Abstract
Background
P wave indices and PR interval from 12-lead electrocardiograms (ECGs) are predictors of cardiovascular morbidity and mortality, but their repeatability has not been examined.
Objectives
Determine the short-term repeatability of P wave indices (P axis, maximum P area and duration, P dispersion and P terminal force in V1) and PR interval.
Methods
Participants (n=63) underwent two standard ECGs at each of two visits, two weeks apart. We calculated the intra-class correlation coefficient (ICC), weighted Kappa, and minimal detectable change and difference.
Results
ICCs were 0.93 for PR interval, 0.78 for P axis, 0.77 for maximum P area, and 0.58 for maximum P duration. Within- and between-visit Kappa were 0.30 and 0.11 for P dispersion, and 0.68 and 0.46 for P terminal force.
Conclusion
Repeatability of PR duration was excellent, that of P wave axis and maximum area was fair, and maximum P wave duration and terminal force was poor. Repeatability of P wave dispersion was fair within visit, yet poor between visits. These results illustrate potential biases when measurement error of some P wave indices is ignored in clinical and epidemiologic studies.
Introduction
P wave indices and PR interval are simple electrocardiographic markers that could be derived from resting, standard 12-lead electrocardiograms (ECGs). P wave indices are measures of atrial conduction while PR interval represents the conduction throughout both the atria and atrioventricular node (AV node). A disturbance in conduction from right to left atrium results in a prolonged P wave duration (1) and heterogeneous conduction patterns of atrial impulses results in an increased P wave dispersion (2).
Abnormal P wave indices and prolonged PR interval have been predictive of atrial fibrillation (AF) (2-4), heart failure (5), stroke (6), and all-cause mortality (7, 8). Novel indices such as P wave area have gained attention and are also predictive of AF (6, 9). Traditionally, abnormalities in P wave indices have been used to define left and right atrial enlargement (10), and screen for chronic obstructive pulmonary disease (11) and emphysema (12, 13).
Despite the emerging renewed interest in P wave indices and PR interval as predictors of outcomes, the repeatability of these ECG measures has not been sufficiently examined. Accurate assessment of the reproducibility of P wave indices is critical to their analysis and interpretation. The aim of this study was to characterize the short-term repeatability, and the minimal detectable change and minimal detectable difference of P wave indices and PR interval estimated from standard 12-lead ECGs in a sample of healthy middle-aged adults.
Materials and Methods
Participants
Our analyses included 63 healthy participants aged 45-64 years recruited from Chapel Hill, NC. Participants were free of diabetes, hypertension, emphysema, chronic obstructive pulmonary disease, congestive heart failure, renal disease, a pacemaker, and were not taking class I or III antiarrhythmics. Participants were asked to avoid intense physical activity, smoking, eating, or drinking alcoholic beverages for 10 hours before the visits. Each participant underwent two standardized visits between 7:30 to 11:30 am one to two weeks apart at the University of North Carolina at Chapel Hill General Clinical Research Center between July and October 2001. Participants provided written informed consent, and the study was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Electrocardiographic methodology
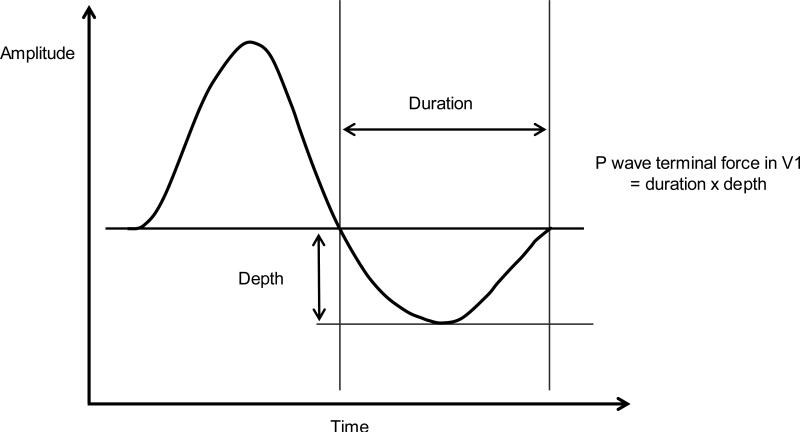
Details of the ECG methodology for this study have been reported elsewhere (14, 15). Briefly, two 10-second standard 12-lead ECG recordings (ECG1 and ECG2 at the initial visit and then ECG3 and ECG4 one to two weeks later) were taken after participants rested for 15 minutes in the supine position. One of four certified technicians obtained the ECGs at each visit following a standardized protocol used in the Atherosclerosis Risk in Communities (ARIC) study (16). The participants were breathing freely and were instructed not to talk during the recording. Technicians positioned the electrodes using the E-V6 Halfpoint Method (17) and a standardized protocol for placing electrodes on women (18). ECGs were recorded using the MAC PC Personal Cardiograph (Marquette Electronics, Inc., Jupiter, FL). The Epidemiology Cardiology Research Center (EPICARE; Wake Forest School of Medicine, Winston Salem, NC) centrally processed the ECGs using the GE Marquette GE 12-SL software 2001 version (GE, Milwaukee, WI). PR interval and P axis were automatically provided by the software as single global measures. On the other hand, P duration, area and amplitude in each of the 12-leads were used to calculate maximum P wave area, maximum duration, dispersion and terminal force in V1. P wave area was multiplied by 19.52 μV*ms to make values comparable to P wave area measured from non-GE software (19). P wave dispersion (ms) was calculated as the maximum P wave duration minus the minimum P wave duration. P wave terminal force (μV*ms) in lead V1 was calculated from the P' duration of the terminal (negative) part of the P wave in lead V1 multiplied by the P' amplitude in lead V1 (Figure 1). We used the absolute value of the P wave terminal force values that were all zero or negative.
Figure 1.
Illustration of P wave terminal force in lead V1
In the PR interval analysis, we used both unadjusted and heart rate-adjusted PR interval (ms) using the Soliman and Rautaharju method (20) where heart rate–adjusted PR interval (PRa) = PR + 0.26 (heart rate − 70) for age < 60 years and PRa = PR + 0.42 (heart rate − 70) for age 60 years or older. We obtained similar results by both methods, thus PR interval without adjustment was presented.
Statistical analysis
Summary measures of P wave indices and PR interval for each ECG measurement were estimated as means and standard deviations (SD). P wave indices with a skewed distribution were expressed as medians and inter-quartile ranges and log transformed for the analysis. The average and absolute difference between pairs of measurements (ECG2 - ECG1 and ECG4 - ECG3) within-and between-visit were calculated (ECG3 - ECG1 and ECG 4 - ECG2). We excluded ECGs that showed evidence of ectopic ventricular complexes (EVC; n=3), and excluded one outlying value for P axis (–25°) since it was greater than four standard deviations away from the mean.
We used a nested random-effects analysis of variance model to parse the variance of the measures into between-participant (σ2p), between-visit (σ2bv), and within visit-components (σ2wv), assuming that the between-visit variation is the same for all participants and that the within-visit variation is the same for all visits and all participants. We calculated the coefficient of variation as 100 X square root of the variance/grand mean, where the variance was σ2p , σ2bv, or σ2wv. To estimate the repeatability of the indices, the intra-class correlation coefficient (ICC) was calculated by dividing the between-participant variance by the total variance [σ2p/σ2p = σ2p/ σ2p+σ2bv+σ2wv]. Maximum P wave area showed a slightly skewed distribution, thus we used the log maximum P area to compute the ICC since the confidence interval is sensitive to departures from normality. We also calculated the standard error of measurement (SEM) as SEM = √(σ2bv+σ2wv). Since P wave terminal force and P wave dispersion showed bi-modal distributions, we used the weighted Kappa (Kw) to evaluate agreement within- and between-visit. Cicchetti-Allison weights (w) were ordered by quartiles of the variables [Kw = Po(w) – Pe(w)/1-Pe(w)].
To aid in the application of these results to study design development and interpretation of results, we estimated changes in P wave indices and PR interval based on the variance and sample size for one- and two-sample study designs. We calculated the minimal detectable change with 95% confidence between two time points for an individual that reflects true change above that of measurement error [MDC95 = SEM*√2*1.96]. For a two-sample study design, we calculated the minimal detectable difference (MDD) between two measurements as MDD = [(√2*σ2total)/N]*(tα(df)+tβ(df)). We also calculated the MDD as a percent of the grand mean. Analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC) and all statistical tests are 2-sided with statistical significance level of P <0.05.
Results
The average age of the participants was 52 years (range 45.1 to 64.6 years old) and the average body mass index (BMI) was 26.9 kg/m2 (range 19.4 to 42.6 kg/m2; Table 1). There were 31 (49.2%) females and 20 (31.8%) non-Caucasian participants. The mean values for PR interval, P axis, maximum P wave area, and maximum P wave duration were consistent within and between visits, but varied for P wave dispersion and P wave terminal force (Table 2). The average within-visit difference was smaller than the between-visit difference for all indices except for P wave axis and maximum P wave duration. The absolute difference between-visit was greater than the absolute difference within-visit for all indices.
Table 1.
Participant characteristics of the ECG repeatability study (N=63)
| Characteristic | Mean ± SD | No. (%) |
|---|---|---|
| Age | 52.0 ± 5.1 | |
| Body mass indexa | 26.9 ± 5.3 | |
| Female | 31 (49.2) | |
| Non-Caucasian | 20 (31.8) | |
| Medication Use | ||
| Anticholinergic | 5 (7.9) | |
| Beta-Blocker | 1 (1.6) | |
| Diuretic | 4 (6.4) | |
| Selective serotonin reuptake inhibitor (SSRI) | 10 (15.9) | |
| Sympathomimetic | 4 (6.4) | |
| Thyroid medication | 3 (4.8) | |
| Protocol deviation at either visitb | 6 (9.5) |
N=62
Protocol deviation defined as eating, drinking beverages other than water, or smoking <10 hours before the study; SD: standard deviation
Table 2.
Descriptive Statistics for PR interval and P wave indices by visit and differences within and between visit by measure
| Visit 1 |
Visit 2 |
Within-Visita |
Between-Visitb |
|||||
|---|---|---|---|---|---|---|---|---|
| Index | ECG1 mean ± SD N=61 | ECG2 mean ± SD N=62 | ECG3 mean ± SD N=62 | ECG4 mean ± SD N=62 | Average Difference mean ± SD N=63 | Absolute Difference mean ± SD N=63 | Average Difference mean ± SD N=62 | Absolute Difference mean ± SD N=62 |
| PR Interval (ms) | 169 ± 29 | 169 ± 29 | 168 ± 27 | 168 ± 27 | 0.1 ± 4.3 | 4.4 ± 4.2 | −0.9 ± 9.0 | 7.2 ± 7.1 |
| Maximum P wave area (μV*ms) | 6,906 ± 2,271 | 7,004 ± 2,398 | 6,807 ± 2,015 | 6,929 ± 2,097 | 130.1 ± 616.9 | 559.3 ± 463.7 | −137.4 ± 1,483.7 | 958.8 ± 1,191.9 |
| P-axis (°)c | 53.0 ± 15.9 | 51.3 ± 17.1 | 52.6 ± 16.2 | 50.9 ± 17.7 | −1.4 ± 6.8 | 5.1 ± 5.8 | −0.2 ± 8.3 | 6.6 ± 7.2 |
| Maximum P wave duration (ms) | 109 ± 11 | 110 ± 12 | 109 ± 11 | 111 ± 13 | 1.6 ± 6.3 | 7.0 ± 7.1 | 0.6 ± 6.8 | 7.7 ± 6.8 |
| P wave dispersion (ms)d | 29 (0, 46) | 17 (0, 44) | 0 (0, 44) | 27 (0, 54) | 1.1 ± 16.9 | 18.7 ± 15.6 | −1.6 ± 22.5 | 23.4 ± 16.1 |
| P wave terminal force at lead V1 (μV*ms)b | 1,450 (0, 2,650) | 1,375 (0, 2,709) | 1,657 (0, 2,709) | 1,496 (0, 2,584) | −49.3 ± 726.5 | 559.2 ± 600.3 | −161.1 ± 1,256.3 | 962.4 ± 970.4 |
ECG2 - ECG1 and ECG4 - ECG3
ECG3 - ECG1 and ECG 4 -ECG2
ECG1 N=59, ECG 2 N=61, between-visit N=61
Median (25th, 75th percentile)
Between-participant variation accounted for the majority of the total variation among all P wave indices (Table 3). The between-visit variation was higher than the within-visit variation for PR interval and maximum P wave area, but lower for P axis and maximum P wave duration. The within-visit coefficient of variation (CV) ranged from 2.9% for PR interval to 13.7% for P axis, and the between-visit CV ranged from undetectable for maximum P wave duration to 13.8% for maximum P wave area.
Table 3.
Components of measurement error for PR interval and P wave indices, based on 247 ECGs
| PR Interval (ms) |
Maximum P wave area (μV*ms) |
P-axis (°)a |
Maximum P wave duration (ms) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | SD | % total variance | CV (%) | SD | % total variance | CV (%) | SD | % total variance | CV (%) | SD | % total variance | CV (%) |
| Between-participant | 26.84 | 93.07 | 15.91 | 1,880.82 | 73.28 | 27.21 | 14.76 | 78.40 | 28.41 | 8.77 | 57.76 | 8.00 |
| Between-visit | 5.45 | 3.83 | 3.23 | 952.05 | 18.78 | 13.77 | 3.03 | 3.30 | 5.83 | 0.00 | 0.00 | 0.00 |
| Within-visit | 4.90 | 3.10 | 2.90 | 619.09 | 7.94 | 8.96 | 7.13 | 18.31 | 13.73 | 7.5 | 42.24 | 6.84 |
| Total | 27.82 | 100.00 | 16.49 | 2,197.08 | 100.00 | 31.79 | 16.67 | 100.00 | 9.00 | 11.54 | 100.00 | 10.52 |
244 ECGs
SD: standard deviation; CV: coefficient of variation = 100 × square root of the variance/grand mean.
Grand means are as follows: 168.69 ms for PR interval, 51.94° for P axis, 6,911.74 μV*ms for maximum P wave area, and 109.64 ms for maximum P wave duration.
The ICC (95% confidence interval) was 0.93 (0.90, 0.96) for PR interval, 0.78 (0.71, 0.86) for P axis, 0.77 (0.67, 0.86) for log maximum P wave area, and 0.58 (0.46, 0.70) for maximum P wave duration (Table 4). For comparison, the ICC for heart rate was 0.84 (0.77, 0.91). The within-visit weighted Kappa (95% confidence limit) was 0.30 (0.16, 0.44) for P wave dispersion and 0.68 (0.58, 0.78) for P wave terminal force. The between-visit weighted Kappa was 0.11 (-0.02, 0.25) for P wave dispersion and 0.46 (0.33, 0.58) for P wave terminal force.
Table 4.
ICCs and 95% CIs for P wave indices
| Index | ICC (95% CI) | SEM | MDC95 |
|---|---|---|---|
| PR Interval (ms) | 0.93 (0.90, 0.96) | 7.33 | 20.31 |
| P-axis (°) | 0.78 (0.71, 0.86) | 7.75 | 21.47 |
| Maximum P wave area (μV*ms) | 0.73 (0.63, 0.84) | 1,135.64 | 3,147.83 |
| Log maximum P wave area (μV*ms) | 0.77 (0.67, 0.86) | 0.06 | 0.18 |
| Maximum P wave duration (ms) | 0.58 (0.46, 0.70) | 7.50 | 20.78 |
ICC: Intraclass Correlation Coefficient; SEM: Standard Error of Measurement; MDC95: Minimal Detectable Change
The MDC95 was 20 ms for PR interval, 21° for P wave area, 3,148 μV*ms for P wave area, and 21 ms for maximum P wave duration (Table 4). As shown in Table 5, the MDD for 50 participants per group was 20 ms for PR interval (12% of the mean), 12° for P wave axis (23% of the mean), 1,602 μV*ms for P wave area (23% of the mean), and 8 ms for maximum P wave duration (8% of the mean). Also shown in this table is the gain in precision for each index achieved by increasing sample size.
Table 5.
Minimal Detectable Difference (MDD) for PR interval and P wave indices
| PR interval (ms) |
P-axis (°) |
Maximum P wave area (μV*ms) |
Maximum P duration (ms) |
|||||
|---|---|---|---|---|---|---|---|---|
| N | MDD | % of mean | MDD | % of mean | MDD | % of mean | MDD | % of mean |
| 5 | 73.31 | 43.46 | 43.91 | 84.54 | 5,788.27 | 83.75 | 30.39 | 27.72 |
| 10 | 47.72 | 28.29 | 28.58 | 55.03 | 3,768.12 | 54.52 | 19.79 | 18.05 |
| 50 | 20.28 | 12.02 | 12.15 | 23.39 | 1,601.68 | 23.17 | 8.41 | 7.67 |
| 100 | 14.26 | 8.46 | 8.54 | 16.45 | 1,126.22 | 16.29 | 5.91 | 5.39 |
| 500 | 6.35 | 3.76 | 3.80 | 7.32 | 501.45 | 7.26 | 2.63 | 2.40 |
| 1,000 | 4.49 | 2.66 | 2.69 | 5.18 | 354.42 | 5.13 | 1.86 | 1.70 |
| 5,000 | 2.00 | 1.19 | 1.20 | 2.31 | 158.42 | 2.29 | 0.83 | 0.76 |
| 10,000 | 1.42 | 0.84 | 0.85 | 1.64 | 112.01 | 1.62 | 0.59 | 0.54 |
| 50,000 | 0.63 | 0.38 | 0.38 | 0.73 | 50.09 | 0.72 | 0.26 | 0.24 |
| 100,000 | 0.45 | 0.27 | 0.27 | 0.52 | 35.42 | 0.51 | 0.19 | 0.17 |
Grand means are as follows: 168.69 ms for PR interval, 51.94° for P axis, 6,911.74 μV*ms for maximum P wave area, and 109.64 ms for maximum P wave duration.
Discussion
To our knowledge this study is the first to report the repeatability of P indices and PR interval from digitized ECGs and automated P wave calculations. Our analysis showed that among healthy adults, the repeatability of PR duration is excellent, that of P wave axis and maximum area is fair, and maximum P wave duration and terminal force is poor. Repeatability of P wave dispersion is fair within visit, yet poor between visits.
Previous studies have focused on the within- and between-observer reproducibility of maximum P wave duration and P wave dispersion from manual or semi-automatic measurements. One such study reported that the intraobserver ICC for maximum P wave duration was 0.80 and the ICC for P wave dispersion was 0.82 (21). The interobserver ICC for maximum P wave duration was 0.56 and the ICC for P wave dispersion was 0.70. However, a single lead acquisition method was used that is less accurate than a simultaneous lead acquisition with digital ECGs. Simultaneous lead acquisition allows for a more accurate determination of the on-set and off-set of the P wave signals (22).
Current technology allows for highly repeatable, automated measurement of P wave indices, which was used in this analysis. Andrikopoulos et al. showed that a computer based semi-automatic method had lower relative errors for maximum P wave duration and P wave dispersion compared to manual methods (23). The interobserver relative errors were 7.8 ± 5.3% for maximum P wave duration and 14.4 ± 6.4% for P wave dispersion. The intraobserver relative errors were 7.3 ± 5.4% for maximum P wave duration and 12.6 ± 6.7% for P wave dispersion.
Our study design used participant and visit to estimate the variance for each P wave index to quantify intra-individual variability (sum of the within- and between-visit variation) and inter-individual variability (between-participant variation) in these indices. The resulting model was as follows: Yijk=μ + Pi + Vj(i) = ek(ij) where Y = the P wave index, μ = the intercept, i = 1,2,3 to the 63rd participant, j =visit one or visit two, and k = the first or second ECG recording, with the assumption that the means of the P wave index do not vary by visit or by order within visit. As expected, the majority of the variation occurred between-participants. In our random-effects analysis of variance models, the between-visit variation represents the additional variation that is added when the ECGs are repeated a week apart above that of the variation that occurs when the ECGs are repeated two minutes apart (within-visit). For PR interval and maximum P wave area, the within-visit variation was lower than the between-visit variation suggesting that more variation was added between-visits. For P axis and maximum P wave duration, the within-visit variation was higher than the between-visit variation suggesting that little variation was added when relating two ECGs from different visits compared to relating ECGs from the same visit.
The between-participant, between-visit and within-visit variation we observed in PR interval and P wave indices could be attributed to biological variation in cardiac electrophysiology, measurement error, technical factors, electrode positioning, and environmental factors. The morphology of the P wave may also contribute to the variation in these measures. Because of the small magnitude of the P wave signal, greater variability might be inherent when determining area from low amplitude short P waves. Although digital ECGs allow for more accurate measurement of the P wave, the onset- and off-set of P waves can be difficult to define.
The estimates of the SEM, MDC95, and MDD for PR interval and P wave indices we report can aid in estimating sample sizes and in the evaluation whether differences in P wave indices are meaningful or statistically significant within participants or between participant groups. The MDC95 for PR interval was 20 ms suggesting that a change of more than 20 ms may be necessary in order to determine whether a change in PR interval exceeds measurement error and intraindividual variability. A 20 ms change in PR interval may be clinically relevant as Magnani et al. reported that a 29 ms increase in PR interval conferred a 13% increase in the 10-year risk of heart failure and incident AF (5).
For the MDD, we show the relation between group size and measurement precision. From this, clinical and population researchers can assess optimal study sizes and possible trade-offs between precision and the costs associated with additional measurements. Given the low repeatability of some P wave indices, attempts to increase the precision of P wave indices by repeating measures, standardizing procedures, and assembling larger sample sizes should be considered. Otherwise, procedures to adjust for measurement error may be required. P wave indices also could be used in combination as shown by Altunkeser et al. in the multivariate prediction of AF from prolonged P wave duration and increased P wave dispersion (24).
ECG-based indices are extensively used in clinical practice, clinical trials, and to evaluate the cardiac safety of drugs. At present, the QT interval is a gold standard measure of cardiac safety used by The Food and Drug Administration (FDA). In some cases, the FDA evaluates PR interval elongation when reviewing clinical trial data. P wave indices may serve as additional indicators of cardiac safety since they represent a cardiac electrophysical biomarker of AV conduction. A maximum P wave duration of ≥ 110 ms (25) and ≥ 120 ms (26) have been used as a threshold for identification of conduction delays, and a P wave dispersion ≥ 40 ms has been used as a marker of non-uniform atrial conduction (25).
In addition to the application to cardiac safety, P wave indices also have potential in risk stratification for AF and pulmonary disease. P wave dispersion has been shown to be a predictor of the progression from paroxysmal to persistent AF (27), and PR interval was associated with the risk of heart failure and AF (5). P wave terminal force has been shown to be a marker of pressure and volume overload in the left atrium (9) and a marker for left atrial enlargement (10). P axis has been associated with chronic obstructive pulmonary disease (11) and emphysema (12), while a threshold of >60° has been used to screen for the latter (28).
Our analysis is limited to short-term repeatability, estimated from ECG measurements at only two time points. ECGs controlled for respiration phase sampled at rates as high as 1200 Hz would be ideal for ascertaining the on- and off-set of the P wave. ECGs in our study were originally sampled at 250 Hz with participants breathing freely; as such, it does not represent the ideal situation but mimics typical clinical practice and research settings. Accounting for respiration and increasing the sampling rate would likely improve the repeatability of the P wave indices. Another limitation is that our study design did not allow for an accounting of technician variation. However, the variance due to technician is likely negligible as reported for heart rate variability in Schroeder et al. (14) and for spatial T-wave axis and QT interval in Vaidean et al. (15), consistent with our use of a standardized protocol and study procedures designed to minimize measurement variability. In a sensitivity analysis, we obtained the same results when we excluded individuals who deviated from the protocol at either visit. It is also important to note that the calculations for the SEM and MDC assume that the measurement error and detectable change is constant across the range of values.
PR interval and P wave indices represent widely used biomarkers to detect abnormal atrial and AV nodal conduction which have implications for clinical research and risk stratification. The excellent repeatability of PR interval supports its use in clinical and epidemiologic studies, but there is a potential for bias when such studies ignore the error with which other P wave indices are measured. Further investigation is needed to tell whether a change in PR interval and P wave indices are clinically meaningful, but we offer estimates to aid in interpreting these measurements.
Acknowledgements
This work was supported by grant (RR00046) from the General Clinical Research Centers program of the Division of Research Resources, National Institutes of Health, and by grants (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C) from the National Heart, Lung, and Blood Institute. M.L.S. and K.S.G was supported by the National Heart, Lung, and Blood Institute T32 training grant HL-007055. The authors thank the staff and participants for their important contributions.
Footnotes
Disclosure statement: none declared.
References
- 1.Cosio FG, Palacios J, Vidal JM, et al. Electrophysiologic studies in atrial fibrillation. Slow conduction of premature impulses: a possible manifestation of the background for reentry. Am J Cardiol. 1983;51(1):122–30. doi: 10.1016/s0002-9149(83)80022-8. [DOI] [PubMed] [Google Scholar]
- 2.Dilaveris PE, Gialafos JE. P-wave dispersion: a novel predictor of paroxysmal atrial fibrillation. Ann Noninvasive Electrocardiol. 2001;6(2):159–65. doi: 10.1111/j.1542-474X.2001.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aytemir K, Ozer N, Atalar E, et al. P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23(7):1109–12. doi: 10.1111/j.1540-8159.2000.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 4.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373(9665):739–45. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnani JW, Wang N, Nelson KP, et al. Electrocardiographic PR interval and adverse outcomes in older adults: the Health, Aging, and Body Composition study. Circ Arrhythm Electrophysiol. 2013;6(1):84–90. doi: 10.1161/CIRCEP.112.975342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soliman EZ, Prineas RJ, Case LD, et al. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40(4):1204–11. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorodeski EZ, Ishwaran H, Kogalur UB, et al. Use of hundreds of electrocardiographic biomarkers for prediction of mortality in postmenopausal women: the Women's Health Initiative. Circ Cardiovasc Qual Outcomes. 2011;4(5):521–32. doi: 10.1161/CIRCOUTCOMES.110.959023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnani JW, Gorodeski EZ, Johnson VM, et al. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm. 2011;8(1):93–100. doi: 10.1016/j.hrthm.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida K, Hayashi H, Miyamoto A, et al. P wave and the development of atrial fibrillation. Heart Rhythm. 2010;7(3):289–94. doi: 10.1016/j.hrthm.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Morris JJ, Jr., Estes EH, Jr., Whalen RE, et al. P-Wave Analysis in Valvular Heart Disease. Circulation. 1964;29:242–52. doi: 10.1161/01.cir.29.2.242. [DOI] [PubMed] [Google Scholar]
- 11.Akturk F, Biyik I, Kocas C, et al. The role of electrocardiography in evaluation of severity of chronic obstructive pulmonary disease in daily clinical practice. Tuberk Toraks. 2013;61(1):38–42. doi: 10.5578/tt.4101. [DOI] [PubMed] [Google Scholar]
- 12.Chhabra L, Sareen P, Perli D, et al. Vertical P-wave axis: the electrocardiographic synonym for pulmonary emphysema and its severity. Indian Heart J. 2012;64(1):40–2. doi: 10.1016/S0019-4832(12)60009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chhabra L, Chaubey VK, Kothagundla C, et al. P-wave indices in patients with pulmonary emphysema: do P-terminal force and interatrial block have confounding effects? Int J Chron Obstruct Pulmon Dis. 2013;8:245–50. doi: 10.2147/COPD.S45127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder EB, Whitsel EA, Evans GW, et al. Repeatability of heart rate variability measures. J Electrocardiol. 2004;37(3):163–72. doi: 10.1016/j.jelectrocard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Vaidean GD, Schroeder EB, Whitsel EA, et al. Short-term repeatability of electrocardiographic spatial T-wave axis and QT interval. J Electrocardiol. 2005;38(2):139–47. doi: 10.1016/j.jelectrocard.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 16.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 17.Rautaharju PM, Wolf HK, Eifler WJ, et al. A simple procedure for positioning precordial ECG and VCG electrodes using an electrode locator. J Electrocardiol. 1976;9(1):35–40. doi: 10.1016/s0022-0736(76)80007-6. [DOI] [PubMed] [Google Scholar]
- 18.Rautaharju PM, Park L, Rautaharju FS, et al. A standardized procedure for locating and documenting ECG chest electrode positions: consideration of the effect of breast tissue on ECG amplitudes in women. J Electrocardiol. 1998;31(1):17–29. doi: 10.1016/s0022-0736(98)90003-6. [DOI] [PubMed] [Google Scholar]
- 19.Soliman EZ. Response to Letter by Dewhurst and Adams. Stroke. 2011;42(2):e20. [Google Scholar]
- 20.Soliman EZ, Rautaharju PM. Heart rate adjustment of PR interval in middle-aged and older adults. J Electrocardiol. 2012;45(1):66–9. doi: 10.1016/j.jelectrocard.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Magnani JW, Mazzini MJ, Sullivan LM, et al. P-wave indices, distribution and quality control assessment (from the Framingham Heart Study). Ann Noninvasive Electrocardiol. 2010;15(1):77–84. doi: 10.1111/j.1542-474X.2009.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pipberger HV, Tanenbaum HL. [The P wave, P-R interval, and Q-T ratio of the normal orthogonal electrocardiogram]. Circulation. 1958;18(6):1175–80. doi: 10.1161/01.cir.18.6.1175. [DOI] [PubMed] [Google Scholar]
- 23.Andrikopoulos GK, Dilaveris PE, Richter DJ, et al. Increased variance of P wave duration on the electrocardiogram distinguishes patients with idiopathic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23(7):1127–32. doi: 10.1111/j.1540-8159.2000.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 24.Altunkeser BB, Ozdemir K, Gok H, et al. Can P wave parameters obtained from 12-lead surface electrocardiogram be a predictor for atrial fibrillation in patients who have structural heart disease? Angiology. 2003;54(4):475–9. doi: 10.1177/000331970305400412. [DOI] [PubMed] [Google Scholar]
- 25.Dilaveris PE, Gialafos EJ, Sideris SK, et al. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135(5 Pt 1):733–8. doi: 10.1016/s0002-8703(98)70030-4. [DOI] [PubMed] [Google Scholar]
- 26.Willems JL, Robles de Medina EO, Bernard R, et al. Criteria for intraventricular conduction disturbances and pre-excitation. World Health Organizational/International Society and Federation for Cardiology Task Force Ad Hoc. J Am Coll Cardiol. 1985;5(6):1261–75. doi: 10.1016/s0735-1097(85)80335-1. [DOI] [PubMed] [Google Scholar]
- 27.Koide Y, Yotsukura M, Ando H, et al. Usefulness of P-wave dispersion in standard twelve-lead electrocardiography to predict transition from paroxysmal to persistent atrial fibrillation. Am J Cardiol. 2008;102(5):573–7. doi: 10.1016/j.amjcard.2008.04.065. [DOI] [PubMed] [Google Scholar]
- 28.Thomas AJ, Apiyasawat S, Spodick DH. Electrocardiographic detection of emphysema. Am J Cardiol. 2011;107(7):1090–2. doi: 10.1016/j.amjcard.2010.11.039. [DOI] [PubMed] [Google Scholar]