Abstract
Purpose
Determining whether potentially therapeutic drug exposure is achieved within brain tumors in an exploratory clinical investigation would provide a rational basis for selecting agents for evaluation in phase II trials. This study investigated the use of microdialysis to assess intratumoral drug distribution in patients with recurrent high grade gliomas (HGG).
Patients and Methods
Microdialysis catheters were placed during surgery for residual HGG 1-day before giving methotrexate (MTX) 12-g/m2 by 4-h i.v. infusion. MTX was measured by Liquid Chromatography/Mass Spectrometry (LC/MS) in plasma and microdialysate during the infusion and for 24-h thereafter. Blood brain barrier (BBB) permeability of tissue in which the microdialysis probe was located was determined by digitally fusing brain CT and contrast enhanced MRI images.
Results
The microdialysis probe was located in contrast enhancing tumor in two patients and nonenhancing tissue in two others. Cerebral drug penetration, as indicated by the ratio of the area under the MTX concentration–time curves in brain extracellular fluid and plasma, was considerably greater in contrast enhancing tumor (0.28–0.31) than nonenhancing tissue (0.032–0.094). Nevertheless, MTX concentrations in ECF exceeded 2-μM, the average concentration for 50% cell kill against glioma cell lines in vitro, for 20–26 h in both regions of the tumor.
Conclusions
Microdialysis is a very informative technique for characterizing the intratumoral pharmacokinetics of drugs, such as MTX, that do not freely penetrate the BBB. Establishing the catheter probe location relative to areas of BBB disruption is required to properly assess the significance of microdialysis data in this context.
Keywords: Glioma, Microdialysis, Drug delivery, Pharmacokinetics
Introduction
Despite recent advances in the treatment of solid tumors, there are few effective treatments for recurrent high grade gliomas (HGG) and survival is often limited to several months [1, 2]. Promising new compounds that exploit novel molecular targets to inhibit tumor growth are currently being evaluated for the treatment of brain tumors in recognition of the genuine need to identify drugs that are effective against this disease [2–4]. Unfortunately, molecularly targeted agents have shown little evidence of activity in patients with HGG to date, including drugs directed at glioma-specific pathways [4, 5].
The effectiveness of chemotherapy depends upon exposing tumor cells to adequate concentrations of the biologically active form of the drug for a sufficient duration of time. This basic tenet of cancer therapeutics is difficult to achieve in most solid tumors due to well known factors such as hypoxia, intratumoral pressure gradients and abnormal vasculature that can limit the distribution of a drug from systemic circulation to interstitial spaces surrounding tumor cells [6, 7]. Delivering drugs to a brain tumors is inherently more complex than solid tumors outside of the central nervous system (CNS) because of the blood brain barrier (BBB). The BBB is a physical structure at the level of brain capillaries that separates cerebral circulation from systemic circulation and effectively prevents many exogenous molecules from entering the CNS [8, 9]. Inadequate penetration of the BBB has been identified as a significant factor contributing to the failure of systemic chemotherapy for malignant brain tumors [9–11].
Assessing drug distribution to brain tumors in patients presents considerable experimental challenges [12, 13]. Drug levels that may be achieved in brain tumors or surrounding normal tissue cannot be inferred from the concentrations measured in plasma or cerebrospinal fluid [14]. Directly measuring the concentration of drug in tumor tissue is not very informative unless it can be given in a manner that provides continuous systemic exposure because surgical specimens cannot be serially obtained. Furthermore, only a small fraction of the compounds entering clinical trials are amenable to detection by non-invasive imaging techniques such as positron emission tomography and magnetic resonance spectroscopy [13, 15]. Consequently, the clinical evaluation of new anticancer drugs in brain cancer patients often proceeds without establishing whether potentially effective concentrations can be achieved and maintained in the tumor for a sufficient period of time.
Microdialysis is a technique that enables low molecular weight, water soluble compounds to be continuously sampled in extracellular fluid (ECF) within a highly localized area of intact tissue. The theoretical considerations and practical application of microdialysis for pharmacokinetic drug level monitoring have been extensively reviewed [13, 16]. Commercially available microdialysis catheters are approved for clinical use to assess endogenous markers of brain injury or ischemia [17]. There is minimal injury to normal brain tissue, alteration of fluid balance, or disruption of the BBB associated with catheter insertion [16, 18, 19]. However, clinical experience with the utilization of microdialysis to characterize drug distribution to brain tumors remains extremely limited [20, 21].
Methotrexate was chosen as the agent for this investigation because the feasibility of using microdialysis to characterize its intratumoral pharmacokinetics had been demonstrated in preclinical studies and assays for MTX detection are well developed [22–25]. The findings described in this report confirm that microdialysis is indeed a practical and highly informative technique in brain tumors in vivo. Moreover, the regional integrity of the BBB, as assessed by gadolinium contrast enhanced MRI, dramatically alters the concentration–time profile of MTX within these tumors.
Patients and methods
Eligibility
Patients were required to have histologically confirmed supratentorial grade III or IV glioma with radiographic evidence of progression after radiation therapy. A planned biopsy or partial resection of the tumor for clinical indications and eligibility for treatment with high dose MTX included: age ≥ 18-years; KPS ≥ 60; MMSE score ≥ 15; ≥3-months since radiation therapy; ≥3-weeks since chemotherapy (6-weeks for chloroethylnitrosoureas); standard clinical pathology tests within normal ranges; and adequate renal function (serum creatinine ≤ 2-mg/dl; creatinine clearance ≥ 50-ml/min). Conditions resulting in exclusion from the study included: concurrent chemotherapy; another malignancy or serious illness; allergy to MTX; ascites or pleural effusions; females who were either pregnant or breast-feeding. Salicylates, nonsteroidal antiinflammatory drugs and sulfonamides could not be used 1-week prior to the administration of MTX. Written informed consent was provided by patients meeting all eligibility criteria.
Study design
The protocol was approved by the NCI and the IRB of each participating site. Patients underwent a biopsy or resection as clinically indicated. Upon pathological confirmation of HGG, a CMA 70 Brain Microdialysis Catheter (CMA Microdialysis, Solna, Sweden) was inserted into brain tissue adjacent to the resection cavity in an area corresponding to contrast enhancement on preoperative MRI using the BrainLab VectorVision Neuronavigation System (BrainLab USA, Moorestone, NJ) or through the biopsy burr hole into the tumor. The catheter was 0.9-mm in diameter with a 100-mm shaft that had a 10-mm dialysis membrane (Mr 20,000 cut-off), and gold thread in the tip. A CT scan without contrast was obtained to assess the surgical site and verify the microdialysis probe position as the gold thread is best visualized by CT. In the absence of postoperative complications, the catheter was perfused with a modified Ringer's solution (i.e., CMA CSF Perfusion Fluid) at 1.0-μl/min for 18–32-h before starting the MTX infusion. During this time, the patient received i.v. hydration with potassium chloride 10-mEq/l and sodium bicarbonate 50-mEq/l in D5W at 0.1-l/h/m2.
After verifying creatinine clearance >100-ml/min/1.73 m2, urine output >100-ml/h, and urine pH >7.5 were achieved for at least 4-h, Methotrexate Sodium for Injection 12-g/m2 reconstituted in D5W (500 ml) was given by continuous i.v. infusion over 4-h. Hydration was continued during the infusion and until achieving successful rescue with leucovorin (described below). Perfusate was collected in 30-min intervals from 1-h before to 24-h after the infusion. Plasma was harvested from heparinized blood samples (3-ml) collected before dosing and at 1, 2, 3, 3.9, 4.1, 4.25, 4.5, 4.75, 6, 8, 10, 12, 16, 20, 24, and 28-h after starting the infusion. Samples were stored at −70°C until thawed for analysis.
The microdialysis catheter was removed at the bedside and a standard contrast enhanced brain MRI was acquired using a 1.5-Tesla scanner with 5-mm slice thickness. Data from the MRI and CT scans were fused using BrainLab BrainSCAN 5.3 software. Twenty-four hours after starting the MTX infusion, calcium leucovorin 25-mg i.v. was given every 6-h until the MTX concentration in plasma (Cp) was <0.2 μM. The dose was then changed to 25-mg p.o. every 6-h for 48-h (Cp 0.05–0.1 μM) or 72-h (Cp 0.1–0.2 μM). Patients were removed from the study 1-week after discharge and were not followed for response or survival. Characterizing the plasma and ECF pharmacokinetics of MTX was the study endpoint; however, eligible patients were permitted to continue receiving high dose MTX until progression.
Analytical methods
A validated liquid chromatography/mass spectrometry (LC/MS) assay was used to determine the concentrations of MTX in plasma and microdialysis perfusate. Methotrexate hydrate (>98%) from Sigma-Aldrich Corp. (St. Louis, MO) was used as the analytical reference. An aminopterin analog, Nα-(4-amino-4-deoxypteroyl)-Nδ-hemiphthaloyl-l-ornithine (NSC-712783), from the NCI was used as the internal standard (IS). Plasma (100-μl) was vigorously mixed with methanol (500-μl) after adding 5-μl of IS working solution (500-μg/ml in dimethyl sulfoxide). After centrifuging (10,000g, 5-min), 50-μl of the supernatant was diluted with 450-μl of 25 mM potassium phosphate buffer, pH 7.0. Microdialysate (5-μl) and 5-μl of IS working solution (25 μg/ml in Ringer's solution) were pipetted into 100-μl of Ringer's solution and vortexed. The prepared sample solutions (plasma, 25-μl; microdialysate, 10-μl) were loaded onto a Luna 5-μm C18(2) 150 × 4.6 mm HPLC column (Phenomenex, Torrance, CA) and separated with an isocratic mobile phase composed of methanol (25%) and 25-mM formic acid adjusted to pH 8.00 ± 0.05 with 1.0-M ammonium hydroxide (75%) delivered at 0.8-ml/min. An Agilent 1100 Series LC/MSD system (Agilent Technologies, Palo Alto, CA) with an atmospheric pressure ionization-electrospray interface was used for detection. Nitrogen was used as the nebulizing gas (60-p.s.i.) and drying gas (12-l/min, 350°C). With a capillary potential of 1,300-V, positive ions corresponding to the protonated molecules for MTX at m/z 455.2 (fragmentor potential, 175-V) and the IS at m/z 574.3 (fragmentor potential, 160-V) were measured by selected-ion monitoring. Average retention times were 4.8 and 6.6-min for MTX and the IS, respectively. Extracted ion chromatograms were integrated to provide peak areas.
Study samples were prepared for analysis daily together with a set of nine calibration standards (0.05–10-μg/ml) and three quality control (QC) samples (0.15, 4,5, 9.0-μg/ml) of MTX in human donor plasma or Ringer's solution. Standard curves constructed by plotting the MTX/IS peak area ratio against the concentration of each calibration standard were analyzed by weighted linear regression and used to calculate the drug concentration in study samples. Interday accuracy of the assay for measuring the QC samples of MTX in human plasma ranged from 100.3% to 105.8% of the known concentrations and the precision was 1.8%–4.0%. Accuracy and precision for measuring QC samples of MTX in Ringer's solution were 98.0%–103.5%, and 4.8%–6.0%, respectively.
In vitro microdialysis recovery
In vitro recovery of MTX closely approximates in vivo recovery as determined by the retrodialysis method in normal tissue and tumor xenografts [23–25]. In vitro recovery was determined at a range of concentrations encompassing the assayed drug concentration in patient microdialysate samples. The probe of a new microdialysis catheter perfused with Ringer's solution at 1.0-μl/min was sequentially inserted into test tubes containing solutions of MTX in Ringer's solution with concentrations of 0, 1.9, 18.3, and 152-μM at 37°C. Perfusate was collected from each tube every 30-min continuously for 3-h. Relative recovery (mean ± SD), calculated by dividing the average MTX concentration for the 6-perfusate samples by the concentration of the bulk solution and multiplying by 100, was 43.1 ± 2.5% at 1.9-μM, 44.3 ± 0.9% at 18.4-μM, and 43.2 ± 4.3% at 152-μM. The overall mean recovery was 43.6 ± 2.8%.
Pharmacokinetic data analysis
Sample times were calculated from the beginning of the MTX infusion to the midpoint of the collection interval for each sample. The MTX concentration in brain microdialysate was corrected for in vitro recovery yielding the estimated concentration of drug in brain ECF (Cecf). Time courses for Cp and Cecf were analyzed independently. The equation for zero-order i.v. drug input and first-order biexponential disposition was fit to the Cp-time data for each patient, as described previously [26]. The Cecf-time data was best described by a model that assumed unidirectional, first-order transfer of MTX from plasma into and first-order elimination directly from brain tumor ECF, as given by the equation:
where k13 is the first-order rate constant for the transfer of MTX from plasma to brain ECF, k30 is the rate constant for elimination of MTX from brain ECF, V1 is the central compartment apparent volume of distribution, and V3 is the apparent volume of distribution in brain ECF. Parameters describing the best-fit equation for Cp-time data for each patient were defined as constants to calculate the predicted Cp at any given time. Nonlinear regression was performed with WinNonlin Professional version 5.0 (Pharsight Corp., Cary, NC) using the Gauss Newton algorithm for analyzing the Cp-time data and the Nelder-Mead simplex algorithm for the Cecf-time data, with y−2 weighting. Final values of the iterated parameters in the best-fit equations were used to calculate the maximum concentration of drug (Cmax), area under the drug concentration-time curve from zero to infinity (AUC), half-life of the apparent terminal phase in the drug concentration-time curve (t1/2,z), and total body clearance (CL).
Results
Ten patients were enrolled into the study. Microdialysis catheters were not placed in two patients because of low grade histology on intra-operative pathology review. One patient did not receive MTX because of postsurgical thrombocytopenia that did not resolve after 30-h. In the seven patients treated with MTX, technical malfunctions resulting in failure to collect any dialysate occurred in two patients. The membrane of the microdialysis catheter ruptured in one case and there was a faulty connection between the syringe pump and tubing in the other case. In another patient, the MTX infusion was stopped after 50-min because of inadequate urine alkalinization, then resumed 6.6-h later. Additional patients were not enrolled because a concurrent ECOG phase II trial of high dose MTX in patients with recurrent HGG was closed to accrual due to ineffectiveness of MTX and it was felt further use of MTX in patients with HGG was not warranted.
Characteristics the four patients who successfully completed the study without deviation are summarized in Table 1. All were male, with a mean age of 47 years (range, 33–72 years). One had glioblastoma multiforme, one had anaplastic astrocytoma and two had anaplastic oligodendroglioma. In each case, prior treatment included surgical resection, radiation therapy, and at least one chemotherapy regimen. All four were taking an enzyme inducing antiseizure drug (phenytoin or oxcarbazepine) and a stable dose of dexamethasone. The placement and removal of the microdialysis catheters was performed without difficulty and there were no complications associated with the procedure. One patient had a biopsy and three had subtotal resections. Postoperative imaging showed that the microdialysis probe was located in contrast enhancing tissue in the biopsy patient. In the partially resected patients, the probe was in noncontrast enhancing tissue in two patients and in contrast enhancing tissue in the third (Fig. 1).
Table 1. Patient characteristics.
| Patient | Age | Tumor characteristics | Surgical procedure | |
|---|---|---|---|---|
|
| ||||
| Type | Location | |||
| A | 72 | GBM | Left occipital | Partial resection |
| B | 43 | AA | Left frontal | Stereotactic biopsy |
| C | 40 | AO | Right frontal | Partial resection |
| D | 33 | AO | Left parietal | Partial resection |
Note: all of the patients were male
Abbreviations: GBM, glioblastoma multiforme; AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma
Fig. 1.
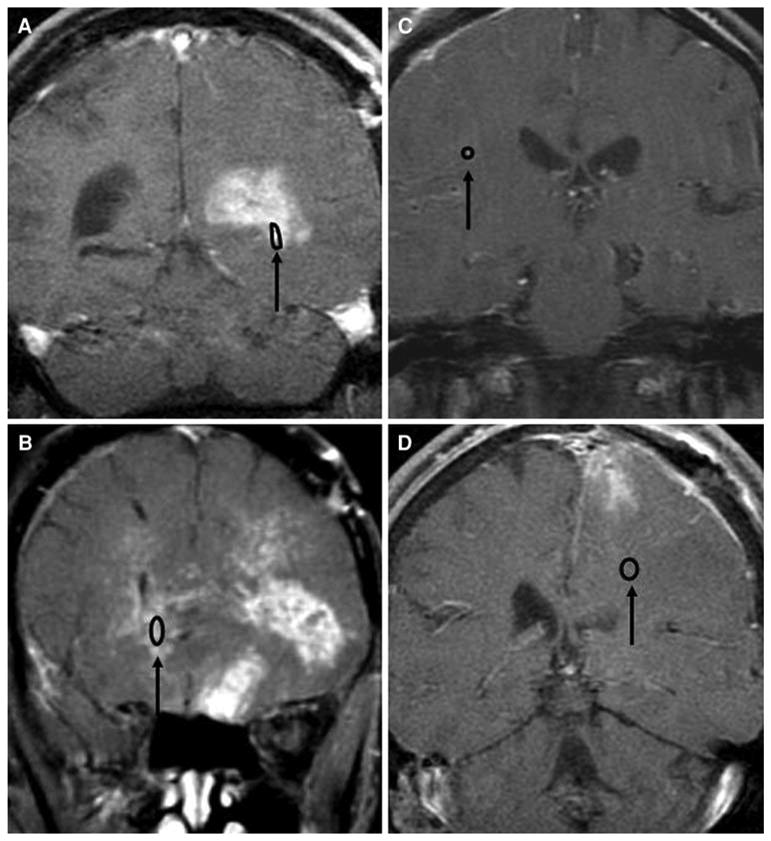
Coronal view of digitally fused gadopentetic acid contrast enhanced brain MRI and nonenhanced head CT images of the four patients who completed the study. A gold filament in the tip of the microdialysis catheter enables visualization of the probe location in the CT scan. The microdialysis probe tip was located in contrast enhancing tissue in patients A and B and in nonenhancing tissue in patients C and D. The catheter was placed in tissue near the margin of the surgical cavity following resection of the tumor in patients A, C, and D, and in tumor accessed through the biopsy burr hole in patient B
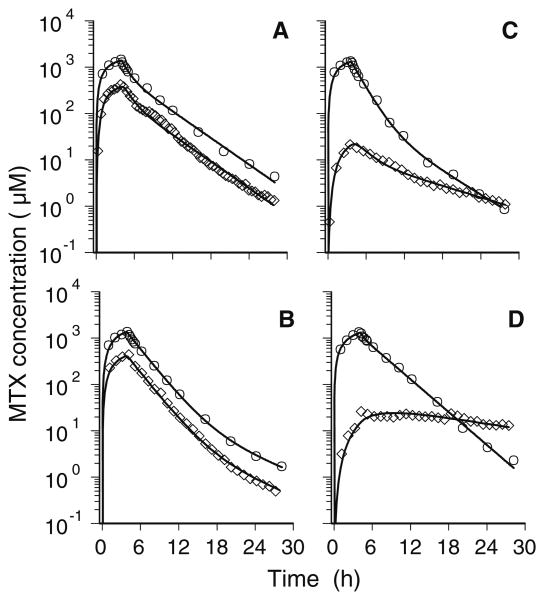
Time courses for Cp and Cecf determined for each patient are shown in Fig. 2. Estimated values of the pharmacokinetic parameters determined by nonlinear regression analysis of the plasma and ECF concentration–time profiles are given in Table 2. The plasma pharmacokinetics of MTX were similar in all four patients and in excellent agreement with data from a previously reported study of high dose MTX [27]. The Cmax in plasma ranged from 1,321 to 1,407 μM and the CL values ranged from 3.44 to 4.58 l/h/m2.
Fig. 2.
Time courses of the MTX concentration in plasma (○) and brain ECF (◊). The plasma profiles are similar in each of the four patients, with peak drug levels ranging from 1,321 to 1,407-μM at the end of the 4-h i.v. infusion of MTX 12 g/m2. Time courses of MTX in ECF are dependent upon whether the probe of the microdialysis catheter was placed in contrast enhancing (patients A and B) or nonenhancing (patients C and D) regions of the tumor
Table 2. Pharmacokinetic parameters of methotrexate in plasma and brain tumor extracellular fluid.
| Compartment | Parameter | Patient | |||
|---|---|---|---|---|---|
|
| |||||
| A | B | C | D | ||
| Plasma | Cmax (μM) | 1,407 | 1,367 | 1,321 | 1,325 |
| AUC (μM·h) | 7,553 | 6,850 | 5,766 | 7,686 | |
| t1/2,z (h) | 3.15 | 5.40 | 3.56 | 2.54 | |
| CL (l/h/m2) | 3.50 | 3.85 | 4.58 | 3.44 | |
| V1 (l/m2) | 6.93 | 9.89 | 8.47 | 7.10 | |
| MRT (h) | 3.12 | 2.75 | 2.10 | 3.28 | |
| ECF | Cmax (μM) | 380 | 407 | 22 | 24 |
| AUC (μM·h) | 2,121 | 2,086 | 186 | 721 | |
| t1/2,z (h) | 3.15 | 4.59 | 7.18 | 14.51 | |
| V3 (l/m2) | 67 | 11 | 21 | 223 | |
| k13 (10−3 h−1) | 6.54 | 5.95 | 0.41 | 0.15 | |
| k30 (h−1) | 2.42 | 17.50 | 0.52 | 0.05 | |
| AUCecf/AUCp | 0.281 | 0.305 | 0.032 | 0.094 | |
Despite the similarity in the plasma pharmacokinetics of MTX among these patients, there were distinct differences in the time course of MTX in brain ECF associated with the microdialysis probe location relative to contrast enhancing tissue. As shown in Fig. 2 (panels A and B), concentrations of the drug in brain ECF closely paralleled the Cp-time profile in both patients in whom the probe was in contrast enhancing tissue. The peak Cecf occurred shortly after the end of the MTX infusion and the Cmax in brain ECF was 27%–30% of the Cmax in plasma for these patients. The t1/2,z of the Cecf-time curves was similar to the Cp-time profiles and cerebral drug penetration, as indicated by the ratio of the AUC in brain ECF-to-plasma, was 0.28–0.31. In contrast, Cecf was much lower than the corresponding Cp in the two patients in whom the probe resided in nonenhancing tissue (Fig. 2, panels C and D). For these patients, the peak Cecf occurred later and was only 1.7%–1.8% of the Cmax in plasma. The t1/2,z of the Cecf-time curves were notably longer than the corresponding Cp-time profiles and cerebral drug penetration was only 0.032–0.094.
Discussion
Until recently, the only clinical application of microdialysis to measure cerebral drug concentrations was a pilot study to determine whether therapeutic levels of the antibiotic rifampacin were achieved in glioblastoma patients [20]. Differences between the concentration of rifampacin in ECF adjacent to the bulk tumor, determined from dialysate collected during a single 2-h interval, and biopsy specimens removed from the tumor and normal brain during this same time were attributed to variability in the functional integrity of the BBB. It is well known that HGGs are diffusely infiltrative tumors in which the BBB is disrupted within the bulk tumor and intact in the well vascularized outer regions [28, 29]. The existence of a gradient of drug concentrations within brain tumors following i.v. administration of various anticancer agents, attributable to the disruption of the BBB within the tumor, has been demonstrated in the analysis of biopsy specimens obtained from patients [30]. Accordingly, the existence of regional differences in BBB permeability is an important consideration that must be taken into account in the design of microdialysis studies to assess drug penetration in brain tumors. The presence of a gold thread embedded in the tip of a commercially available microdialysis catheter readily enables the physical location of the probe to be visualized by CT. Enhancement in brain MRI scans after i.v. administration of the contrast agent gadopentetic acid, currently the technique of choice for the radiographic assessment of HGGs, is effectively a measure of BBB disruption within the tumor [31]. This report describes the first study in which the integrity of the BBB in the region of the tumor in which the microdialysis probe resides was established by digitally fusing CT and contrast enhanced MRI scans.
In our experience, inserting the microdialysis catheter added little complexity to the normal surgical procedure and the patients had no complications related to its placement or removal, as previously reported [20, 21, 32]. However, the devices malfunctioned in two of seven patients in whom catheters were implanted precluding the collection of dialysate. This is similar to the failure rate in another microdialysis study in glioma patients [21]. Nevertheless, prominent regional differences in the distribution of MTX from systemic circulation to ECF within brain tumors associated with BBB integrity were clearly identified among the four patients who were successfully evaluated. Cerebral drug penetration was considerably greater in contrast enhancing regions of the tumor (28%–31%) than nonenhancing tissue (3.2%–9.4%). Interestingly, these findings are very similar to the cerebral penetration of MTX determined by microdialysis in brain tumor and normal brain tissue in rats [22]. Considering that MTX is 54%–59% protein bound in human plasma [33, 34] it appears that interstitial concentrations achieved in regions of BBB disruption are effectively limited by the free fraction of drug in systemic blood.
It was also observed that the concentration of MTX in ECF sampled from contrast enhancing regions of the tumor declined in parallel with drug levels in plasma, but at a much slower rate in ECF within nonenhancing tissue. This behavior further suggests that unbound drug molecules are readily exchanged between blood and brain ECF in regions of the tumor where the BBB is disrupted, as opposed to nearby tissue where the BBB is functionally intact. Moreover, diffusion of MTX from regions of high interstitial concentration to tissue in close proximity with an intact BBB appears to be inconsequential. These differences in the intratumoral pharmacokinetics of MTX are qualitatively similar to those described in a recent report of a microdialysis study of p-boronophenylalanine in glioblastoma patients [21]. However, the investigators could only speculate that differences in the uptake and elimination of boron in the bulk tumor, brain adjacent to tumor and normal brain were attributable to variations in BBB integrity because correlative contrast enhanced MRI scans were not obtained.
The average MTX concentration required for 50% cell kill against a variety of glioma cell lines in vitro after 72-h of incubation is 2.4-μM [35]. Considerably greater peak concentrations of MTX in ECF were achieved in contrast enhancing (average, 393-μM) than in nonenhancing (average, 23-μM) regions of the tumor in patients evaluated in this study. Despite the nearly 12-fold difference between the peak drug levels, the time that interstitial MTX concentrations exceeded 2-μM ranged from 20 to 26-h in both regions of the tumor. Achieving cytotoxic drug concentrations in nonenhancing tissue is of particular importance for the treatment of HGG because tumor cells are widely disseminated beyond the region of gadolinium contrast enhancement [28]. Although the value of MTX in the treatment of malignant gliomas has not been determined, these findings show that the minimum requirement of achieving a potentially cytotoxic concentration within the tumor is achieved with high dose MTX. This would theoretically support undertaking a clinical trial to evaluate the efficacy of high dose MTX against various forms of brain tumors.
This investigation has served to further demonstrate that microdialysis can provide unique and highly informative quantitative data on the distribution of anticancer drugs to brain tumors in a study involving a small number of patients. Moreover, the results of this study highlight the critical importance of determining the location of the microdialysis probe within a brain tumor to properly assess the significance of intratumoral pharmacokinetic data. Indeed, microdialysis data can be misleading without establishing the location of the probe relative to areas of BBB disruption as indicated by gadolinium contrast enhancement. Employing microdialysis to assess intratumoral drug distribution during the early clinical development of investigational drugs being considered for the treatment of brain tumors is highly advantageous. Phase II studies to evaluate the efficacy of compounds against HGG would not be recommended in the absence of achieving potentially therapeutic concentrations in tumor ECF. Preliminary characterization of intratumoral drug distribution would prevent patients from being enrolled into a clinical trial of an agent that has little chance of providing a therapeutic effect and permit the allocation of limited resources to studying other compounds that offer a better chance for success.
Acknowledgments
This study was supported by grants U01-CA62475, U01-CA105689, and P30-CA0516 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland. Microdialysis catheters were generously donated by CMA Microdialysis AB (North Chelmsford, MA).
Footnotes
Preliminary results of this study were presented at the 42nd annual meeting of the American Society of Clinical Oncology in Atlanta, GA, June 2–6, 2006.
Contributor Information
Jaishri O. Blakeley, Email: jblakel3@jhmi.edu, Johns Hopkins University School of Medicine, Cancer Research Building II, 1550 Orleans Street, Suite 1M16, Baltimore, MD 21231, USA.
Jeffrey Olson, Emory University School of Medicine, Atlanta, GA, USA.
Stuart A. Grossman, Johns Hopkins University School of Medicine, Cancer Research Building II, 1550 Orleans Street, Suite 1M16, Baltimore, MD 21231, USA
Xiaoying He, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Jon Weingart, Johns Hopkins University School of Medicine, Cancer Research Building II, 1550 Orleans Street, Suite 1M16, Baltimore, MD 21231, USA.
Jeffrey G. Supko, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
References
- 1.Butowski NA, Sneed PK, Chang SM. Diagnosis and treatment of recurrent high-grade astrocytoma. J Clin Oncol. 2006;24(8):1273–1280. doi: 10.1200/JCO.2005.04.7522. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Gilbert MR, et al. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25(26):4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 3.Reardon DA, Rich JN, Friedman HS, et al. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol. 2006;24(8):1253–1265. doi: 10.1200/JCO.2005.04.5302. [DOI] [PubMed] [Google Scholar]
- 4.Brandsma D, van den Bent MJ. Molecular targeted therapies and chemotherapy in malignant gliomas. Curr Opin Oncol. 2007;19(6):598–605. doi: 10.1097/CCO.0b013e3282f0313b. [DOI] [PubMed] [Google Scholar]
- 5.Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007;6(7):1909–1919. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271(1):58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 7.Netti PA, Baxter LT, Boucher Y, et al. Time-dependent behavior of interstitial fluid pressure in solid tumors: implications for drug delivery. Cancer Res. 1995;55(22):5451–5458. [PubMed] [Google Scholar]
- 8.Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54(1):131–140. doi: 10.1227/01.neu.0000097715.11966.8e. 10.1227/01.NEU. 0000097715.11966.8E. discussion 141–2. [DOI] [PubMed] [Google Scholar]
- 9.Motl S, Zhuang Y, Waters CM, et al. Pharmacokinetic considerations in the treatment of CNS tumours. Clin Pharmacokinet. 2006;45(9):871–903. doi: 10.2165/00003088-200645090-00002. [DOI] [PubMed] [Google Scholar]
- 10.Doolittle ND, Peereboom DM, Christoforidis GA, et al. Delivery of chemotherapy and antibodies across the blood-brain-barrier and the role of chemoprotection, in primary and metastatic brain tumors: report of the eleventh annual blood-brain barrier consortium meeting. J Neurooncol. 2007;81(1):81–91. doi: 10.1007/s11060-006-9209-y. [DOI] [PubMed] [Google Scholar]
- 11.Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 12.Nicolazzo JA, Charman SA, Charman WN. Methods to assess drug permeability across the blood-brain barrier. J Pharm Pharmacol. 2006;58(3):281–293. doi: 10.1211/jpp.58.3.0001. [DOI] [PubMed] [Google Scholar]
- 13.de Lange EC, Danhof M, de Boer AG, et al. Methodological considerations of intracerebral microdialysis in pharmacokinetic studies on drug transport across the blood-brain barrier. Brain Res Brain Res Rev. 1997;25(1):27–49. doi: 10.1016/S0165-0173(97)00014-3. [DOI] [PubMed] [Google Scholar]
- 14.de Lange EC, Danhof M. Considerations in the use of cerebrospinal fluid pharmacokinetics to predict brain target concentrations in the clinical setting: implications of the barriers between blood and brain. Clin Pharmacokinet. 2002;41(10):691–703. doi: 10.2165/00003088-200241100-00001. [DOI] [PubMed] [Google Scholar]
- 15.Perkins AC, Frier M. Radionuclide imaging in drug development. Curr Pharm Des. 2004;10(24):2907–2921. doi: 10.2174/1381612043383476. [DOI] [PubMed] [Google Scholar]
- 16.Johansen MJ, Newman RA, Madden T. The use of microdialysis in pharmacokinetics and pharmacodynamics. Pharmacotherapy. 1997;17(3):464–481. [PubMed] [Google Scholar]
- 17.Siddiqui MM, Shuaib A. Intracerebral microdialysis and its clinical application: a review. Methods. 2001;23(1):83–94. doi: 10.1006/meth.2000.1108. [DOI] [PubMed] [Google Scholar]
- 18.Major O, Shdanova T, Duffek L, et al. Continuous monitoring of blood-brain barrier opening to Cr51-EDTA by microdialysis following probe injury. Acta Neurochir Suppl (Wien) 1990;51:46–48. doi: 10.1007/978-3-7091-9115-6_16. [DOI] [PubMed] [Google Scholar]
- 19.Ungerstedt U, Rostami E. Microdialysis in neurointensive care. Curr Pharm Des. 2004;10(18):2145–2152. doi: 10.2174/1381612043384105. 10.2174/138161 2043384105. [DOI] [PubMed] [Google Scholar]
- 20.Mindermann T, Zimmerli W, Gratzl O. Rifampin concentrations in various compartments of the human brain: a novel method for determining drug levels in the cerebral extracellular space. Antimicrob Agents Chemother. 1998;42(10):2626–2629. doi: 10.1128/aac.42.10.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergenheim AT, Capala J, Roslin M, et al. Distribution of BPA and metabolic assessment in glioblastoma patients during BNCT treatment: a microdialysis study. J Neurooncol. 2005;71(3):287–293. doi: 10.1007/s11060-004-1724-0. [DOI] [PubMed] [Google Scholar]
- 22.de Lange EC, de Vries JD, Zurcher C, et al. The use of intracerebral microdialysis for the determination of pharmacokinetic profiles of anticancer drugs in tumor-bearing rat brain. Pharm Res. 1995;12(12):1924–1931. doi: 10.1023/A:1016239822287. [DOI] [PubMed] [Google Scholar]
- 23.Devineni D, Klein-Szanto A, Gallo JM. In vivo microdialysis to characterize drug transport in brain tumors: analysis of methotrexate uptake in rat glioma-2 (RG-2)-bearing rats. Cancer Chemother Pharmacol. 1996;38(6):499–507. doi: 10.1007/s002800050518. 10.1007/s0028000 50518. [DOI] [PubMed] [Google Scholar]
- 24.Dukic S, Heurtaux T, Kaltenbach ML, et al. Pharmacokinetics of methotrexate in the extracellular fluid of brain C6-glioma after intravenous infusion in rats. Pharm Res. 1999;16(8):1219–1225. doi: 10.1023/A:1018945529611. [DOI] [PubMed] [Google Scholar]
- 25.Dukic S, Kaltenbach ML, Gourdier B, et al. Determination of free extracellular levels of methotrexate by microdialysis in muscle and solid tumor of the rabbit. Pharm Res. 1998;15(1):133–138. doi: 10.1023/A:1011973409022. [DOI] [PubMed] [Google Scholar]
- 26.Eder JP, Jr, Supko JG, Lynch T, et al. Phase I trial of the colloidal dispersion formulation of 9-amino-20(S)-camptothecin administered as a 72-hour continuous intravenous infusion. Clin Cancer Res. 1998;4(2):317–324. [PubMed] [Google Scholar]
- 27.Comandone A, Passera R, Boglione A, Tagini V, Ferrari Sand, Cattel L. High dose methotrexate in adult patients with osteosarcoma: Clinical and pharmacokinetic results. Acta Oncol. 2005;44:406–411. doi: 10.1080/02841860510029770. [DOI] [PubMed] [Google Scholar]
- 28.Earnest FIV, Kelly PJ, Scheithauer BW, et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology. 1988;166(3):823–827. doi: 10.1148/radiology.166.3.2829270. [DOI] [PubMed] [Google Scholar]
- 29.McKnight TR, von dem Bussche MH, Vigneron DB, et al. Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg. 2002;97(4):794–802. doi: 10.3171/jns.2002.97.4.0794. [DOI] [PubMed] [Google Scholar]
- 30.Donelli MG, Zucchetti M, D'Incalci M. Do anticancer agents reach the tumor target in the human brain? Cancer Chemother Pharmacol. 1992;30(4):251–260. doi: 10.1007/BF00686291. [DOI] [PubMed] [Google Scholar]
- 31.Hesselink JR, Press GA. MR contrast enhancement of intracranial lesions with Gd-DTPA. Radiol Clin North Am. 1988;26(4):873–887. [PubMed] [Google Scholar]
- 32.Flannery T, McConnell RS, McQuaid S, et al. Detection of cathepsin S cysteine protease in human brain tumour microdialysates in vivo. Br J Neurosurg. 2007;21(2):204–209. doi: 10.1080/02688690701248190. [DOI] [PubMed] [Google Scholar]
- 33.Rochas MA, Tufenkji AE, Levillain P, et al. Protein binding of methotrexate to human albumin and serum. A first derivative spectroscopic analysis. Arzneimittelforschung. 1991;41(12):1286–1288. [PubMed] [Google Scholar]
- 34.Maia MB, Saivin S, Chatelut E, et al. In vitro and in vivo protein binding of methotrexate assessed by microdialysis. Int J Clin Pharmacol Ther. 1996;34(8):335–341. [PubMed] [Google Scholar]
- 35.Wolff JE, Trilling T, Molenkamp G, Egeler RM, Jurgens H. Chemosensitivity of glioma cells in vitro: a metaanalysis. J Cancer Res Clin Oncol. 1999;125:481–486. doi: 10.1007/s004320050305. [DOI] [PMC free article] [PubMed] [Google Scholar]