Abstract
Tamoxifen is an unlikely pioneering medicine in medical oncology. Nevertheless, the medicine has continued to surprise us, perform and save lives for the past 40 years. Unlike any other medicine in oncology, it is used to treat all stages of breast cancer, ductal carcinoma in situ, male breast cancer, pioneered the use of chemoprevention by reducing the incidence of breast cancer in women at high risk and induces ovulation in subfertile women! The impact of tamoxifen is ubiquitous. However, the power to save lives from this unlikely success story came from the first laboratory studies which defined that “longer was going to be better” when tamoxifen was being considered as an adjuvant therapy (Jordan 1978 Use of the DMBA-induced rat mammary carcinoma system for the evaluation of tamoxifen as a potential adjuvant therapy Reviews in Endocrine Related Cancer. October Supplement: 49–55.). This is that success story, with a focus on the interdependent components of: excellence in drug discovery, investment in self-selecting young investigators, a conversation with Nature, a conversation between the laboratory and the clinic, and the creation of the Oxford Overview Analysis. Each of these factors was essential to propel the progress of tamoxifen to evolve as an essential part of the fabric of society.
“Science is adventure, discovery, new horizons, insight into our world, a means of predicting the future and enormous power to help others”(Hoagland 1990).
- Mahlon Hoagland, MD. Director, Worcester Foundation for Experimental Biology (1970–85)
Tamoxifen (ICI 46,474) (Cole, et al. 1971; Harper and Walpole 1967; Klopper and Hall 1971) is an old medicine with origins unlikely to predict pioneer or breakthrough status(Jordan 2003, 2006; Maximov PY, et al. 2013). I was the least likely schoolboy to go to university (University of Leeds) but subsequently selected a career path “to help develop a drug to treat cancer” (Poirot 2011). At the time, this was not a popular or even reasonable career path as treatments were primitive and invariably unsuccessful (except for childhood leukemia). Tamoxifen and I became the “odd couple”, but nobody cared in the 1970s, as combination cytotoxic chemotherapy was predicted to cure cancer. Be that as it may, tamoxifen slowly “arrived” and advanced on the clinical scene in the 1970’s but only as an orphan drug after all but being abandoned by the pharmaceutical industry. This old medicine never went away and continues to provide surprises (Davies, et al. 2013; The aTTom Collaborative Group 2013). Through the application of experimental science in cancer therapeutics, (I was, and remain, a pharmacologist first) questions were asked, but Nature’s replies were unanticipated. However, Nature does not lie, and if the controls are correct, and it is reproducible, then one in compelled to re-evaluate the implications for medicine. The science of tamoxifen became “a means of predicting the future and enormous power to help others”(Hoagland 1990). This is that story.
In 1977, I presented an invited lecture at an Imperial Chemical Industries (ICI) Pharmaceuticals Division Medical symposium at King’s College, Cambridge. I described a new strategy to treat breast cancer (Jordan 1978). This was to use tamoxifen, a palliative agent then used in the final stages of breast cancer as a long term adjuvant therapy but this was not the fashion. Already adjuvant therapy with cytotoxic chemotherapy was showing promise (Bonadonna, et al. 1976; Fisher, et al. 1975) on the way to cures. The clinical strategy was considered sound. The primary tumor is first removed with a mastectomy then nonspecific cytotoxic chemotherapy is given for many months afterwards to destroy the micrometastases scattered unseen around the patient’s body. Destruction of micrometastases would produce cures.
During the 1970’s I was supported by both ICI Pharmaceuticals Division and the Yorkshire Cancer Research campaign to explore the mechanism of action and clinical opportunities for ICI’s orphan drug tamoxifen(Jordan 2006). Tamoxifen, a nonsteroidal antiestrogen, was no better than high dose estrogen or androgen therapy (Cole et al. 1971; Ingle, et al. 1981; Morgan, et al. 1976; Ward 1973) as a treatment for metastatic breast cancer and was available as a palliative therapy in the UK and other countries (except the USA) to treat metastatic breast cancer in postmenopausal women. Only “fewer side effects”, and higher cost, separated tamoxifen from the other “hormone therapies” (Cole et al. 1971; Ingle et al. 1981; Ward 1973). No cures were anticipated as the “hormone therapies”, as they were then called, were only effective in 30% of patients for a year or two. The medicine would not be approved in the United States for the treatment of metastatic breast cancer until December 1977 and chances for economic success for ICI Pharmaceuticals Division were hovering just above zero.
The experimental results I presented(Jordan 1978) at the medical symposium at Kings College demonstrated that long term tamoxifen treatment was superior to short term treatment in suppressing rat mammary tumorigenesis (Fig. 1). At the time numerous adjuvant trials of one year adjuvant tamoxifen were proposed for the simple reason that tamoxifen treatment only controlled breast cancer for a year (Cummings, et al. 1985; Hubay, et al. 1980; Ludwig Breast Cancer Study Group 1984; Ribeiro and Palmer 1983; Ribeiro and Swindell 1985; Rose, et al. 1985). The new concept presented presaged any clinical trials of more than one year of adjuvant tamoxifen and proposed that an appropriate clinical strategy for adjuvant tamoxifen treatment would be for extended or indefinite tamoxifen administration. My catch phrase at medical meetings was “tamoxifen forever”. However, the proposal was immediately controversial. Attendees at the conference (Fig. 2) challenged the fidelity of the dimethyl benzanthracene (DMBA)-induced rat mammary carcinoma model I was using as it did not replicate human micrometastatic dissemination. Worse still, “your strategy is dangerous!” It was universally known by the clinical community that tamoxifen would only be effective for less than two years in one third of patients when used to treat metastatic disease in postmenopausal women. “You’re proposing we give long term or indefinite adjuvant tamoxifen to women, some of whom are already cured, so you can prevent a recurrence. Your treatment strategy may, infact, encourage premature drug resistance and we will have wasted a valuable palliative drug by using it too soon.”
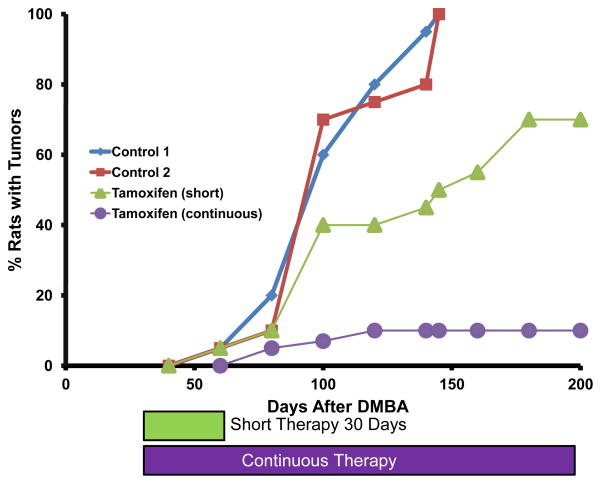
Figure 1.
The use of the dimethyl benzanthracene (DMBA)-induced rat mammary carcinoma model to demonstrate that longer or continuous therapy with daily tamoxifen (50μg subcutaneous injection) was superior at preventing the appearance of mammary tumors when compared to short therapy of 30 days. Fifty day old female Sprague Dawley rats were each given 20mg DMBA by gavage in 2 mls peanut oil. In nontreated control groups of 20 animals, all rats had multiple palpable tumors by 150 days. The model design for therapy groups first administered the DMBA at 50 days of age but the 30 day or continuous treatment was delayed for 30 days to permit mammary carcinogenesis of initiation and promotion to occur. The goal was to establish whether a short 30 day course of tamoxifen (estimated to be equivalent to 1 year of adjuvant tamoxifen in patients) could destroy the deranged microscopic cancer cells in the mammary glands or whether continuous therapy was required for complete tumor control and suppression. Continuous therapy is necessary. The strategy was to use tamoxifen only in the patients with ER positive tumors (Jordan and Koerner 1975) and use continuous therapy. This new strategy was first reported at the King College Cambridge, ICI Pharmaceuticals Division, Medical Symposium September 1977.
Figure 2.

The participants at the King College, Cambridge ICI Pharmaceuticals Division Medical Symposium September 1977. The author (top) presented the new strategy, Professor Michael Baum (right) was the session chair and leader of the proposed NATO trial that was planned to advance the current 1 year adjuvant tamoxifen trials to a 2 year treatment period. Helen Stewart (left), was in the audience and had plans to compare placebo and tamoxifen at first recurrence with 5 years of immediate adjuvant tamoxifen in the Scottish trial. Both trials (NATO and the Scottish trial) were to demonstrate, for the first true, survival advantages for adjuvant tamoxifen used for longer than 1 year.
Immediately after the King’s College meeting, in October of 1977, I had been invited to visit the University of Wisconsin Clinical Cancer Center in Madison by Paul Carbone (Director) and Doug Tormey (Head of the breast program) to spend several months doing collaborative research. I presented my ideas about long term adjuvant tamoxifen therapy – a new strategy with a drug that was not yet on the market in the United States! I was immediately offered a job at the Cancer Center and asked to move to Madison. Doug Tormey, based on my lecture, decided to continue his patients on indefinite tamoxifen(Tormey and Jordan 1984; Tormey, et al. 1987) and an Eastern Cooperative Oncology Group adjuvant protocol of indefinite tamoxifen was subsequently approved (Falkson, et al. 1990). But first, I spent a year designing and creating a Ludwig Institute for Cancer Research in Bern, Switzerland. I was provided with a large travel budget as I was asked to quality control estrogen receptor (ER) assays (Jordan, et al. 1983)for the Ludwig Adjuvant Tamoxifen Trials (regrettably only one year(Ludwig Breast Cancer Study Group 1984)). As a gamble, I decided to submit an abstract to the Adjuvant Therapy of Cancer II meeting in Tucson Arizona organized by the late Syd Salmon and Steve Jones. We now had much more data to support the proposal to use long term tamoxifen as a long term adjuvant therapy and I hoped, maybe, I would be lucky and get my abstract accepted for presentation. Imagine my surprise to find myself in the opening session sandwiched between the clinical greats of cancer research. The talk went well and was quickly published (Jordan, et al. 1979)for global distribution to the clinical community. At the meeting, I was able to enlarge my circle of colleagues in clinical breast cancer research but one “premonition” is worthy of mention. After my talk, Lois Trench, who we will meet again later, turned to her colleagues in the marketing department of ICI Americas and exclaimed “you have no idea what Dr. Jordan has just announced with his talk on indefinite adjuvant tamoxifen. This will be a blockbuster!” And so it was. Those first animal experiments provided a scientific justification and road map for all subsequent long term adjuvant clinical trials with tamoxifen that were to show unanticipated large survival advantages for patients(EBCTCG 1998, EBCTCG 2005) and consistent decreases in death rates from breast cancer in national statistics(Berry, et al. 2005; Peto, et al. 2000).
There is no better example of the value of long term adjuvant tamoxifen therapy than the recent reports of the Adjuvant Tamoxifen Longer Against Shorter (ATLAS) (Davies et al. 2013) and adjuvant Tamoxifen Treatment offers more (aTTom)(The aTTom Collaborative Group 2013). Until these trials of 10 years of adjuvant tamoxifen it was well established that five years of tamoxifen is dramatically superior to no treatment(Davies, et al. 2011) but ATLAS and aTTom compare 5 vs. 10 years of tamoxifen. The conclusion is that 10 years of adjuvant tamoxifen causes a superior decrease in mortality than 5 years of adjuvant tamoxifen(Davies et al. 2013). However, the question that must be raised is why mortality only decreases for the 10 year treatment group in the decade after tamoxifen is stopped? To seek the answer to this paradox, that should not occur with a palliative non-steroidal antiestrogen that blocks ER-mediated estrogen-stimulated growth of micrometastases(no drug, no action!)we have to return to the origins of tamoxifen, follow the interconnected events in translational research, and identify the factors that allowed tamoxifen to triumph.
In retrospect, the essential components to achieve the full potential of tamoxifen in the clinic were: a commitment to excellence in drug discovery, investment in a young self-selecting investigator, keeping an open mind with the conversation with Nature, and maintaining an active conservation between the laboratory and the clinical investigators. Laid over all of these essentials was the creation of the recurrent Oxford overview analyses of adjuvant trials by Sir Richard Peto and his team. This process formed the fundamental foundation to create the ATLAS trial based on firm clinical evidence and acts as a continuing catalyst to provide scientific support for aTTom. Finally, there is another dimension best described as seeing an opportunity, being in the right place at the right time and be willing to train yourself to be talent spotted. Slightly different circumstances or a different decision or meeting can change everything: the play of professional chance. “Sliding Doors” starring Gwyneth Paltrow and John Hannah is an excellent film based on the premise that by just missing or catching a tube train in London, a life can be altered forever. The film then portrays two parallel lives to the conclusion. In the spirit of “Sliding Doors” I will retell the progression of the aforementioned interconnecting components that created the tamoxifen of today.
A commitment to Excellence in Drug Discovery
Following the chance discovery of the first non-steroidal antiestrogen ethamoxy triphetol (MER25) by Lerner and coworkers(Lerner, et al. 1958) at the William S. Merrell company in Cincinnati and the finding that there was post-coital antifertility activity in laboratory animals (Segal and Nelson 1958) numerous companies immediately began synthesizing and screening for suitable compounds for use as “morning after pills”. Contraceptive research was the “hot” topic and fashion in the wake of the approval of the oral steroid contraceptive “to regulate the menstrual cycle” in 1960. A range of nonsteroidal compounds became available but one, clomiphene, induced ovulation in women – it guaranteed what it was planned to prevent! Clomiphene (Greenblatt, et al. 1961)subsequently found sustained use in medicine for the induction of ovulation after a 5 day course, in subfertile women. However, clomiphene increases demosterol levels, which is associated with cataract formation(Avigan, et al. 1960; Laughlin and Carey 1962)and there was no further development for long term therapy.
Drs. Mike Harper and Arthur Walpole (Fig. 3) tested the antifertility properties of a range of compounds related to clomiphene at ICI Pharmaceuticals laboratory at Alderley Park near Macclesfield, Chesire. The compounds were made by a talented organic chemist, Dr. Dora Richardson (Fig. 3). Compound ICI 46,474, the antiestrogenic trans isomer of a substituted triphenylethylene did not increase desmosterol(Harper and Walpole 1967) but like clomiphene was also found to induce ovulation(Klopper and Hall 1971). By coincidence, I was a summer student working in the nascent cancer research laboratory opposite Dr. Walpole’s fertility control laboratory in 1967. Alderley Park is just 10 miles from my home where I grew up in Cheshire. Walpole was the Head of the Fertility Control program at ICI Pharmaceuticals Division but was subsequently to play an essential role to ensure the successful development of ICI 46,474 as a cancer treatment. This was because Walpole had long standing interest in cancer research (Jordan 1988)though he was required to work in what was judged to be the more fertile field of contraception. I was to meet Walpole again 5 years later in 1972 but this time he was the examiner of my PhD thesis entitled “A study of the structure function relationship of substituted triphenylethylenes and triphenylethanes”.
Figure 3.

The principal players in the discovery of ICI 46,474 at ICI Pharmaceuticals Division, Cheshire, UK in the 1960’s that eventually evolved into tamoxifen a decade later. Arthur Walpole (Walop) (left) was the head of the Fertility Control Program tasked with the mission to discover safer compounds to “regulate the sexual cycle”. Dora Richardson (center), the team organic chemist who synthesized all of the isomers of the triphenylethylene derivatives that would be tested as antifertility agents in rats by Mike Harper, the team reproductive endocrinologist. Arthur Walpole would be the author’s PhD examiner, scientific supporter and administrative link to ICI until his untimely death on July 2nd 1977. Dora Richardson would provide the metabolites of tamoxifen to the author to be tested as anticancer agents and Mike Harper would offer the author a two year BTA (Been to America) at the Worcester Foundation, MA. Each individual was generous with important opportunities, investment and support for a young investigator starting their adventure to investigate “failed morning after pills” as future important therapeutic agents in women’s health.
Self-Selecting Young Investigator
I started my lifelong “love affair” with triphenylethylenes in 1969 when I chose to accept a PhD project to crystallize and study the x-ray crystallography of the ER complex liganded with an estrogen and antiestrogen. Jack Gorski (Toft and Gorski 1966; Toft, et al. 1967) had just published a series of papers in the Proceedings of the National Academy of Sciences showing that the ER protein could easily be extracted from rat uteri. My PhD supervisor, in the Department of Pharmacology at Leeds University, was Dr. Edward (Ted) Clark, a brilliant and exciting lecturer in medicinal chemistry with encyclopedic knowledge and a long-standing interest in estrogens. “It will be simple” he said. “You will extract and purify the rat uterine ER and crystallize it with an estrogen and an antiestrogen and do the x-ray crystallography up the road at the Astbury Department of Biophysics”. Well that did not work (the whole ER complex has yet to be crystallized!) and I switched to study the structure function relationships of triphenylethylene antiestrogens – the failed contraceptives. Although this would prove to be a sound foundation for a future, at the time no one was recommending careers in failed contraceptives!
During the 3 years of my PhD studies (1969–72), armed with a Medical Research Council scholarship, I was talent-spotted by Professor Michael Barrett, the new Chair of Pharmacology (a cardiovascular pharmacologist from ICI Pharmaceuticals Division) appointed in 1970. As an undergraduate, I had created, organized, and led our student society, named the Medean Society after the sorceress Medea who created magic potions to protect Jason (of Argonaut fame) from death as he completed his impossible tasks to retrieve the Golden Fleece. She was, it seems the first to create effective chemopreventive agents!
Professor Barrett recognized I had talent for organization in science and, as a graduate student, I chose to create lectures for parent teacher organizations in the Leeds area schools on drug abuse. I strongly believed in public service by reinvestment in the community was important to “pay back” for my free education. These lectures were also presented, at Professor Barrett’s insistence, to the undergraduates as I was also closely connected with the Leeds City Police Drug Squad as an advisor. Thirdly, Professor Barrett was aware I had been talent spotted to be on the advisory staff for the Deputy Chief Scientist (Army) and one of my duties was to present Drug Abuse lectures for Army units throughout the country. In this role, I was Reserve Army Officer. I was focused on perils of drug abuse and worked with the police. As a PhD student I was researching, the regulation of the sexual cycle with pharmacological agents, and I was an Army Officer advisor to the Deputy Chief Scientist (Army). In America (1972–74) I would often be asked to give talks in the community (the English accent went over well!) so I would preface my talks by stating that my career was based on sex, drugs, and violence” (with apologies to the “sex, drugs and rock and roll” in the sixties; I was however, actually a drummer in a rock band as a teenager!).
In 1972, Professor Barrett now saw potential in me as a new staff member in his new Department of Pharmacology. I found myself as a prospective lecturer in Pharmacology, but first I had to complete my PhD in “failed contraceptives”. During my interview for the lecturer’s job, it was stated and required that I should spend two years in America to acquire new scientific skills and return to invest the new knowledge back in Leeds University following my BTA (Been to America).
Professor Barrett and the administration were however challenged to find an examiner for my PhD on “failed contraceptives”. All approaches were declined - nobody cared as this was a topic considered of no significance. Professor Barrett turned to his former colleague at ICI Pharmaceuticals Division, Dr. Arthur Walpole, head of the Fertility Control Programme to be my external examiner. The University administration was initially resistant to having “someone from industry” as an examiner; but, fortunately for me and, perhaps, the future of tamoxifen, the administration finally agreed. Indirectly, the door had opened for the development of ICI 46,464, the failed contraceptive to evolve into the “gold standard” tamoxifen for the adjuvant treatment of breast cancer.
Dr. Mike Harper, the reproductive endocrinologist at ICI Pharmaceuticals Division who had completed all of the biology of ICI 46,474, was Mike Barrett’s friend but was now heading a research program at the Worcester Foundation for Experimental Biology in Massachusetts. I remember my transatlantic telephone call with Mike Harper. “Can you come in September, will $12,000 a year be OK, and will you work on prostaglandins?” “Yes, yes, yes” I replied and went off to the library to find out what prostaglandins were! My examination with Arthur Walpole went well, but I had not anticipated that our lives would be intertwined for the remaining years of his life. I now found myself off to America for two years as a Visiting Scientist (1972–74).
I arrived at the Worcester Foundation, the home of the oral contraceptive, with the invitation and plan to work with Mike Harper on a “once a month contraceptive”. However, when I arrived I found he had accepted a job as Head of Reproduction at the World Health Organization in Geneva, Switzerland. I was told I could do any research I liked for the next two years as long as some of it involved prostaglandins. I was confronted with a daunting task as a brand new PhD graduate – start my own laboratory as an independent investigator, find my own funds and hire and train a technician. Her name was Susan Koerner and she was spectacular. She was included as an author on my early papers.
I had always wanted to be involved in the discovery and development of drugs to treat cancer so perhaps here was my opportunity. I was a pharmacologist, but do what you know and all I knew about was triphenylethylenes and the ER so a phone call to Arthur Walpole gained his support to aid in turning ICI 46,474 into a prospective breast cancer drug. What I did not know at that time was that ICI Pharmaceuticals Divisions had reviewed all the clinical data on ICI 46,474 in March 1972, and the decision was made to stop development for clinical use as there was no financial reward to be accrued for the treatment of metastatic breast cancer or as another inducer of ovulation(Jordan 2006). Arthur Walpole had tendered his resignation and sought early retirement. He would, however, remain at Alderley Park if ICI 46,474 was advanced for approval for clinical use as an orphan drug for the treatment of metastatic breast cancer and the induction of ovulation. “Sliding Doors” occurred in September in 1972 with Mike Harper going to Geneva and me calling Arthur Walpole. Walpole supported me to receive an unrestricted research grant from ICI Americas and introduced me to the lady who became my lifelong friend - Lois Trench. She was the new drug monitor in charge of developing ICI 46,474 in the United States and she succeeded. She recruited me as the scientific consultant for ICI Americas to advocate tamoxifen to clinical trials groups (ECOG and the NSABP) for clinical testing. I returned to Leeds University in September 1974 as a lecturer in pharmacology with much work to accomplish. I had omitted to publish my work and had to catch up. Remember: if you do not publish, it never happened and you cannot claim the credit (only in your mind!).
Investment in Young Investigators
In 1974, Dr. Roy Cotton was the clinician in charge of the development of Nolvadex (ICI 46,474, tamoxifen) for ICI Pharmaceuticals Division. He was my contact person with an agenda to devise a way for the Clinical Department to support my work at Leeds. He was inspirational and through his innovation advanced tamoxifen to become a “pioneering medicine”. He devised a way for “flexible support” that had minimal cost for ICI Pharmaceuticals Division or his clinical budget, but was to create a foundation for a blockbuster medicine for women’s health. Roy Cotton provided hundreds of rats from Alderley Park stocks in Cheshire for my work at Leeds University. He arranged for continuous supplies of rats to be chauffeured to Leeds Medical School every week between 1975 and 1978 to complete dozens of experiments on the mechanism of action of tamoxifen, metabolism, the strategy to deploy tamoxifen as the first chemopreventive, and as the first targeted long term antiestrogenic adjuvant therapy. The paper entitled, “Use of the DMBA-induced rat mammary carcinoma system for the evaluation of tamoxifen as a potential adjuvant therapy”(Jordan 1978), was the first to propose publically that “longer was better than shorter adjuvant therapy” published in the Reviews of Endocrine-Related Cancer. The Yorkshire Cancer Research Campaign also provided essential support to this young investigator, without which we could not have, supported our staff and students and bought essential equipment that demonstrated tamoxifen bound to the ER(Jordan and Prestwich 1977). Strange as this seems today, the ER was an unpopular and unproven mechanism of tamoxifen action for the clinical community in the United Kingdom and for the next 10 years, the ER assay we use today was not accepted in the 1970s–80s in the UK. The good news was that instead of doing an ER assay, every breast cancer patient received tamoxifen anyway, and as a result untold numbers of lives were saved with tamoxifen from the beginning.
Conversation with Nature
In 1975, Marc Lippman published (Lippman and Bolan 1975)that tamoxifen was a competitive inhibitor of estrogen stimulated growth of MCF-7 breast cancer cells. Lois Trench in America had provided me with a selection of frozen breast cancers to measure ER, and in 1975 we showed that tamoxifen blocks estradiol binding to the human tumor ER(Jordan and Koerner 1975). Now back at Leeds, I was refining another publication, started at Worcester Foundation(Jordan 1974) that tamoxifen prevented rat mammary carcinogenesis(Jordan 1976b).At that time, chemoprevention of breast cancer was a “forlorn hope”. Indeed Michael Sporn had only just invented the new word(Sporn, et al. 1976). I decided instead to turn to the issue of adjuvant therapy with tamoxifen. Marc Lippman stated in a line of his paper(Lippman and Bolan 1975) that high doses of tamoxifen were tumoricidal for MCF-7 cells, so we decided to put it to the test in vivo.
When I was at the Worcester Foundation, I spent a day (and dinner) with the late Elwood Jensen, then the Director of the Ben May Laboratory for Cancer Research in Chicago, when he visited the Foundation in September 1972. He was a new member of the Scientific Advisory Board for the Foundation, appointed by Mahlon Hoagland, the new Director in 1970. I accepted Elwood’s offer to go to Chicago in summer of 1973 to learn ER assay techniques and the DMBA-induced rat mammary carcinogen model. Both techniques were essential for the job to be completed; to find new and novel clinical strategies fortamoxifen.
Back at Leeds some 3 years later, I devised a model that, in my naïve view, would replicate adjuvant therapy with tamoxifen despite the fact that it was not a real model of human disease. There was no real model, so there was no choice but to use what was available. My reasoning was as follows. If DMBA was administered to 50 day old Sprague Dawley rats, then all animals would develop tumors within 150 days. I planned two strategies initially: give the DMBA at 50 days of age and then treat daily with increasing doses of tamoxifen starting 30 days after DMBA but only for one month. A month in a rat-life is about a year for a humanie: what was proposed for current adjuvant trials with tamoxifen(Cummings et al. 1985; Hubay et al. 1980; Ludwig Breast Cancer Study Group 1984; Ribeiro and Palmer 1983; Ribeiro and Swindell 1985; Rose et al. 1985). The results show there was a delay in tumorigenesis but then tumors appeared later with at least one tumor per rat(Jordan 1983; Jordan and Allen 1980).However, there was a clue as the higher the daily dose, the larger the delay in tumorigenesis. As it was known that tamoxifen had a long biological half-life (Fromson, et al. 1973a, b) then I reasoned that tumorigenesis proceeded only after the drug was cleared following short term treatment. We tried another approach, earlier or later after DMBA – earlier was better to prevent tumorigenesis(Jordan et al. 1979). So if the drug needs to be there to prevent the microfoci of deranged rat mammary epithelial cells from growing into tumors, then is long term tamoxifen treatment superior to short term therapy? The results showed that indefinite tamoxifen vs. shorter tamoxifen is illustrated in Fig. 1(Jordan 1978; Jordan 1983; Jordan et al. 1979). We had asked the question of what is the best way to give “adjuvant tamoxifen” in the DMBA-model and we did not get back the answer we expected but it was a consistent answer. No drug, no antiestrogen action – long term therapy was the way to go. Conversion of the rat model to clinical practice: five or more years of adjuvant tamoxifen would be a superior adjuvant strategy than the planned 1 year of treatment.
Neither did we get the answer we anticipated when we tested the potent metabolite of tamoxifen 4-hydroxy tamoxifen (Jordan, et al. 1977) in the same model against tamoxifen.(Jordan and Allen 1980). We had initially discovered that tamoxifen could be metabolically activated by 4-hydroxy tamoxifen in our collaboration with ICI Pharmaceuticals Division but I agreed to a delay in my publications for a year (Jordan et al. 1977) while ICI Pharmaceuticals Division sought to patent the metabolites. It was anticipated that there was little likelihood of successful development of tamoxifen to a financially rewarding product so there had been no need to follow protocol, waste time and money and patent the metabolites. I was told years later, that the clinical staff at the beginning of the ‘70’s was told not to spend too much time on tamoxifen!
We tested the better antiestrogen, 4-hydroxy tamoxifen just in case we had found a better breast cancer drug. However, it turned out to be a less effective antitumor agent than tamoxifen in our model(Jordan and Allen 1980). The hydroxylated metabolite was cleared too quickly, simple pharmacology. Tamoxifen can be detected for up to 6 weeks after treatment stops. So it seems that tamoxifen maintained a supply of the active metabolite as the potent drug but the less potent parent acts as the depot that saturates a patient’s body. Nevertheless, the metabolite experiments with 4-hydroxy tamoxifen again showed that longer was better than shorter(Jordan and Allen 1980). Keep the drug there constantly: no drug present, no action. This was the principle that we advanced to the clinical community starting that day at King’s College, Cambridge in 1977.
A Conversation Between the Laboratory and the Clinic
My love of chemistry was always focused on what organic chemistry can do to create medicines to defeat disease. That for me was the guiding principle first created by Professor Paul Ehrlich at the dawn of the 20th century when he created the first chemical therapy (chemotherapy) to cure syphilis(Baumler 1984). I seized upon the principle with alacrity in my teens with the desire to find molecules to treat cancer. This was pharmacology and “failed contraceptives” were both my “Sliding Doors” and my opportunity. But unless you train yourself and learn to be ready to seize the opportunity it will vanish as quickly as it appeared. It is a moment in time governed by factors that you cannot control but determination and discipline will aid your quest for success. In my case, the topic was definitely not fashionable so nobody cared or very few. The “few” were happy amateurs who wanted to contribute to human health when the majority considered “another hormone therapy” a waste of time and resources. In my case, it was said I had poor career judgment because more than once the topic would crop up that if tamoxifen failed, then I would have nothing. It is true that tamoxifen would most certainly fail today as tamoxifen was unexpectedly proven to cause liver cancer in rats in the early 1990’s(Greaves, et al. 1993). This was some 20 years after clinical use started! Testing of the toxicology of an agent for cancer is trivial but for a medicine for healthy women (chemoprevention) the rules rightly change and major long term toxicity testing occurs. No company today would develop tamoxifen knowing it caused cancer. But Nature gave the right answer if you were a rat (Greaves et al. 1993)and the right answer for women in the invaluable Overview Analyses that show no increase in liver cancer (Early Breast Cancer Trialists’ Collaborative Group 1992, Early Breast Cancer Trialists’ Collaborative Group 2005).
Because pharmacology is about “the enormous power to help others”, I chose to move my career into clinical cancer research through clinical cancer centers in the United States. The opportunities to learn and contribute to oncology at the University of Wisconsin Comprehensive Cancer Center are a tribute to Paul Carbone, Doug Tormey, and David Rose each making my recruitment happen. I chose to train myself. Actually, it was Lois Trench who initiated all of the process back in 1977 and funded studies through ICI Americas for me to travel to Madison for 3 months to if I could be recruited. ICI Pharmaceutical Division also deserves the credit for encouraging my career development into clinical research. They provided a decade of support for my laboratory (1973–1983), to pay staff, students’ scholarships, (Clive Dix rose rapidly to be research Director for Glaxo, and Anna Tate Riegel, is an endowed Professor in Oncology at Georgetown) laboratory supplies, “free rats”, and most importantly Arthur Walpole did not take early retirement but remained at ICI Pharmaceuticals Division as my link for my University of Leeds/ICI Pharmaceuticals Division Joint Research Scheme until his untimely death on July 20, 1977. He never saw the success of tamoxifen; but, our connection made the possibility of success a certainty (this is however only the wisdom of hindsight!)
At the King College meeting around this time, I met Professor Michael Baum (Fig. 2) who was now to chair my session and introduce me. In the discussion of my paper, he mentioned that he had arbitrarily planned to use 2 years of adjuvant tamoxifen, thereby advancing ahead of the numerous 1 year trials(Cummings et al. 1985; Hubay et al. 1980; Ludwig Breast Cancer Study Group 1984; Ribeiro and Palmer 1983; Ribeiro and Swindell 1985; Rose et al. 1985). Bernie Fisher in America planned to do the same and advance to 2 years following the NSABP symposium in Key Biscayne Florida organized by Lois Trench in 1976. I gave the pharmacology of tamoxifen talk(Jordan 1976a), but I promised ICI Pharmaceuticals Division I would not speak about “metabolites”. Tamoxifen, as I mentioned earlier, was not to be FDA approved until December 1977 in America so that step was a priority for the company and I strongly believed this was also a priority for women’s health with breast cancer.
Michael Baum and John Patterson, now the clinician responsible for tamoxifen, taking over from Roy Cotton, worked to come up with an imaginative acronym for this group’s adjuvant 2 year trial to be sponsored by ICI Pharmaceuticals Division. It was called the NATO group to make American clinicians think it was an American trial and read the results. The acronym stands for “Nolvadex Adjuvant Trial Organization” and the NATO group has the distinction of being the first to detect a survival advantage for patients taking adjuvant tamoxifen(Baum, et al. 1983; Nolvadex Adjuvant Trial Organisation 1983). Helen Stewart (Fig. 2) was in the audience at King’s College in 1977. As it turned out, she would be running what was to be known as the Scottish trial led by Sir Patrick Forest and sponsored by the Medical Research Council (the same group who sponsored my PhD at Leeds University “failed contraceptives”; I will forever be grateful as their investment really paid off!). The Scottish trialists were in the process of deciding whether patients could tolerate 5 years of tamoxifen. If so, their trial was then to start accruing patients to be randomized to 5 years of adjuvant tamoxifen or placebo and tamoxifen at first recurrence. Their results were published on the 25th July 1987(Scottish Cancer Trials Office (MRC) 1987)(coincidentally my birthday!) with significant survival advantages for early tamoxifen vs. later use of tamoxifen upon recurrence. The animal studies therefore were “a means of predicting the future” when presented at Kings College a decade earlier. For me, the “power to help others” was important as I subsequently traveled to speak at literally hundreds of clinical meetings worldwide. The clinical colleagues who became lifelong friends are too numerous to list but those close friends and colleagues in breast cancer research, Bill McGuire, Monica Morrow, and Gabriel Hortobagyide serve special recognition here for the part each was to play in my life.
By the mid 1980’s, clinical trials slowly started to demonstrate some benefit for tamoxifen but in the main, the trials were too small to declare “breakthrough” as “hormone therapy” was not curing everyone – chemotherapy would do that. Well perhaps but now enter the meta-analysis.
The Oxford Overview Analysis
Dr. Craig Henderson tells the story of the first overview analysis(Henderson 1999). The overview was conducted by Sir Richard Peto, Sir Rory Collins, Richard Gray, and the team from the Clinical Trials Unit Oxford University in 1984. There were two main camps of randomized trials: the Europeans were cautious about the toxicity of cytotoxic chemotherapy and the American skeptical that a palliative “hormone therapy” could aid survival. The results presented in a hotel at the Heathrow Airport in the mid-80’s showed that chemotherapy or tamoxifen improved disease free survival and overall survival to about the same extent but in premenopausal and postmenopausal patients respectively. Since then, analyses have occurred in 1990 and 1995 and at regular intervals thereafter to this day. The value of seeing an analysis of all the data permitted the prevention trials with tamoxifen to advance as inhibition of contralateral breast cancer in adjuvant tamoxifen trials was consistently at 50% and safety with endometrial cancer in postmenopausal women was much less significant than feared. Also, the concern about tamoxifen-induced rat hepatocarcinogenesis was not translated to human treatment trials. The trends observed with 1, 2, and 5 years of adjuvant tamoxifen predicted “even more” was going to be better. There would have been no ATLAS trial or a focus on unanticipated outcomes without the overview analysis. Nature was also to tell us something unanticipated about decreasing mortality with tamoxifen. If tamoxifen is classified as a nonsteroidal antiestrogen that blocks estrogen stimulated growth of micrometastases as a cytostatic agent, then why does stopping tamoxifen at 5 years not cause recurrence? No drug, no effect. Instead it causes a continuing decrease in mortality after stopping the antiestrogen. We know that stopping tamoxifen too soon ie: at one or two years, regrettably reduces the numbers of lives saved. But why?
The Legacy of Long Term Adjuvant Tamoxifen
The full story of tamoxifen has recently been told(Maximov PY et al. 2013). Through study of the pharmacology of tamoxifen, its metabolites, and its ubiquitous use for the treatment and prevention of breast cancer, several other significant advances in therapeutics and women’s health have occurred.
The introduction of long term adjuvant tamoxifen therapy mandated an examination of the development of acquired resistance to tamoxifen in the laboratory. At the time, in the mid 1980’s, there were some cell culture studies of resistance, but the finding that opened the door to understand the evolution of acquire resistance to tamoxifen treatment was the transplantable model of acquires resistance in athymic mice (Gottardis and Jordan 1988; Gottardis, et al. 1989). These studies also lead to the discovery that tamoxifen could control the growth of breast cancer but cause the growth of pre-existing endometrial cancer(Gottardis, et al. 1988). Different tissues responded to tamoxifen in different ways: in the breast it was an antiestrogen; but in the bones, endometrium and the regulation of circulating cholesterol estrogenic actions were predominant (Jordan 2001; Lerner and Jordan 1990). These observations gave medicine selective ER modulators (SERMs). There were no SERMs in 1990, only tamoxifen classified as a nonsteroidal antiestrogen to treat breast cancer(Jordan 1984). Today there are numerous SERMs (tamoxifen, raloxifene, bazedoxifene, toremifene, ospemifene, and lasofoxifene) for a wide variety of indications. That story has recently been told(Jordan 2013).
An understanding of the evolution of acquired resistance to tamoxifen(Jordan 2004, 2008; Yao, et al. 2000) also led to the discovery of the new biology of estrogen-induced apoptosis that not only has clinical applications to treat antihormone resistant breast cancer(Ellis, et al. 2009) and explain how estrogen replacement therapy can reduce the incidence of breast cancer in long term estrogen-deprived (>10 years post menopause) women(Anderson, et al. 2012)but, also, can explain the reason why tamoxifen therapy for >5 years can dramatically reduce mortality after therapy stops. The woman’s own estrogen may destroy selected and vulnerable clonal micrometastases(Wolf and Jordan 1993).
The idea that longer therapy with adjuvant tamoxifen in patients with ER positive breast cancer was not fashionable at the start. This is the way it is with most new concepts in any discipline. The clinical strategies of using one year of adjuvant tamoxifen(Cummings et al. 1985; Hubay et al. 1980; Ludwig Breast Cancer Study Group 1984; Ribeiro and Palmer 1983; Ribeiro and Swindell 1985; Rose et al. 1985) were clinically sound in the late 1970’s because clinical experience using tamoxifen to treat metastatic breast cancer showed that treatment was successful in a minority of unselected for less than 2 years. Suggesting a treatment strategy for indefinite adjuvant tamoxifen treatment was destined to fail at 2 years – but it did not. I believe that the reason lies in the fact that metastatic disease is too established and can readily subvert the stress caused by preventing estrogen stimulated growth. It is also a matter of bulk and vascularization that aid the survival of breast cancer cells in metastatic disease. But micrometastatic disease is apparently indolent and not well established but survives through slow and deliberate microscopic steps to select cells with acquired resistance that evolves very slowly through phases of resistance to reach unstable and vulnerable clonal populations over 5 years of treatment. It takes this long in the laboratory (Yao et al. 2000) and physiologic estrogen will now cause rapid tumor regression (Wolf and Jordan 1993; Yao et al. 2000). But what if estrogen from the patient now triggers estrogen-induced apoptosis in the adjuvant tamoxifen trial of 5 years or more (Davies et al. 2013; Early Breast Cancer Trialists’ Collaborative Group 1998; Jordan 2008)?
Is there direct evidence that the new biology of estrogen that causes apoptosis give us profound mortality decreases after tamoxifen is stopped? Yes, I believe so. We know (Anderson et al. 2012) from the Women’s Health Initiative (WHI) estrogen-only trial that there is a profound decrease in the incidence of breast cancer and mortality for women treated with estrogen in their 60’s when compared to placebo. Estrogen kills estrogen-deprived occult cancer cells more than a decade post menopause (Obiorah and Jordan 2013). None of this science would have been revealed but for the fact that long term adjuvant tamoxifen advanced from a laboratory concept in the late 1970’s (Jordan 1978; Jordan et al. 1979), through clinical trials, to be enhanced as a reality by the Oxford overview analyses (Davies et al. 2011). Today we have a successful clinical strategy with the results of ATLAS (Davies et al. 2013) and aTTom(The aTTom Collaborative Group 2013). Further lives are saved with a cheap effective medicine that never went away. The science of long-term adjuvant tamoxifen was indeed an adventure, discovery, new horizons, insights into our world, a means of predicting the future and enormous power to help others(Hoagland 1990).
Acknowledgments
I thank all my Tamoxifen Teams for their remarkable gift of transforming ideas, through laboratory experiments to become lives saved. I especially wish to thank my Laboratory Manager Russell McDaniel MS and Fadeke Agboke for their dedicated work to create this manuscript for publication. This work was supported by the Department of Defense Breast Program (award number W81XWH-06-1-0590) Center of Excellence, Susan G. Komen for the Cure Foundation (award number SAC100009). Comprehensive Cancer Center Support Grant (core grant NIH P30 CA051008). The views and opinions of the author do not reflect those of the US Army or the Department of Defense.
References
- Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, Bluhm E, Connelly S, Hubbell FA, Lane D, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avigan J, Steinberg D, Vroman HE, Thompson MJ, Mosettig E. Studies of cholesterol biosynthesis. I. The identification of desmosterol in serum and tissues of animals and man treated with MER-29. J Biol Chem. 1960;235:3123–3126. [PubMed] [Google Scholar]
- Baum M, Brinkley DM, Dossett JA, McPherson K, Patterson JS, Rubens RD, Smiddy FG, Stoll BA, Wilson A, Lea JC, et al. Improved survival among patients treated with adjuvant tamoxifen after mastectomy for early breast cancer. Lancet. 1983;2:450. doi: 10.1016/s0140-6736(83)90406-3. [DOI] [PubMed] [Google Scholar]
- Baumler E. Paul Ehrlich: Scientist for Life. Holmes & Meier Pub; 1984. [Google Scholar]
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, De Lena M, Tancini G, Bajetta E, Musumeci R, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI 46474. Br J Cancer. 1971;25:270–275. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings FJ, Gray R, Davis TE, Tormey DC, Harris JE, Falkson G, Arseneau J. Adjuvant tamoxifen treatment of elderly women with stage II breast cancer. A double-blind comparison with placebo. Ann Intern Med. 1985;103:324–329. doi: 10.7326/0003-4819-103-3-324. [DOI] [PubMed] [Google Scholar]
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Alencar VH, Badran A, Bonfill X, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy 133 randomised trials involving 31, 000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:71–85. [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, Dickler MN, Silverman P, Fleming GF, Kommareddy A, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkson HC, Gray R, Wolberg WH, Gillchrist KW, Harris JE, Tormey DC, Falkson G. Adjuvant trial of 12 cycles of CMFPT followed by observation or continuous tamoxifen versus four cycles of CMFPT in postmenopausal women with breast cancer: an Eastern Cooperative Oncology Group phase III study. J Clin Oncol. 1990;8:599–607. doi: 10.1200/JCO.1990.8.4.599. [DOI] [PubMed] [Google Scholar]
- Fisher B, Carbone P, Economou SG, Frelick R, Glass A, Lerner H, Redmond C, Zelen M, Band P, Katrych DL, et al. 1-Phenylalanine mustard (L-PAM) in the management of primary breast cancer. A report of early findings. N Engl J Med. 1975;292:117–122. doi: 10.1056/NEJM197501162920301. [DOI] [PubMed] [Google Scholar]
- Fromson JM, Pearson S, Bramah S. The metabolism of tamoxifen (I.C.I. 46,474). I. In laboratory animals. Xenobiotica. 1973a;3:693–709. doi: 10.3109/00498257309151594. [DOI] [PubMed] [Google Scholar]
- Fromson JM, Pearson S, Bramah S. The metabolism of tamoxifen (I.C.I. 46,474). II. In female patients. Xenobiotica. 1973b;3:711–714. doi: 10.3109/00498257309151595. [DOI] [PubMed] [Google Scholar]
- Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–5187. [PubMed] [Google Scholar]
- Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–815. [PubMed] [Google Scholar]
- Gottardis MM, Wagner RJ, Borden EC, Jordan VC. Differential ability of antiestrogens to stimulate breast cancer cell (MCF-7) growth in vivo and in vitro. Cancer Res. 1989;49:4765–4769. [PubMed] [Google Scholar]
- Greaves P, Goonetilleke R, Nunn G, Topham J, Orton T. Two-year carcinogenicity study of tamoxifen in Alderley Park Wistar-derived rats. Cancer Res. 1993;53:3919–3924. [PubMed] [Google Scholar]
- Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. Preliminary report. JAMA. 1961;178:101–104. doi: 10.1001/jama.1961.03040410001001. [DOI] [PubMed] [Google Scholar]
- Harper MJ, Walpole AL. A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil. 1967;13:101–119. doi: 10.1530/jrf.0.0130101. [DOI] [PubMed] [Google Scholar]
- Henderson IC. Early Overviews of Adjuvant Tamoxifen Therapy. In: Jordan VC, Melville, editors. Tamoxifen for the Treatment and Prevention of Breast Cancer. New York: PRR, Inc; 1999. pp. 57–77. [Google Scholar]
- Hoagland M. Towards the Habbit of Truth. New York: The Commonwealth Fund Book Program. W. W. Norton & Company; 1990. [Google Scholar]
- Hubay CA, Pearson OH, Marshall JS, Rhodes RS, DeBanne SM, Rosenblatt J, Mansour EG, Hermann RE, Jones JC, Flynn WJ, et al. Adjuvant chemotherapy, antiestrogen therapy and immunotherapy for stage II breast cancer: 45-month follow-up of a prospective, randomized clinical trial. Cancer. 1980;46:2805–2808. doi: 10.1002/1097-0142(19801215)46:12+<2805::aid-cncr2820461413>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, Nichols WC, Creagan ET, Hahn RG, Rubin J, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304:16–21. doi: 10.1056/NEJM198101013040104. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Antitumour activity of the antioestrogen ICI 46,474 (tamoxifen) in the dimethyl benzanthracene (DMBA)-induced rat mammary carcinoma model. J Steroid Biochem. 1974;5:354. [Google Scholar]
- Jordan VC. Antiestrogenic and antitumor properties of tamoxifen in laboratory animals. Cancer Treat Rep. 1976a;60:1409–1419. [PubMed] [Google Scholar]
- Jordan VC. Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA-induced rat mammary carcinomata. Eur J Cancer. 1976b;12:419–424. doi: 10.1016/0014-2964(76)90030-x. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Use of the DMBA-induced rat mammary carcinoma system for the evaluation of tamoxifen as a potential adjuvant therapy. Reviews on Endocrine-related Cancer (October supplement) 1978:49–55. [Google Scholar]
- Jordan VC. Laboratory studies to develop general principles for the adjuvant treatment of breast cancer with antiestrogens: problems and potential for future clinical applications. Breast Cancer Res Treat. 1983;3 (Suppl):S73–86. doi: 10.1007/BF01855131. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984;36:245–276. [PubMed] [Google Scholar]
- Jordan VC. The development of tamoxifen for breast cancer therapy: a tribute to the late Arthur L. Walpole. Breast Cancer Res Treat. 1988;11:197–209. doi: 10.1007/BF01807278. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Selective estrogen receptor modulation: a personal perspective. Cancer Res. 2001;61:5683–5687. [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5:207–213. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen (ICI 46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol. 2006;147 (Suppl 1):S269–276. doi: 10.1038/sj.bjp.0706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26:3073–3082. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- Jordan VC, editor. Estrogen Action, Selective Estrogen Receptor Modulators and Women’s Health: Progress and Promise. London: Imperial College Press; 2013. [Google Scholar]
- Jordan VC, Allen KE. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxy tamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16:239–251. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75:305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Dix CJ, Allen KE. The effectiveness of long term tamoxifen treatment in a laboratory model for adjuvant hormone therapy of breast cancer. In: Salmon SE, Jones SE, editors. Adjuvant Therapy II. Grune & Stratton; 1979. pp. 19–26. [Google Scholar]
- Jordan VC, Koerner S. Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J Cancer. 1975;11:205–206. doi: 10.1016/0014-2964(75)90119-x. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Prestwich G. Binding of [3H]tamoxifen in rat uterine cytosols: a comparison of swinging bucket and vertical tube rotor sucrose density gradient analysis. Mol Cell Endocrinol. 1977;8:179–188. doi: 10.1016/0303-7207(77)90090-9. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Zava DT, Eppenburger U, Kiser A, Sebek S, Dowdle E, Krozowski Z, Bennett RC, Funder J, Holdaway IM, et al. Reliability of steroid hormone receptor assays: an international study. Eur J Cancer Clin Oncol. 1983;19:357–363. doi: 10.1016/0277-5379(83)90133-5. [DOI] [PubMed] [Google Scholar]
- Klopper A, Hall M. New synthetic agent for the induction of ovulation: preliminary trials in women. Br Med J. 1971;1:152–154. doi: 10.1136/bmj.1.5741.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin RC, Carey TF. Cataracts in patients treated with triparanol. JAMA. 1962;181:339–340. doi: 10.1001/jama.1962.03050300059020a. [DOI] [PubMed] [Google Scholar]
- Lerner LJ, Holthaus FJ, Jr, Thompson CR. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958;63:295–318. doi: 10.1210/endo-63-3-295. [DOI] [PubMed] [Google Scholar]
- Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990;50:4177–4189. [PubMed] [Google Scholar]
- Lippman ME, Bolan G. Oestrogen-responsive human breast cancer in long term tissue culture. Nature. 1975;256:592–593. doi: 10.1038/256592a0. [DOI] [PubMed] [Google Scholar]
- Ludwig Breast Cancer Study Group. Randomised trial of chemo-endocrine therapy, endocrine therapy, and mastectomy alone in postmenopausal patients with operable breast cancer and axillary node metastasis. Lancet. 1984;1:1256–1260. [PubMed] [Google Scholar]
- Maximov PY, McDaniel RE, Jordan VC. Milestones in Drug Therapy. Basel: Springer; 2013 Tamoxifen: Pioneering Medicine in Breast Cancer. [Google Scholar]
- Morgan LR, Jr, Schein PS, Woolley PV, Hoth D, Macdonald J, Lippman M, Posey LE, Beazley RW. Therapeutic use of tamoxifen in advanced breast cancer: correlation with biochemical parameters. Cancer Treat Rep. 1976;60:1437–1443. [PubMed] [Google Scholar]
- Nolvadex Adjuvant Trial Organisation. Controlled trial of tamoxifen as adjuvant agent in management of early breast cancer. Interim analysis at four years. Lancet. 1983;1:257–261. [PubMed] [Google Scholar]
- Obiorah I, Jordan VC. Scientific rationale for postmenopause delay in the use of conjugated equine estrogens among postmenopausal women that causes reduction in breast cancer incidence and mortality. Menopause. 2013;20:372–382. doi: 10.1097/GME.0b013e31828865a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- Poirot M. Four decades of discovery in breast cancer research and treatment –an interview with V. Craig Jordan. Int J Dev Biol. 2011;55:703–712. doi: 10.1387/ijdb.113418mp. [DOI] [PubMed] [Google Scholar]
- Ribeiro G, Palmer MK. Adjuvant tamoxifen for operable carcinoma of the breast: report of clinical trial by the Christie Hospital and Holt Radium Institute. Br Med J (Clin Res Ed) 1983;286:827–830. doi: 10.1136/bmj.286.6368.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro G, Swindell R. The Christie Hospital tamoxifen (Nolvadex) adjuvant trial for operable breast carcinoma--7-yr results. Eur J Cancer Clin Oncol. 1985;21:897–900. doi: 10.1016/0277-5379(85)90104-x. [DOI] [PubMed] [Google Scholar]
- Rose C, Thorpe SM, Andersen KW, Pedersen BV, Mouridsen HT, Blichert-Toft M, Rasmussen BB. Beneficial effect of adjuvant tamoxifen therapy in primary breast cancer patients with high oestrogen receptor values. Lancet. 1985;1:16–19. doi: 10.1016/s0140-6736(85)90966-3. [DOI] [PubMed] [Google Scholar]
- Scottish Cancer Trials Office (MRC) Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Edinburgh. Lancet. 1987;2:171–175. [PubMed] [Google Scholar]
- Segal SJ, Nelson WO. An orally active compound with anti-fertility effects in rats. Proc Soc Exp Biol Med. 1958;98:431–436. doi: 10.3181/00379727-98-24068. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- The aTTom Collaborative Group. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. Journal of Clinical Oncology. 2013;31(No 18_supplement) [Google Scholar]
- Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966;55:1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft D, Shyamala G, Gorski J. A receptor molecule for estrogens: studies using a cell-free system. Proc Natl Acad Sci U S A. 1967;57:1740–1743. doi: 10.1073/pnas.57.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormey DC, Jordan VC. Long-term tamoxifen adjuvant therapy in node-positive breast cancer: a metabolic and pilot clinical study. Breast Cancer Res Treat. 1984;4:297–302. doi: 10.1007/BF01806042. [DOI] [PubMed] [Google Scholar]
- Tormey DC, Rasmussen P, Jordan VC. Long-term adjuvant tamoxifen study: clinical update. Breast Cancer Res Treat. 1987;9:157–158. doi: 10.1007/BF01807370. [DOI] [PubMed] [Google Scholar]
- Ward HW. Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels. Br Med J. 1973;1:13–14. doi: 10.1136/bmj.1.5844.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DM, Jordan VC. A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res. 1993;127:23–33. doi: 10.1007/978-3-642-84745-5_4. [DOI] [PubMed] [Google Scholar]
- Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O’Regan RM, Jordan VC. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–2036. [PubMed] [Google Scholar]