Abstract
OncoGel™ incorporates paclitaxel, a mitotic inhibitor, into ReGel™, a thermosensitive gel depot system to provide local delivery, enhance efficacy and limit systemic toxicity. In previous studies the alkylating agent temozolomide (TMZ) incorporated into a polymer, pCPP:SA, also for local delivery, and OncoGel were individually shown to increase efficacy in a rat glioma model. We investigated the effects of OncoGel with oral TMZ or locally delivered TMZ polymer, with and without radiotherapy (XRT) in rats with intracranial gliosarcoma. Eighty-nine animals were intracranially implanted with a 9L gliosarcoma tumor and divided into 12 groups that received various combinations of 4 treatment options; OncoGel 6.3 mg/ml (Day 0), 20 Gy XRT (Day 5), 50 % TMZ–pCPP:SA (Day 5), or oral TMZ (50 mg/kg, qd, Days 5–9). Animals were followed for survival for 120 days.
Median survival for untreated controls, XRT alone or oral TMZ alone was 15, 19 and 28 days, respectively. OncoGel 6.3 or TMZ polymer alone extended median survival to 33 and 35 days, respectively (p = 0.0005; p < 0.0001, vs. untreated controls) with 50 % living greater than 120 days (LTS) in both groups. Oral TMZ/XRT extended median survival to 36 days (p = 0.0002), with no LTS. The group that received OncoGel and Oral TMZ did not reach median survival with 57 % LTS (p = 0.0002). All other combination groups [OncoGel/XRT], [TMZ polymer/XRT], [OncoGel/TMZ polymer], [OncoGel/TMZ polymer/XRT], and [OncoGel/oral TMZ/XRT] yielded greater than 50 % LTS (p < 0.0001 for each combination as compared to controls), therefore median survival was not reached. OncoGel/TMZ polymer and OncoGel/oral TMZ/XRT had 100 % LTS (p < 0.0001 and p = 0.0001 vs. oral TMZ/XRT, respectively). These results indicate that OncoGel given locally with oral or locally delivered TMZ and/or XRT significantly increased the number of LTS and improved median survival compared to oral TMZ and XRT given alone or in combination in a rodent intracranial gliosarcoma model.
Keywords: Glioma, Paclitaxel, Temozolomide, Radiation therapy
Introduction
Despite advances in medical therapy for the treatment of brain tumors, with recent therapies increasing the median survival (MS) from 9 to 21 months [1], there remains a need for further improvement in the treatment of glioblastoma multiforme (GBM). Ongoing research in tumor biology has identified that multiple signaling pathways play a key role in tumor progression. Utilizing combinations therapies that synergistically target different pathways, therefore, appears to be a logical strategy for improving the efficacy of current therapies.
Temozolomide (TMZ) is an FDA approved alkylating agent that has been shown to improve survival in patients with newly diagnosed glioblastoma [2] or recurrent anaplastic astrocytomas [3] when administered orally. TMZ is typically used for the treatment of malignant glioma in conjunction with radiation therapy and following surgical resection. When dose escalation studies revealed severe hematologic toxicity at higher doses [4–6] with no neurological side effects observed at the highest doses [2, 7], we developed a method for local delivery of TMZ using a biodegradable polymer and found this to have enhanced efficacy when compared to oral TMZ in a rodent model of glioma [8, 9].
Paclitaxel is a mitotic inhibitor with established efficacy against glioma in vitro [10, 11] and is known to sensitize glioma cells to radiation therapy [12, 13]. However, successful translation to clinical use has been impeded by its poor blood brain barrier permeability and consequently its inability to reach an adequate concentration within the CNS at non-toxic doses [14–16]. To overcome these issues, we and other investigators have explored the local delivery of paclitaxel to the brain by various methods such as polymeric as well as convection-enhanced delivery [17–20]. We recently reported a novel method of local paclitaxel delivery by stereotactic injection of OncoGel, a thermal gel depot containing paclitaxel [21]. In this study, OncoGel was found to be safe for intracranial injection in rodents, effective against an intracranial rodent glioma model and provided a synergistic effect with radiation therapy in rats challenged with 9L gliosarcoma. We also recently demonstrated that intramedullary injection of OncoGel is safe and improves survival and functional outcome in a rodent model of intramedullary spinal cord gliosarcoma [22].
Since TMZ and paclitaxel have different mechanisms of action, we hypothesized that the combination of TMZ and OncoGel might have greater efficacy than either drug's effect alone. In this report, we examine the possible synergistic effect of OncoGel and oral or locally delivered TMZ in a rodent model of gliosarcoma. Since nearly all patients with glioma receive radiation therapy, we also examined the effect of these combination chemotherapies when given along with adjuvant radiotherapy.
Methods
Study materials
OncoGel contains [6.3 mg paclitaxel/ml ReGel, where ReGel is a thermosensitive, biodegradable triblock copolymer composed of poly (D,L-lactide-co-glycolide) and poly (ethylene glycol)], and was provided by Protherics Salt Lake City Inc., and stored at —20 °C. It was thawed at 4 °C for 72 h before each experiment and subsequently maintained on ice until use. Stereotactic injections were performed with a Hamilton syringe (Model 700, Hamilton Company) with a Luer lock tip and a modified shortened needle (∼ 1.5 cm long).
Temozolomide, provided by the National Institute of Health/National Cancer Institute (Bethesda, MD), was incorporated into a polyanhydride poly (1,3-bis-[p-carboxyphenoxy propane]-co-[sebacic anhydride]) (CPP:SA) polymer by methods described previously [23].
9L gliosarcoma cells were obtained from the Brain Tumor Research Center (University of California at San Francisco, CA).
Study animals
Female Fischer 344 rats, weighing 125-175 g each, were purchased from Harlan Bioproducts (Indiana, Indianapolis), housed in standard facilities and given free access to food and water. All animals were treated in accordance with the policies and guidelines of the Johns Hopkins University Animal Care and Use Committee.
Efficacy study
For intracranial implantation of the 9L gliosarcoma, 87 F344 female rats were anesthetized and implanted with tumor as described previously [9, 21] and then were divided into the following groups- control (no treatment) (n = 7); 35 μl OncoGel (6.3 mg/ml) intracranially injected on Day 0 (n = 6); 50 % TMZ CPP:SA polymer implanted on Day 5 (n = 8); Oral TMZ (50 mg/kg) given p.o. Days 5-9 (n = 7); Radiation therapy (XRT) alone—20 Gy on Day 5 (n = 7); 35 μl OncoGel (6.3 mg/ml) intracranially injected on Day 0 + XRT on Day 5 (n = 7); 50 % TMZ CPP:SA polymer implanted on Day 5 + XRT on Day 5 (n = 8); Oral TMZ (50 mg/kg) given p.o. Days 5–9 + XRT on Day 5 (n = 8); 35 μl OncoGel (6.3 mg/ml) intracranially injected on Day 0 + 50 % TMZ CPP:SA polymer implanted on Day 5 (n = 8); 35 μl OncoGel (6.3 mg/ml) intracranially injected on Day 0 + Oral TMZ (50 mg/kg) given p.o. Days 5–9 (n = 7); 35 μl OncoGel (6.3 mg/ml) intracranially injected on Day 0 + 50 % TMZ CPP:SA polymer implanted on Day 5 + XRT on Day 5 (n = 8); and 35 μl OncoGel (6.3 mg/ml) intracranially injected on Day 0 + Oral TMZ (50 mg/kg) given p.o. Days 5–9 + XRT on Day 5 (n = 6). The experiment was continued for 120 days and animals that survived to that point were deemed to be long term survivors (LTS).
Radiation therapy
Anesthetized rats were placed at a fixed distance from the radiation source in a Cs137 laboratory irradiator (Mark 1 Irradiator, model 68; J. L. Shepherd Associates, San Fernando, CA), and shielded with a square primary collimator (1 cm in diameter) centered over the tumor implantation site. The dose rate at this setting was 2.94 Gy/min and the body was externally shielded from the radiation. External beam single-dose radiation treatment was subsequently delivered at a dose of 20 Gy to XRT treatment animals 5 days after tumor placement.
Histological analyses
At the time of death or sacrifice at the end of the experiment (Day 120), brains were extracted, stored in formalin, and sectioned for histological analysis. Sections were examined to confirm the presence or absence of tumor growth and to determine if there was any adverse effect of OncoGel, local TMZ or XRT.
Statistical analyses
The primary outcome variable measured was the time to death from the day of tumor implantation (Day 0). Kaplan– Meier survival curves were generated and groups were compared using Kruskal–Wallis ANOVA followed by Wilcoxon rank-sum test since the data are nonparametric. GraphPad Prism 4.0 (GraphPad Software, Inc.) was used for all analyses. A probability value < 0.05 was considered statistically significant for all comparisons.
Results
Effects of no treatment and single treatment options
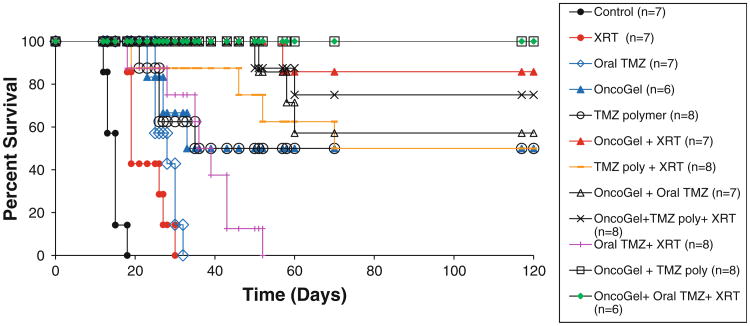
In our 9L rodent gliosarcoma model, rats that did not receive any treatment (control, n = 7) had a MS of 15 days (with a range of 12–18 days) (Fig. 1; Table 1). Treatment with XRT alone resulted in a modest increase in survival (MS = 19 days, p = 0.0002 vs. control) (with a range of 18–30 days). Oral TMZ significantly improved survival (MS = 28 days) when compared to control rats (p = 0.0002) (with a range of 25–32 days). However, there were no LTS with oral TMZ treatment. Adjuvant radiotherapy (Oral TMZ + XRT) extended the MS to 36 days (p = 0.0002 vs. control) (with a range of 18–52 days) also with no LTS in this group either. Delivery of TMZ via local polymer implantation extended the MS to 35 days (p < 0.0001 vs. control) with 50 % of the rats being LTS (a range of 21–120 days). The survival benefit conferred by local TMZ polymer treatment was also significantly better than oral TMZ administration (p = 0.0415). Adjuvant radiation therapy (Local TMZ + XRT) further increased the survival (MS not reached; p < 0.0001 vs. control) (with a range of 19–120 days) and there were 62.5 % LTS. However, there was no statistically significant increase in survival when compared to treatment with local TMZ alone (p = 0.8052). Administration of OncoGel extended the MS to 33 days (p = 0.0005 vs. control) (with a range of 23–120 days) and 50 % of the rats were LTS. Combination with radiation therapy (OncoGel + XRT) further extended the survival (MS not reached, p < 0.0001 vs. control) (with a range of 57–120 days) and increased the number of LTS to 87.5 %. This combination also resulted in a significantly higher survival than Oral TMZ + XRT (p < 0.0001).
Fig. 1.
Intracranial efficacy of TMZ given IC or PO in combination with OncoGel 6.3 mg/ml with and without XRT for the treatment of experimental malignant gliosarcoma. F344 rats were intracranially implanted with 9L tumor. Controls (n = 7) received no further treatment (filled circle) and had a MS of 15 days. Animals receiving XRT (20 Gy) on Day 5 (n = 7) (filled circle) and animals that received oral TMZ on Days 5–9 (n = 7) (open diamond) had MS of 19 and 28 days, respectively. Animals receiving OncoGel 6.3 on Day 0 (n = 6) (filled triangle) had a MS of 33 days. Animals receiving a TMZ polymer on Day 5 (n = 8) (open circle) had a MS of 35 days. Animals that received OncoGel 6.3 and XRT (n = 7) (filled triangle) did not reach MS with 85 % long term survivors (LTS). Animals that received a TMZ polymer and XRT (n = 8) (emdashed line) reached MS on Day 70 with 50 % LTS. Animals that received OncoGel 6.3 and Oral TMZ (n = 7) (open triangle) did not reach MS with 57 % LTS. Animals receiving OncoGel 6.3, TMZ polymer and XRT (n = 8) (cross symbol) did not reach MS with 75 % LTS. Animals that received Oral TMZ and XRT (n = 8) (vertical line) had a MS of 35 days. Animals that received either OncoGel 6.3 and TMZ polymer (n = 8) (open square) or the triple combination of OncoGel 6.3, oral TMZ and XRT (n = 6) (filled circle) had no deaths with both groups having 100 % LTS
Table 1. Intracranial Efficacy of Temozolomide (TMZ) in combination with OncoGel 6.3 mg/ml with and without Radiation therapy (XRT) for the treatment of experimental malignant gliosarcoma.
| Treatment group | Number of animals | Median survival (days) (range of survival) | Long term survivors (%) | Statistical significance relative to no treatment | Statistical significance relative to XRT + Oral TMZ |
|---|---|---|---|---|---|
| No treatment | 7 | 15 (12–18) | 0 | 1 | |
| XRT | 7 | 19 (18–30) | 0 | p = 0.0002 | |
| Oral TMZ | 7 | 28 (25–32) | 0 | p = 0.0002 | |
| XRT + Oral TMZ | 8 | 36 (18–52) | 0 | p = 0.0002 | |
| TMZ polymer | 8 | 35 (21–120) | 50 | p < 0.0001 | |
| OncoGel | 6 | 33 (23–120) | 50 | p = 0.0005 | |
| XRT + TMZ polymer | 8 | Not reached (19–120) | 62.50 | p < 0.0001 | |
| OncoGel + XRT | 7 | Not reached (57–120) | 87.50 | p < 0.0001 | p < 0.0001 |
| OncoGel + Oral TMZ | 7 | Not reached (51–120) | 57.14 | p = 0.0002 | p = 0.0003 |
| OncoGel + TMZ polymer | 8 | Not reached (No deaths) | 100 | p < 0.0001 | p < 0.0001 |
| OncoGel + Oral TMZ + XRT | 6 | Not reached (No deaths) | 100 | p < 0.0001 | p = 0.0001 |
| OncoGel + TMZ polymer + XRT | 8 | Not reached (50–120) | 75 | p < 0.0001 | p = 0.0001 |
In vivo efficacy of the combination of OncoGel and oral TMZ
Combination of OncoGel with oral TMZ significantly increased the survival of tumor bearing animals compared to control rats (MS not reached; p = 0.0002 vs. control) (with a range of 51–120 days) as well as individual treatment groups (p = 0.0001 vs. Oral TMZ, p = 0.0001 vs. XRT). Also, 57 % rats were LTS. The survival in this group was also significantly better than rats that received Oral TMZ and XRT, a clinically utilized treatment regimen (OncoGel + Oral TMZ:MS not reached, vs. Oral TMZ + XRT:MS = 36 days, p = 0.0003). Moreover, the combination group (OncoGel + Oral TMZ) had 57 % LTS (120 days) (with a range of 51–120 days) while all rats in the group treated with Oral TMZ and XRT died by 52 days (with a range of 18–52 days).
Addition of XRT to the combination regimen resulted in 100 % LTS and again, MS was not reached (p < 0.0001 vs. control; p = 0.0001 vs. Oral TMZ + XRT). This triple combination therapy also resulted in significantly better survival when compared to any of the agents alone (p = 0.0004 vs. Oral TMZ, p = 0.027 vs. OncoGel, p = 0.025 vs. Local TMZ and p < 0.0001 vs. XRT), the current standard of treatment (p = 0.0001 vs. Oral TMZ + XRT) and, the same combination without radiation therapy (p = 0.04 vs. OncoGel + Oral TMZ).
In vivo efficacy of the combination of OncoGel and intracranial TMZ
Median survival was not reached in the group that received the combination of OncoGel and intracranial TMZ polymer with 100 % rats being LTS (n = 8, p < 0.0001 vs. control). The survival in this group was significantly higher than any of the individual treatments (p < 0.0001 vs. Oral TMZ, p = 0.027 vs. OncoGel, p = 0.025 vs. Local TMZ, p < 0.0001 vs. XRT). This group also had a significantly higher survival than rats treated with Oral TMZ and XRT (p < 0.0001 vs. Oral TMZ + XRT). In the group where OncoGel and local TMZ were combined with adjuvant radiation therapy (OncoGel + local TMZ + XRT), MS was not reached (with a range of 50–120 days) (p < 0.0001 vs. control; p = 0.0001 vs. Oral TMZ + XRT) and 75 % rats were LTS.
Histological analyses
All brains were examined histologically, including those that underwent spontaneous death as well as those sacrificed at 120 days and deemed LTS. Upon histological analysis, all untreated control rats were found to have tumor (Fig. 2a). Additionally, all rats that died within the experimental time frame (<120 days) were found to have tumor. No tumor was seen in the animals euthanized on Day 120, however, there were some distinguishing features present, including dystrophic calcification, hemosiderin-laden macrophages surrounded by reactive gliosis (Fig. 2c), and some residual wafer in those animals that had been implanted with TMZ wafers. All of these histological findings were consistent with local treatment for brain tumors and were not lethal.
Fig. 2.
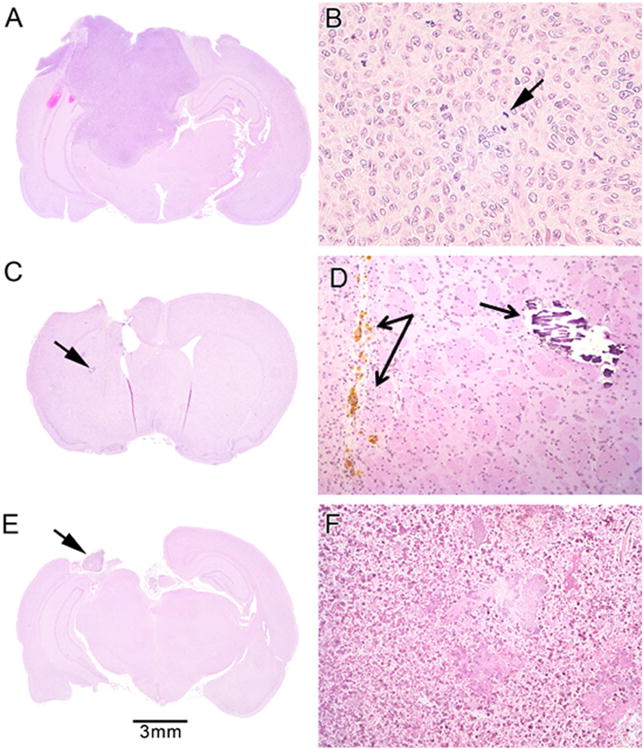
a–b Tumor infiltration of untreated rat brain from control group (a macroscopic view) with mitotic figures (arrow) in neoplastic cells (b × 160 magnification); c–d rat brain from OncoGel + oral TMZ + XRT group with no tumor (c macroscopic view). 2D shows the area marked by the arrow in 2C, demonstrating hemosiderin-laden macrophages within the wound tract surrounded by reactive gliosis (left arrows) and dystrophic calcification (right arrow, ×160 magnification); e–f rat brain from OncoGel + 50 % TMZ + XRT group with no tumor (e macroscopic view) but showing inflammation, acellular debris, and residual polymer (arrow and f ×64 magnification)
Discussion
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor in adults. Though current treatment—maximal safe surgical resection combined with radiotherapy and alkylating chemotherapy—prolongs survival, improvements need to be made [1, 2, 24]. In a large subset of patients, tumor cells express the DNA repair enzyme O(6)-methylguanine-DNA methyltransferase which confers resistance to alkylating agents, such as TMZ and carmustine, thereby causing these patients to respond poorly [25]. It is well known that the combination of two chemotherapeutic drugs with different mechanisms of action or different mechanisms of resistance can result in an additive or synergistic tumoricidal effect. For example, a recent retrospective review of 108 GBM patients treated with either Gliadel alone or Gliadel and Temodar showed an increase in survival in the combination group from 9 to 21 months [1]. This demonstrates that combinatorial drug therapies are a promising strategy for the treatment of GBM. Carmustine and TMZ, however, are both alkylating agents with similar mechanisms of action. To overcome this limitation, we explored the potential anti-tumor effect of the combination chemotherapy of TMZ and paclitaxel. These two chemotherapeutic drugs have: (1) Different mechanisms of action; (2) different resistance mechanisms; (3) non-overlapping toxicity profiles; (4) established efficacy in glioma; and (5) known synergistic effect in melanoma [26–28].
Consistent with our previous results, this study showed that local TMZ delivery was superior to controls as well as superior to oral TMZ in prolonging the survival of rats bearing 9L gliosarcoma. Also, confirming previous findings, rats treated with OncoGel alone performed significantly better than controls and the addition of radiation therapy further extended the survival [21]. One of the advantages of locally placing OncoGel at the tumor site is that the gel has a slow clearance rate from the cavity, with delivery times ranging from 1 to 6 weeks [29]. Recurrence of malignant gliomas occurs most often within 2 cm of the original site of resection [30]. Matthes et al. [31] injected OncoGel in a porcine pancreatic model and demonstrated that therapeutic concentrations of paclitaxel were detected at a distance of 3–5 cm from the depot. This is encouraging data for this aggressive and invasive tumor type.
When considering combination chemotherapy, it is essential to ensure that resistance to one cytotoxic agent does not induce cross-resistance to other potentially useful agents [32]. In this regard, it has been shown that the administration of paclitaxel has no effect on the in vitro sensitivity of different glioma cell lines to alkylating agents including TMZ [33]. Recent clinical studies have demonstrated that the combination of TMZ with Taxanes (paclitaxel or docetaxel) has a synergistic effect in metastatic melanoma [26–28]. Consistent with our expectations, all combinations of OncoGel with TMZ significantly prolonged survival in the rodent tumor model and no signs of systemic or neurological toxicity were observed. The combination of OncoGel with oral or local TMZ resulted in 57 and 100 % LTS, respectively, and therefore neither group reached MS whereas the MS for rats that received no treatment was 15 days with all control rats dead by Day 18. The efficacy of the combination of OncoGel and TMZ is further evident when compared to the MS of 36 days and no LTS in the group that received one of the current clinical treatments of Oral TMZ and XRT. The combination of OncoGel with oral or local TMZ resulted in higher survival than any single agent treatments. Also, the number of LTS was higher in the group treated with OncoGel and local TMZ when compared to rats treated with OncoGel and oral TMZ, thereby suggesting that maximum synergistic effect is observed with the former combination. We thus demonstrate that there is a synergistic effect of the combination of an alkylating agent (TMZ) with a Taxane (paclitaxel) against gliosarcomas in vivo and that this combination does not produce local or systemic toxicity.
In clinical practice, almost all patients with malignant gliomas receive some form of radiation therapy [34, 35]. It is therefore imperative to determine the in vivo effect of any new chemotherapeutic strategy when administered in conjunction with radiation therapy. We examined the effect of adjuvant XRT on combinations of OncoGel with oral or local TMZ. Consistent with our hypothesis, the combined regimen of adjuvant XRT, OncoGel and either mode of TMZ administration (oral or local) significantly increased survival when compared to both the control group and the oral TMZ and XRT combination group. The addition of OncoGel to the TMZ/XRT combinations did not add any demonstrable toxicity. Furthermore, in the group that received OncoGel, Oral TMZ and XRT, all rats survived to 120 days and the survival in this group was significantly better than treatment with any of the individual agents (Oral TMZ, Local TMZ or OncoGel), thus indicating a strong therapeutic effect of this treatment regimen. This triple combination group showed statistically longer survival (p < 0.0001) than the clinically comparable combination of oral TMZ and XRT. The increase in efficacy of the OncoGel and TMZ combination when administered along with radiotherapy could be attributed to the polymerization of microtubules by paclitaxel, which substantially increases the fraction of cells in G2/M phase, the optimal time period for radiosensitization during the cell cycle [36, 37].
Some limitations of our study should be noted. First, we used rodent glioma cell lines, whose characteristics may be different from human glioma. In particular, the 9L gliosarcoma line used in our study is a highly immunogenic tumor due to which the anti-tumor response of experimental therapeutic strategies may be amplified. Second, our in vivo efficacy studies utilized only a single rodent glioma model. Future studies examining the efficacy of the combination therapy of OncoGel and TMZ should be performed in complementary glioma models such as orthotopic xenografts from human glioma tissue. Third, in our study, rats were administered OncoGel on Day 0, while TMZ wafers were implanted on Day 5. Direct translation of this strategy into clinical trials may be limited, as this would necessitate two surgical procedures. Therefore, our proof of concept study should be expanded upon by identifying the therapeutic window when Oncogel and TMZ can be administered simultaneously, in a clinically applicable paradigm.
Conclusion
The combination of OncoGel (paclitaxel) with oral or local TMZ is safe, effective and synergistic in the treatment of the rodent 9L gliosarcoma model. Adjuvant radiation therapy further increases the efficacy of this combination therapy. Future studies examining the efficacy of this combination in other glioma models are needed before moving forward into clinical trials.
Acknowledgments
Funding This work was supported by the Research Scholar Grant 116293-RSG-08-119-01-CCE from the American Cancer Society.
Contributor Information
Ananth K. Vellimana, Department of Neurosurgery, Johns Hopkins University School, of Medicine, 1550 Orleans Street CRB2 2M41, Baltimore, MD, 21231, USA
Violette Renard Recinos, Department of Neurosurgery, Johns Hopkins University School, of Medicine, 1550 Orleans Street CRB2 2M41, Baltimore, MD, 21231, USA.
Lee Hwang, Department of Neurosurgery, Johns Hopkins University School, of Medicine, 1550 Orleans Street CRB2 2M41, Baltimore, MD, 21231, USA.
Kirk D. Fowers, Protherics Salt Lake City Inc., Salt Lake City, UT, USA
Khan W. Li, Department of Neurosurgery, Johns Hopkins University School, of Medicine, 1550 Orleans Street CRB2 2M41, Baltimore, MD, 21231, USA
Yonggang Zhang, Radiation Oncology and Molecular Radiation Services, Baltimore, MD 21231, USA.
Saint Okonma, Department of Neurosurgery, Johns Hopkins University School, of Medicine, 1550 Orleans Street CRB2 2M41, Baltimore, MD, 21231, USA.
Charles G. Eberhart, Department of Ophthalmology, Johns Hopkins University, School of Medicine, Baltimore, MD 21231, USA
Henry Brem, Department of Neurosurgery, Johns Hopkins University School, of Medicine, 1550 Orleans Street CRB2 2M41, Baltimore, MD, 21231, USA; Department of Ophthalmology, Johns Hopkins University, School of Medicine, Baltimore, MD 21231, USA; Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD 21231, USA.
Betty M. Tyler, Email: btyler@jhmi.edu, Department of Neurosurgery, Johns Hopkins University School, of Medicine, 1550 Orleans Street CRB2 2M41, Baltimore, MD, 21231, USA.
References
- 1.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quinones-Hinojosa A. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O'Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 4.Hammond LA, Eckardt JR, Baker SD, Eckhardt SG, Dugan M, Forral K, Reidenberg P, Statkevich P, Weiss GR, Rinaldi DA, Von Hoff DD, Rowinsky EK. Phase I and pharmacokinetic study of temozolomide on a daily-for-5-days schedule in patients with advanced solid malignancies. J Clin Oncol. 1999;17:2604–2613. doi: 10.1200/JCO.1999.17.8.2604. [DOI] [PubMed] [Google Scholar]
- 5.Newlands ES, Blackledge GR, Slack JA, Rustin GJ, Smith DB, Stuart NS, Quarterman CP, Hoffman R, Stevens MF, Brampton MH, et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856) Br J Cancer. 1992;65:287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudek MA, Donehower RC, Statkevich P, Batra VK, Cutler DL, Baker SD. Temozolomide in patients with advanced cancer: phase I and pharmacokinetic study. Pharmacotherapy. 2004;24:16–25. doi: 10.1592/phco.24.1.16.34800. [DOI] [PubMed] [Google Scholar]
- 7.Brock CS, Newlands ES, Wedge SR, Bower M, Evans H, Colquhoun I, Roddie M, Glaser M, Brampton MH, Rustin GJ. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res. 1998;58:4363–4367. [PubMed] [Google Scholar]
- 8.Brem S, Tyler B, Li K, Pradilla G, Legnani F, Caplan J, Brem H. Local delivery of temozolomide by biodegradable polymers is superior to oral administration in a rodent glioma model. Cancer Chemother Pharmacol. 2007;60:643–650. doi: 10.1007/s00280-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 9.Recinos VR, Tyler BM, Bekelis K, Sunshine SB, Vellimana A, Li KW, Brem H. Combination of intracranial temozolomide with intracranial carmustine improves survival when compared with either treatment alone in a rodent glioma model. Neurosurgery. 2010;66:530–537. doi: 10.1227/01.NEU.0000365263.14725.39. discussion 537. [DOI] [PubMed] [Google Scholar]
- 10.Silbergeld DL, Chicoine MR, Madsen CL. In vitro assessment of taxol for human glioblastoma: chemosensitivity and cellular locomotion. Anticancer Drugs. 1995;6:270–276. doi: 10.1097/00001813-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Cahan MA, Walter KA, Colvin OM, Brem H. Cytotoxicity of taxol in vitro against human and rat malignant brain tumors. Cancer Chemother Pharmacol. 1994;33:441–444. doi: 10.1007/BF00686276. [DOI] [PubMed] [Google Scholar]
- 12.Gupta N, Hu LJ, Deen DF. Cytotoxicity and cell-cycle effects of paclitaxel when used as a single agent and in combination with ionizing radiation. Int J Radiat Oncol Biol Phys. 1997;37:885–895. doi: 10.1016/s0360-3016(96)00535-4. [DOI] [PubMed] [Google Scholar]
- 13.Tishler RB, Geard CR, Hall EJ, Schiff PB. Taxol sensitizes human astrocytoma cells to radiation. Cancer Res. 1992;52:3495–3497. [PubMed] [Google Scholar]
- 14.Heimans JJ, Vermorken JB, Wolbers JG, Eeltink CM, Meijer OW, Taphoorn MJ, Beijnen JH. Paclitaxel (taxol) concentrations in brain tumor tissue. Ann Oncol. 1994;5:951–953. doi: 10.1093/oxfordjournals.annonc.a058736. [DOI] [PubMed] [Google Scholar]
- 15.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, Bernhardt G, Graeff C, Farber L, Gschaidmeier H, Buschauer A, Fricker G. Transport of paclitaxel (taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glantz MJ, Choy H, Kearns CM, Mills PC, Wahlberg LU, Zuhowski EG, Calabresi P, Egorin MJ. Paclitaxel disposition in plasma and central nervous systems of humans and rats with brain tumors. J Natl Cancer Inst. 1995;87:1077–1081. doi: 10.1093/jnci/87.14.1077. [DOI] [PubMed] [Google Scholar]
- 17.Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, Hadani M, Ram Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 18.Pradilla G, Wang PP, Gabikian P, Li K, Magee CA, Walter KA, Brem H. Local intracerebral administration of Paclitaxel with the paclimer delivery system: toxicity study in a canine model. J Neurooncol. 2006;76:131–138. doi: 10.1007/s11060-005-5531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter KA, Cahan MA, Gur A, Tyler B, Hilton J, Colvin OM, Burger PC, Domb A, Brem H. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res. 1994;54:2207–2212. [PubMed] [Google Scholar]
- 20.Wang Z, Hu X, Yue J, Jing X. Experimental study on biodegradable polymer-paclitaxel conjugate micelles for chemotherapy of C6 glioma. J Control Release. 2011;152(Suppl 1):e41–e42. doi: 10.1016/j.jconrel.2011.08.110. [DOI] [PubMed] [Google Scholar]
- 21.Tyler B, Fowers KD, Li KW, Recinos VR, Caplan JM, Hdeib A, Grossman R, Basaldella L, Bekelis K, Pradilla G, Legnani F, Brem H. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. J Neurosurg. 2010;113:210–217. doi: 10.3171/2009.11.JNS08162. [DOI] [PubMed] [Google Scholar]
- 22.Tyler BM, Hdeib A, Caplan J, Legnani FG, Fowers KD, Brem H, Jallo G, Pradilla G. Delayed onset of paresis in rats with experimental intramedullary spinal cord gliosarcoma following intratumoral administration of the paclitaxel delivery system OncoGel. J Neurosurg Spine. 2012;16:93–101. doi: 10.3171/2011.9.SPINE11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamargo RJ, Myseros JS, Epstein JI, Yang MB, Chasin M, Brem H. Interstitial chemotherapy of the 9L gliosarcoma: controlled release polymers for drug delivery in the brain. Cancer Res. 1993;53:329–333. [PubMed] [Google Scholar]
- 24.McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, Olivi A, Brem H, Quinones-Hinojosa A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700–707. doi: 10.1227/01.NEU.0000325729.41085.73. author reply 707-708. [DOI] [PubMed] [Google Scholar]
- 25.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 26.Azzabi A, Hughes AN, Calvert PM, Plummer ER, Todd R, Griffin MJ, Lind MJ, Maraveyas A, Kelly C, Fishwick K, Calvert AH, Boddy AV. Phase I study of temozolomide plus paclitaxel in patients with advanced malignant melanoma and associated in vitro investigations. Br J Cancer. 2005;92:1006–1012. doi: 10.1038/sj.bjc.6602438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bafaloukos D, Gogas H, Georgoulias V, Briassoulis E, Fountzilas G, Samantas E, Kalofonos C, Skarlos D, Karabelis A, Kosmidis P. Temozolomide in combination with docetaxel in patients with advanced melanoma: a phase II study of the Hellenic Cooperative Oncology Group. J Clin Oncol. 2002;20:420–425. doi: 10.1200/JCO.2002.20.2.420. [DOI] [PubMed] [Google Scholar]
- 28.Yoon C, Papadopoulos NE, Camacho LH, McIntyre S, Alvarado GC, Bedikian AY, Hwu P, Kim KB. The clinical efficacy of combination of docetaxel and temozolomide in previously treated patients with stage IV melanoma. Melanoma Res. 2010;20:43–47. doi: 10.1097/CMR.0b013e3283324e2e. [DOI] [PubMed] [Google Scholar]
- 29.Zentner GM, Rathi R, Shih C, McRea JC, Seo MH, Oh H, Rhee BG, Mestecky J, Moldoveanu Z, Morgan M, Weitman S. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 1992;72(1-3):203–215. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 30.Gaspar LE, Fisher BJ, Macdonald DR, LeBer DV, Halperin EC, Schold SC, Jr, Cairncross JG. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24:55–57. doi: 10.1016/0360-3016(92)91021-e. [DOI] [PubMed] [Google Scholar]
- 31.Matthes K, Mino-Kenudson M, Sahani DV, Holalkere N, Fowers KD, Rathi R, Brugge WR. EUS-guided injection of paclitaxel (OncoGel) provides therapeutic drug concentrations in the porcine pancreas (with video) Gastrointest Endosc. 2007;65(3):448–453. doi: 10.1016/j.gie.2006.06.030. Epub 2006 Dec 14. [DOI] [PubMed] [Google Scholar]
- 32.Jensen PB, Holm B, Sorensen M, Christensen IJ, Sehested M. In vitro cross-resistance and collateral sensitivity in seven resistant small-cell lung cancer cell lines: preclinical identification of suitable drug partners to taxotere, taxol, topotecan and gemcitabin. Br J Cancer. 1997;75:869–877. doi: 10.1038/bjc.1997.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng SH, Bobola MS, Berger MS, Silber JR. Characterization of paclitaxel (taxol) sensitivity in human glioma- and medulloblastoma-derived cell lines. Neuro Oncol. 1999;1:101–108. doi: 10.1093/neuonc/1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro MG, Cowen R, Williamson IK, David A, Jimenez-Dalmaroni MJ, Yuan X, Bigliari A, Williams JC, Hu J, Lowenstein PR. Current and future strategies for the treatment of malignant brain tumors. Pharmacol Ther. 2003;98:71–108. doi: 10.1016/s0163-7258(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 35.Chang SM, Parney IF, Huang W, Anderson FA, Jr, Asher AL, Bernstein M, Lillehei KO, Brem H, Berger MS, Laws ER. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557–564. doi: 10.1001/jama.293.5.557. [DOI] [PubMed] [Google Scholar]
- 36.Liebmann J, Cook JA, Fisher J, Teague D, Mitchell JB. In vitro studies of taxol as a radiation sensitizer in human tumor cells. J Natl Cancer Inst. 1994;86:441–446. doi: 10.1093/jnci/86.6.441. [DOI] [PubMed] [Google Scholar]
- 37.Elomaa L, Joensuu H, Kulmala J, Klemi P, Grenman R. Squamous cell carcinoma is highly sensitive to taxol, a possible new radiation sensitizer. Acta Otolaryngol. 1995;115:340–344. doi: 10.3109/00016489509139325. [DOI] [PubMed] [Google Scholar]