Abstract
Chronic inflammation is now known to contribute to several forms of human cancer, with an estimated 20% of adult cancers attributable to chronic inflammatory conditions caused by infectious agents, chronic noninfectious inflammatory diseases and / or other environmental factors. Indeed, chronic inflammation is now regarded as an ‘enabling characteristic’ of human cancer. The aim of this review is to summarize the current literature on the evidence for a role for chronic inflammation in prostate cancer aetiology, with a specific focus on recent advances regarding the following: (i) potential stimuli for prostatic inflammation; (ii) prostate cancer immunobiology; (iii) inflammatory pathways and cytokines in prostate cancer risk and development; (iv) proliferative inflammatory atrophy (PIA) as a risk factor lesion to prostate cancer development; and (v) the role of nutritional or other antiinflammatory compounds in reducing prostate cancer risk.
Keywords: cytokines, diet, infections, inflammation, proliferative inflammatory atrophy, prostate cancer
Introduction
Prostate cancer will be diagnosed in approximately 240 890 US men in 2011, and an estimated 33 720 men will die from this disease.1 While the death rate has been decreasing somewhat over the last decade, the absolute number of men with prostate cancer is projected to increase substantially as a result of the ageing baby boomer population. Thus, there is a critical need for a better understanding of the aetiological factors that drive prostate cancer development; knowledge which may be utilized for cancer prevention and treatment strategies.
The most well-recognized risk factors for the development of prostate cancer are advanced age, family history and African American ancestry; however, there is also a distinct geographic distribution to prostate cancer incidence, and an apparent increase in risk with the adoption of a ‘westernized’ lifestyle. Clearly, the pathogenesis of prostate cancer involves environmental factors in addition to hereditary factors. One such potential environmental factor which has gained a great deal of recent attention is the development of chronic inflammation in the prostate due to a number of potential causes including infections, dietary factors, hormonal changes and / or other unknown environmental exposures.2 The aim of this review is to summarize the current literature regarding a role for chronic inflammation in prostate cancer aetiology, with a specific focus on potential stimuli for prostatic inflammation, prostate cancer immunobiology, inflammatory pathways and cytokines in prostate cancer risk and development, proliferative inflammatory atrophy (PIA) as a risk factor lesion to prostate cancer development and the role of nutritional or other anti-inflammatory compounds in reducing prostate cancer risk.
Prevalence of prostatic inflammation
There are multiple different lines of evidence suggesting that inflammation is very common within the adult prostate. Prostatitis is a heterogeneous and complex entity which the National Institutes of Health (NIH) consensus classification refers to as chronic prostatitis / chronic pelvic pain syndrome (CPPS). CPPS is divided into the following four categories, the first three of which relate to men with symptoms of disease: (I) acute bacterial prostatitis; (II) chronic bacterial prostatitis; (III) chronic prostatitis / CPPS; and (IV) asymptomatic inflammatory prostatitis.3 Bacterial prostatitis accounts for only an estimated 5–10% of prostatitis cases, with the most commonly implicated microorganisms being Escherichia coli and Enterococcus spp.4,5 In terms of symptomatic ‘prostatitis’ (e.g. NIH categories I–III), it is estimated that up to 16% of men in the US population are afflicted at some time in their life.4,6 The prevalence of asymptomatic prostatic inflammation (i.e. ‘histological prostatitis’) appears to be in fact much higher, as evidenced by studies examining men who undergo biopsy for prostate cancer due to elevated prostate-specific antigen (PSA) levels and test negative for cancer,7–10 autopsy studies11 and findings from transurethral resections for benign prostatic hyperplasia (BPH).12 A recent example of this stems from results published from the baseline data of the REDUCE (REduction by DUtasteride of prostate Cancer Events) trial, where 80% of patient biopsies were found to have some degree of inflammation. 13 Similarly, results from a prospective randomized controlled trial of 328 men with PSA levels between 2.5 and 10 ng / ml and normal digital rectal examination (DRE) indicated that more than 45% of the patients had leucocytes in expressed prostatic secretions (EPS).9 Finally, histological specimens of prostate cancer tissue frequently exhibit unexplained acute and chronic inflammation and inflammation-associated lesions.2
Evidence suggests there is also a racial and geographical difference in the prevalence of prostatic inflammation in adult men, which falls in line with the geographic distribution difference in prostate cancer incidence. For example, studies have reported an increased incidence of inflammation in biopsy specimens 14 and increased expression of immune-related genes in tumour tissues15 from African American men compared to European American men. Also, recent findings from our own group from an autopsy study revealed less inflammation in the prostates of Asian men as opposed to European American men (C. Joshu, A.M. De Marzo, M.S. Lucia, J.K. Parsons, C. Maggi-Galluzzi and E.A. Platz, unpublished data).
As will be discussed below in the section regarding the immunobiology of prostate inflammation, preliminary work indicates that there may also be a difference in the prevalence of prostatic inflammation which correlates to risk of high-grade prostate cancer (B. Gurel, M.S. Lucia, E.A. Platz and A.M. De Marzo, manuscript in preparation). These preliminary studies revealed that chronic inflammation in benign tissue of a needle biopsy was predictive of a higher risk for prostate cancer diagnosis and specifically with higher-grade (Gleason score 7–10) disease.
Contributors to prostatic inflammation
Multiple different aetiological agents are thought to contribute to the initiation of prostatic inflammation, including infections, dietary factors, corpora amylacea (and associated physical trauma), hormonal changes and urine reflux.2 Here we focus on recent evidence regarding the role of infections, diet and corpora amylacea in prostatic inflammation and cancer development.
A POTENTIAL LINK BETWEEN PROSTATITIS, PROSTATE INFECTIONS AND PROSTATE CANCER
Multiple different bacterial species are known to infect the human prostate and induce inflammation, and many of these organisms have been identified from studying patients with bacterial prostatitis. Interestingly, in the study referred to above by Ugurlu et al.9 regarding the incidence of leucocytes in EPS from patients with elevated PSA levels, the patients with leucocyte-positive EPS were randomized into antibiotic (levofloxacin), anti-inflammatory (naproxen sodium) and control treatment groups. Only the antibiotic-treated patients exhibited a significant decrease in PSA levels, suggesting a potential contribution from an unrecognized prostatic infection.
As mentioned previously, the most commonly implicated microorganisms in bacterial prostatitis are E. coli and Enterococcus spp.; however, additional organisms such as Pseudomonas spp., Proteus mirabilis, Klebsiella spp. and Serratia spp. have also been identified.4,5 Several sexually transmitted organisms have also been implicated in bacterial prostatitis or prostatic inflammation, and these include Chlamydia trachomatis, Gonococcal organisms, Trichomonas vaginalis and Treponema pallidum (reviewed in Ref. 2,16). Mycoplasma spp. have also been implicated in chronic prostatitis.17,18
Studies attempting to define a potential correlation between prostatitis and prostate cancer risk have reported both positive19,20 and negative results21 (also reviewed in Ref. 22). A very recent study performed in a large, multiracial and ethnic cohort as part of the California Men’s Health Study (CMHS) found an increase in risk for prostate cancer with a history of prostatitis [relative risk (RR) = 1.30; 95% confidence interval (CI): 1.10–1.54] and long duration of prostatitis symptoms.23 This study also found that a self-reported reported history of sexually transmitted disease (STD) was not associated with overall prostate cancer risk; however, Latinos reporting a history of STDs had an increased risk of prostate cancer compared to Latinos with no STD history. Furthermore, foreign-born Latinos were found to have a greater risk of prostate cancer associated with STD history than US-born Latinos.23 Although the authors report that this study could have potentially been confounded by detection bias (e.g. men with symptomatic prostatitis may seek medical attention, which may in turn lead to a greater chance for testing and incidental detection of prostate cancer), the association between prostatitis and prostate cancer risk certainly remains an important area for further research.
There are also several lines of evidence that support a potential role for asymptomatic (i.e. subclinical) prostatic inflammation caused by infectious microorganisms and prostate cancer development. An organism of particular interest in this respect is E. coli. Apart from being one of the most frequently isolated microorganisms from patients with bacterial prostatitis, E. coli has also been identified in both BPH and prostate cancer tissues using both culture-dependent and culture-independent molecular techniques.24,25 In mouse models, infection of the prostate with uropathogenic strains of E. coli (UPEC) has been reported to induce epithelial proliferation and reactive hyperplasia,26 dysplasia and oxidative DNA damage27 and a marked reduction of the haploinsufficient prostate cancer tumour suppressor, NKX3.1.28 A recent study in Wistar rats described prostatic epithelial hypertrophy and atrophy in response to UPEC E. coli infection, with a transient upregulation of ErbB2 [human epidermal growth factor receptor 2 (HER2 / neu)].29 Different strains of UPEC E. coli produce a number of virulence factors, such as cytotoxic necrotizing factor 1 (CNF1), which has been shown to promote tissue damage in a rat model of prostatitis.30 Furthermore, strains of pathogenic E. coli which harbour a ‘pks’ genomic island responsible for synthesis of a peptide–polyketide hybrid cytotoxin (termed colibactin) have been shown to induce DNA double-strand breaks (DSBs) in human cells.31 Strains of E. coli which are implicated in urosepsis have been shown to frequently harbour both CNF1 and the pks island.32 Intriguingly, in a recent study examining E. coli isolates from patients presenting with acute bacterial prostatitis, more than 70% of isolates were found to express colibactin and one strain carried the cytolethal distending toxin (CDT) gene cluster.33 The authors of this study posit that the production of genotoxic toxins by E. coli implicated in acute bacterial prostatitis could contribute to subsequent carcinogenesis and potentially explain the epidemiological data suggesting an increased risk for prostate cancer with previous history of prostatitis.33 For reasons such as those described here, E. coli should remain an organism of particular interest in regards to a potential link between prostatic inflammation and prostate cancer development.
Additional microorganisms (both bacterial and viral) have been implicated in stimulating prostatic inflammation that may contribute to the development of prostate carcinogenesis. For example, C. trachomatis has been detected in prostatitis,34 BPH35 and prostate cancer,36 although multiple epidemiological studies have reported no association between C. trachomatis seropositivity and prostate cancer risk.37–39 Studies have shown that C. trachomatis can infect rat prostate epithelial cells in vitro, leading to the production of proinflammatory cytokines and chemokines.40,41
Another bacterial species that has gained recent attention in the prostate cancer field is Propionibacterium acnes, a proinflammatory bacterium that is considered to be the aetiological agent in the skin condition acne, as well as several other inflammatory conditions including endocarditis and post-surgical infections.42 P. acnes was first reported in association with prostate inflammation and cancer in 2005,43 and several subsequent studies have also identified P. acnes in prostate specimens.24,44–46 The potential association between acne and / or plasma antibodies to P. acnes and prostate cancer incidence and outcomes has been examined in multiple epidemiological studies.47–49 In 2005, Galobardes et al.47 reported on an investigation involving the Glasgow Alumni Cohort Study whereby a reported history of acne in young adulthood was associated with an increased risk of prostate cancer mortality [hazard ratio (HR) = 1.67, 95% CI: 0.79–3.55]. In 2007, Sutcliffe et al.48 reported that men with a history of tetracycline use for four or more years (as a measure of previous history of severe acne) had a significantly higher risk of prostate cancer (multivariable-adjusted RR = 1.70, 95% CI: 1.03–2.80). In contrast, a study in 2010 by Severi et al.49 reported an inverse association between P. acnes antibody titres and prostate cancer risk [e.g. higher concentrations of circulating P. acnes antibodies were associated with decreased prostate cancer risk, odds ratio (OR) = 0.73, 95% CI: 0.58–0.91], which was especially pronounced for advanced prostate cancer (OR = 0.59, 95% CI: 0.43–0.81). Here, the authors assert that the potential role that P. acnes may play in prostate cancer might be a protective one, in that immune responses elicited against P. acnes may be tumoricidal.
Recent evidence suggests that infection by the protozoan T. vaginalis, the causative sexually transmitted infection (STI) agent of trichomoniasis, may contribute to asymptomatic prostatic inflammation as well as prostate cancer risk (reviewed in Ref. 16). A positive association between T. vaginalis serostatus and both overall prostate cancer risk (OR = 1.43, 95% CI: 1.00–2.03) as well as high-grade disease (OR = 1.76, 95% CI: 0.97–3.18) was first reported by Sutcliffe et al.50 in 2006 in a case–control study in the Health Professionals Follow-up Study (HPFS). This association was not present in a subsequent study conducted in the Prostate Cancer Prevention Trial (PCPT)51; however, a positive association with measures of advanced cancer (extraprostatic extension, metastatic prostate cancer) was again observed in the Physicians’ Health Study (PHS).52 Although attempts to detect T. vaginalis via molecular techniques do not indicate that the organism is readily detectable in ex vivo prostate tissue biopsies from prostate cancer patients,24 the epidemiological findings may warrant further investigations into potential mechanisms by which T. vaginalis might contribute to prostate cancer risk, and specifically risk of advanced disease.
Another indication that STIs may infect the prostate and contribute to prostatic inflammation stems from recent studies suggesting an increase in serum PSA levels in men with a confirmed diagnosis of an STI. In 2006, Sutcliffe et al.53 reported that men with laboratory-confirmed exudative STIs, including gonorrhoea, chlamydia and trichomonosis, were significantly more likely to have increased PSA levels. In another recent study in 2011, Sutcliffe et al.54 again reported that men with active infections of chlamydia and gonorrhoea were likely to have a large rise in PSA, further indicating prostate involvement with STIs. While it is not entirely clear why serum PSA levels are elevated after infection with these organisms, the most straight-forward mechanism would be that infection of the prostate leads to prostatic inflammation and damage to prostate epithelial cells, resulting in release of PSA extracellularly that, in turn, is released into the circulation.
It has been suggested that a newly discovered gammaretrovirus, termed xenotropic murine leukaemia virus-related virus (XMRV), may also act as an aetiological agent in prostatic inflammation.55 This virus was first reported in association with prostate cancer in 2006;56 however, the likelihood that XMRV ever establishes a true infection in humans has become the subject of intense controversy.57,58 Indeed, very recent evidence suggests that XMRV originated from a recombination event between two endogenous murine retroviruses during the passage of the CWR22 prostate cancer xenograft in mice.59,60 Furthermore, mouse DNA contamination of samples and common laboratory reagents has now been shown to be a potential source of false-positive polymerase chain reaction (PCR) results for XMRV.61–64 Currently, therefore, we suggest that the likelihood that XMRV is playing any role in human prostate cancer initiation or progression is exceedingly small.
DIET
Although a later section of this review will focus on the evidence for anti-inflammatory dietary components in prostate cancer prevention, evidence also exists that suggests a potential role for certain dietary factors in promoting prostatic inflammation and prostate cancer development.65 In this regard, much attention has been given to a class of dietary mutagens called heterocyclic amines (HCAs), to which humans are exposed under common dietary practices. HCAs are generated in abundance in meats cooked under high temperature conditions and have been linked to cancer of multiple organs including prostate, colon and breast.66 In prostate cancer, a particular focus has been given to the cancer-inducing capabilities of the heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[ 4,5-b]pyridine (PhIP). Although not all studies are positive, a number of epidemiological studies have linked the consumption of meat and very well-cooked meat with risk of overall prostate cancer and / or aggressive prostate cancer.66–71 In rodents, dietary intake of PhIP represents one of the few animal models which results in prostatic intraepithelial neoplasia (PIN) lesions and intraductal carcinoma,72,73 providing further evidence of a potential role of dietary intake of PhIP in human prostate cancer. In a recent study, conducted in a transgenic Fisher344 (Big Blue) rat model, PhIP consumption was shown to induce elevated mutation frequencies in all lobes of the prostate.73 PhIP-induced cancerous lesions are restricted to the ventral lobe of the rat prostate, however, and in this study an increase in stromal mast cells and macrophages that was restricted to the ventral prostate was observed. This study represents experimental evidence whereby lobe-specific inflammation was shown to be induced in response to PhIP consumption, and it was suggested that this represents a potential new mechanism by which dietary PhIP can increase cancer risk (i.e. by prompting inflammation). Interestingly, while the inflammation did not appear restricted to the ventral lobe, Borowsky et al.74 also reported that PhIP-induced PIN lesions in the rat prostate were preceded by inflammatory infiltrates.
Another line of indirect experimental evidence which supports a role for dietary-induced inflammation in prostate cancer development is contained in reported results from the PCPT. Here, study participants who consumed the highest amounts of polyunsaturated fats were found to have an increased risk of high-grade (Gleason score 8–10) prostate cancer.75 Whereas several recent large-scale US and European cohort studies have not found a positive association between high fat intake and prostate cancer risk,76–78 a study in heavy smokers with a family history of prostate cancer found a substantially increased risk of prostate cancer associated with high polyunsaturated fat consumption.79 A major constituent of dietary polyunsaturated fats in western diets are n-6 fatty acids, primarily in the form of linoleic acid (LA).80 LA is metabolized into arachidonic acid, which can in turn be transformed into the proinflammatory eicosanoids prostaglandin E2 (PGE2) and leukotriene B4 (LTB4). The potential increase in prostate cancer risk found with high dietary intake of n-6 fatty acids may, therefore, be mediated through their effect on oxidative stress and inflammation.
CORPORA AMYLACEA AND CALCULI
Prostatic corpora amylacea and calculi are tiny laminated bodies and calcified stones, respectively, which are observed frequently in the adult prostate. Both corpora amylacea and calculi, which apparently represent calcified corpora amylacea, are postulated to cause physical trauma to glandular epithelium with the subsequent induction of focal acute and chronic inflammation and gland occlusion (Figure 1A).2,81 A study published in 2009 reported that the organic matrix of corpora amylacea and calculi is largely comprised of proteins involved in acute inflammation, and specifically proteins contained at high levels in neutrophil granules including lactoferrin, calprotectin, myeloperoxidase and α-defensins (Figure 1B,C).82 These findings suggest that corpora amylacea and calculi, which are highly prevalent in prostates of older men, may represent lasting remnants of past acute inflammatory events. Another study published a few months later reported largely similar findings regarding the identification of proteins involved in acute inflammatory pathways in corpora amylacea.83 Intriguingly, this study also identified both DNA and proteins from E. coli in corpora amylacea samples.
Figure 1.
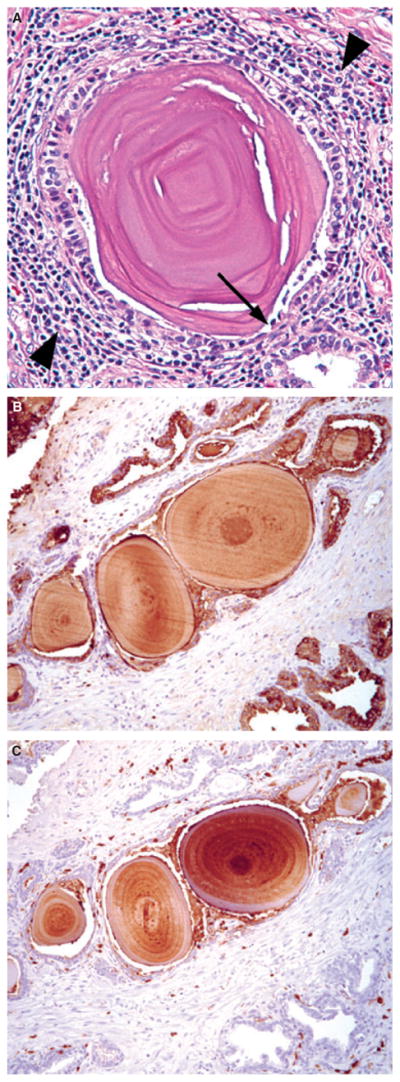
(A), Example of prostatic corpora amylacea. Note physical trauma to glandular epithelium (arrow) and associated surrounding focal chronic inflammation (arrowheads). Immunohistochemistry (IHC) for lactoferrin (B) and calprotectin (C) on prostate tissue sections containing corpora amylacea.
The immunobiology of prostate inflammation
The inflammation identified histologically in prostate cancer tissues is most commonly chronic, being chiefly comprised of lymphocytes as well as macrophages, and less frequently of plasma cells and eosinophils. Acute inflammation is present to a lesser extent and is comprised primarily of neutrophils. Just as the stimuli for prostatic inflammation are largely yet to be defined, our understanding of prostate immunobiology is still relatively poor. Over the past few years, however, there have been several recent advances in the characterization of the inflammatory cell types infiltrating the prostate.
IMMUNE REGULATORY CELLS
Regulatory T cells (Treg) are a subset of CD4+ T cells which act in the suppression of autoreactive T cells and prevention of autoimmunity. Treg were identified initially by high coexpression of CD4 and CD25 surface markers (CD4+CD25high cells); however, CD25 is also elevated on activated T cells. Treg are currently defined by the expression of the forkhead family transcription factor FoxP3. In light of their role in immune suppression, Treg have been investigated for a role as suppressors of antitumour immune responses.84 Infiltrating Treg have been identified in a number of solid tumours, and in some instances the presence of these cells correlates with poor prognostic outcome (reviewed in Ref. 85). CD4+CD25high T cells were first reported in tumour tissues and peripheral blood of prostate cancer patients in 2006.86 In this study, T cells outgrown from prostate tumour tissues were found to have elevated numbers of CD4+CD25high T cells in comparison to matched normal prostate tissues. Furthermore, this study reported elevated numbers of CD4+CD25high T cells in the peripheral blood of prostate cancer patients compared to normal donors. A subsequent study published in 2008 confirmed these findings, showing a relative enrichment of CD4+FoxP3+ Treg in prostate tissues of cancer patients with respect to peripheral blood.87 Another study published in 2009 demonstrated CD25+ and FoxP3+ cells in lymphocyte clusters surrounding prostate cancer lesions via immunohistochemistry (IHC).88 Additional studies have reported an increase in the suppressive function of Treg isolated from the peripheral blood of prostate cancer patients as opposed to normal donors,89 the expression of FoxP3 in prostate cancer cell lines90 and the presence of CD8+ FoxP3+ cells in prostate tumours.91
While the significance of elevated levels of Treg in the prostate remains unclear, the presence of infiltrating Treg in prostate tumours may have significant implications with regard to the design and potential efficacy of prostate cancer immunotherapy strategies. In the same respect, another molecule of potential interest is the inhibitory receptor programmed death 1 (PD-1), a cell-surface protein associated with inhibition of T cell responses. A number of human tumours have been found to express PD-1L (B7-H1),92 and expression of PD-1 on cytotoxic T lymphocytes (CTL) inhibits antitumour effector function. 93 Very promising results are arising from clinical trials based on treatment strategies utilizing monoclonal antibodies for PD-1 blockade for multiple forms of advanced malignancies.94,95 Anti-PD-1 treatment strategies may also prove to be beneficial in prostate cancer therapeutic strategies, as prostate-infiltrating lymphocytes have been found to express PD-1.88,96 The first study to demonstrate the presence of PD-1+ cells in prostate cancer tissues utilized IHC with an anti-PD-1 antibody to demonstrate that lymphocyte clusters surrounding prostate tumours contained both PD-1+ and B7-H1+ cells.88 A second study specifically analysed tumour-infiltrating CD8+ T cells and demonstrated by flow cytometry that PD-1 was up-regulated on prostate-infiltrating CD8+ cells in comparison to matched peripheral blood samples. In some patients, up to 90% of prostate-infiltrating CD8+ T cells expressed PD-1,96 with potentially major implications in prostate cancer immunotherapy strategies.97
T HELPER TYPE 17 (TH17) CELLS
A newly discovered CD4+ effector T cell lineage, termed Th17 cells, has been described which develops through distinct cytokine signals [specifically interleukin (IL)-23] and are characterized by the production of IL-17.98 Th17 cells are thought to be the key mediators in a number of autoimmune diseases, and may play a role in inflammation-associated cancer.98,99 The role of Th17 cells in cancer is currently a topic of significant debate, as there appear to be conflicting reports as to whether Th17 cells promote carcinogenesis or play a role in antitumour immunity.100 In prostate cancer, increased expression of IL-17 at the mRNA level was shown in tissue from both prostate cancer and BPH before it was known that Th17 T cells represent a distinct CD4 effector T cell lineage.101 A study published in 2008 reported a significant skewing of prostate-infiltrating CD4+ T cells towards a Th17 phenotype.87 Interestingly, this study found a statistically significant inverse correlation between Th17 skewing and tumour grade. Although this study was conducted in a relatively small patient set (n = 20),87 these findings are in agreement with other studies that find a protective role for Th17 cells with regard to tumour immunity (reviewed in Ref. 100). Another recent study found that the generation of an IL-17-associated progressive autoimmune response resulted in rejection of the TC2 tumour in C57BL / 6 mice.102 In contrast, in a recent study in patients with advanced prostate cancer who were undergoing immunotherapy, higher levels of CCR4−IL-17+CD4+ T cells in the patient’s peripheral blood pre-therapy corresponded significantly to decreased time to disease progression. 103 Although the potential relationship between elevated levels of Th17 cells in the peripheral blood, which can also be due to underlying infection or inflammation, and prostate tumour-infiltrating lymphocyte populations is unclear, these studies warrant further evaluation of the protective versus tumour-promoting roles that Th17 cells may play with regard to prostate cancer immunobiology.
In terms of inflammation being associated with a procarcinogenic environment, a number of studies have suggested that inflammation in and around prostate cancer is associated with worse disease outcome.104,105 Further, recent work found that chronic inflammation in benign tissue of a patient biopsy was predictive of a higher risk for prostate cancer diagnosis (OR = 1.79, 95% CI: 1.06–3.04), and specifically with higher-grade (Gleason score 7–10) disease (OR = 2.41, 95% CI: 1.17–4.95). The risk of prostate cancer and high-grade prostate cancer also increased with the number of biopsies that were found to contain chronic inflammation (B. Gurel, M.S. Lucia, E.A. Platz and A.M. De Marzo, manuscript in preparation). These data raise the intriguing possibility that chronic inflammation in benign areas of the prostate may contribute to the development of high-grade disease. Further evidence for a role for chronic inflammation and prostate cancer development has been presented above in the context of inflammatory stimuli in the prostate and will be discussed in further detail below in the context of genetic polymorphisms in inflammatory pathways and cytokines in prostate cancer.
Inflammation pathways and cytokines in prostate cancer
A great deal of literature has addressed the role of genetic polymorphisms in inflammation pathways and the production of inflammatory cytokines with regard to prostate cancer risk and promotion. In the following sections we will address recent literature in these areas, as opposed to summarizing the body of literature as a whole, for which there are many relevant review articles.2,22,106,107
GENETIC POLYMORPHISMS IN INFLAMMATIONRELATED GENES AND PATHWAYS
Over the past few years, and with recent advances in sequencing and genotyping technologies, the number of studies that have reported on the association between one or multiple single nucleotide polymorphisms (SNPs) in inflammation-related pathways and prostate cancer risk has greatly increased. Licastro et al.108 recently reported an interesting association between a SNP (GG genotype) in the promoter region of alpha-1-antichymotrypsin (ACT) and increased risk of prostate cancer (age-adjusted OR = 2.676, CI: 1.375–5.205). A correlation between circulating levels of PSA and the ACT GG genotype was also reported in younger prostate cancer patients. ACT is an acute-phase protein which is up-regulated in response to inflammation. ACT is also a serine protease inhibitor, and most circulating PSA is bound to ACT. Another recent case–control study in the Risk Factors for Prostate Cancer Study examined a cytokine-rich region at 5q31.1 which has been implicated previously as a potential prostate cancer risk loci, and found a modest association between two alleles of IL-4 and prostate cancer risk and no association between IL-5 or IL-13.109 The associations with IL-4 were not present in another large case–control study (the Melbourne Collaborative Cohort Study); however, the authors report that one of the IL-4 alleles (rs2243250 genotype) led to a decrease in IL-4 activity, potentially pointing to an antitumour function of IL-4 in prostate cancer risk.109
A number of recent studies have also attempted to correlate the potential interaction of SNPs between multiple different cytokines in conferring an increased risk of prostate cancer. In 2008, Zabaleta et al.110 examined nine SNPs in three cytokines [IL-1B, IL-10, tumour necrosis factor (TNF)] in a case–control study of African American versus Caucasian men. This study found an increased combined risk of prostate cancer in African Americans carrying variants in IL-1B (IL1B–511CT / TT) and IL-10 (IL10–592CC) (OR = 2.56, 95% CI: 1.09–6.02). Similarly, in Caucasians, a higher risk of prostate cancer was associated with the combinations of variants of IL-1B (IL1B–31TT / TC) and IL-10 (IL10–1082AA) (OR = 2.92, 95% CI: 1.13–7.55), as well as IL-10 (IL10–592AA) and TNF (TNF–238GG) (OR = 2.14, 95% CI: 1.05–4.38). In 2009, this same group evaluated 15 SNPs in five cytokine genes (IL-1B, IL-10, TNF-α, IL-6 and IL-8) in relation to risk of aggressive prostate cancer (Gleason sum ≥8 or PSA >20 ng / ml or Gleason sum ≥7 and clinical stages T3–T4) in African Americans and Caucasians.111 Using the multivariate adaptive regression splines (MARS) form of regression analysis, this study found an association between aggressive prostate cancer and an IL-8 (IL8–47CT) genotype (OR = 3.50; 95% CI: 1.13–10.88) as well as an increased risk with combined genotypes in IL-1B (IL1B–511CC) and IL-10 (IL10–1082GG) (OR = 3.38; 95% CI: 1.70–6.71) in Caucasian men. Unfortunately, the numbers of African American men in this study were not large enough for analysis. Interestingly, both studies found an association between increased risk for prostate cancer or aggressive prostate cancer and the IL10–1082GG variant of IL-10 alone.110,111 Another recent study which undertook a similar approach examined 143 SNPs in 16 inflammation-related genes [CXC ligand 12 (CXCL12), IL-4, IL-6, IL-6ST, prostaglandin-endoperoxide synthase 2 (PTGS2), signal transducer and activator of transcription 3 (STAT3), TNF, protein kinase B (AKT1), CXCR4, IL-6R, IL-8, IL-10, nuclear factor kappa B (NFκB), phosphatidylinositol 3-kinase (PIK3)R1, PTGS1 and vascular endothelial growth factor (VEGF)] in a case–control study of African American versus Caucasian men.112 The authors reported that SNPs in IL-4, IL-6ST, PTGS2 and STAT3 were associated significantly and independently with prostate cancer susceptibility, and that SNPs in AKT1, PIK3R1 and STAT3 were associated with aggressive prostate cancer. Furthermore, this study reported that men carrying multiple ‘high-risk’ alleles are at an elevated risk for prostate cancer development. These studies collectively underline the potential importance of the interactions between inflammatory cytokines and inflammation pathways in conferring prostate cancer risk.
COX-2
Another inflammation-related molecule that has generated a great deal of interest with regard to prostate cancer is cyclooxygenase 2 (COX-2, also known as PTGS2). This enzyme is an inducible isoform of the enzymes that convert arachidonic acid to proinflammatory prostaglandins (see previous discussion regarding n-6 fatty acids). Previous reports have indicated that COX-2 may be overexpressed in prostate cancer;113,114 however, overexpression of this enzyme may in fact be limited to areas of proliferative inflammatory atrophy (PIA), a predicted risk factor lesion to prostate cancer, 115 which will be discussed in detail below. A large range of recent literature has been published regarding the association of SNPs in PTGS2 and prostate cancer risk, and a summary of this literature is provided in Table 1.112,116–124 The diagnostic and prognostic value of CpG island hypermethylation at PTGS2 is also an area of active current investigation.125–129 COX-2 remains a molecule of particular interest in the link between inflammation and prostate cancer.
Table 1.
Summary of single nucleotide polymorphism (SNP) studies involving cyclooxygenase 2 (COX-2) in prostate cancer
| Study population | Size* | SNP(s) | Result | OR, CI | References |
|---|---|---|---|---|---|
| Caucasian | 1309, 1266 | rs2206593 (G / A) | + | 1.69, 1.14–2.5 | 112 |
| rs2745557 (G / A) | − | 0.78, 0.62–0.97 | |||
| rs6685280 (A / C) | + | 1.16, 1.01–1.33 | |||
| African American | 149, 85 | rs2206593, rs2745557, rs6685280 | NA | ||
| South African | 151, 134 | rs3918304 (AG and GG) | + | 3.53, 2.14–5.90† | 116 |
| rs20415 (CT and TT) | + | 3.01, 1.82–5.02† | |||
| rs5270 | NA | ||||
| Caucasian, non-Hispanic | 2321, 2560 | rs5277, rs20432, rs4648276, rs5275, rs689470 | NA | 117 | |
| Primarily Caucasian | 8008, 8604 | rs5275, rs5277, rs20417, rs689466, rs2206593, rs2383529, rs2745557, rs4648261, rs4648298, rs7550380, rs10911902, rs12042763 | NA | 118 | |
| Primarily Caucasian | 1608, 3058 | rs20417 | NA | 119 | |
| Caucasian (non-obese) | 585, 585 | rs2745557 | NA | 120 | |
| Sicilian | 50, 125 | rs20417 | NA‡ | 121 | |
| Taiwanese | 218, 436 | rs689466, rs5275, rs2745557, rs16825748, rs2066826 | NA | 122 | |
| rs20417 (GC) | − | 0.52, 0.31–0.88 | |||
| Caucasian | 416, 417 | rs689466, rs20417, rs5277, rs2066826, rs5275, rs689470, rs4648310 | NA | 123 | |
| rs2745557 (GA) | − | 0.67, 0.50–0.90 | |||
| rs2206593 (CT) | − | 0.58, 0.38–0.89 | |||
| African American | 89, 88 | rs689466, rs20417, rs2745557, rs5277, rs2066826, rs5275, rs2206593, rs4648310 | NA | ||
| rs689470 (AA) | + | 2.79, 1.17–6.64 | |||
| Caucasian | 92, 92 | ss5112604, ss5112605, ss5112606 | NA | 124 | |
| ss5112607 (CG / GG) | − | 0.33, 0.1–0.9 | |||
| African American | 124, 164 | ss5112604 | NA | ||
| ss5112605 (GA / AA) | + | 2.72, 1.3–5.8 | |||
| ss5112606 (GC / CC) | + | 3.67, 1.4–9.9 | |||
| ss5112607 (CG / GG) | − | 0.51, 0.2–0.9 | |||
| Nigerian | 154, 110 | ss5112604, ss5112605, ss5112606, ss5112607 | NA |
+: Positive association; −: inverse association; NA, no association.
Cases, controls.
Age-adjusted.
No association between cases and age-matched controls.
An association was observed between cases and centenarians [odds ratio (OR) = 2.1, 95% confidence interval (CI): 1.1–3.9].
CYTOKINES IN PROSTATE CANCER
Aside from genotyping studies, multiple inflammatory cytokines have been identified as potential mediators in an interplay between prostatic inflammation and prostate carcinogenesis. One such cytokine is macrophage inhibitory cytokine 1 (MIC-1), a member of the transforming growth factor-β (TGF-β) superfamily identified initially using a subtraction cloning approach used to discover genes associated with macrophage activation.130 Studies have demonstrated the up-regulation of MIC-1 in prostate cancer,131,132 and recent evidence also suggests that circulating levels of MIC-1 predicts poor prostate cancer prognosis.133 It has been suggested that MIC-1 may be a key molecule linking macrophages to prostate cancer pathogenesis.134
IL-6 is a multifunctional cytokine which is involved in numerous innate and adaptive inflammatory processes, including B cell activation, acute-phase inflammatory response and thrombopoiesis.135 IL-6 is produced by multiple cell types, including macrophages, endothelial cells and T lymphocytes. Deregulation of IL-6 is also known to play a role in multiple disease processes, including autoimmune disorders, rheumatoid arthritis, osteoporosis, psoriasis, diabetes, atherosclerosis and cancer.136,137 In prostate cancer, multiple lines of evidence point to a contributory role of IL-6 in cancer initiation and / or progression: (i) IL-6 and IL6-R can be produced by prostate cells and up-regulation of IL-6 and IL-6R has been detected in malignant epithelium and high-grade prostatic intraepithelial neoplasia (PIN),138 (ii) circulating levels of IL-6 are elevated in patients with metastatic prostate cancer and hormone-refractory prostate cancer (reviewed in Ref. 139), (iii) IL-6 has been shown to be correlated with measures of prostate cancer morbidity140 and (iv) IL-6 may function in activation of androgen receptor (AR).141 Furthermore, an intriguing study was published recently by Iliopoulos et al.142 that presented evidence for a positive feedback loop between inflammation and IL-6 activation, STAT3 activation and NF-κB activation in cancer which maintains cells in an ‘epigenetic transformed’ state. IL-6 remains a particular cytokine of interest in prostate cancer aetiology, and especially with regard to a potential contribution of deregulated and / or systemic IL-6 levels to advanced prostate cancer and disease progression.
In 2010, a study from the laboratory of Michael Karin was published regarding a potential role for B cell-derived lymphotoxin in the development of hormone-refractory prostate cancer.143 This group found that IKK-β ablation in prostate epithelial cells in the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model had no effect on the development or progression of androgen-independent cancer. In contrast, ablation of IKK-β in bone marrow-derived cells in animals allografted with myc-CaP cells delayed the development of castration-resistant cancer after castration. The authors discovered that regressing tumours in castrated animals were infiltrated with T and B lymphocytes. Interestingly, previous studies in human prostate cancer patients have also demonstrated that androgen ablation results in the infiltration of leucocytes into the prostate.144 It was determined that IKK-β ablation in bone marrow-derived cells abrogated lymphotoxin expression by B cells. Further studies involving transplanted bone marrow from mice lacking lymphotoxin in either B or T cells into irradiated mice demonstrated that lymphotoxin-β ablation in B cells delayed the growth of castration-dependent cancer.143 Very few studies regarding lymphotoxin and prostate cancer had been published prior to the Nature paper in 2010. Intriguingly, a study published by Zhou et al.145 in 2009 demonstrated that a targeted deletion of lymphotoxin-α in T cells in TRAMP mice inhibited the development of spontaneous tumours and had a profound impact on metastasis. A study by Liu et al. in 2006146 reported that a functional polymorphism in the lymphotoxin-α gene conferred increased protection from prostate cancer development with non-steroidal anti-inflammatory drug (NSAID) use. Lymphotoxin may, therefore, be an important inflammatory cytokine driving prostate cancer progression.
PIA as a precursor lesion to prostate cancer
In response to unknown stimuli, regions of prostatic atrophy, which are generally associated with inflammatory cell infiltrates, develop at a very high frequency to encompass large regions of the prostate in some men (Figures 2 and 3). These regions, referred to as proliferative inflammatory atrophy (PIA), contain atrophic epithelial cells that appear to be regenerating in response to cellular damage.147 Furthermore, these atrophic lesions, which frequently merge with the recognized direct precursor to many prostatic adenocarcinomas, high-grade PIN, have some of the hallmark somatic genome alterations found in prostate cancer and PIN and have been proposed as ‘risk factor lesions’ for the development of prostate cancer.2,148,149 Morphological transitions between PIA, PIN and prostate cancer have been described previously.149 In 2009, a report by Wang et al.150 also documented the morphological transition between PIA and PIN as well as PIA and prostate cancer. In this study, serial sections of whole-mount prostates were examined from 50 cases, and 17% of PIN lesions were found to be in the morphological process of merging with PIA in 70% of the prostates examined. Furthermore, instances of PIA directly merging with cancer were identified in 28% of the cases. The authors state that merging lesions were ‘closely adjacent to areas with chronic inflammation, suggesting that it may have a role to play in this process’.150
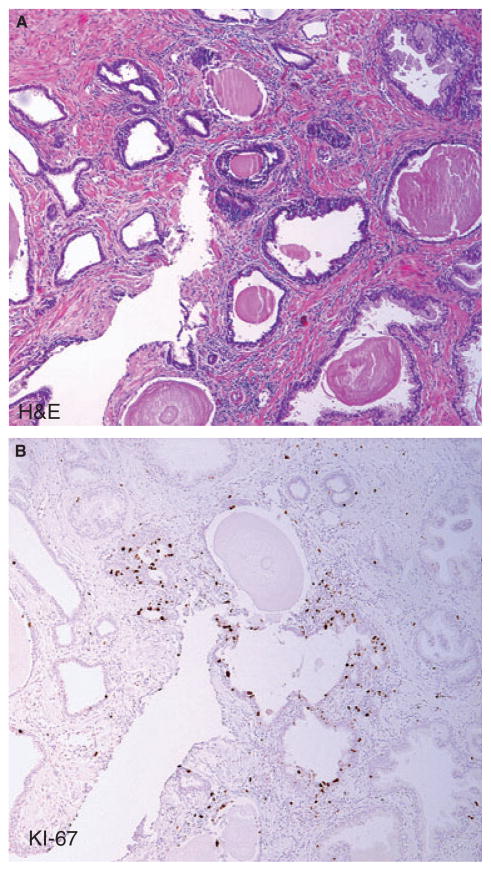
Figure 2.
Example of proliferative inflammatory atrophy (PIA) lesion. A, Haematoxylin and eosin (H&E) and B, immunohistochemistry (IHC) for Ki-67 proliferation marker shows relatively high fraction of luminal epithelial cells staining (compared to normal appearing epithelium which is not shown).
Figure 3. Example of post-atrophic hyperplasia (PAH), a form of PIA.
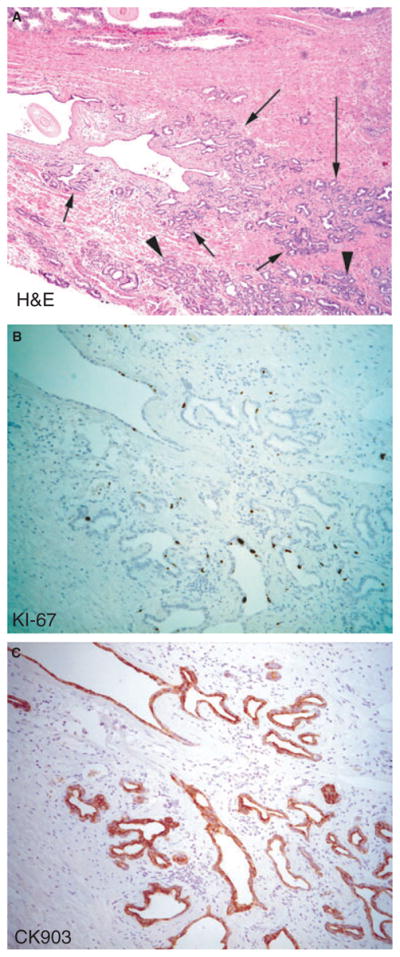
A, Haematoxylin and eosin (H&E) arrows point to PAH, which shows mild stromal inflammation. Arrowheads point to carcinoma.
B, Immunohistochemistry (IHC) for Ki-67. C, IHC for ‘basal specific’ cytokeratin (CK903). Note that, as is characteristic of most forms of prostatic atrophy, many cells in the luminal cell layer stain positive with this antibody as well as other antibodies against keratin 5.
As mentioned previously, further evidence that PIA may be a direct precursor to PIN and / or cancer stems from the fact that PIA has some of the hallmark gene expression changes found in prostate cancer and PIN. For example, two genes which are highly expressed in normal prostate epithelium and frequently down-regulated or absent in PIN and prostate cancer, NKX3.1 and p27, are down-regulated in prostate atrophy. 2,147,151 Another recent study reported increased p53 immunostaining in PIA lesions, especially in areas of acute inflammation.152 Although the technique used could not differentiate between wild type and mutated p53, the authors showed that p53-positive areas correspond to a high proliferation index (Ki-67), CK5 expression, overexpression of COX-2 and high levels of glutathione S-transferase-π (GSTP1) in the same lesion. Nakayama et al.153 found previously that methylation of deoxycytidine residues within the cytosine–guanine– dinucleotide (CpG) island near the GSTP1 promoter region, which occurs in nearly all prostatic adenocarcinomas (approximately 90% in this study) and most PIN lesions (approximately 70%), occurred in 6.4% of atrophy lesions. While this is relatively infrequent, as atrophy / PIA is highly prevalent and often quite extensive in the peripheral zone of the prostate these findings are consistent with the possibility that a significant fraction of PIN and / or adenocarcinoma lesions may indeed originate in these atrophic lesions.153
Anti-inflammatory compounds and prostate cancer risk
DIETARY ANTIOXIDANTS
Some intriguing epidemiological evidence for the role of inflammation in prostate cancer aetiology comes from studying the correlation between dietary or medicinal intake of anti-inflammatory compounds and prostate cancer risk. In this respect, both soy and green tea have anti-inflammatory properties and have been shown to be associated with decreased prostate cancer risk in human epidemiology studies and to decrease prostate cancer in animal studies (reviewed in Ref. 154). Interestingly, a recent study in prostate cancer cell lines demonstrated that treatment with phytoestrogens (genistein and daidzein) resulted in demethylation of GSTP1 and ephrin B2 (EPHB2) promoter regions.155 The authors of this study suggest that the protective effects of soy in prostate cancer prevention may involve epigenetic modifications to DNA. Previous studies have also shown a significant correlation between the consumption of tomato products and decreased prostate cancer risk (reviewed in in Ref. 156). A study published in 2010 reported increased survival, delayed progression from PIN to cancer and decreased incidence of poorly differentiated cancer in TRAMP mice fed a diet enriched with processed whole tomatoes.157 Interestingly, this study found that serum concentrations of several cytokines (such as IL-6-family cytokines IL-6, oncostatin M and IL-11) were reduced significantly in animals fed the tomato-enriched diet. Just as proinflammatory n-6 polyunsaturated fatty acids (PUFA) may confer an increased risk of prostate cancer, the anti-inflammatory n-3 (omega 3) PUFA has been associated with a decreased risk (reviewed in Ref. 158), although dietary n-3 polyunsaturated fatty acids failed to reduce prostate tumorigenesis in a preclinical mouse model.159 A recent case–control study found a strong association between high intake of long chain n-3 PUFA and decreased risk of aggressive prostate cancer.158 Furthermore, this study identified a SNP in the COX-2 gene that, along with low dietary intake of n-3 PUFA, had an increased risk of prostate cancer (OR = 5.49; 95% CI: 1.80–16.7). Interestingly, this association was decreased with increased intake of n-3 PUFA.158
NSAIDS
Multiple epidemiological studies have reported a reduced risk of prostate cancer with NSAID use, possibly by inhibition of the COX-2 enzyme. However, other studies have reported no association. A recent meta-analysis of the reported literature on this topic concluded that NSAID use may reduce the risk of prostate cancer; however, the effect is small.160 Another large case–control study recently found a reduction in prostate cancer risk for current aspirin users, daily users of low-dose aspirin and long-term users of aspirin, as opposed to non-users.161 There was an effect modification identified for a COX-2 SNP; however, prostate cancer risk was not reduced by any other form of NSAID or acetaminophen.
Summary and conclusions
In summary, a number of separate lines of research have pointed to a potential role for inflammation in prostatic carcinogenesis and tumour progression. These lines involve epidemiological, genetic, histopathological, molecular pathological and animal studies. Future studies in all of these areas will be needed to ultimately elucidate the precise mechanisms that may prove useful for the development of novel strategies to target the inflammatory process in preventing either prostate cancer outright or preventing progression of established prostate cancer.
Abbreviations
- ACT
alpha-1-antichymotrypsin
- BPH
benign prostatic hyperplasia
- CNF1
cytotoxic necrotizing factor 1
- CPPS
chronic pelvic pain syndrome
- DRE
digital rectal examination
- EPS
expressed prostatic secretins
- HCA
heterocyclic amine
- IHC
immunohistochemistry
- LA
linoleic acid
- MIC-1
macrophage inhibitory cytokine 1
- NSAID
non-steroidal anti-inflammatory drug
- PAH
post-atrophic hyperplasia
- PCPT
Prostate Cancer Prevention Trial
- PD-1
programmed death 1
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- PIA
proliferative inflammatory atrophy
- PIN
prostatic intraepithelial neoplasia
- PSA
prostate-specific antigen
- PUFA
polyunsaturated fatty acids
- SNP
single nucleotide polymorphism
- STD
sexually transmitted disease
- STI
sexually transmitted infection
- UPEC
uropathogenic strains of E. coli
- XMRV
xenotropic murine leukaemia virus-related virus
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieger JN, Nyberg L, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 4.Brede CM, Shoskes DA. The etiology and management of acute prostatitis. Nat Rev Urol. 2011;8:207–212. doi: 10.1038/nrurol.2011.22. [DOI] [PubMed] [Google Scholar]
- 5.Cai T, Mazzoli S, Meacci F, et al. Epidemiological features and resistance pattern in uropathogens isolated from chronic bacterial prostatitis. J Microbiol. 2011;49:448–454. doi: 10.1007/s12275-011-0391-z. [DOI] [PubMed] [Google Scholar]
- 6.Collins MM, Meigs JB, Barry MJ, Corkery EW, Giovannucci E, Kawachi I. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol. 2002;167:1363–1366. [PubMed] [Google Scholar]
- 7.Stimac G, Reljic A, Spajic B, et al. Aggressiveness of inflammation in histological prostatitis – correlation with total and free prostate specific antigen levels in men with biochemical criteria for prostate biopsy. Scott Med J. 2009;54:8–12. doi: 10.1258/RSMSMJ.54.3.8. [DOI] [PubMed] [Google Scholar]
- 8.Gui-zhong LI, Libo M, Guanglin H, Jianwei W. The correlation of extent and grade of inflammation with serum PSA levels in patients with IV prostatitis. Int Urol Nephrol. 2011;43:295–301. doi: 10.1007/s11255-010-9825-5. [DOI] [PubMed] [Google Scholar]
- 9.Ugurlu O, Yaris M, Oztekin CV, Kosan TM, Adsan O, Cetinkaya M. Impacts of antibiotic and anti-inflammatory therapies on serum prostate-specific antigen levels in the presence of prostatic inflammation: a prospective randomized controlled trial. Urol Int. 2010;84:185–190. doi: 10.1159/000277596. [DOI] [PubMed] [Google Scholar]
- 10.Fujita K, Hosomi M, Tanigawa G, Okumi M, Fushimi H, Yamaguchi S. Prostatic inflammation detected in initial biopsy specimens and urinary pyuria are predictors of negative repeat prostate biopsy. J Urol. 2011;185:1722–1727. doi: 10.1016/j.juro.2010.12.058. [DOI] [PubMed] [Google Scholar]
- 11.Delongchamps NB, de la Roza G, Chandan V, et al. Evaluation of prostatitis in autopsied prostates – is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008;179:1736–1740. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and / or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 13.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eastham JA, May RA, Whatley T, Crow A, Venable DD, Sartor O. Clinical characteristics and biopsy specimen features in African-American and white men without prostate cancer. J Natl Cancer Inst. 1998;90:756–760. doi: 10.1093/jnci/90.10.756. [DOI] [PubMed] [Google Scholar]
- 15.Wallace TA, Prueitt RL, Yi M, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927–936. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 16.Sutcliffe S. Sexually transmitted infections and risk of prostate cancer: review of historical and emerging hypotheses. Future Oncol. 2010;6:1289–1311. doi: 10.2217/fon.10.95. [DOI] [PubMed] [Google Scholar]
- 17.Weidner W, Schiefer HG, Krauss H. Role of Chlamydia trachomatis and mycoplasmas in chronic prostatitis. A review. Urol Int. 1988;43:167–173. doi: 10.1159/000281331. [DOI] [PubMed] [Google Scholar]
- 18.Mandar R, Raukas E, Turk S, Korrovits P, Punab M. Mycoplasmas in semen of chronic prostatitis patients. Scand J Urol Nephrol. 2005;39:479–482. doi: 10.1080/00365590500199822. [DOI] [PubMed] [Google Scholar]
- 19.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 20.Sarma AV, McLaughlin JC, Wallner LP, et al. Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. J Urol. 2006;176:1108–1113. doi: 10.1016/j.juro.2006.04.075. [DOI] [PubMed] [Google Scholar]
- 21.Sutcliffe S, Giovannucci E, De Marzo AM, Leitzmann MF, Willett WC, Platz EA. Gonorrhea, syphilis, clinical prostatitis, and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2160–2166. doi: 10.1158/1055-9965.EPI-05-0913. [DOI] [PubMed] [Google Scholar]
- 22.Palapattu GS, Sutcliffe S, Bastian PJ, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 23.Cheng I, Witte JS, Jacobsen SJ, et al. Prostatitis, sexually transmitted diseases, and prostate cancer: the California Men’s Health Study. PLoS ONE. 2010;5:e8736. doi: 10.1371/journal.pone.0008736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sfanos KS, Sauvageot J, Fedor HL, Dick JD, De Marzo AM, Isaacs WB. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate. 2008;68:306–320. doi: 10.1002/pros.20680. [DOI] [PubMed] [Google Scholar]
- 25.Bergh J, Marklund I, Thellenberg-Karls C, Gronberg H, Elgh F, Alexeyev OA. Detection of Escherichia coli 16S RNA and cytotoxic necrotizing factor 1 gene in benign prostate hyperplasia. Eur Urol. 2007;51:457–462. doi: 10.1016/j.eururo.2006.06.008. discussion 462–453. [DOI] [PubMed] [Google Scholar]
- 26.Boehm BJ, Colopy SA, Jerde TJ, Loftus CJ, Bushman W. Acute bacterial inflammation of the mouse prostate. Prostate. 2011 doi: 10.1002/pros.21433. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkahwaji JE, Hauke RJ, Brawner CM. Chronic bacterial inflammation induces prostatic intraepithelial neoplasia in mouse prostate. Br J Cancer. 2009;101:1740–1748. doi: 10.1038/sj.bjc.6605370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalili M, Mutton LN, Gurel B, Hicks JL, De Marzo AM, Bieberich CJ. Loss of Nkx3.1 expression in bacterial prostatitis: a potential link between inflammation and neoplasia. Am J Pathol. 2010;176:2259–2268. doi: 10.2353/ajpath.2010.080747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintar AA, Doll A, Leimgruber C, et al. Acute inflammation promotes early cellular stimulation of the epithelial and stromal compartments of the rat prostate. Prostate. 2010;70:1153–1165. doi: 10.1002/pros.21150. [DOI] [PubMed] [Google Scholar]
- 30.Rippere-Lampe KE, Lang M, Ceri H, Olson M, Lockman HA, O’Brien AD. Cytotoxic necrotizing factor type 1-positive Escherichia coli causes increased inflammation and tissue damage to the prostate in a rat prostatitis model. Infect Immun. 2001;69:6515–6519. doi: 10.1128/IAI.69.10.6515-6519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nougayrede J-P, Homburg S, Taieb F, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 32.Dubois D, Delmas J, Cady A, et al. Cyclomodulins in urosepsis strains of Escherichia coli. J Clin Microbiol. 2010;48:2122–2129. doi: 10.1128/JCM.02365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieger JN, Dobrindt U, Riley DE, Oswald E. Acute Escherichia coli prostatitis in previously health young men: bacterial virulence factors, antimicrobial resistance, and clinical outcomes. Urology. 2011;77:1420–1425. doi: 10.1016/j.urology.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 34.Ostaszewska I, Zdrodowska-Stefanow B, Badyda J, Pucilo K, Trybula J, Bulhak V. Chlamydia trachomatis: probable cause of prostatitis. Int J STD AIDS. 1998;9:350–353. doi: 10.1258/0956462981922395. [DOI] [PubMed] [Google Scholar]
- 35.Corradi G, Bucsek M, Panovics J, et al. Detection of Chlamydia trachomatis in the prostate by in-situ hybridization and by transmission electron microscopy. Int J Androl. 1996;19:109–112. doi: 10.1111/j.1365-2605.1996.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 36.Bielecki R, Zdrodowska-Stefan B, Ostaszewska-Pucha I, Baltaziak M, Kozlowski R. Subclinical prostatic inflammation attributable to Chlamydia trachomatis in a patient with prostate cancer. Med Wieku Rozwoj. 2005;9:87–91. [PubMed] [Google Scholar]
- 37.Sutcliffe S, Giovannucci E, Gaydos CA, et al. Plasma antibodies against Chlamydia trachomatis, human papillomavirus, and human herpesvirus type 8 in relation to prostate cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2007;16:1573–1580. doi: 10.1158/1055-9965.EPI-07-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang W-Y, Hayes R, Pfeiffer R, et al. Sexually transmissible infections and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2374–2381. doi: 10.1158/1055-9965.EPI-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anttila T, Tenkanen L, Lumme S, et al. Chlamydial antibodies and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:385–389. doi: 10.1158/1055-9965.EPI-03-0325. [DOI] [PubMed] [Google Scholar]
- 40.Mackern-Oberti JP, Maccioni M, Breser ML, Eley A, Miethke T, Rivero VE. Innate immunity in the male genital tract: Chlamydia trachomatis induces keratinocyte-derived chemokine production in prostate, seminal vesicle and epididymis / vas deferens primary cultures. J Med Microbiol. 2011;60:307–316. doi: 10.1099/jmm.0.024877-0. [DOI] [PubMed] [Google Scholar]
- 41.Mackern-Oberti JP, Maccioni M, Cuffini C, Gatti G, Rivero VE. Susceptibility of prostate epithelial cells to Chlamydia muridarum infection and their role in innate immunity by recruitment of intracellular Toll-like receptors 4 and 2 and MyD88 to the inclusion. Infect Immun. 2006;74:6973–6981. doi: 10.1128/IAI.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakab E, Zbinden R, Gubler J, Ruef C, von Graevenitz A, Krause M. Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. Yale J Biol Med. 1996;69:477–482. [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen RJ, Shannon BA, McNeal JE, Shannon TOM, Garrett KL. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol. 2005;173:1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 44.Fassi Fehri L, Mak TN, Laube B, et al. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int J Med Microbiol. 2011;301:69–78. doi: 10.1016/j.ijmm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Alexeyev O, Bergh J, Marklund I, et al. Association between the presence of bacterial 16SRNAin prostate specimens taken during transurethral resection of prostate and subsequent risk of prostate cancer (Sweden) Cancer Causes Control. 2006;17:1127–1133. doi: 10.1007/s10552-006-0054-2. [DOI] [PubMed] [Google Scholar]
- 46.Alexeyev OA, Marklund I, Shannon B, et al. Direct visualization of Propionibacterium acnes in prostate tissue by multicolor fluorescent in situ hybridization assay. J Clin Microbiol. 2007;45:3721–3728. doi: 10.1128/JCM.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galobardes B, Smith GD, Jeffreys M, Kinra S, McCarron P. Acne in adolescence and cause-specific mortality: lower coronary heart disease but higher prostate cancer mortality. Am J Epidemiol. 2005;161:1094–1101. doi: 10.1093/aje/kwi147. [DOI] [PubMed] [Google Scholar]
- 48.Sutcliffe S, Giovannucci E, Isaacs WB, Willett WC, Platz EA. Acne and risk of prostate cancer. Int J Cancer. 2007;121:2688–2692. doi: 10.1002/ijc.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Severi G, Shannon BA, Hoang HN, et al. Plasma concentration of Propionibacterium acnes antibodies and prostate cancer risk: results from an Australian population-based case–control study. Br J Cancer. 2010;103:411–415. doi: 10.1038/sj.bjc.6605757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutcliffe S, Giovannucci E, Alderete JF, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiology Biomarkers & Prevention. 2006;15:939–945. doi: 10.1158/1055-9965.EPI-05-0781. [DOI] [PubMed] [Google Scholar]
- 51.Sutcliffe S, Alderete JF, Till C, et al. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Int J Cancer. 2009;124:2082–2087. doi: 10.1002/ijc.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stark JR, Judson G, Alderete JF, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J Natl Cancer Inst. 2009;101:1406–1411. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutcliffe S, Zenilman JM, Ghanem KG, et al. Sexually transmitted infections and prostatic inflammation / cell damage as measured by serum prostate specific antigen concentration. J Urol. 2006;175:1937–1942. doi: 10.1016/S0022-5347(05)00892-X. [DOI] [PubMed] [Google Scholar]
- 54.Sutcliffe S, Nevin RL, Pakpahan R, et al. Prostate involvement during sexually transmitted infections as measured by prostate-specific antigen concentration. Br J Cancer. 2011;105:602–605. doi: 10.1038/bjc.2011.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klein EA, Silverman R. Inflammation, infection, and prostate cancer. Curr Opin Urol. 2008;18:315–319. doi: 10.1097/MOU.0b013e3282f9b3b7. [DOI] [PubMed] [Google Scholar]
- 56.Urisman A, Molinaro RJ, Fischer N, et al. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2:e25. doi: 10.1371/journal.ppat.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Aloia AL, Sfanos KS, Isaacs WB, et al. XMRV: a new virus in prostate cancer? Cancer Res. 2010;70:10028–10033. doi: 10.1158/0008-5472.CAN-10-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farley SJ. Prostate cancer: XMRV-contaminant, not cause? Nat Rev Urol. 2011;8:409. doi: 10.1038/nrurol.2011.101. [DOI] [PubMed] [Google Scholar]
- 59.Paprotka T, Delviks-Frankenberry KA, Cingoz O, et al. Recombinant origin of the retrovirus XMRV. Science. 2011;333:97–101. doi: 10.1126/science.1205292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J, Battacharya P, Singhal R, Kandel ES. Xenotropic murine leukemia virus-related virus (XMRV) in prostate cancer cells likely represents a laboratory artifact. Oncotarget. 2011;2:358–362. doi: 10.18632/oncotarget.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oakes B, Tai AK, Cingoz O, et al. Contamination of human DNA samples with mouse DNA can lead to false detection of XMRV-like sequences. Retrovirology. 2010;7:109. doi: 10.1186/1742-4690-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato E, Furuta RA, Miyazawa T. An endogenous murine leukemia viral genome contaminant in a commercial RT–PCR kit is amplified using standard primers for XMRV. Retrovirology. 2010;7:110. doi: 10.1186/1742-4690-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson MJ, Erlwein OW, Kaye S, et al. Mouse DNA contamination in human tissue tested for XMRV. Retrovirology. 2010;7:108. doi: 10.1186/1742-4690-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erlwein O, Robinson MJ, Dustan S, Weber J, Kaye S, McClure MO. DNA extraction columns contaminated with murine sequences. PLoS ONE. 2011;6:e23484. doi: 10.1371/journal.pone.0023484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nelson WG, DeWeese TL, DeMarzo AM. The diet, prostate inflammation, and the development of prostate cancer. Cancer Metastasis Rev. 2002;21:3–16. doi: 10.1023/a:1020110718701. [DOI] [PubMed] [Google Scholar]
- 66.Schut HAJ, Snyderwine EG. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 1999;20:353–368. doi: 10.1093/carcin/20.3.353. [DOI] [PubMed] [Google Scholar]
- 67.Sander A, Linseisen J, Rohrmann S. Intake of heterocyclic aromatic amines and the risk of prostate cancer in the EPIC-Heidelberg cohort. Cancer Causes Control. 2011;22:109–114. doi: 10.1007/s10552-010-9680-9. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez C, McCullough ML, Mondul AM, et al. Meat consumption among black and white men and risk of prostate cancer in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:211–216. doi: 10.1158/1055-9965.EPI-05-0614. [DOI] [PubMed] [Google Scholar]
- 69.Sinha R, Park Y, Graubard BI, et al. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol. 2009;170:1165–1177. doi: 10.1093/aje/kwp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koutros S, Cross AJ, Sandler DP, et al. Meat and meat mutagens and risk of prostate cancer in the agricultural health study. Cancer Epidemiol Biomarkers Prev. 2008;17:80–87. doi: 10.1158/1055-9965.EPI-07-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.John EM, Stern MC, Sinha R, Koo J. Meat consumption, cooking practices, meat mutagens, and risk of prostate cancer. Nutr Cancer. 2011;63:525–537. doi: 10.1080/01635581.2011.539311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirai T, Sano M, Tamano S, et al. The prostate: a target for carcinogenicity of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–198. [PubMed] [Google Scholar]
- 73.Nakai Y, Nelson WG, De Marzo AM. The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007;67:1378–1384. doi: 10.1158/0008-5472.CAN-06-1336. [DOI] [PubMed] [Google Scholar]
- 74.Borowsky AD, Dingley KH, Ubick E, Turteltaub KW, Cardiff RD, Devere-White R. Inflammation and atrophy precede prostatic neoplasia in a PhIP-induced rat model. Neoplasia. 2006;8:708–715. doi: 10.1593/neo.06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kristal AR, Arnold KB, Neuhouser ML, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2010;172:566–577. doi: 10.1093/aje/kwq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park S-Y, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2007;121:1339–1345. doi: 10.1002/ijc.22805. [DOI] [PubMed] [Google Scholar]
- 77.Wallström P, Bjartell A, Gullberg B, Olsson H, Wirfält E. A prospective study on dietary fat and incidence of prostate cancer (Malmö, Sweden) Cancer Causes Control. 2007;18:1107–1121. doi: 10.1007/s10552-007-9050-4. [DOI] [PubMed] [Google Scholar]
- 78.Crowe FL, Key TJ, Appleby PN, et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;87:1405–1413. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- 79.Neuhouser ML, Barnett MJ, Kristal AR, et al. (n-6) PUFA increase and dairy foods decrease prostate cancer risk in heavy smokers. J Nutr. 2007;137:1821–1827. doi: 10.1093/jn/137.7.1821. [DOI] [PubMed] [Google Scholar]
- 80.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 81.Klimas R, Bennett B, Gardner WA. Prostatic calculi: a review. Prostate. 1985;7:91–96. doi: 10.1002/pros.2990070110. [DOI] [PubMed] [Google Scholar]
- 82.Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci USA. 2009;106:3443–3448. doi: 10.1073/pnas.0810473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yanamandra K, Alexeyev O, Zamotin V, et al. Amyloid formation by the pro-inflammatory S100A8 / A9 proteins in the ageing prostate. PLoS ONE. 2009;4:e5562. doi: 10.1371/journal.pone.0005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 85.Dranoff G. The therapeutic implications of intratumoral regulatory T cells. Clin Cancer Res. 2005;11:8226–8229. doi: 10.1158/1078-0432.CCR-05-2035. [DOI] [PubMed] [Google Scholar]
- 86.Miller AM, Lundberg K, Ozenci V, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 87.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ebelt K, Babaryka G, Frankenberger B, et al. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur J Cancer. 2009;45:1664–1672. doi: 10.1016/j.ejca.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 89.Yokokawa J, Cereda V, Remondo C, et al. Enhanced functionality of CD4+CD25highFoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 90.Ebert LM, Tan BS, Browning J, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68:3001–3009. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- 91.Kiniwa Y, Miyahara Y, Wang HY, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 92.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 93.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 94.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 95.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.May KF, Gulley JL, Drake CG, Dranoff G, Kantoff PW. Prostate cancer immunotherapy. Clin Cancer Res. 2011;17:5233–5238. doi: 10.1158/1078-0432.CCR-10-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 99.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 100.Wilke CM, Kryczek I, Wei S, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steiner GE, Newman ME, Paikl D, et al. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56:171–182. doi: 10.1002/pros.10238. [DOI] [PubMed] [Google Scholar]
- 102.Kottke T, Sanchez-Perez L, Diaz RM, et al. Induction of hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007;67:11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 103.Derhovanessian E, Adams V, Hähnel K, et al. Pretreatment frequency of circulating IL-17+CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 104.Karja V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, Mokka R. Tumour-infiltrating lymphocytes: a prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005;25:4435–4438. [PubMed] [Google Scholar]
- 105.Nonomura N, Takayama H, Nakayama M, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107:1918–1922. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 106.Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16:663–673. doi: 10.1677/ERC-09-0109. [DOI] [PubMed] [Google Scholar]
- 107.Hughes C, Murphy A, Martin C, Sheils O, O’Leary J. Molecular pathology of prostate cancer. J Clin Pathol. 2005;58:673–684. doi: 10.1136/jcp.2002.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Licastro F, Bertaccini A, Porcellini E, et al. Alpha 1 antichymotrypsin genotype is associated with increased risk of prostate carcinoma and PSA levels. Anticancer Res. 2008;28:395–399. [PubMed] [Google Scholar]
- 109.Tindall EA, Severi G, Hoang HN, et al. Comprehensive analysis of the cytokine-rich chromosome 5q31. 1 region suggests a role for IL-4 gene variants in prostate cancer risk. Carcinogenesis. 2010;31:1748–1754. doi: 10.1093/carcin/bgq081. [DOI] [PubMed] [Google Scholar]
- 110.Zabaleta J, Lin H-Y, Sierra RA, et al. Interactions of cytokine gene polymorphisms in prostate cancer risk. Carcinogenesis. 2008;29:573–578. doi: 10.1093/carcin/bgm277. [DOI] [PubMed] [Google Scholar]
- 111.Zabaleta J, Su LJ, Lin H-Y, et al. Cytokine genetic polymorphisms and prostate cancer aggressiveness. Carcinogenesis. 2009;30:1358–1362. doi: 10.1093/carcin/bgp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kwon EM, Salinas CA, Kolb S, et al. Genetic polymorphisms in inflammation pathway genes and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:923–933. doi: 10.1158/1055-9965.EPI-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 114.Kirschenbaum A, Klausner AP, Lee R, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56:671–676. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 115.Zha S, Gage WR, Sauvageot J, et al. Cyclooxygenase-2 is upregulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- 116.Fernandez P, de Beer PM, van der Merwe L, Heyns CF. COX-2 promoter polymorphisms and the association with prostate cancer risk in South African men. Carcinogenesis. 2008;29:2347–2350. doi: 10.1093/carcin/bgn245. [DOI] [PubMed] [Google Scholar]
- 117.Danforth KN, Hayes RB, Rodriguez C, et al. Polymorphic variants in PTGS2 and prostate cancer risk: results from two large nested case–control studies. Carcinogenesis. 2008;29:568–572. doi: 10.1093/carcin/bgm253. [DOI] [PubMed] [Google Scholar]
- 118.Dossus L, Kaaks R, Canzian F, et al. PTGS2 and IL6 genetic variation and risk of breast and prostate cancer: results from the Breast and Prostate Cancer Cohort Consortium (BPC3) Carcinogenesis. 2010;31:455–461. doi: 10.1093/carcin/bgp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murad A, Lewis SJ, Smith GD, et al. PTGS2-899G>C and prostate cancer risk: a population-based nested case–control study (ProtecT) and a systematic review with meta-analysis. Prostate Cancer Prostatic Dis. 2009;12:296–300. doi: 10.1038/pcan.2009.18. [DOI] [PubMed] [Google Scholar]
- 120.Amirian ES, Ittmann MM, Scheurer ME. Associations between arachidonic acid metabolism gene polymorphisms and prostate cancer risk. Prostate. 2011 doi: 10.1002/pros.21354. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balistreri CR, Caruso C, Carruba G, et al. A pilot study on prostate cancer risk and pro-inflammatory genotypes: pathophysiology and therapeutic implications. Curr Pharm Des. 2010;16:718–724. doi: 10.2174/138161210790883877. [DOI] [PubMed] [Google Scholar]
- 122.Wu H-C, Chang C-H, Ke H-L, et al. Association of cyclooxygenase 2 polymorphic genotypes with prostate cancer in Taiwan. Anticancer Res. 2011;31:221–225. [PubMed] [Google Scholar]
- 123.Cheng I, Liu X, Plummer SJ, Krumroy LM, Casey G, Witte JS. COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br J Cancer. 2007;97:557–561. doi: 10.1038/sj.bjc.6603874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panguluri RCK, Long LO, Chen W, et al. COX-2 gene promoter haplotypes and prostate cancer risk. Carcinogenesis. 2004;25:961–966. doi: 10.1093/carcin/bgh100. [DOI] [PubMed] [Google Scholar]
- 125.Yegnasubramanian S, Kowalski J, Gonzalgo ML, et al. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64:1975–1986. doi: 10.1158/0008-5472.can-03-3972. [DOI] [PubMed] [Google Scholar]
- 126.Woodson K, O’Reilly KJ, Ward DE, et al. CD44 and PTGS2 methylation are independent prognostic markers for biochemical recurrence among prostate cancer patients with clinically localized disease. Epigenetics. 2006;1:183–186. doi: 10.4161/epi.1.4.3530. [DOI] [PubMed] [Google Scholar]
- 127.Bastian PJ, Ellinger J, Heukamp LC, Kahl P, Muller SC, von Rucker A. Prognostic value of CpG island hypermethylation at PTGS2, RAR-beta, EDNRB, and other gene loci in patients undergoing radical prostatectomy. Eur Urol. 2007;51:665–674. doi: 10.1016/j.eururo.2006.08.008. discussion 674. [DOI] [PubMed] [Google Scholar]
- 128.Ellinger J, Bastian PJ, Jurgan T, et al. CpG island hypermethylation at multiple gene sites in diagnosis and prognosis of prostate cancer. Urology. 2008;71:161–167. doi: 10.1016/j.urology.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 129.Bastian PJ, Palapattu GS, Yegnasubramanian S, et al. CpG island hypermethylation profile in the serum of men with clinically localized and hormone refractory metastatic prostate cancer. J Urol. 2008;179:529–534. doi: 10.1016/j.juro.2007.09.038. discussion 534–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakamura T, Scorilas A, Stephan C, et al. Quantitative analysis of macrophage inhibitory cytokine-1 (MIC-1) gene expression in human prostatic tissues. Br J Cancer. 2003;88:1101–1104. doi: 10.1038/sj.bjc.6600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheung PK, Woolcock B, Adomat H, et al. Protein profiling of microdissected prostate tissue links growth differentiation factor 15 to prostate carcinogenesis. Cancer Res. 2004;64:5929–5933. doi: 10.1158/0008-5472.CAN-04-1216. [DOI] [PubMed] [Google Scholar]
- 133.Brown DA, Lindmark F, Stattin P, et al. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin Cancer Res. 2009;15:6658–6664. doi: 10.1158/1078-0432.CCR-08-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karan D, Holzbeierlein J, Thrasher JB. Macrophage inhibitory cytokine-1: possible bridge molecule of inflammation and prostate cancer. Cancer Res. 2009;69:2–5. doi: 10.1158/0008-5472.CAN-08-1230. [DOI] [PubMed] [Google Scholar]
- 135.Hirano T. The biology of interleukin-6. Chem Immunol. 1992;51:153–180. [PubMed] [Google Scholar]
- 136.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 137.Kishimoto T. Interleukin-6: from basic science to medicine-40 years in immunology. Ann Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 138.Hobisch A, Rogatsch H, Hittmair A, et al. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol. 2000;191:239–244. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH633>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 139.Smith PC, Hobisch A, Lin D-L, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001;12:33–40. doi: 10.1016/s1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 140.Twillie DA, Eisenberger MA, Carducci MA, Hseih W-S, Kim WY, Simons JW. Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology. 1995;45:542–549. doi: 10.1016/S0090-4295(99)80034-X. [DOI] [PubMed] [Google Scholar]
- 141.Culig Z, Puhr M. Interleukin-6: a multifunctional targetable cytokine in human prostate cancer. Mol Cell Endocrinol. 2011 doi: 10.1016/j.mce.2011.05.033. Epub ahead of print. doi:org/10.1016/j.mce.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-[kappa]B, Lin28, Let-7 microRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ammirante M, Luo J-L, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou P, Fang X, McNally BA, et al. Targeting lymphotoxin-mediated negative selection to prevent prostate cancer in mice with genetic predisposition. Proc Natl Acad Sci USA. 2009;106:17134–17139. doi: 10.1073/pnas.0905707106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu X, Plummer SJ, Nock NL, Casey G, Witte JS. Nonsteroidal Antiinflammatory Drugs and Decreased Risk of Advanced Prostate Cancer: Modification by Lymphotoxin Alpha. American Journal of Epidemiology. 2006;164:984–989. doi: 10.1093/aje/kwj294. [DOI] [PubMed] [Google Scholar]
- 147.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 149.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 150.Wang W, Bergh A, Damber J-E. Morphological transition of proliferative inflammatory atrophy to high-grade intraepithelial neoplasia and cancer in human prostate. Prostate. 2009;69:1378–1386. doi: 10.1002/pros.20992. [DOI] [PubMed] [Google Scholar]
- 151.Bethel CR, Faith D, Li X, et al. Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: association with Gleason score and chromosome 8p deletion. Cancer Res. 2006;66:10683–10690. doi: 10.1158/0008-5472.CAN-06-0963. [DOI] [PubMed] [Google Scholar]
- 152.Wang W, Bergh A, Damber J-E. Increased p53 immunoreactivity in proliferative inflammatory atrophy of prostate is related to focal acute inflammation. APMIS. 2009;117:185–195. doi: 10.1111/j.1600-0463.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- 153.Nakayama M, Bennett CJ, Hicks JL, et al. Hypermethylation of the human glutathione S-transferase-[pi] gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection. Am J Pathol. 2003;163:923–933. doi: 10.1016/s0002-9440(10)63452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hsu A, Bray TM, Ho E. Anti-inflammatory activity of soy and tea in prostate cancer prevention. Exp Biol Med. 2010;235:659–667. doi: 10.1258/ebm.2010.009335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vardi A, Bosviel R, Rabiau N, et al. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells. In Vivo. 2010;24:393–400. [PubMed] [Google Scholar]
- 156.Hadley CW, Miller EC, Schwartz SJ, Clinton SK. Tomatoes, lycopene, and prostate cancer: progress and promise. Exp Biol Med. 2002;227:869–880. doi: 10.1177/153537020222701006. [DOI] [PubMed] [Google Scholar]
- 157.Pannellini T, Iezzi M, Liberatore M, et al. A dietary tomato supplement prevents prostate cancer in TRAMP mice. Cancer Prev Res. 2010;3:1284–1291. doi: 10.1158/1940-6207.CAPR-09-0237. [DOI] [PubMed] [Google Scholar]
- 158.Fradet V, Cheng I, Casey G, Witte JS. Dietary omega-3 fatty acids, cyclooxygenase-2 genetic variation, and aggressive prostate cancer risk. Clin Cancer Res. 2009;15:2559–2566. doi: 10.1158/1078-0432.CCR-08-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vissapragada S, Ghosh A, Ringer L, et al. Dietary n-3 polyunsaturated fatty acids fail to reduce prostate tumorigenesis in the PB-ErbB-2 × Pten preclinical mouse model. Cell Cycle. 2010;9:1824–1829. doi: 10.4161/cc.9.9.11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jafari S, Etminan M, Afshar K. Nonsteroidal anti-inflammatory drugs and prostate cancer: a systematic review of the literature and meta-analysis. Can Urol Assoc J. 2009;3:323–330. doi: 10.5489/cuaj.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salinas CA, Kwon EM, FitzGerald LM, et al. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol. 2010;172:578–590. doi: 10.1093/aje/kwq175. [DOI] [PMC free article] [PubMed] [Google Scholar]