Abstract
The kallikreins are a family of serine proteases with a range of tissue-specific and essential proteolytic functions. Among the most well-studied are the prostate tissue specific KLK2 and KLK3 genes and their secreted protease products, hk2 and PSA. Members of the so-called classic kallikreins, these highly active trypsin-like serine proteases play established roles in human reproduction. Both hK2 and PSA expression is regulated by the androgen receptor, whose activity has a fundamental role in prostate tissue development and progression of disease. This feature, combined with the ability to sensitively detect different forms of these proteins in blood and biopsies, result in a crucially important biomarker for the presence and recurrence of cancer. Emerging evidence has begun to suggest a role for these kallikreins in critical vascular events. This review discusses the established and developing biological roles of hK2 and PSA, as well as the historical and advanced use of their detection to accurately and non-invasively detect and guide treatment of prostatic disease.
Introduction
Tissue kallikrein and kallikrein-related peptidases (KLKs) comprise a family of 15 homologous secreted trypsin- or chymotrypsin-like serine proteases. These enzymes play important roles in a variety of complex processes including reproductive function, inflammation, skin homeostasis, blood clotting, fibrinolysis, and possibly cancer. Kallikreins are expressed in numerous tissues, are commonly co-expressed [1], and are localized predominantly in the cytoplasm of glandular epithelia. The multiplicity of these enzymes and their diverse roles has attracted considerable interest for use as diagnostic markers and for therapeutic targets across many pathologies. Despite their importance, many of the functions of this family of proteases remain unknown. The presence and forms of the kallikreins found in glandular excretions, such as breast milk, seminal fluid, and other fluids, such as blood and urine, have long been investigated in order to better understand the vital functions of KLK-gene products and to detect disease.
KLK2 and KLK3 have the most organ restricted expression profile of all KLKs; specifically, they are abundantly expressed in the luminal epithelium of the prostate. KLK2-KLK3 constitute two of the few highly expressed genes and secreted proteins produced by this gland. While low expression may be detected in other organs, the levels found in extra-prostatic tissue are many orders of magnitude lower, and the biological significance of KLK2-KLK3 in these tissues remains uncertain. The abundant production in the prostate helps to explain why the KLK3-gene product is commonly called Prostate-Specific Antigen or PSA. By convention, we will refer to KLK2 as “human Kallikrein 2” (or hK2).
KLK2 and KLK3 are also unique in that they are regulated by androgens, with expression levels reflecting the functional status and activity of the nuclear androgen receptor (AR) and its response to supply of testosterone or other androgens [2]. The unique combination of tissue-specificity and androgen-driven expression profile of KLK2 and KLK3 provide a straightforward and biologically relevant read-out of the activity of the prostate epithelium. This is particularly useful in the context of detecting and monitoring prostate cancer. This review will focus on these two most well studied and prostate-specific kallikreins, PSA and hK2. We will introduce and discuss their roles in prostate and prostate cancer biology, in detecting prostate cancer, the most commonly diagnosed malignancy in men [3] and their potential contribution to vascular disorders.
Kallikrein Genetics
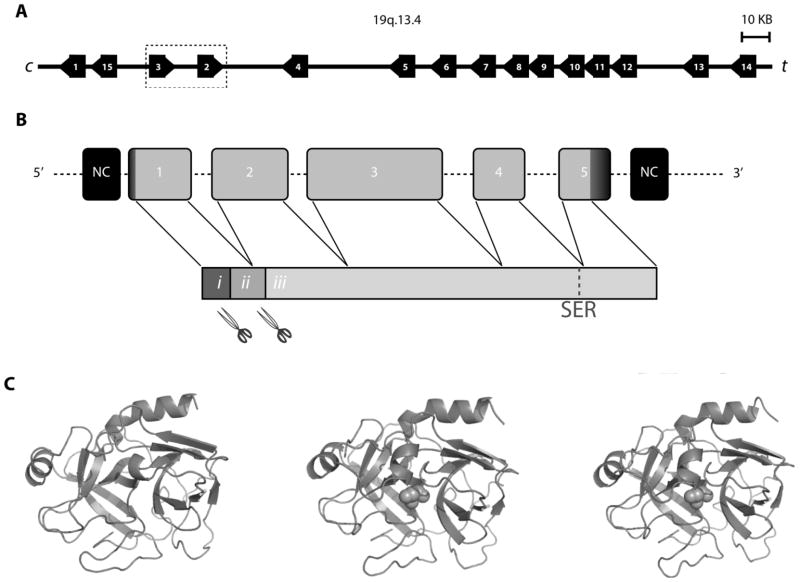
Located in series at chromosomal region 19q13.3-q13.4, the kallikreins comprise the largest cluster of peptidases in the human genome [4] (Fig. 1A). Each of the tissue kallikrein genes shares similar encoding features. This includes five exons of similar size separated by four introns of varying sizes [5] (Fig. 1B). KLK1-3 are considered the “classic kallikreins” due to phylogenetic analysis showing a distinct evolutionary history from the other human kallikreins. It is likely that a duplication of KLK1 created the progenitor of KLK2 in the early evolution of eutherian mammals, with subsequent primate-specific duplication of the predecessor that gave rise to KLK3 and KLK2 [6, 7]. The progenitor of KLK2 is a non-functional pseudogene in many mammals including rodents, but not in canines in which expression is regulated by AR and abundant in the canine prostate [6]. Distinct from KLK4-15, KLK1-3 also share a unique surface loop called the “kallikrein loop”. The evolutionary relationship is also reflected in the amino acid sequences; the identity in amino acid sequence with KLK1 is much higher for KLK2 or KLK3 (62–67%) than for KLK4-15 (27–39%). KLK2 and KLK3 manifest 80% identity in amino acid sequence, making them the most closely homologous KLK-gene products.
Figure 1.
KLK2,3 Activity
The KLK family of proteases has important functions in a range of tissues. Therefore, regulation of the enzymes’ activities may be essential for the maintenance of health, and dysregulation of such activity can be used as a marker for disease (Table 1, and following sections). This perhaps explains why the proteases are produced as non-catalytic preproenzymes which requiring multiple posttranslational modifications to result in a catalytically active form (Fig. 1B). To obtain the catalytic PSA or hK2, the proteolytic cleavage of the signal sequence by a signal peptidase precedes the proteolytic release of the short N-terminal activation peptide by trypsin-like peptidase(s) converting the non-catalytic zymogen to an enzymatically active 237-amino acid single-chain form of PSA or hK2 [8]. The trypsin-like tertiary structure differs between each kallikrein, resulting in variation in specificity and cleaving/activating motifs of physiologic substrates (Fig. 1C).
Table 1.
| Tissue Kallikrein | Alternative Names | Protease Specificity | Tissue Expression | Disease Biomarker | Prostate-Specific KLKs Vascular Substrates |
|---|---|---|---|---|---|
| KLK1 | Pancreastic/renal Kallikrein (hPRK), hK1 | Trypsin/Chymotrypsin | A, K | P′, B′, K′ | |
| KLK2 | Human (glandular Kallikrein 2 (hK2; hGK1) | Trypsin | P | P′, B′ | Fibronectin |
| KLK3 | Prostate Specific Antigen (PSA; hK3, APS) | Chymotrypsin | B, P | P′, B′ | Plasminogen, Fibronectin |
| KLK4 | Kallikrein-like 1 (KLK-L1), PRSS17, Enamel Matrix Serine Protease 1 (EMSP1), Androgen- Regulated Message 1 (ARM1), hK4, Prostase Serine-like Antigen | Trypsin | CNS, G, H, P, T | P′, O′, B′ | |
| KLK5 | Kallikrein-like 2 (KLK-L2), Human Stratum Corneum Tryptic Enzyme (HSCTE), hK5 | Trypsin | B, D, K, S | P′, O′, B′, T′, K′ | |
| KLK6 | Serine Protease 9, Serine Protease 18, Zyme, Neurosin, Protease M, Myelencephalo n-specific Protease, hK6 | Trypsin | CNS | B′, O′, K′ | |
| KLK7 | Human Stratum Corneum Chymotryptic Enzyme, hK7 | Chymotrypsin | A, C, D, K, L, O | O′, K′ | |
| KLK8 | Ovasin, Neuropsin, Brain Serine Protease 1 (BSP1), hK8 | Trypsin | B, CNS, D, K, S | O′ | |
| KLK9 | Kallikrein-like 3 (KLK-L3), hK9 | unknown | CNS, D, O, P, T | O′ | |
| KLK10 | Normal Epithelial Cell- Specific 1 (NES1), hK10 | Trypsin/Chymotrypsin | B, K, O, P | P′, O′, B′, T′, K′ | |
| KLK11 | Hippostasin, Trypsin-like Serine Protease (TLSP), hK11 | Trypsin/Chymotrypsin | B, CNS, D, F, K, P | O′, P′, K′ | |
| KLK12 | Kallikrein-like 5 (KLK-L5), hK12 | Trypsin | I, P, S, X | B′ | |
| KLK13 | Kallikrein-like 4 (KLK-L4), hK13 | Trypsin | K, S | B′ | |
| KLK14 | Kallikrein-like 6 (KLK-L6), hK14 | Trypsin | B, CNS, K, P, X | B′, O′, T′ | |
| KLK 15 | Prostinogen, ACO protease, hK15 | Trypsin | P, C, T | P′, O′, B′, K′ |
Catalytically active PSA is secreted into the seminal fluid [9]. It is there, along with secreted and possibly auto-activated hK2 [10], that the main gel forming proteins (SEMG1 and SEMG2) contributed by the seminal vesicles of the ejaculatory mix can be proteolytically degraded by KLK3 (possibly also KLK2) to liquefy the seminal gel [9, 11, 12]. This critical action results in the freeing of spermatozoa for the necessary translocation to the womb to fertilize the egg.
Cleavage of semenogelin and fibronectin in the ejaculate suggest that these serine proteases could degrade extracellular matrix proteins elsewhere [11]. Accordingly, investigations have focused on a potential link between KLK2-3 function and the invasion and growth of prostate cancer. While the explicit role(s) of the prostate-tissue kallikreins in prostate cancer development and progression remainis unclear, there is considerable interest in determining if such links exist. For example, the activation of urokinase by hK2 provides a scenario in which this enzyme initiates a proteolytic cascade to degrade the tissues surrounding a tumour and contributing to expansion [13]. In addition to degrading the surrounding tissue, it has been reported that hK2 and PSA activity can directly promote cancer cell growth. Studies have shown that the kallikreins are able to cleave insulin-like growth factor proteins, yielding a growth factor for prostate cancer [14–16] and may regulate the activity of PTHrP in vitro [17].
Described in detail in the following section, catalytically active PSA interacts with native α2-macroglobulin (A2M) to form stable covalent complexes [18], while A2M has been shown to bind cell-surface glucose-regulated protein (GRP78). This activates canonical proliferative and survival pathways such as Akt, MEK and ERK signaling cascades. This work suggests a critical role for PSA in driving aggressive disease as it induces a feed-forward loop for the production of more of the PSA-activated α2-macroglobulin complex [19]. Recently, Galectin-3 in the seminal fluid has been identified as a substrate for PSA [20]. This may explain the presence of cleaved galectin-3 at sites of prostate cancer, with potentially important consequences for tumour development and angiogenesis by this biologically important lectin [21].
An important role for hK1 (the product of the KLK1 gene) involves the regulation of blood pressure homeostasis and inflammation. hK1 acts on kinonogen to release the vasoactive peptide lys-bradykinin [22]. At present, there exists no evidence that the catalytic action of PSA or hK2 could contribute a critical role in the kallikrein/kinin system. While hK2 has been shown to have some activity, it is strictly limited by the 1000-fold lower kinin cleavage relative to hK1 [23].
Emerging work has evaluated the role of PSA activity on endothelial cell proliferation and migration, suggesting an anti-angiogenic effect. In vitro experiments have shown that human endothelial umbilical cord (HUVEC) proliferation and invasion was inhibited by addition of PSA to culture media. In vivo, using a metastatic melanoma model, high concentrations of systemically administered PSA reduced the number of metastases detected [24, 25]. Indeed, in vitro studies have shown that inhibition of PSA activity (by small molecules [26], antibodies [27] and peptides [28]) negates the putative anti-angiogenic effects of PSA. With continually refined models of kallikrein expression in cancer prone and healthy mice, it is expected that greater clarity of the putative effects of the PSA and hK2 on the vasculature will be gained in the near future.
Prostate Cancer and PSA and hK2 as Biomarkers
Prostate cancer is highly dependent on AR signaling for both cancer cell proliferation and survival. There is no definitive explanation for how the generally slow-growing adenocarcinoma forms. However, the dependence of the disease on AR can be utilized for the treatment of the disease through androgen deprivation therapy. Inevitably, the disease progresses to a lethal castration-resistant phenotype.
The disease is most commonly diagnosed by digital rectal examination or PSA-testing in blood. The serum PSA test, which is the most commonly used cancer biomarker assay, refers to the measuring of the “total” immune-reactive concentration of PSA in serum or plasma. Normally, the architecture of the prostate gland maintains the highly abundant levels of PSA (10–100 micromoles per liter) tightly confined to the glandular ducts and the secretory epithelium. In this healthy state, PSA occurs in the blood circulation at approximately one-millionth of the intra-ductal levels, with median blood levels in healthy adult males below age 50 close to 0.6 ng/mL [29, 30]. Disease processes such as malignant transformation of the prostate epithelium may result in degradation of the basement membrane, loss of basal cell layer and glandular architecture, but experimental evidence is lacking whether this leads to increased release of PSA into the blood circulation where the levels may elevate up to 10,000-fold. However, a large body of evidence suggests that the detection of increased levels of PSA in blood constitutes a highly sensitive (albeit not very specific) means of detecting risk or the presence of prostate disease, and is an effective means to monitor recurrence following treatment [30].
Due to the catalytic activity of PSA and the 10,000-fold molar excess of proteinase inhibitors present in extra-cellular fluids, different forms of PSA are detected in blood [31]. When released into blood, enzymatically-active PSA forms irreversible and covalently linked complexes with extra-cellular protease inhibitors such as α1-antichymotrypsin (ACT or SERPINA3), α2-macroglobulin (A2M), pregnancy-zone protein (PZP), α1-antitrypsin (SERPINA1) [32], or the protein C inhibitor (SERPINA5) [33]. PSA bound to ACT constitutes the predominant fraction of the immunoreactive forms of PSA in blood [31, 34, 35].
In addition to these irreversibly linked complexes of the activated PSA, there are non-catalytic PSA-forms [19, 35]. These proteins are not bound by any of the inhibitors and commonly referred to as “free PSA” and are present at proportions varying from less than 5% up to greater than 45% of the “total” immunoreactive PSA present in blood. The free PSA is proteolytically processed to an inactive form and is therefore not able to form complexes with inhibitors noted above, nor is it thought to have a role in cleaving vascular substrates. The different inactive free PSA-forms have been thoroughly characterized and comprise proprotein subtypes with varying extensions of the N-terminal propeptide (e.g. [−2] proPSA) [36], single-chain form(s) with truncated N-terminal [37], and non-catalytic single-chain protein, nicked PSA, which contains internal peptide bond cleavage(s) at Lys145 or Lys146, resulting in the loss of an antigenic epitope critical for high-affinity antibody binding to the non-catalytic single-chain free PSA-forms referred to as intact PSA (iPSA) [38]. Tests have been developed that are able to distinguish many of the forms [39–41] and “total PSA” refers to the sum of the different forms of detectable PSA [19]. With respect to hK2, levels in blood are commonly 10−2 of those determined for total PSA. Here, the majority of the non-catalytic hK2-forms in blood appear to be unbound to any of the inhibitors discussed above [42].
The measurement of the several fractions of PSA has been proposed with the aim of improving the specificity and sensitivity of total PSA. Multiple studies, including several meta-analyses, have showed that the determination of the percentage of free PSA (%fPSA) is useful to improve the detection of prostate cancer [43]. More recently, several research teams have been successful in increasing accuracy by also including the subforms iPSA or [−2] proPSA, and hereby creating detections systems called “indexes” or “panels”. The Prostate Health Index (phi) is a mathematical combination with [−2] proPSA according to the formula [− 2] proPSA/fPSA) x√ tPSA [44]. The panel of four kallikrein markers includes intact PSA and hK2 [45, 46]. The underlying hypothesis of this approach is that the relative amounts of the different prostate-kallikrein subtypes are indicative of underlying disease biology, with implications for patient selection and management. While promising, a rigorous comparison, or combination, of these systems has yet not been completed.
PSA Screening, Validation and Concerns
Historically, the only early detection strategy for prostate cancer was digital rectal exam (DRE). However, as a screening tool DRE is far from perfect most notably for finding many tumours that are no longer curable by the time they are palpable [47]. Hence, there was a need for a better test that could detect asymptomatic disease at an earlier stage. This call was met with the discovery and implementation of the PSA blood test in the late 1980’s [48, 49]. This marked a significant global shift towards earlier detection and improved management of prostate cancer. Over the past two decades, the age-adjusted death rate from prostate cancer has decreased by 40% in the U.S., owing to the combination of early detection with improvements in treatment [3].
The utility of PSA detection as a screening tool has been evaluated in trials in Europe and the U.S. The world’s largest randomized controlled PSA screening trial, with nearly 180,000 participants in eight countries was initiated in 1995 as the European Randomized Study of Screening for Prostate Cancer (ERSPC) [50]. In January 1995, the Göteborg Randomized Population-based Prostate Cancer Screening Trial [51] began in Sweden, inviting 10,000 men for biennial PSA screening. This trial was integrated with the ERSPC in 1996. These two high-quality trials have demonstrated significant reductions in prostate-cancer mortality by screening men 55–69 years every two-four years and men 50–64 years every two years. The results show a 21% prostate-cancer mortality reduction in favor of screening after 11 years of follow-up and 44% after 14 years. [51, 52]
The largest U.S. trial, the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial, was also initiated in the early 1990’s. However, in contrast to Europe, where PSA testing was not recommended and very few men had taken a PSA-test, opportunistic PSA testing had already become widespread in the U.S. by this time [53]. About half of the men in the control arm of the PLCO trial had a PSA test during the first years of the trial and 30% of men in the screening arm were pre-screened [53–55]. This so-called contamination diluted the PLCO’s capacity to distinguishing any significant differences in prostate-cancer mortality between the groups. Accordingly, at 13 years of follow-up, there was no statistically significant difference in prostate-cancer mortality risk between the study groups (risk ratio 1.09; 95% CI, 0.87–1.36) [56].
The evidence of reduced prostate cancer mortality at the population level may not translate to survival advantages at the individual level, generating considerable controversy. The benefits of PSA screening for enhanced detection include early detection and enhanced cure, prevention of metastatic disease [57] and reduced prostate cancer mortality. However, these benefits must be weighed against potential harm to patient management in a generally slow growing disease. Impairments include anxiety/distress, the potential for false-positives, unnecessary prostate biopsies, over-diagnosis, infectious complications from biopsies and side-effects from treatment such as impotence and urinary incontinence with subsequent impact on quality of life [58]. This imbalance between benefit and harm, is one of the reasons why the United States Preventive Services Task Force recommended against PSA screening in 2012 [59].
Although the relative cost-benefit ratio for the PSA testing continues to be examined, incorporating long-term follow-up data of studies such as the ERSPC, it is obvious that advanced screening strategies are needed to reduce disadvantages. Improvements to sensitivity and specificity can include reduction in false-positives and over-diagnosis. The way forward involves risk stratification approaches, where PSA-testing is used together with other clinical risk factors and markers, to predict individual risk – and takes into account the individual’s preferences [60]. Many men demand the PSA-test and most guideline groups and professional urological organizations agree on and emphasize shared decision-making, stipulating that “PSA screening must be preceded by a discussion regarding risks and benefits” [60, 61] and that well-informed men suitable for screening should have access to PSA testing upon request [62].
Advanced Screening with KLK2 and KLK3
Population representation, storage conditions of samples, completeness of follow-up and reproducibility are some of many important factors that need to be considered with respect to interpreting outcomes from retrospective biomarker studies. One of the few cohorts that meet these stringent criteria, and has a very low rate of PSA testing, is the Malmö Preventive Medicine cohort (MPP). The MPP is a large, representative, population-based study of cardiovascular risk factors that took place in Malmö, Sweden between 1974 and 1992 [63]. The study included 22,444 men (participation rate 71.2%), of whom 21,277 were ≤50 years of age at the baseline blood draw. Through the end of 1999, 498 of the 21,277 participants who were aged less than 50 years at study participation had been diagnosed with prostate cancer.
Strikingly, studies based on the MPP data demonstrate that a single measurement of either PSA, or hK2, measured at or before age 50 predicts advanced prostate cancer diagnosed up to 30 years in advance [64–66]. Interestingly, total PSA (the sum of PSA complexed with ACT and the free, uncomplexed form) is a stronger predictor in younger men, while percent free PSA and hK2 add important predictive value in older men and much closer to diagnosis [67]. In summary, these studies show that the use of early PSA to stratify risk would allow a large group of low-risk men to be screened less often but increase frequency of testing on a more limited number of high-risk men.
Beyond serum ELISAs: Emerging technologies to utilize PSA activity and subforms
Kallikrein Inhibitors
A deeper understanding of the molecular properties of PSA has engendered new approaches to target this protein for prostate cancer diagnostics and therapy. A seminal example is how the discovery of PSA biochemistry provoked several fruitful endeavors to identify active site directed ligands. Indeed, upon the discovery that PSA is a serine protease, many groups drew from the prior medicinal chemistry literature on this enzyme family to design and synthesize avid and selective peptide-based substrate mimetics. As expected, incrementally more potent binding (and inhibition) was achieved by grafting electrophiles (e.g. aldehydes or boronic acids) onto the peptide leads to engage PSA’s catalytic serine nucleophile [68, 69]. In addition, small molecules bearing an electrophilic chemotype known to react with activated serine nucleophiles (in this case, a β-lactam) have been shown to irreversibly inhibit PSA catalytic activity [26]. Several of these compounds suppress angiogenesis in preclinical models, further underscoring the potential value of curbing PSA proteolytic activity. The inhibition of other kallikrein-activities has already been translated to the clinic (Aprotinin; to reduce peri-operative bleeding in cardiac surgery [70]). Pending first in man studies, the ultimate clinical utility of this strategy for PSA can be assessed.
PSA possesses a unique substrate specificity among serine proteases (to hydrolyze amide bonds on peptides bearing a glutamine residue at the P1 position [69]). This feature has inspired some creative chemistry to develop pro-drugs for the selective activation of therapies at the prostate pre-neoplastic or tumour microenvironment. In such a strategy, cytotoxic chemotherapies are rendered inactive by conjugation to a short peptide sequence (HSSKLQ). This sequence is specifically cleaved by PSA in situ with an efficiency vastly exceeding that of other abundant serine proteases [71–73]. This then enables site-specific activation and delivery of the chemotherapeutic agent. Among these drug candidates, preclinical trials have shown that systemic administration of a HSSKLQ-doxorubicin adduct incurs no obvious toxicity in mice at doses several fold higher than the MTD for doxorubicin. This pro-drug system demonstrated tumour regression in a prostate cancer model that endogenously produces and secretes catalytically active PSA. Again, the promise of these preclinical findings argues strongly for a human trial to assess the therapeutic potential of this strategy.
Imaging Free PSA
The complex posttranslational regulation of PSA protein has also born a clever strategy to image PSA expression at a prostate tumour. Much like the thinking that motivated the pro-drug technology, Ulmert et al. reasoned that tumour imaging could be achieved by selectively targeting the pool of PSA uncomplexed to serpins (“free” PSA) known to transiently exist in pericellular space [74]. As PSA in blood is overwhelmingly bound to serpins, this strategy circumvents the inherent challenges in imaging a tumour biomarker with an abundant circulating isoform (in principle, the circulating isoform could impair tumour visualization by binding a majority of the systemically administered imaging tool). Consistent with this hypothesis, a radiolabeled monoclonal antibody to an epitope on PSA that is masked by bound serpins (89Zr-labeled 5A10) produced high contrast images of PSA-secreting prostate cancer tumours in animal models with positron emission tomography (PET). This radiotracer also detected small orthotopic tumours in the bone, suggesting it could be a powerful tool for prostate cancer staging at diagnosis. Of note, some of the aforementioned substrate mimetics have been successfully conjugated to the radionuclide 99mTc, and the promise of 89Zr-5A10 PET suggests these radiolabeled ligands may demarcate prostate tumours as well [75].
Ironically, current gaps in our knowledge of PSA biology have also inspired new technologies to expand the utility of this biomarker. Specifically, there is a strong need to understand the paradoxical observation that declines in serum PSA do not lead to a survival benefit from androgen deprivation therapy in castration-resistant prostate cancer (CRPC). It is thought that a more refined monitoring of AR activity, as discussed below in tumours or single cells, rather than an average serum value, could shed light on this phenomenon. The rationale for this hypothesis is based on the widely accepted observation that the concentration of intratumoural PSA vastly exceeds that present in serum, and there is no known role for AR in the rate limiting steps of PSA secretion or leakage into serum. In this regard, one can reasonably speculate that relative changes in secreted PSA levels need not necessarily reflect corresponding changes in AR activity, and by extension, a technology that measures a pool of PSA more closely linked to AR transcriptional activity could provide a higher fidelity readout of AR signaling.
This concept was formally introduced to the literature by comparing 89Zr-5A10 PET to serum PSA levels post MDV3100 therapy in preclinical models of CRPC. Downregulation of 89Zr-5A10 localization to a prostate cancer tumour post MDV3100 treatment was observed without a corresponding change in serum PSA levels from the respective animals over an acute treatment interval. Although far short of validating this concept, these findings nevertheless support the promise in exploring PSA measurements orthogonal to the serum ELISA.
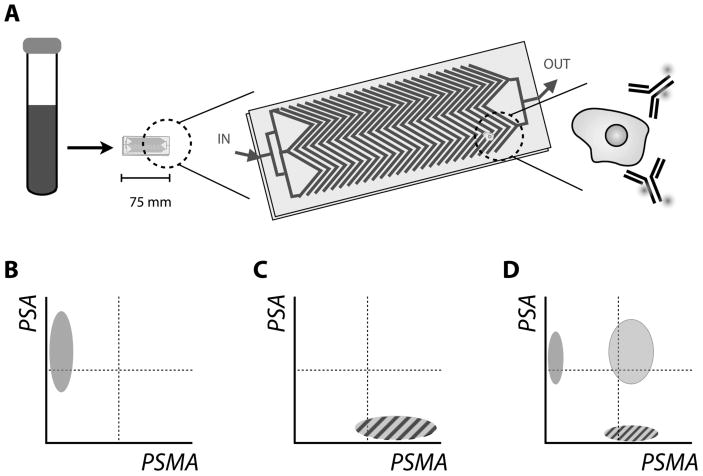
A second example of using PSA measurements to determine AR activity has utilized a novel microfluidics platform to capture and analyze CTCs on a per cell basis. Miyamoto et al. have reported that PSA expression (and that of other markers) can be effectively quantified in CTCs by staining with fluorescently labeled monoclonal antibodies and subsequent immunofluorescent analysis to determine AR status [76] (Fig. 2A). This enables single-cell molecular characterization within and across patients. Significant (and expected) changes in the molecular profile of PSA expression were detected from the CTCs assayed from patients undergoing therapy with Lupron™ (an androgen deprivation therapy) or abiraterone acetate (an androgen biosynthesis inhibitor). In this proof-of-concept report, no pairwise comparison was made between the predictive value of the molecular changes in CTCs versus serum PSA levels of patients. However, the results clearly showed that the CTCs from patient with previously untreated disease show an “AR-on” phenotype, which turned “AR-off” after initial treatment, which also eliminated the CTCs from circulation. Upon progression to CRPC and reemergence of CTCs, the molecular profiling reveals a mixture of phenotypes (Fig. 2B). This surprising finding sits in contrast to current popular opinion of the role AR plays in CRPC. It also adds to the discourse regarding inter- or intra-patient tumour heterogeneity at this stage of the disease and may yield molecularly distinguished populations for enhanced patient management.
Figure 2.
The relative ease with which blood samples can be acquired from large groups of patients following therapy for such evaluation foreshadows a potentially landmark study for the field. To this debate, serum PSA measurements unfortunately offer little clarity, as it by nature represents an averaged sum of production from lesions in the body. In this regard, it is also important to highlight how developing new technologies for even established serum biomarkers like PSA might enhance our understanding of the fundamental biology of clinical disease.
Conclusion
PSA and hK2 are serine proteases in the kallikrein family that are highly and specifically expressed in the prostate. Their detection in the general blood circulation has become an established biomarker for the detection of prostate cancer. Increasing interest is being directed to their role in regulation of angiogenic and vascular events. While debate continues on the weight that PSA level should have in directing options for treatment, advancements in the accuracy and information that can be determined continue to be made. New technologies have recently demonstrated the visualization of PSA pools in vivo with high-resolution, quantitative and non-invasive techniques. Expansion of the preclinical successes of 89Zr-5A10 to man may ultimately result in the same paradigmatic shift in patient management as occurred with the advent of the modern PSA screening era.
Acknowledgments
DLJT was supported by US National Institutes of Health (NIH) through the R25T Molecular Imaging Fellowship: Molecular Imaging Training in Oncology (5R25CA096945-07; Prinicpal investigator H. Hricack) and Steve Wynn through the Young Investigator Award of the Prostate Cancer Foundation (PCF). MJE was supported by the Imaging and Radiation Sciences Bridge Program and the Experimental Therapeutics Center of MSKCC, Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the David H. Koch Young Investigator Award from the PCF, and NIH (K99CA172695, R01CA176671). SC was supported by the Swedish Cancer Society, the Sweden America Foundation, the Swedish Council for Working Life and Social Research, and the Swedish Society for Medical Research. Additional support was provided from NIH [R01 CA160816, R33 CA 127768-03, P50-CA92629]; Swedish Cancer Society [11-0624]; the Sidney Kimmel Center for Prostate and Urologic Cancers; David H. Koch through the PCF (DU); the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford; FiDIPro-program award from TEKES (Finland) and Fundación Federico SA.
Footnotes
Conflicts of Interest:
Dr. Lilja holds patents for free PSA, intact PSA, and hK2 assays. Dr. Lilja is named as co-inventor on a patent application for a statistical method to predict the result of prostate biopsy.
References
- 1.Shaw JL, Diamandis EP. Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem. 2007;53(8):1423–32. doi: 10.1373/clinchem.2007.088104. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Coetzee GA. Prostate specific antigen gene regulation by androgen receptor. J Cell Biochem. 2004;93(2):233–41. doi: 10.1002/jcb.20228. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Lundwall A, et al. A comprehensive nomenclature for serine proteases with homology to tissue kallikreins. Biol Chem. 2006;387(6):637–41. doi: 10.1515/BC.2006.082. [DOI] [PubMed] [Google Scholar]
- 5.Clements JA, et al. The tissue kallikrein family of serine proteases: functional roles in human disease and potential as clinical biomarkers. Crit Rev Clin Lab Sci. 2004;41(3):265–312. doi: 10.1080/10408360490471931. [DOI] [PubMed] [Google Scholar]
- 6.Olsson AY, Lilja H, Lundwall A. Taxon-specific evolution of glandular kallikrein genes and identification of a progenitor of prostate-specific antigen. Genomics. 2004;84(1):147–56. doi: 10.1016/j.ygeno.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Marques PI, et al. Birth-and-death of KLK3 and KLK2 in primates: evolution driven by reproductive biology. Genome Biol Evol. 2012;4(12):1331–8. doi: 10.1093/gbe/evs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovgren J, et al. Activation of the zymogen form of prostate-specific antigen by human glandular kallikrein 2. Biochem Biophys Res Commun. 1997;238(2):549–55. doi: 10.1006/bbrc.1997.7333. [DOI] [PubMed] [Google Scholar]
- 9.Lilja H, Laurell CB. The predominant protein in human seminal coagulate. Scand J Clin Lab Invest. 1985;45(7):635–41. doi: 10.3109/00365518509155271. [DOI] [PubMed] [Google Scholar]
- 10.Denmeade SR, et al. Activation of latent protease function of pro-hK2, but not pro-PSA, involves autoprocessing. Prostate. 2001;48(2):122–6. doi: 10.1002/pros.1088. [DOI] [PubMed] [Google Scholar]
- 11.Lilja H, et al. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest. 1987;80(2):281–5. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lilja H, Abrahamsson PA, Lundwall A. Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem. 1989;264(3):1894–900. [PubMed] [Google Scholar]
- 13.Frenette G, et al. Prostatic kallikrein hK2, but not prostate-specific antigen (hK3), activates single-chain urokinase-type plasminogen activator. Int J Cancer. 1997;71(5):897–9. doi: 10.1002/(sici)1097-0215(19970529)71:5<897::aid-ijc31>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Rehault S, et al. Insulin-like growth factor binding proteins (IGFBPs) as potential physiological substrates for human kallikreins hK2 and hK3. Eur J Biochem. 2001;268(10):2960–8. doi: 10.1046/j.1432-1327.2001.02185.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen P, et al. Biological effects of prostate specific antigen as an insulin-like growth factor binding protein-3 protease. J Endocrinol. 1994;142(3):407–15. doi: 10.1677/joe.0.1420407. [DOI] [PubMed] [Google Scholar]
- 16.Cohen P, et al. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab. 1992;75(4):1046–53. doi: 10.1210/jcem.75.4.1383255. [DOI] [PubMed] [Google Scholar]
- 17.Iwamura M, et al. Alteration of the hormonal bioactivity of parathyroid hormone-related protein (PTHrP) as a result of limited proteolysis by prostate-specific antigen. Urology. 1996;48(2):317–25. doi: 10.1016/S0090-4295(96)00182-3. [DOI] [PubMed] [Google Scholar]
- 18.Christensson A, Laurell CB, Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990;194(3):755–63. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- 19.Misra UK, Payne S, Pizzo SV. Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop: a role for secreted prostate-specific antigen. J Biol Chem. 2011;286(2):1248– 59. doi: 10.1074/jbc.M110.129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saraswati S, et al. Galectin-3 is a substrate for prostate specific antigen (PSA) in human seminal plasma. Prostate. 2011;71(2):197–208. doi: 10.1002/pros.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nangia-Makker P, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156(3):899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahabeer R, Bhoola KD. Kallikrein and kinin receptor genes. Pharmacol Ther. 2000;88(1):77–89. doi: 10.1016/s0163-7258(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 23.Deperthes D, et al. Human kallikrein hK2 has low kininogenase activity while prostate-specific antigen (hK3) has none. Biochim Biophys Acta. 1997;1343(1):102–6. doi: 10.1016/s0167-4838(97)00135-0. [DOI] [PubMed] [Google Scholar]
- 24.Fortier AH, et al. Recombinant prostate specific antigen inhibits angiogenesis in vitro and in vivo. Prostate. 2003;56(3):212–9. doi: 10.1002/pros.10256. [DOI] [PubMed] [Google Scholar]
- 25.Fortier AH, et al. Antiangiogenic activity of prostate-specific antigen. J Natl Cancer Inst. 1999;91(19):1635–40. doi: 10.1093/jnci/91.19.1635. [DOI] [PubMed] [Google Scholar]
- 26.Koistinen H, et al. Novel small molecule inhibitors for prostate-specific antigen. Prostate. 2008;68(11):1143–51. doi: 10.1002/pros.20773. [DOI] [PubMed] [Google Scholar]
- 27.Mattsson JM, et al. The antiangiogenic role of prostate-specific antigen. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; Washington, DC: AACR; 2010. [Google Scholar]
- 28.Mattsson JM, et al. Peptides binding to prostate-specific antigen enhance its antiangiogenic activity. Prostate. 2012;72(14):1588–94. doi: 10.1002/pros.22512. [DOI] [PubMed] [Google Scholar]
- 29.Savblom C, et al. Blood levels of free-PSA but not complex-PSA significantly correlates to prostate release of PSA in semen in young men, while blood levels of complex-PSA, but not free-PSA increase with age. Prostate. 2005;65(1):66–72. doi: 10.1002/pros.20254. [DOI] [PubMed] [Google Scholar]
- 30.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8(4):268–78. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 31.Lilja H, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37(9):1618– 25. [PubMed] [Google Scholar]
- 32.Zhang WM, et al. Prostate-specific antigen forms a complex with and cleaves alpha 1-protease inhibitor in vitro. Prostate. 1997;33(2):87–96. doi: 10.1002/(sici)1097-0045(19971001)33:2<87::aid-pros2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 33.Christensson A, Lilja H. Complex formation between protein C inhibitor and prostate-specific antigen in vitro and in human semen. Eur J Biochem. 1994;220(1):45–53. doi: 10.1111/j.1432-1033.1994.tb18597.x. [DOI] [PubMed] [Google Scholar]
- 34.Stenman UH, et al. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51(1):222–6. [PubMed] [Google Scholar]
- 35.Christensson A, et al. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150(1):100–5. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 36.Mikolajczyk SD, et al. A truncated precursor form of prostate-specific antigen is a more specific serum marker of prostate cancer. Cancer Res. 2001;61(18):6958–63. [PubMed] [Google Scholar]
- 37.Vaisanen V, et al. Characterization and processing of prostate specific antigen (hK3) and human glandular kallikrein (hK2) secreted by LNCaP cells. Prostate Cancer Prostatic Dis. 1999;2(2):91–97. doi: 10.1038/sj.pcan.4500289. [DOI] [PubMed] [Google Scholar]
- 38.Nurmikko P, et al. Production and characterization of novel anti-prostate-specific antigen (PSA) monoclonal antibodies that do not detect internally cleaved Lys145-Lys146 inactive PSA. Clin Chem. 2000;46(10):1610–8. [PubMed] [Google Scholar]
- 39.Vaisanen V, et al. Intact free prostate-specific antigen and free and total human glandular kallikrein 2. Elimination of assay interference by enzymatic digestion of antibodies to F(ab′)2 fragments. Anal Chem. 2006;78(22):7809–15. doi: 10.1021/ac061201+. [DOI] [PubMed] [Google Scholar]
- 40.Nurmikko P, et al. Discrimination of prostate cancer from benign disease by plasma measurement of intact, free prostate-specific antigen lacking an internal cleavage site at Lys145-Lys146. Clin Chem. 2001;47(8):1415–23. [PubMed] [Google Scholar]
- 41.Mikolajczyk SD, et al. Free prostate-specific antigen in serum is becoming more complex. Urology. 2002;59(6):797–802. doi: 10.1016/s0090-4295(01)01605-3. [DOI] [PubMed] [Google Scholar]
- 42.Steuber T, et al. Comparison of free and total forms of serum human kallikrein 2 and prostate-specific antigen for prediction of locally advanced and recurrent prostate cancer. Clin Chem. 2007;53(2):233–40. doi: 10.1373/clinchem.2006.074963. [DOI] [PubMed] [Google Scholar]
- 43.Roddam AW, et al. Use of prostate-specific antigen (PSA) isoforms for the detection of prostate cancer in men with a PSA level of 2–10 ng/ml: systematic review and meta-analysis. Eur Urol. 2005;48(3):386–99. doi: 10.1016/j.eururo.2005.04.015. discussion 398–9. [DOI] [PubMed] [Google Scholar]
- 44.Filella X, Gimenez N. Evaluation of [−2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med. 2013;51(4):729–39. doi: 10.1515/cclm-2012-0410. [DOI] [PubMed] [Google Scholar]
- 45.Vickers AJ, et al. A panel of kallikrein marker predicts prostate cancer in a large, population-based cohort followed for 15 years without screening. Cancer Epidemiol Biomarkers Prev. 2011;20(2):255–61. doi: 10.1158/1055-9965.EPI-10-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metoclopramide in variceal bleeding. Indian J Gastroenterol. 1991;10(2):71–2. [PubMed] [Google Scholar]
- 47.Ankerst DP. Prostate cancer screening. 2. New York: Humana Press; 2009. [Google Scholar]
- 48.Stamey TA, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–16. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 49.Catalona WJ, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156–61. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 50.de Koning HJ, et al. Large-scale randomized prostate cancer screening trials: program performances in the European Randomized Screening for Prostate Cancer trial and the Prostate, Lung, Colorectal and Ovary cancer trial. Int J Cancer. 2002;97(2):237–44. doi: 10.1002/ijc.1588. [DOI] [PubMed] [Google Scholar]
- 51.Hugosson J, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11(8):725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroder FH, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schroder FH, Roobol MJ. ERSPC and PLCO prostate cancer screening studies: what are the differences? Eur Urol. 2010;58(1):46–52. doi: 10.1016/j.eururo.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 54.Andriole GL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulati R, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes Control. 2012;23(6):827–35. doi: 10.1007/s10552-012-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andriole GL, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroder FH, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2012;62(5):745–52. doi: 10.1016/j.eururo.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 58.Heijnsdijk EA, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367(7):595–605. doi: 10.1056/NEJMoa1201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moyer VA and U.S.P.S.T. Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 60.Roobol MJ, Carlsson SV. Risk stratification in prostate cancer screening. Nat Rev Urol. 2012;10(1):38–48. doi: 10.1038/nrurol.2012.225. [DOI] [PubMed] [Google Scholar]
- 61.Gomella LG, et al. Screening for prostate cancer: the current evidence and guidelines controversy. Can J Urol. 2011;18(5):5875–83. [PubMed] [Google Scholar]
- 62.Horwich AHJ, de Reijke T, Wiegel T, Fizazi K, Kataja V Panel Members: Sigrid Carlsson. Annals of Oncology; ESMO Consensus Conference Guidelines 2012.; 2013. pp. 1–22. [DOI] [PubMed] [Google Scholar]
- 63.Nilsson PM, et al. Social mobility, marital status, and mortality risk in an adult life course perspective: the Malmo Preventive Project. Scand J Public Health. 2005;33(6):412–23. doi: 10.1080/14034940510005905. [DOI] [PubMed] [Google Scholar]
- 64.Ulmert D, et al. Prostate-specific antigen at or before age 50 as a predictor of advanced prostate cancer diagnosed up to 25 years later: a case-control study. BMC Med. 2008;6:6. doi: 10.1186/1741-7015-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lilja H, et al. Long-term prediction of prostate cancer up to 25 years before diagnosis of prostate cancer using prostate kallikreins measured at age 44 to 50 years. J Clin Oncol. 2007;25(4):431–6. doi: 10.1200/JCO.2006.06.9351. [DOI] [PubMed] [Google Scholar]
- 66.Lilja H, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011;117(6):1210–9. doi: 10.1002/cncr.25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vickers AJ, et al. The predictive value of prostate cancer biomarkers depends on age and time to diagnosis: towards a biologically-based screening strategy. Int J Cancer. 2007;121(10):2212–7. doi: 10.1002/ijc.22956. [DOI] [PubMed] [Google Scholar]
- 68.LeBeau AM, et al. Potent and selective peptidyl boronic acid inhibitors of the serine protease prostate-specific antigen. Chem Biol. 2008;15(7):665–74. doi: 10.1016/j.chembiol.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeBeau AM, et al. Prostate-specific antigen is a “chymotrypsin-like” serine protease with unique P1 substrate specificity. Biochemistry. 2009;48(15):3490–6. doi: 10.1021/bi9001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cosgrove DM, 3rd, et al. Aprotinin therapy for reoperative myocardial revascularization: a placebo-controlled study. Ann Thorac Surg. 1992;54(6):1031–6. doi: 10.1016/0003-4975(92)90066-d. discussion 1036–8. [DOI] [PubMed] [Google Scholar]
- 71.Denmeade SR, et al. Specific and efficient peptide substrates for assaying the proteolytic activity of prostate-specific antigen. Cancer Res. 1997;57(21):4924–30. [PMC free article] [PubMed] [Google Scholar]
- 72.Denmeade SR, et al. Enzymatic activation of a doxorubicin-peptide prodrug by prostate-specific antigen. Cancer Res. 1998;58(12):2537–40. [PubMed] [Google Scholar]
- 73.Denmeade SR, et al. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst. 2003;95(13):990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- 74.Ulmert D, et al. Imaging androgen receptor signaling with a radiotracer targeting free prostate-specific antigen. Cancer Discov. 2012;2(4):320–7. doi: 10.1158/2159-8290.CD-11-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.LeBeau AM, et al. Optimization of peptide-based inhibitors of prostate-specific antigen (PSA) as targeted imaging agents for prostate cancer. Bioorg Med Chem. 2009;17(14):4888–93. doi: 10.1016/j.bmc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyamoto DT, et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2012;2(11):995–1003. doi: 10.1158/2159-8290.CD-12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]