shigella pathogenesis by protective stimulation of host defense mechanisms.
4. APPLICATIONS OF SCFA
4.1. Food safety
The food industry has been taking advantage of the toxic effect of SCFA on microbes to enhance food safety. SCFA can be added to food products as preservatives that will inhibit bacterial growth (Carpenter & Broadbent, 2009; Ricke, 2003). Moreover, as contaminated poultry is believed to be the main source of human Salmonella infections (Callaway, Edrington, Anderson, Byrd, & Nisbet, 2008), many research efforts have investigated the effects of adding SCFA into poultry feed to control Salmonella colonization in poultry (Cox & Pavic, 2010; Defoirdt, Boon, Sorgeloos, Verstraete, & Bossier, 2009; Dibner & Buttin, 2002; Jones, 2011; Ricke, 2003; Van Immerseel et al., 2006; Wales et al., 2010). In this regard, addition of SCFA in animal feed in theory has the potential to prevent colonization and shedding of pathogenic organisms, thereby lowering the initial risk of contamination in the food production line. However, other hygienic controls are also important considering that SCFA additives in feed at best only reduce but do not eliminate Salmonella colonization (Van Immerseel et al., 2005).
4.2. Prebiotics
The concept of prebiotics was introduced by Gibson et al. and defined as a food ingredient that can modulate the gut microbiota to confer health benefits (Gibson, Probert, Loo, Rastall, & Roberfroid, 2004; Gibson & Roberfroid, 1995). Inulin, fructo-oligosaccharides, and galato-oligosaccharides, which are complex carbohydrates nondigestible by humans, represent the best-studied types of prebiotics. As recommended by the World Gastroenterology Organisation, dietary supplementation with these prebiotics can confer significant health benefits and often leads to enrichment in selective members of the gut microbiota, mainly bifidobacteria and Lactobacillus species and increases in the level of SCFA (Macfarlane, Steed, & Macfarlane, 2008). The health benefits of prebiotics shown in these studies do not dismiss the concern that individual variation in gut microbiota composition (Schloissnig et al., 2013) may make it difficult to predict the efficacy of prebiotics that target specific community members of the microbiota. This is particularly relevant in diseased individuals that may lack the target microbiota members and therefore will not benefit from prebiotic supplementation. One solution to this challenge is the concept of “synbiotics” where prebiotics are provided simultaneously with live commensal bacteria or “probiotics,” to ensure the presence of the desired species. Further research in this field may reveal novel and beneficial strategies to prevent disease and promote human health.
5. PERSPECTIVES
SCFA exert protective effects against enteric pathogen colonization and infection by multiple mechanisms and can act to regulate virulence in different pathogens as diagrammed in Fig. 3.3. The chemical nature of SCFA allows easy penetration into bacterial cells and subsequent incorporation into common metabolic pathways. Therefore, the effects of SCFA on bacterial virulence may vary depending on the metabolic processes involved in different pathogens. For example, it is possible that C. jejuni will respond to SCFA differently than other enteric pathogens because of its inability to utilize carbohydrates (Dasti, Tareen, Lugert, Zautner, & Groß, 2010) and may be better adapted to utilize SCFA as a source of carbon and energy in the intestines. While metabolism of intracellular bacteria has received increasing attention and has an established role in intracellular pathogenesis (Eisenreich, Dandekar, Heesemann, & Goebel, 2010; Muñoz-Elías & McKinney, 2006), defining metabolism of extracellular pathogens while inside the host (Alteri & Mobley, 2012) is equally crucial. Defining the relationship between SCFA metabolism and SCFA-dependent virulence responses will enhance our understanding in bacterial virulence processes in the context of the host environment and its resident microbiota.
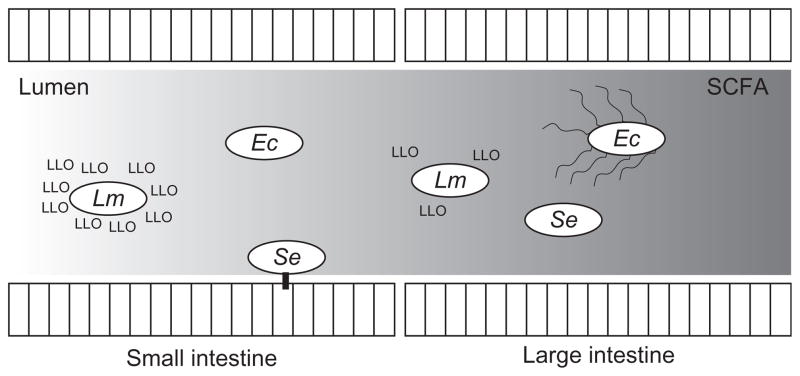
Figure 3.3.
A model depicting virulence functions of representative enteric pathogen in response to an intestinal gradient of short-chain fatty acids (SCFA). Ec, Enterohaemorrhagic Escherichia coli, upregulates flagella synthesis in response to butyrate. Lm, Listeria mono-cytogenes, reduces production of the pore-forming toxin, listeriolysin O, in response to butyrate. Se, Salmonella enterica, decreases production of Type III Secretion System in response to colonic mixtures of SCFA.
The recognition of SCFA as a signal for virulence regulation in enteric pathogens and as a potential health determinant conferring protection against enteric infections argues for a closer look at the importance of chemical homeostasis in the intestinal environment. As most intestinal levels of SCFA are reported based on bulk analysis, their values likely do not reflect the microenvironment experienced by enteric pathogens. Moreover, there is likely a cross-sectional SCFA gradient that cannot be revealed by bulk analysis. The gradient can be established because SCFA are produced in the lumen and absorbed by the epithelium. The aerobic environment near the epithelium also provides a thermodynamically more favorable condition than the anaerobic lumen to promote complete oxidation of the same carbon source, thereby potentially reducing the production of fermentation products. The chemical environment near the epithelium is further complicated by the fact that absorption rates for individual SCFA are different and might lead to distinct local pools of SCFA. Consequently, it will be important to develop better in vivo tools to measure local levels of SCFA and to determine if the SCFA concentration near the host epithelium still maintains modulatory activity on the virulence regulation of enteric pathogens.
6. CONCLUSION
The multifaceted interaction between the gut microbiota and its host exerts profound influence in many aspects of host development and physiology. The close association of the gut microbiota with human health and disease is now widely accepted, but the mechanistic details involved in how the microbiota contributes to human health require much more in-depth analysis. Nevertheless, these early studies of the chemical messages that mediate interactions between intestinal bacteria and their host have led to a more comprehensive picture of human biology. In this review, we have focused on the role of a particular class of chemical messages, microbiota-derived SCFA, during interactions between the host and enteric pathogens. Based on the literature summarized in this review, SCFA provide an important resistance mechanism against pathogen by exerting toxic acid stress. However, some enteric pathogens have adapted to the intestinal gradient of SCFA and have evolved mechanisms to regulate virulence gene expression that allow successful colonization of the host. In summary, SCFA provide a key link between the microbiota, the host, and invading enteric pathogens. It is likely that the studies reviewed here are just a small representation of the many chemical interactions of the microbiota that drive health and disease. Future studies that further characterize the role of SCFA in the complex interactions taking place in the intestine will enhance our ability to control and prevent food contamination and to improve human digestive health.
Acknowledgments
We acknowledge the excellent research that has been done in this field and apologize to colleagues whose work could not be cited due to space limitations. This work was made possible by support from the USDA National Institute of Food and Agriculture Postdoctoral Fellowship 2011-67012-30682 (to Y. S.) and from the National Institute for Allergy and Infectious Disease (NIH AI101777 to M. X. D. O.).
References
- Alteri CJ, Mobley HLT. Escherichia coli physiology and metabolism dictates adaptation to diverse host microenvironments. Current Opinion in Microbiology. 2012;15:3–9. doi: 10.1016/j.mib.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Ordóñez A, Begley M, Prieto M, Messens W, López M, Bernardo A, et al. Salmonella spp. survival strategies within the host gastrointestinal tract. Microbiology. 2011;157:3268–3281. doi: 10.1099/mic.0.050351-0. [DOI] [PubMed] [Google Scholar]
- Archambaud C, Nahori MA, Soubigou G, Bécavin C, Laval L, Lechat P, et al. Impact of lactobacilli on orally acquired listeriosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16684–16689. doi: 10.1073/pnas.1212809109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axe DD, Bailey JE. Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnology and Bioengineering. 1995;47:8–19. doi: 10.1002/bit.260470103. [DOI] [PubMed] [Google Scholar]
- Bambirra FHS, Lima KGC, Franco BDGM, Cara DC, Nardi RMD, Barbosa FHF, et al. Protective effect of Lactobacillus sakei 2a against experimental challenge with Listeria monocytogenes in gnotobiotic mice. Letters in Applied Microbiology. 2007;45:663–667. doi: 10.1111/j.1472-765X.2007.02250.x. [DOI] [PubMed] [Google Scholar]
- Baskett RC, Hentges DJ. Shigella flexneri inhibition by acetic acid. Infection and Immunity. 1973;8:91–97. doi: 10.1128/iai.8.1.91-97.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeim O. Toxicity of intestinal volatile fatty acids for yeast and Esch. coli. Journal of Infectious Diseases. 1940;66:222–234. [Google Scholar]
- Berndt BE, Zhang M, Owyang SY, Cole TS, Wang TW, Luther J, et al. Butyrate increases IL-23 production by stimulated dendritic cells. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;303:G1384–G1392. doi: 10.1152/ajpgi.00540.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiological Reviews. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2006;290:G30–G35. doi: 10.1152/ajpgi.00302.2005. [DOI] [PubMed] [Google Scholar]
- Bowman JP, Hages E, Nilsson RE, Kocharunchitt C, Ross T. Investigation of the Listeria monocytogenes Scott A acid tolerance response and associated physiological and phenotypic features via whole proteome analysis. Journal of Proteome Research. 2012;11:2409–2426. doi: 10.1021/pr201137c. [DOI] [PubMed] [Google Scholar]
- Bowman JP, Lee Chang KJ, Pinfold T, Ross T. Transcriptomic and phenotypic responses of Listeria monocytogenes strains possessing different growth efficiencies under acidic conditions. Applied and Environmental Microbiology. 2010;76:4836–4850. doi: 10.1128/AEM.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyen F, Haesebrouck F, Vanparys A, Volf J, Mahu M, Van Immerseel F, et al. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Veterinary Microbiology. 2008;132:319–327. doi: 10.1016/j.vetmic.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Brinkman AB, Ettema TJ, de Vos WM, van der Oost J. The Lrp family of transcriptional regulators. Molecular Microbiology. 2003;48:287–294. doi: 10.1046/j.1365-2958.2003.03442.x. [DOI] [PubMed] [Google Scholar]
- Callaway TR, Edrington TS, Anderson RC, Byrd JA, Nisbet DJ. Gastrointestinal microbial ecology and the safety of our food supply as related to Salmonella. Journal of Animal Science. 2008;86:E163–E172. doi: 10.2527/jas.2007-0457. [DOI] [PubMed] [Google Scholar]
- Calvo JM, Matthews RG. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiological Reviews. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JM, Fahey GC, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. Journal of Nutrition. 1997;127:130–136. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- Cardenal-Muñoz E, Ramos-Morales F. Analysis of the expression, secretion and translocation of the Salmonella enterica Type III Secretion System Effector SteA. PLoS One. 2011;6:e26930. doi: 10.1371/journal.pone.0026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CE, Broadbent JR. External concentration of organic acid anions and pH: Key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods. Journal of Food Science. 2009;74:R12–R15. doi: 10.1111/j.1750-3841.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- Chang DE, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proceedings of the National Academy of Sciences. 2004;101:7427–7432. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington CA, Hinton M, Chopra I. Effect of short-chain organic acids on macromolecular synthesis in Escherichia coli. Journal of Applied Bacteriology. 1990;68:69–74. doi: 10.1111/j.1365-2672.1990.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Cherrington CA, Hinton M, Mead G, Chopra I. Organic acids: Chemistry, antibacterial activity and practical applications. Advances in Microbial Physiology. 1991;32:87–108. doi: 10.1016/s0065-2911(08)60006-5. [DOI] [PubMed] [Google Scholar]
- Cobbold RN, Desmarchelier PM. In vitro studies on the colonization of bovine colonic mucosa by Shiga-toxigenic Escherichia coli (STEC) Epidemiology and Infection. 2004;132:87–94. doi: 10.1017/s0950268803001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte MP, Petrone G, Di Biase AM, Ammendolia MG, Superti F, Seganti L. Acid tolerance in Listeria monocytogenes influences invasiveness of enterocyte-like cells and macrophage-like cells. Microbial Pathogenesis. 2000;29:137–144. doi: 10.1006/mpat.2000.0379. [DOI] [PubMed] [Google Scholar]
- Conte MP, Petrone G, Di Biase AM, Longhi C, Penta M, Tinari A, et al. Effect of acid adaptation on the fate of Listeria monocytogenes in THP-1 human macrophages activated by gamma interferon. Infection and Immunity. 2002;70:4369–4378. doi: 10.1128/IAI.70.8.4369-4378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr SC, Hill C, Gahan CGM. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. In: Taylor SL, editor. Advances in food and nutrition research. Vol. 56. San Diego: Elsevier Academic Press Inc; 2009. pp. 1–15. [DOI] [PubMed] [Google Scholar]
- Corr SC, Li Y, Riedel CU, O’Toole PW, Hill Colin, Gahan CGM. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PD, Gahan CG, Hill C. Analysis of the role of the Listeria monocytogenes F0F1-ATPase operon in the acid tolerance response. International Journal of Food Microbiology. 2000;60:137–146. doi: 10.1016/s0168-1605(00)00305-6. [DOI] [PubMed] [Google Scholar]
- Cotter PD, Gahan CG, Hill C. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Molecular Microbiology. 2001;40:465–475. doi: 10.1046/j.1365-2958.2001.02398.x. [DOI] [PubMed] [Google Scholar]
- Cotter PD, Hill C. Surviving the acid test: Responses of Gram-positive bacteria to low pH. Microbiology and Molecular Biology Reviews. 2003;67:429–453. doi: 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PD, O’Reilly K, Hill C. Role of the glutamate decarboxylase acid resistance system in the survival of Listeria monocytogenes LO28 in low pH foods. Journal of Food Protection. 2001;64:1362–1368. doi: 10.4315/0362-028x-64.9.1362. [DOI] [PubMed] [Google Scholar]
- Cotter PD, Ryan S, Gahan CG, Hill C. Presence of GadD1 glutamate decarboxylase in selected Listeria monocytogenes strains is associated with an ability to grow at low pH. Applied and Environmental Microbiology. 2005;71:2832–2839. doi: 10.1128/AEM.71.6.2832-2839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JM, Pavic A. Advances in enteropathogen control in poultry production. Journal of Applied Microbiology. 2010;108:745–755. doi: 10.1111/j.1365-2672.2009.04456.x. [DOI] [PubMed] [Google Scholar]
- Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. Journal of Applied Bacteriology. 1991;70:443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Cursino L, Smajs D, Smarda J, Nardi RMD, Nicoli JR, Chartone-Souza E, et al. Exoproducts of the Escherichia coli strain H22 inhibiting some enteric pathogens both in vitro and in vivo. Journal of Applied Microbiology. 2006;100:821–829. doi: 10.1111/j.1365-2672.2006.02834.x. [DOI] [PubMed] [Google Scholar]
- Czuprynski CJ, Balish E. Pathogenesis of Listeria monocytogenes for gnotobiotic rats. Infection and Immunity. 1981;32:323–331. doi: 10.1128/iai.32.1.323-331.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasti JI, Tareen AM, Lugert R, Zautner AE, Groß U. Campylobacter jejuni: A brief overview on pathogenicity-associated factors and disease-mediating mechanisms. International Journal of Medical Microbiology. 2010;300:205–211. doi: 10.1016/j.ijmm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Datta AR, Benjamin MM. Factors controlling acid tolerance of Listeria monocytogenes: Effects of nisin and other ionophores. Applied and Environmental Microbiology. 1997;63:4123–4126. doi: 10.1128/aem.63.10.4123-4126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Coote PJ, O’Byrne CP. Acid tolerance in Listeria monocytogenes: The adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology. 1996;142:2975–2982. doi: 10.1099/13500872-142-10-2975. [DOI] [PubMed] [Google Scholar]
- Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Short-chain fatty acids and poly-β-hydroxyalkanoates: (New) Biocontrol agents for a sustainable animal production. Biotechnology Advances. 2009;27:680–685. doi: 10.1016/j.biotechadv.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Dibner JJ, Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. Journal of Applied Poultry Research. 2002;11:453–463. [Google Scholar]
- Diez-Gonzalez F, Russell JB. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiology. 1997;143:1175–1180. doi: 10.1099/00221287-143-4-1175. [DOI] [PubMed] [Google Scholar]
- Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: A probiotic trait? Applied and Environmental Microbiology. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Molecular Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabolism. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos LM, Santos MM, de Souza Silva HP, Arantes RME, Nicoli JR, Vieira LQ. Monoassociation with probiotic Lactobacillus delbrueckii UFV-H2b20 stimulates the immune system and protects germfree mice against Listeria monocytogenes infection. Medical Microbiology and Immunology. 2011;200:29–38. doi: 10.1007/s00430-010-0170-1. [DOI] [PubMed] [Google Scholar]
- Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ. Acetate utilization and butyryl coenzyme A (CoA): Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Applied and Environmental Microbiology. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant JA, Corrier DE, Ricke SC. Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella typhimurium. Journal of Food Protection. 2000;63:573–578. doi: 10.4315/0362-028x-63.5.573. [DOI] [PubMed] [Google Scholar]
- Durant JA, Lowry VK, Nisbet DJ, Stanker LH, Corrier DE, Ricke SC. Short-chain fatty acids affect cell-association and invasion of HEp-2 cells by Salmonella typhimurium. Journal of Environmental Science and Health. Part. B. 1999;34:1083–1099. doi: 10.1080/03601239909373246. [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Dandekar T, Heesemann J, Goebel W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nature Reviews. Microbiology. 2010;8:401–412. doi: 10.1038/nrmicro2351. [DOI] [PubMed] [Google Scholar]
- El-Gedaily A, Paesold G, Krause M. Expression profile and subcellular location of the plasmid-encoded virulence (Spv) proteins in wild-type Salmonella dublin. Infection and Immunity. 1997;65:3406–3411. doi: 10.1128/iai.65.8.3406-3411.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Sue D, O’Byrne CP, Boor KJ. Role of Listeria monocytogenes Sigma(B) in survival of lethal acidic conditions and in the acquired acid tolerance response. Applied and Environmental Microbiology. 2003;69:2692–2698. doi: 10.1128/AEM.69.5.2692-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Sonnenburg JL. Eating for two: How metabolism establishes interspecies interactions in the gut. Cell Host & Microbe. 2011;10:336–347. doi: 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E, Sheu CW, Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973;241:321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- Galan JE. Salmonella interactions with host cells: Type III secretion at work. Annual Review of Cell and Developmental Biology. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nature Reviews. Immunology. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Hautefort I, Thompson A, et al. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Applied and Environmental Microbiology. 2006;72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CD, Antonopoulos DA, Wagner B, Duhamel GE, Keresztes I, Ross DA, et al. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infection and Immunity. 2009;77:2691–2702. doi: 10.1128/IAI.01570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutrition Research Reviews. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Gibson G, Roberfroid M. Dietary modulation of the human colonic microbiota—Introducing the concept of prebiotics. Journal of Nutrition. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Goepfert JM, Hicks R. Effect of volatile fatty acids on Salmonella typhimurium. Journal of Bacteriology. 1969;97:956–958. doi: 10.1128/jb.97.2.956-958.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Su J, Bai Y, Miao L, Kim K, Yang Y, et al. Characterization of the expression of Salmonella Type III secretion system factor PrgI, SipA, SipB, SopE2, SpaO, and SptP in cultures and in mice. BMC Microbiology. 2009;9:73. doi: 10.1186/1471-2180-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmanis M, Gatenbeck S. Intermediary metabolism in Clostridium acetobutylicum—Levels of enzymes involved in the formation of acetate and butyrate. Applied and Environmental Microbiology. 1984;47:1277–1283. doi: 10.1128/aem.47.6.1277-1283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges D. Influence of pH on inhibitory activity of formic and acetic acids for Shigella. Journal of Bacteriology. 1967;93:2029–2030. doi: 10.1128/jb.93.6.2029-2030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heres L, Engel B, Urlings HAP, Wagenaar JA, van Knapen F. Effect of acidified feed on susceptibility of broiler chickens to intestinal infection by Campylobacter and Salmonella. Veterinary Microbiology. 2004;99:259–267. doi: 10.1016/j.vetmic.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Heres L, Engel B, Van Knapen F, Wagenaar JA, Urlings BA. Effect of fermented feed on the susceptibility for Campylobacter jejuni colonisation in broiler chickens with and without concurrent inoculation of Salmonella enteritidis. International Journal of Food Microbiology. 2003;87:75–86. doi: 10.1016/s0168-1605(03)00055-2. [DOI] [PubMed] [Google Scholar]
- Hermans D, Van Deun K, Messens W, Martel A, Van Immerseel F, Haesebrouck F, et al. Campylobacter control in poultry by current intervention measures ineffective: Urgent need for intensified fundamental research. Veterinary Microbiology. 2011;152:219–228. doi: 10.1016/j.vetmic.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Herold S, Paton JC, Srimanote P, Paton AW. Differential effects of short-chain fatty acids and iron on expression of iha in Shiga-toxigenic Escherichia coli. Microbiology. 2009;155:3554–3563. doi: 10.1099/mic.0.029454-0. [DOI] [PubMed] [Google Scholar]
- Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annual Review of Nutrition. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- Horswill AR, Escalante-Semerena JC. Salmonella typhimurium LT2 catabolizes propionate via the 2-methylcitric acid cycle. Journal of Bacteriology. 1999;181:5615–5623. doi: 10.1128/jb.181.18.5615-5623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutrition Reviews. 2011;69:245–258. doi: 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Suyemoto M, Garner CD, Cicconi KM, Altier C. Formate acts as a diffusible signal to induce Salmonella invasion. Journal of Bacteriology. 2008;190:4233–4241. doi: 10.1128/JB.00205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CC, Garner CD, Slauch JM, Dwyer ZW, Lawhon SD, Frye JG, et al. The intestinal fatty acid propionate inhibits Salmonella invasion through the post-translational control of HilD. Molecular Microbiology. 2013;87:1045–1060. doi: 10.1111/mmi.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, et al. Downregulation of bactericidal peptides in enteric infections: A novel immune escape mechanism with bacterial DNA as a potential regulator. Nature Medicine. 2001;7:180–185. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- Jones FT. A review of practical Salmonella control measures in animal feed. Journal of Applied Poultry Research. 2011;20:102–113. [Google Scholar]
- Julotok M, Singh AK, Gatto C, Wilkinson BJ. Influence of fatty acid precursors, including food preservatives, on the growth and fatty acid composition of Listeria monocytogenes at 37 and 10°C. Applied and Environmental Microbiology. 2010;76:1423–1432. doi: 10.1128/AEM.01592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C, Maurer LM, Oyelakin NE, Yoncheva YN, Maurer R, Slonczewski JL. Acetate and formate stress: Opposite responses in the proteome of Escherichia coli. Journal of Bacteriology. 2001;183:6466–6477. doi: 10.1128/JB.183.21.6466-6477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll RG, Patchett RA. Induced acid tolerance in Listeria monocytogenes. Letters in Applied Microbiology. 1992;14:224–227. doi: 10.1111/j.1472-765x.1996.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Lambert RJ, Stratford M. Weak-acid preservatives: Modelling microbial inhibition and response. Journal of Applied Microbiology. 1999;86:157–164. doi: 10.1046/j.1365-2672.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- Lawhon S, Maurer R, Suyemoto M, Altier C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/ SirA. Molecular Microbiology. 2002;46:1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138:1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL., Jr Short chain fatty acids and their receptors: New metabolic targets. Translational Research. 2013;161:131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Leatham MP, Banerjee S, Autieri SM, Mercado-Lubo R, Conway T, Cohen PS. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the Streptomycin-treated mouse intestine. Infection and Immunity. 2009;77:2876–2886. doi: 10.1128/IAI.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Frassetto A, Kowalik EJ, Jr, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host & Microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. Journal of Bacteriology. 2004;186:2099–2106. doi: 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. Journal of Applied Bacteriology. 1992;72:57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. Journal of Applied Microbiology. 2008;104:305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- Macy JM, Ljungdahl LG, Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. Journal of Bacteriology. 1978;134:84–91. doi: 10.1128/jb.134.1.84-91.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron L, Emerson N, Gahan CG, Hill C. A mutant of Listeria monocytogenes LO28 unable to induce an acid tolerance response displays diminished virulence in a murine model. Applied and Environmental Microbiology. 1997;63:4945–4947. doi: 10.1128/aem.63.12.4945-4947.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronicolis SK, Berberi A, Diakogiannis I, Petrova E, Kiaki I, Baltzi T, et al. Alteration of the phospho- or neutral lipid content and fatty acid composition in Listeria monocytogenes due to acid adaptation mechanisms for hydrochloric, acetic and lactic acids at pH 5.5 or benzoic acid at neutral pH. Antonie Van Leeuwenhoek. 2010;98:307–316. doi: 10.1007/s10482-010-9439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHan F, Shotts EB. Effect of feeding selected short-chain fatty acids on the in vivo attachment of Salmonella typhimurium in chick ceca. Avian Diseases. 1992;36:139–142. [PubMed] [Google Scholar]
- McHan F, Shotts EB. Effect of short-chain fatty acids on the growth of Salmonella typhimurium in an in vitro system. Avian Diseases. 1993;37:396–398. [PubMed] [Google Scholar]
- Merrigan MM, Sambol SP, Johnson S, Gerding DN. Prevention of fatal Clostridium difficile-associated disease during continuous administration of clindamycin in Hamsters. Journal of Infectious Diseases. 2003;188:1922–1927. doi: 10.1086/379836. [DOI] [PubMed] [Google Scholar]
- Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Applied and Environmental Microbiology. 1996;62:1589–1592. doi: 10.1128/aem.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millette M, Cornut G, Dupont C, Shareck F, Archambault D, Lacroix M. Capacity of human nisin- and pediocin-producing lactic acid bacteria to reduce intestinal colonization by vancomycin-resistant Enterococci. Applied and Environmental Microbiology. 2008;74:1997–2003. doi: 10.1128/AEM.02150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Elías EJ, McKinney JD. Carbon metabolism of intracellular bacteria. Cellular Microbiology. 2006;8:10–22. doi: 10.1111/j.1462-5822.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kuda T, An C, Kanno T, Takahashi H, Kimura B. Inhibitory effects of Leuconostoc mesenteroides 1RM3 isolated from narezushi, a fermented fish with rice, on Listeria monocytogenes infection to Caco-2 cells and A/J mice. Anaerobe. 2012;18:19–24. doi: 10.1016/j.anaerobe.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Tashiro K, Kuhara S, Hayashi T, Sugimoto N, Tobe T. Regulation of virulence by butyrate sensing in enterohaemorrhagic Escherichia coli. Microbiology (UK) 2009;155:521–530. doi: 10.1099/mic.0.023499-0. [DOI] [PubMed] [Google Scholar]
- Nava GM, Friedrichsen HJ, Stappenbeck TS. Spatial organization of intestinal microbiota in the mouse ascending colon. ISME Journal. 2011;5:627–638. doi: 10.1038/ismej.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EB, Lin R. Leucine-responsive regulatory protein: A global regulator of gene expression in E. coli. Annual Review of Microbiology. 1995;49:747–775. doi: 10.1146/annurev.mi.49.100195.003531. [DOI] [PubMed] [Google Scholar]
- O’Driscoll B, Gahan CG, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: Isolation of an acid-tolerant mutant which demonstrates increased virulence. Applied and Environmental Microbiology. 1996;62:1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll B, Gahan C, Hill C. Two-dimensional polyacrylamide gel electrophoresis analysis of the acid tolerance response in Listeria monocytogenes LO28. Applied and Environmental Microbiology. 1997;63:2679–2685. doi: 10.1128/aem.63.7.2679-2685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedron T, Mulet C, Dauga C, Frangeul L, Chervaux C, Grompone G, et al. A crypt-specific core microbiota resides in the mouse colon. mBio. 2012;3:e00116–12. doi: 10.1128/mBio.00116-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan-Thanh L, Mahouin F. A proteomic approach to study the acid response in Listeria monocytogenes. Electrophoresis. 1999;20:2214–2224. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2214::AID-ELPS2214>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Rabbani GH, Albert MJ, Hamidur Rahman AS, Moyenul Isalm M, Nasirul Islam KM, Alam K. Short-chain fatty acids improve clinical, pathologic, and microbiologic features of experimental shigellosis. Journal of Infectious Diseases. 1999;179:390–397. doi: 10.1086/314584. [DOI] [PubMed] [Google Scholar]
- Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9178–9183. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Sarker P, Mily A, Alam NH, Arifuzzaman ASM, Rekha RS, et al. Efficacy of sodium butyrate adjunct therapy in shigellosis: A randomized, double-blind, placebo-controlled clinical trial. BMC Infectious Diseases. 2012;12:111. doi: 10.1186/1471-2334-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Beardall J. The intrinsic permeability of biological membranes to H+: Significance for the efficiency of low rates of energy transformation. FEMS Microbiology Letters. 1981;10:1–5. [Google Scholar]
- Rechkemmer G, Rönnau K, Engelhardt W. Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comparative Biochemistry and Physiology. Part A. 1988;90:563–568. doi: 10.1016/0300-9629(88)90668-8. [DOI] [PubMed] [Google Scholar]
- Repaske DR, Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. Journal of Bacteriology. 1981;145:1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, et al. Dissecting the in vivo metabolic potential of two human gut acetogens. Journal of Biological Chemistry. 2010;285:22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke SC. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Science. 2003;82:632–639. doi: 10.1093/ps/82.4.632. [DOI] [PubMed] [Google Scholar]
- Roe AJ, McLaggan D, Davidson I, O’Byrne C, Booth IR. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. Journal of Bacteriology. 1998;180:767–772. doi: 10.1128/jb.180.4.767-772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AJ, O’Byrne C, McLaggan D, Booth IR. Inhibition of Escherichia coli growth by acetic acid: A problem with methionine biosynthesis and homocysteine toxicity. Microbiology (UK) 2002;148:2215–2222. doi: 10.1099/00221287-148-7-2215. [DOI] [PubMed] [Google Scholar]
- Ross RP, Mills S, Hill C, Fitzgerald GF, Stanton C. Specific metabolite production by gut microbiota as a basis for probiotic function. International Dairy Journal. 2010;20:269–276. [Google Scholar]
- Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: Ready for prime time? Nutrition in Clinical Practice. 2006;21:351–366. doi: 10.1177/0115426506021004351. [DOI] [PubMed] [Google Scholar]
- Russell JB. Intracellular pH of acid-tolerant ruminal bacteria. Applied and Environmental Microbiology. 1991;57:3383–3384. doi: 10.1128/aem.57.11.3383-3384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JB. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. Journal of Applied Microbiology. 1992;73:363–370. [Google Scholar]
- Ryan S, Begley M, Gahan CGM, Hill C. Molecular characterization of the arginine deiminase system in Listeria monocytogenes: Regulation and role in acid tolerance. Environmental Microbiology. 2009;11:432–445. doi: 10.1111/j.1462-2920.2008.01782.x. [DOI] [PubMed] [Google Scholar]
- Ryan S, Hill C, Gahan CGM. Acid stress responses in Listeria monocytogenes. Advances in Applied Microbiology. 2008;65:67–91. doi: 10.1016/S0065-2164(08)00603-5. [DOI] [PubMed] [Google Scholar]
- Salmond CV, Kroll RG, Booth IR. The effect of food preservatives on pH homeostasis in Escherichia coli. Journal of General Microbiology. 1984;130:2845–2850. doi: 10.1099/00221287-130-11-2845. [DOI] [PubMed] [Google Scholar]
- Sambol SP, Merrigan MM, Tang JK, Johnson S, Gerding DN. Colonization for the prevention of Clostridium difficile disease in hamsters. Journal of Infectious Diseases. 2002;186:1781–1789. doi: 10.1086/345676. [DOI] [PubMed] [Google Scholar]
- Schamberger GP, Diez-Gonzalez F. Characterization of colicinogenic Escherichia coli strains inhibitory to enterohemorrhagic Escherichia coli. Journal of Food Protection. 2004;67:486–492. doi: 10.4315/0362-028x-67.3.486. [DOI] [PubMed] [Google Scholar]
- Schloissnig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A, et al. Genomic variation landscape of the human gut microbiome. Nature. 2013;493:45–50. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala L, Budde B, Ross T, Siegumfeldt H, Jakobsen M, McMeekin T. Responses of Listeria monocytogenes to acid stress and glucose availability revealed by a novel combination of fluorescence microscopy and microelectrode ion-selective techniques. Applied and Environmental Microbiology. 2002;68:1794–1802. doi: 10.1128/AEM.68.4.1794-1802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. Journal of Biological Chemistry. 2010;285:27601–27608. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Advances in Microbial Physiology. 2009;55(1–79):317. doi: 10.1016/S0065-2911(09)05501-5. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wilkinson BJ, Standiford TJ, Akinbi HT, O’Riordan MXD. Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. Journal of Bacteriology. 2012;194:5274–5284. doi: 10.1128/JB.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, et al. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6:e27225. doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara LT, Jiang W, Zhang G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS One. 2012;7:e49558. doi: 10.1371/journal.pone.0049558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termén S, Tollin M, Rodriguez E, Sveinsdóttir SH, Jóhannesson B, Cederlund A, et al. PU.1 and bacterial metabolites regulate the human gene CAMP encoding antimicrobial peptide LL-37 in colon epithelial cells. Molecular Immunology. 2008;45:3947–3955. doi: 10.1016/j.molimm.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Tobe T, Nakanishi N, Sugimoto N. Activation of motility by sensing short-chain fatty acids via two steps in a flagellar gene regulatory cascade in enterohemorrhagic Escherichia coli. Infection and Immunity. 2011;79:1016–1024. doi: 10.1128/IAI.00927-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiological Reviews. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- Van Deun K, Haesebrouck F, Van Immerseel F, Ducatelle R, Pasmans F. Short-chain fatty acids and L-lactate as feed additives to control Campylobacter jejuni infections in broilers. Avian Pathology. 2008;37:379–383. doi: 10.1080/03079450802216603. [DOI] [PubMed] [Google Scholar]
- Van Deun K, Pasmans F, Van Immerseel F, Ducatelle R, Haesebrouck F. Butyrate protects Caco-2 cells from Campylobacter jejuni invasion and translocation. British Journal of Nutrition. 2008;100:480–484. doi: 10.1017/S0007114508921693. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F, Boyen F, Gantois I, Timbermont L, Bohez L, Pasmans F, et al. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poultry Science. 2005;84:1851–1856. doi: 10.1093/ps/84.12.1851. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F, De Buck J, De Smet I, Pasmans F, Haesebrouck F, Ducatelle R. Interactions of butyric acid- and acetic acid-treated Salmonella with chicken primary cecal epithelial cells in vitro. Avian Diseases. 2004;48:384–391. doi: 10.1637/7094. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F, De Buck J, Pasmans F, Velge P, Bottreau E, Fievez V, et al. Invasion of Salmonella enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids. International Journal of Food Microbiology. 2003;85:237–248. doi: 10.1016/s0168-1605(02)00542-1. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F, Russell JB, Flythe MD, Gantois I, Timbermont L, Pasmans F, et al. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathology. 2006;35:182–188. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- Vieira LQ, dos Santos LM, Neumann E, da Silva AP, Moura LN, Nicoli JR. Probiotics protect mice against experimental infections. Journal of Clinical Gastro-enterology. 2008;42:S168–S169. doi: 10.1097/MCG.0b013e31818063d4. [DOI] [PubMed] [Google Scholar]
- Wales AD, Allen VM, Davies RH. Chemical treatment of animal feed and water for the control of Salmonella. Foodborne Pathogens and Disease. 2010;7:3–15. doi: 10.1089/fpd.2009.0373. [DOI] [PubMed] [Google Scholar]
- Walter J, Ley R. The human gut microbiome: Ecology and recent evolutionary changes. In: Gottesman S, Harwood CS, editors. Annual review of microbiology. Vol. 65. Palo Alto: Annual Reviews; 2011. pp. 411–429. [DOI] [PubMed] [Google Scholar]
- Weiner N, Draskoczy P. The effects of organic acids on the oxidative metabolism of intact and disrupted E. coli. Journal of Pharmacology and Experimental Therapeutics. 1961;132:299–305. [PubMed] [Google Scholar]
- Wiedmann M, Arvik TJ, Hurley RJ, Boor KJ. General stress transcription factor ζB and its role in acid tolerance and virulence of Listeria monocytogenes. Journal of Bacteriology. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: Fermentation and short chain fatty acids. Journal of Clinical Gastroenterology. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Wong ARC, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: Even more subversive elements. Molecular Microbiology. 2011;80:1420–1438. doi: 10.1111/j.1365-2958.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, Ishijima SA, Clowney L, Koike H, Aramaki H, Tanaka C, et al. Feast/famine regulatory proteins (FFRPs): Escherichia coli Lrp, AsnC and related archaeal transcription factors. FEMS Microbiology Reviews. 2006;30:89–108. doi: 10.1111/j.1574-6976.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- Zabala Díaz IB, Ricke SC. Influence of short chain fatty acids and lysine on Salmonella typhimurium cadA expression. Antonie Van Leeuwenhoek. 2004;85:45–51. doi: 10.1023/B:ANTO.0000020271.11533.71. [DOI] [PubMed] [Google Scholar]
- Zachar Z, Savage DC. Microbial interference and colonization of the murine gastrointestinal tract by Listeria monocytogenes. Infection and Immunity. 1979;23:168–174. doi: 10.1128/iai.23.1.168-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, et al. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;302:G1405–G1415. doi: 10.1152/ajpgi.00543.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]