Summary
Dysregulation of the mammalian target of rapamycin and hypoxia-induced pathways has been consistently identified in clear cell renal cell carcinomas. However, experience with non–clear cell renal cell carcinoma subtypes is scant. In this study, we evaluated the immunohistochemical expression of upstream (PTEN and phosphorylated AKT) and downstream (phosphorylated S6 and 4EBP1) effectors of the mammalian target of rapamycin pathway, as well as related cell-cycle proteins (p27 and c-MYC), and a member of the hypoxia-induced pathway (HIF-1α) in 54 patients with papillary renal cell carcinoma treated by nephrectomy. PTEN was lower in tumor than in normal kidney, and loss of PTEN expression was found in 48% of the patients. In tumor tissues, phosphorylated S6, 4EBP1, and HIF-1α were higher than in normal kidney. Conversely, scores of p27 were lower in tumor than in normal kidney. Finally, scores of c-MYC and phosphorylated AKT were similar in tumor and in normal kidney. Overall mortality and cancer-specific mortality were 24% and 11%, respectively. Tumor progression was observed in 17% of the patients. None of the tested biomarkers predicted cancer-specific mortality or tumor progression. As expected, patients with high T-stage tumors had higher hazard ratios for cancer-specific mortality (hazard ratio, 6.9) and tumor progression (hazard ratio, 6.7). Patients with higher Fuhrman grades also had higher risks for cancer-specific mortality (hazard ratio, 11.4) and tumor progression (hazard ratio, 4.5). In summary, our study provides evidence of dysregulation of the mammalian target of rapamycin and hypoxia-induced pathways in papillary renal cell carcinoma. Immunohistochemistry for members of the mammalian target of rapamycin pathway and for HIF-1α lacked prognostic significance in our cohort.
Keywords: Papillary renal cell carcinoma, mTOR, PTEN, AKT, S6, 4EBP1, HIF-1α
1. Introduction
Dysregulation of the mammalian target of rapamycin (mTOR) and hypoxia-induced pathways has been consistently identified in clear cell renal cell carcinomas (RCCs) [1,2]. Currently, inhibitors of the mTOR and vascular endothelial growth factor (VEGF) pathways are being used in patients with advanced clear cell RCC, either as first-line options or in refractory disease [3,4]. However, few clinical trials are addressing the role of mTOR inhibitors and VEGF antagonists in papillary RCC [3,5]. In addition, previous studies have analyzed the expression status and prognostic significance of members of the mTOR and hypoxia-induced pathways in RCC [2,6–8], but none has focused exclusively on papillary RCC. Considering that papillary RCC is the second most common subtype of RCC [9] with no established therapy for disseminated disease, such studies are needed.
In this study, we evaluated the immunohistochemical expression of upstream (phosthatase and tensin homolog [PTEN] and phosphorylated AKT [phos-AKT]) and downstream (phosphorylated S6 [phos-S6] and 4E-binding protein 1 [4EBP1]) effectors of the mTOR pathway, as well as related proteins (p27 and c-MYC), and a member of the hypoxia-induced pathway (hypoxia-inducible factor1 alpha [HIF1 α]) in patients with papillary RCC. First, we compared the expression of these biomarkers between normal kidney and tumor tissue. Second, we analyzed the associations between biomarkers and pathologic features of the primary tumor. Third, we evaluated the prognostic impact that these biomarkers may have on the outcome of patients with papillary RCC.
2. Materials and methods
The present study includes tissue samples from 54 consecutive patients with papillary RCC treated at the Johns Hopkins Medical Institutions (Baltimore, MD) between January 2004 and December 2006. All patients were treated by partial/radical nephrectomy without adjuvant therapy. After approval by the institutional review board, a retrospective study was performed with outcome assessment based on the chart review of clinical and pathologic data. Histologic slides were retrieved and reviewed by 2 urologic pathologists (R.A. and G.J.N.) for confirmation of the original diagnosis and pathologic staging, in compliance with the American Joint Committee on Cancer 2009 Classification [10]. Using a previously described procedure [11], 2 tissue microarrays were build. Four cores of tumor tissue and 4 cores of paired normal kidney tissue were spotted from each specimen. Patients were followed up from the date of surgery (mean, 55 months; median, 60 months; range, 10–91 months). For outcome analysis, end points included cancer-related death, tumor progression, and overall mortality. Tumor progression was defined as the presence of pelvic recurrence or metastasis to distant sites. Overall mortality refers to all-cause death.
2.1. Immunohistochemistry
Immunohistochemistry was performed for the following proteins: PTEN, phos-AKT, phos-S6, 4EBP1, c-MYC, p27, and HIF-1α. Immunostaining was performed on formalin-fixed, paraffin-embedded tissue sections using the Power-Vision Poly-HRP IHC Detection Systems (Leica Microsystems, Bannockburn, IL). Sections were deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval with a buffer solution using a steamer. Sections were then incubated with the appropriate primary antibody. After the application of an antirabbit or antimouse poly-HRP secondary (except for c-MYC, for which the Dako Catalyzed Signal Amplification System Kit was used [Dako, Carpinteria, CA]), the slides were developed using 3-3′-diaminobenzidine chromogen and counterstained with hematoxylin. Proper cell lines were used as external controls, and internal controls were checked for negative and positive immunohistochemical expression. For HIF-1α, the protocol described by Tickoo et al [12] was used. Table 1 lists information regarding antibodies and vendors.
Table 1.
Summary of antibodies used for immunohistochemical analysis
| Vendor | Clone | Pretreatment | Dilution | |
|---|---|---|---|---|
| PTEN | Cell Signaling (Beverly, MA) | D4.3 | EDTA, 45 min | 1:100 |
| c-MYC | Epitomics (Burlingame, CA) | Y69 | EDTA, 45 min | 1:300 |
| p27 | Transduction Lab (Sparks, MD) | 57 | Citrate, 25 min | 1:4000 |
| phos-AKT a | Cell Signaling | 736E11 | EDTA, 45 min | 1:50 |
| phos-S6 b | Cell Signaling | Polyclonal | EDTA, 45 min | 1:200 |
| 4EBP-1 | ProSci (San Diego, CA) | Polyclonal | Citrate, 25 min | 1:250 |
| HIF-1α | Novus Biologicals (Littleton, CO) | NB100-123 | Heat (oven) at 62°C, 60 min | 1:1600 |
Phosphorylation site at Ser473.
Phosphorylation site at Ser235/236.
2.2. Scoring system
Immunohistochemistry staining was evaluated using a previously validated methodology [2]. Both tumor cells and normal epithelial cells from proximal and distal renal tubules were evaluated for pattern of staining (nuclear versus cytoplasmic), extent (percent of positive cells), and intensity (0 to 3+). Nuclear staining was considered positive for HIF-1α, phos-AKT, c-MYC, and p27. For PTEN, phos-S6, and 4EBP1, cytoplasmic stains were considered positive. An H score was calculated for each tissue microarray spot as the sum of the products of the intensity (0 for negative, 1 for weakly positive, 2 for moderately positive, and 3 for strongly positive) by the extent of immunoexpression (0–100%). The overall score used for subsequent statistical analysis was the pooled mean from the 4 spots of normal kidney and of tumor tissue.
2.3. Statistical analysis
Because of the non-gaussian distribution of the population (D’Agostino-Royston tests for normality, P < .001), nonparametric tests were used in all instances. Scores of paired normal kidney and tumor were compared using the Wilcoxon matched-pairs signed rank test. Differences in scores of tumor tissue were stratified by clinicopathologic variables and compared using the Mann-Whitney U test or the Fisher exact test. Unadjusted and adjusted Cox models were built to evaluate the hazard ratio of clinicopathologic variables and biomarkers expression as prognosticators of outcome. Cumulative hazards were plotted using Nelson-Aalen estimates. Survival functions were compared using the log-rank (Mantel-Cox) test. For statistical significance, a 2-tailed P < .05 was required for bivariate analysis. The threshold was adjusted using the Šidák correction when more than 1 test was applied to the same set of data. Hazard ratios were interpreted using 95% confidence intervals. Data were analyzed using STATA release 11 (StataCorp Inc, College Station, TX).
3. Results
Clinicopathologic features of all patients are shown in Table 2. In papillary RCC, overall mortality and cancer-specific mortality were 24% and 11%, respectively. Tumor progression was observed in 17% of the patients.
Table 2.
Demographic and clinicopathologic features of 54 patients with papillary RCC treated by nephrectomy
| No. of cases | |
|---|---|
| Age at nephrectomy (y) | |
| Mean (SD) | 60.1 (12.6) |
| Median (IQR) | 61.5 (15) |
| Range | 29–83 |
| Ethnicity (%) | |
| White | 30 (55.5) |
| African American | 21 (39) |
| Other | 3 (5.5) |
| Sex (%) | |
| Male | 44 (81) |
| Female | 10 (19) |
| Pathologic stage T (%) a | |
| T1a | 24 (45) |
| T1b | 9 (17) |
| T2a | 5 (9) |
| T2b | 3 (6) |
| T3a | 9 (17) |
| T3b | 3 (6) |
| Tumor size (cm) | |
| Mean (SD) | 4.8 (3.2) |
| Median (IQR) | 3.6 (4.4) |
| Range | 0.6–14 |
| Fuhrman grade (%) | |
| I | 2 (4) |
| II | 35 (65) |
| III | 16 (30) |
| IV | 1 (2) |
| Papillary type (%) | |
| I | 52 (96) |
| II | 2 (4) |
| Multifocality (%) | 10 (19) |
| Positive surgical margins (%) | 5 (9) |
Abbreviation: IQR, interquartile range.
In 1 case, staging was not possible due to fragmented specimen.
3.1. Expression of biomarkers in papillary RCC and paired normal kidney
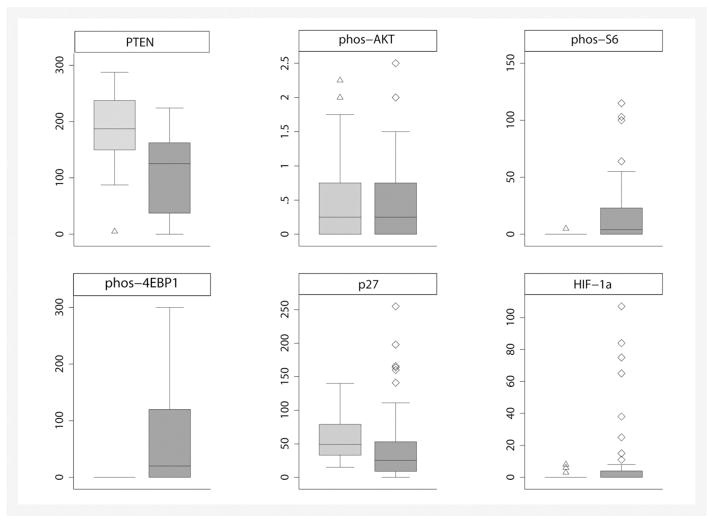
Fig. 1 shows the expression patterns of PTEN, phos-AKT, phos-S6, 4EBP1, c-MYC, and p27. Overall, striking differences were noted in the scores of most biomarkers between normal kidney and tumor tissue (Table 3). PTEN was lower in tumor than in normal kidney, and loss of PTEN expression was found in 26 (48%) of 54 patients. Of the 26 tumors with PTEN loss, 24 and 19 showed positive immunoexpression of HIF-1α and phos-S6, respectively. Scores of phos-S6, 4EBP1, and HIF-1α were higher in tumor than in normal kidney. Conversely, scores of p27 were lower in tumor than in normal kidney. Finally, scores of c-MYC and phos-AKT were similar in tumor and in normal kidney. These patterns of expression are shown in Fig. 2.
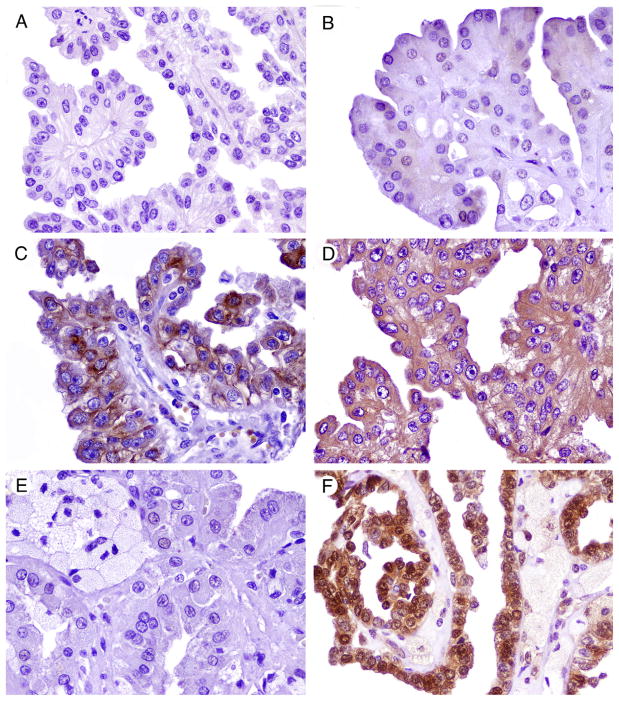
Fig. 1.
Immunohistochemistry for members of the mTOR pathway and related proteins in papillary RCCs. A, Loss of PTEN immunoexpression. B, Weak and sparse nuclear phos-AKT positivity. C, Phos-S6 cytoplasmic positivity. D, 4EBP1 cytoplasmic positivity. E, Negative c-MYC expression. F, Nuclear and cytoplasmic p27 positivity.
Table 3.
Comparison of H score means and 95% CI in papillary RCC and paired normal kidney
| Papillary RCC | Normal kidney | P a | |
|---|---|---|---|
| PTEN | 100.9 (81.9–119.9) | 190.1 (175.6–204.6) | <.001 |
| phos-AKT | 0.5 (0.3–0.6) | 0.5 (0.3–0.6) | .64 |
| phos-S6 | 16.7 (9.3–24) | 0.1 (0–0.3) | <.001 |
| 4EBP1 | 67.2 (44.2–90.2) | 0 b | <.001 |
| c-MYC | 0.1 (0–0.2) | 0.1 (0–0.2) | .11 |
| p27 | 44.7 (29.5–59.8) | 56.3 (47.3–65.2) | .04 |
| HIF-1α | 9.8 (3.4–16.2) | 0.7 (0.1–1.4) | .005 |
Wilcoxon matched-pairs test.
Constant H score.
Fig. 2.
Patterns of immunohistochemical expression of members of the mTOR and hypoxia-induced pathways in papillary RCCs. Scale axis corresponds to H scores. Light gray box plots correspond to normal kidney. Dark gray box plots correspond to tumor tissue. Outliers are represented by hollow triangles (for normal kidney) and hollow diamonds (for tumor tissue). The box plot for c-MYC expression was omitted because of the small number of patients with H scores higher than 0.
3.2. Predictors of outcome in papillary RCC
On bivariate analysis of pathologic features and biomarkers, only HIF-1α was associated with tumor size (Table 4). The same trend was observed for HIF-1α and T stage. None of the other biomarkers were associated with T stage, Fuhrman grade, or tumor size. In addition, no association was identified between biomarkers and surgical margins or multifocality (P ≥ .06 and P ≥ .16, respectively; data not shown).
Table 4.
| T stage c | Fuhrman grade c | Tumor size d | |
|---|---|---|---|
| PTEN | .54 | .23 | .77 |
| phos-AKT | .30 | .99 | .64 |
| phos-S6 | .59 | .99 | .43 |
| 4EBP1 | .27 | .33 | .80 |
| c-MYC | .99 | .35 | .12 |
| p27 | .70 | .35 | .53 |
| HIF-1α | .03 | .65 | .01 |
Biomarkers categorized as positive vs negative using an H score higher than 0 as the threshold for all biomarkers, except for PTEN. Immunoexpression of PTEN was categorizes as “PTEN loss” vs “no PTEN loss” using an H score of 10 or lower for PTEN loss.
Significance level was adjusted to α = .02 using Šidák correction.
Values provided correspond to P values estimated using the Fisher exact test.
Values provided correspond to P values estimated using the Mann-Whitney U test.
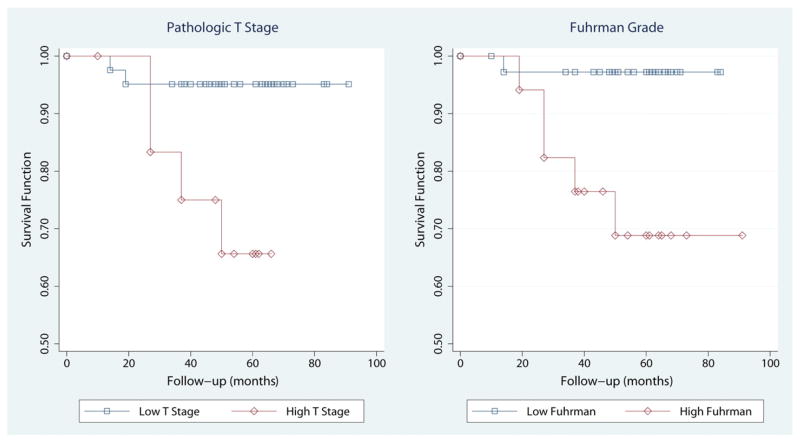
Patients with high T-stage (≥T3a) tumors were at higher risks for overall mortality, cancer-specific mortality, and tumor progression (Table 5). Hazard ratios varied from 6.1 to 6.9, and 95% CIs were similar for all outcomes. In addition, patients with higher Fuhrman grades (III or IV) also had higher risks for cancer-specific mortality and tumor progression. Hazard ratios were higher for cancer-specific mortality (11.4) than for tumor progression (4.5). Fig. 3 shows the cumulative hazards for cancer-specific death stratified by T stage and Fuhrman grade. However, tumor size was not a reliable predictor of outcome, and hazard ratios remained around 1 for all outcomes. Furthermore, none of the biomarkers predicted overall mortality, cancer-specific mortality, and tumor progression in unadjusted (“univariate”) analyses (see Table 5). Similar results were obtained for biomarkers as predictors of outcome when Cox models were adjusted (“multivariate” analysis) for T stage, Fuhrman grade, and tumor size (Table 6).
Table 5.
Unadjusted Cox models for clinicopathologic features and immunoexpression of biomarkers as prognosticators of outcome in papillary RCC a
| Overall mortality
|
Cancer-specific mortality
|
Tumor progression
|
||||
|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | |
| Pathologic features | ||||||
| T stage ≥T3a | 6.09 (1.69–22) | .006 | 6.88 (1.26–37.61) | .03 | 6.70 (1.57–28.69) | .01 |
| Fuhrman grade ≥III | 2.59 (0.86–7.79) | .09 | 11.38 (1.33–97.56) | .03 | 4.52 (1.12–18.30) | .04 |
| Tumor size (cm) | 1.01 (0.86–1.18) | .94 | 1.13 (0.93–1.38) | .22 | 1.10 (0.92–1.31) | .29 |
| Biomarkers b | ||||||
| PTEN | 1.00 (0.99–1.01) | .68 | 1.00 (0.99–1.02) | .51 | 1.00 (0.99–1.01) | .86 |
| phos-AKT | 0.35 (0.05–2.19) | .26 | 0.01 (0.01–3.61) | .10 | 0.23 (0.02–2.77) | .24 |
| phos-S6 | 1.01 (1.00–1.03) | .16 | 1.02 (1.00–1.04) | .07 | 1.01 (1.00–1.03) | .11 |
| phos-4EBP1 | 1.00 (1.00–1.01) | .10 | 1.00 (1.00–1.01) | .09 | 1.01 (1.00–1.01) | .03 |
| p27 | 1.00 (0.99–1.01) | .90 | 0.99 (0.98–1.01) | .58 | 1.00 (0.99–1.01) | .80 |
| HIF-1α | 1.01 (0.99–1.03) | .34 | 1.03 (0.99–1.04) | .31 | 1.01 (0.99–1.03) | .43 |
Values provided correspond to hazard ratios (HRs) with 95% CIs in parenthesis. Variables were entered separately.
HRs for c-MYC were excluded due to extremely low values.
Fig. 3.
Survival curves using the Kaplan-Meier method for cancer-specific mortality by pathologic T stage (left panel) and Fuhrman grade (right panel). Pathologic T stages were grouped in high (≥T3a) and low (≤T2b) categories. Fuhrman grades were grouped in high (3 and 4) and low (1 and 2) categories. Using these groups, survival functions, compared by the log-rank test, were significantly different for pathologic stage (P = .009) and Fuhrman grade (P = .005).
Table 6.
Adjusted Cox model for immunoexpression of biomarkers as prognosticators of outcome in papillary RCC a
| Biomarkers b,c | Overall mortality
|
Cancer-specific mortality
|
Tumor progression
|
|||
|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | |
| PTEN | 1.00 (0.99–1.01) | .73 | 1.00 (0.99–1.01) | .97 | 1.00 (0.99–1.01) | .38 |
| phos-AKT | 0.28 (0.05–1.68) | .16 | 0.01 (0.01–6.61) | .13 | 0.22 (0.02–2.55) | .22 |
| phos-S6 | 1.00 (0.99–1.02) | .73 | 1.01 (0.99–1.03) | .38 | 1.01 (0.99–1.03) | .45 |
| phos-4EBP1 | 1.00 (0.99–1.01) | .84 | 1.00 (0.99–1.01) | .43 | 1.00 (0.99–1.01) | .92 |
| p27 | 1.01 (1.00–1.02) | .19 | 1.01 (0.99–1.03) | .35 | 1.01 (1.00–1.03) | .05 |
| HIF-1α | 1.01 (0.99–1.04) | .37 | 1.02 (0.98–1.05) | .44 | 1.01 (0.97–1.04) | .68 |
Values provided correspond to hazard ratios (HRs) with 95% CIs in parenthesis. Each marker was entered separately.
Adjusted for T stage, Fuhrman grade, and tumor size.
HRs for c-MYC were excluded due to extremely low values.
4. Discussion
In this study, we analyzed the immunohistochemical expression of several members of the mTOR and hypoxia-induced pathways in papillary RCC. To the best of our knowledge, this is the largest series of papillary RCC to date in which these biomarkers are evaluated for expression status and prognostic usefulness. PTEN scores were lower in tumor tissues than in normal kidney, and loss of immunohistochemical expression of PTEN was observed in approximately one half of the patients. We have recently demonstrated that loss of PTEN immunoexpression in prostate cancer is associated in most cases with PTEN genomic loss [13]. Whether similar genetic alterations are responsible for observed PTEN loss of expression in papillary RCC remains to be determined. In addition, scores of downstream effectors phos-S6 and 4EBP1 were higher in tumor tissues than in normal kidney. Similar trends were noted for HIF-1α, a transcription factor of the hypoxia-induced pathway linked to mTOR activation. In addition, p27 scores were higher in normal kidney than in tumor tissues, whereas scores of phos-AKT and c-MYC were similar in both. Almost all tumors with PTEN loss expressed HIF-1α, and most of them also expressed phos-S6. This observation suggests that, in papillary RCC, phos-S6 activation is driven by PTEN loss. Thus, our results may also lend support to the role of interactive effects in the activation of mTOR and hypoxia-induced pathways for the pathogenesis of papillary RCC. Despite these differences, the immunohistochemical expression of these mTOR and hypoxia-induced members seems to bear no impact on the prognosis of papillary RCC. Indeed, our results show that immunohistochemistry adds no value to pathologic T stage or Fuhrman grade in predicting outcome. However, adverse outcome was a rare event in our cohort, with progression rate of 17% and cancer-specific mortality of 24%.
Our study supports the prognostic value of pathologic T stage and Fuhrman grade in papillary RCC. Either both or at least 1 of these variables has been reported as predictors of outcome in a recently published large series of papillary RCCs [14–17]. In addition to the results of our study, the aforementioned series highlight the importance of T stage and Fuhrman grade in defining the prognosis of patients with papillary RCC. Moreover, variables such as patient’s age, sex, and multifocality were consistently irrelevant for prognosis in ours and other studies [14–16]. Finally, another pathologic feature that has gained attention recently as a putative prognosticator of outcome is the subtype of papillary RCC, according to the classification of Delahunt and Eble [18]. Indeed, some studies have found a more aggressive biologic behavior in type 2 compared with type 1 papillary RCC [19,20]. Unfortunately, we were not able to evaluate the prognostic value of subtypes of papillary RCC because of the small number of patients with type 2 tumors.
Our results are comparable with what we have found in clear cell RCC, using the same immunohistochemical approach and scoring system [2]. Patterns of immunoexpression were akin between clear cell and papillary RCC in normal kidneys and tumor tissues, with the sole exception of p27 scores, which were lower in tumor tissues in the current series. The similarities suggest that current therapies involving mTOR inhibitors might also be beneficial for patients with papillary RCC. Nonetheless, experience with mTOR inhibitors in non–clear cell RCC is scant, mostly because patients with non–clear cell subtypes are excluded from clinical trials evaluating these drugs [5]. In a randomized phase III clinical trial, Dutcher et al [21] evaluated the response of 55 patients with advanced papillary RCC treated with either temsirolimus or interferon-α. They found that patients receiving temsirolimus had prolonged overall survival (11.6 months versus 4.3 months, respectively) and progression-free survival (7 months versus 1.8 months) compared with those treated with interferon-α. Furthermore, a prospective phase II trial of everolimus as monotherapy in advanced papillary RCC is currently ongoing in Europe [5]. Our results lend support to the rationale of these studies, by demonstrating immunohistochemical evidence of dysregulation of the mTOR pathway in papillary RCC.
We also found increased HIF-1α in tumor tissues compared with normal kidneys, and higher HIF-1α scores were associated with pathologic features such as tumor size and T stage, marginally in the latter. These findings are consistent with what we have found in clear cell RCC [2] and suggest that antagonists of the VEGF pathway could also be beneficial for patients with papillary RCC. In advanced papillary RCC, VEGF antagonists have been studied more extensively than mTOR inhibitors [5]. However, results have been disappointing so far. Stadler et al [22] reported a partial response in 3% of 107 patients with advanced papillary RCC treated with sorafenib. Low response rates (5%) were also reported by Choueiri et al [23] in a series of 41 patients with metastatic papillary RCC treated with either sunitinib or sorafenib. In addition, no responses were observed by Molina et al [24] in a phase II trial including 8 patients with metastatic papillary RCC treated with sunitinib. Furthermore, in 2 abstracts presented at the recent Annual Meetings of the American Society of Clinical Oncology, the response rates of 51 patients with advanced papillary RCC treated with sunitinib were very low (4%) or null [25,26]. In the current study, we found evidence of overexpression of both HIF-1α and downstream effectors of the mTOR pathway, further suggesting a potential role for combined targeted treatments.
The prognostic value of immunohistochemistry for members of the mTOR and hypoxia-induced pathways to predict the outcome of patients with RCC has been previously evaluated. In an earlier study, we focused on the analysis of 176 patients with clear cell RCC [2]. We found that, after adjusting for clinicopathologic features, phos-S6 was independently associated with disease-free survival and tumor progression. In another series including 375 RCCs, 40 of which were papillary RCC, Pantuck et al [8] found that phos-AKT, PTEN, and phos-S6 were independent predictors of outcome. On the other hand, in study including 176 patients with RCC (without further specification), Merseburger et al [7] found that neither PTEN nor phos-AKT predicted survival. In the current study, none of the biomarkers were associated with cancer-specific survival or tumor progression. In addition, overexpression of HIF-1α has been associated with worse outcome in patients with clear cell RCC [2,6]. However, in the current series, HIF-1α was not associated with outcome. Nevertheless, higher HIF-1α expression was noted in tumor tissues compared with normal kidneys, and tumors with HIF-1α expression were larger than HIF-1α–negative tumors. In this regard, our results indicate that the hypoxia-induced pathway is dysregulated also in papillary RCC.
Although our study suggests that the biomarkers we analyzed lack prognostic use, Cho et al [27] found that high immunohistochemical expression of phos-S6 is associated with an objective response to temsirolimus in patients with RCC. Furthermore, in the series of Cho et al, only patients with high phos-S6 expression experienced objective responses. As evidenced by the aforementioned study, prognostic models (ie, models seeking to predict outcome) should not be used as surrogates for predictive models (ie, models seeking to predict response to treatment). Biomarkers that fail as prognosticators of outcome could still perform better as predictors of therapeutic response.
In summary, we analyzed the expression status and prognostic significance of members of the mTOR and hypoxia-induced pathways in 54 patients with papillary RCC. We found lower PTEN expression and higher phos-S6, 4EBP1, and HIF-1α expressions in tumor tissues compared with normal kidneys. In patients with papillary RCC, prognosis was dictated mainly by pathologic T stage and Fuhrman grade. None of the biomarkers analyzed was a useful predictor of cancer-specific death or tumor progression. Our study provides evidence of dysregulation of the mTOR and hypoxia-induced pathways in papillary RCC. However, biomarkers of these pathways seem to have limited usefulness as prognosticators of outcome in patients with these tumors. Additional studies with similar design would be required to further confirm our results.
Footnotes
Disclosure: This work was partially supported by The Brady Urological Institute–Johns Hopkins Medicine Patana Fund (Baltimore, MD, USA).
References
- 1.Klatte T, Pantuck AJ. Molecular biology of renal cortical tumors. Urol Clin North Aml. 2008;35:573–80. doi: 10.1016/j.ucl.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Schultz L, Chaux A, Albadine R, et al. Immunoexpression status and prognostic value of mTOR and hypoxia-induced pathway members in primary and metastatic clear cell renal cell carcinomas. Am J Surg Pathol. 2011;35:1549–56. doi: 10.1097/PAS.0b013e31822895e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 2011;108:1556–63. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 4.Mulders P. Vascular endothelial growth factor and mTOR pathways in renal cell carcinoma: differences and synergies of two targeted mechanisms. BJU Int. 2009;104:1585–9. doi: 10.1111/j.1464-410X.2009.08987.x. [DOI] [PubMed] [Google Scholar]
- 5.Singer EA, Bratslavsky G, Linehan WM, Srinivasan R. Targeted therapies for non–clear renal cell carcinoma. Target Oncol. 2010;5:119–29. doi: 10.1007/s11523-010-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klatte T, Seligson DB, Riggs SB, et al. Hypoxia-inducible factor 1 alpha in clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:7388–93. doi: 10.1158/1078-0432.CCR-07-0411. [DOI] [PubMed] [Google Scholar]
- 7.Merseburger AS, Hennenlotter J, Kuehs U, et al. Activation of PI3K is associated with reduced survival in renal cell carcinoma. Urol Int. 2008;80:372–7. doi: 10.1159/000132694. [DOI] [PubMed] [Google Scholar]
- 8.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–67. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 9.Tickoo SK, Reuter VE. Differential diagnosis of renal tumors with papillary architecture. Adv Anat Pathol. 2011;18:120–32. doi: 10.1097/PAP.0b013e31820cb3dd. [DOI] [PubMed] [Google Scholar]
- 10.Eggener S. TNM staging for renal cell carcinoma: time for a new method. Eur Urol. 2010;58:517–9. doi: 10.1016/j.eururo.2010.08.007. discussion 9–21. [DOI] [PubMed] [Google Scholar]
- 11.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89–101. doi: 10.1385/1-59259-780-7:089. [DOI] [PubMed] [Google Scholar]
- 12.Tickoo SK, Alden D, Olgac S, et al. Immunohistochemical expression of hypoxia inducible factor-1alpha and its downstream molecules in sarcomatoid renal cell carcinoma. J Urol. 2007;177:1258–63. doi: 10.1016/j.juro.2006.11.100. [DOI] [PubMed] [Google Scholar]
- 13.Lotan TL, Gurel B, Sutcliffe S, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011;17:6563–73. doi: 10.1158/1078-0432.CCR-11-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann E, Trojan L, Becker F, et al. Prognostic factors of papillary renal cell carcinoma: results from a multi-institutional series after pathological review. J Urol. 2010;183:460–6. doi: 10.1016/j.juro.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Klatte T, Remzi M, Zigeuner RE, et al. Development and external validation of a nomogram predicting disease specific survival after nephrectomy for papillary renal cell carcinoma. J Urol. 2010;184:53–8. doi: 10.1016/j.juro.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Zucchi A, Novara G, Costantini E, et al. Prognostic factors in a large multi-institutional series of papillary renal cell carcinoma. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10517.x. Epub ahead of print on Aug 22. [DOI] [PubMed] [Google Scholar]
- 17.Klatte T, Anterasian C, Said JW, et al. Fuhrman grade provides higher prognostic accuracy than nucleolar grade for papillary renal cell carcinoma. J Urol. 2010;183:2143–7. doi: 10.1016/j.juro.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997;10:537–44. [PubMed] [Google Scholar]
- 19.Antonelli A, Tardanico R, Balzarini P, et al. Cytogenetic features, clinical significance and prognostic impact of type 1 and type 2 papillary renal cell carcinoma. Cancer Genet Cytogenet. 2010;199:128–33. doi: 10.1016/j.cancergencyto.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Waldert M, Haitel A, Marberger M, et al. Comparison of type I and II papillary renal cell carcinoma (RCC) and clear cell RCC. BJU Int. 2008;102:1381–4. doi: 10.1111/j.1464-410X.2008.07999.x. [DOI] [PubMed] [Google Scholar]
- 21.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–9. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 22.Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. 2010;116:1272–80. doi: 10.1002/cncr.24864. [DOI] [PubMed] [Google Scholar]
- 23.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–31. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 24.Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9491-6. Epub ahead of print on Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plimack ER, Jonasch E, Bekele BN, Qiao W, Ng CS, Tannir NM. Sunitinib in papillary renal cell carcinoma (pRCC): results from a single-arm phase II study [abstract] J Clin Oncol. 2010:28. Abstract #4604. [Google Scholar]
- 26.Ravaud A, Oudard S, Gravis-Mescam G, et al. First-line sunitinib in type I and II papillary renal cell carcinoma (PRCC): SUPAP, a phase II study of the French Genito-Urinary Group (GETUG) and the Group of Early Phase trials (GEP) [abstract] J Clin Oncol. 2009:27. Abstract #5146. [Google Scholar]
- 27.Cho D, Signoretti S, Dabora S, et al. Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2007;5:379–85. doi: 10.3816/CGC.2007.n.020. [DOI] [PubMed] [Google Scholar]