Abstract
XMRV was first described in 2006, when it was identified in samples isolated from prostate cancer tissues. However, studies have since shown that XMRV arose in the laboratory and was formed by genetic recombination between two viral genomes carried in the germline DNA of mice used during serial transplantation of the CWR22 prostate cancer xenograft. These new findings strongly imply that XMRV does not circulate in humans, but is only present in the laboratory. Thus, there is no reason to believe that it has any role in the etiology of prostate cancer or other diseases.
Introduction
Several classes of viruses, including retroviruses, are known to cause cancer in humans and animals. The possibility that additional types of cancer could be caused by as yet unidentified viruses holds great appeal. However, the attractiveness of this hypothesis has also led to cancer causation being mistakenly attributed to viruses.1,2 It has long been postulated that viruses could have a role in prostate cancer etiology, and reports of viruses in association with prostate cancer date back over 30 years. Thus far, however, no virus has been causally linked to prostate cancer. The latest entry into this catalog is xenotropic murine leukemia virus-related virus (XMRV). This Perspectives article will critically evaluate studies that seemed to identify XMRV in prostate cancer samples, address the known sources of false-positive results in XMRV assays, and describe and explain in depth the findings and the implications of a study that showed that XMRV was formed by a rare recombination event in the laboratory. Thus, we will chart the rise and fall of XMRV from its initial detection in 2006, via claims of its role in prostate cancer, to the 2011 report of its recombinant origin.
Reports of XMRV in prostate cancer
XMRV was first described in 2006, in a study that used a novel DNA microarray (termed the Virochip) containing oligonucleotides covering conserved sequences from reference viral genomes to analyze RNA isolated from prostate cancer tissues.3 Positive hybridization signals were observed in eight of 19 samples in regions of the microarray that contained sequences from known gammaretroviruses. Interestingly, seven of these eight positive samples were from individuals harboring a homozygous R462Q (QQ) variant of the RNASEL gene. This finding was of interest because RNASEL functions in antiviral responses and the R462Q variant of RNASEL had been previously associated with prostate cancer risk.4 Viral genome sequencing identified the virus as a murine leukemia virus (MLV) (Box 1). XMRV was detected in approximately 1% of stromal cells in prostate cancer tissues using both fluorescence in situ hybridization (FISH) with large segments of the XMRV genome as probes and immunohistochemistry (IHC) using a monoclonal antibody against an MLV protein, but it was not found in tumor cells.
Box 1. Murine leukemia viruses.
Murine leukemia viruses (MLVs) are prototypical gammaretroviruses.65,66 Their overall structure and replication cycle are similar to those of other retroviruses, including HIV-1. The virus particle is roughly spherical, ~100 nm in diameter, and bounded by a lipid bilayer. It is constructed from the virus-coded Gag proteins and also contains the viral enzymes protease, reverse transcriptase, and integrase (known together as Pol) as well as the Env proteins that mediate the entry of the particle into new host cells and two copies of the viral RNA genome. Proteins from the virus-producing cell are also present within the particle. MLVs are among the simplest retroviruses, encoding only the three polyproteins—Gag, Pol and Env—that will be assembled into progeny virus particles. After the virus particle is released from the virus-producing cell, each of the polyproteins is cleaved by the viral protease. This maturation step is essential for infectivity
When an MLV particle infects a new cell, its reverse transcriptase copies the viral RNA into double-stranded DNA. Integrase physically inserts this DNA copy into the chromosomal DNA of the cell, and it is then replicated as part of the cell’s genetic material and is transcribed and translated by normal cellular machinery. Productive infection is generally harmless for the cell
MLVs are found in mice in the wild and have been studied extensively in the laboratory. They have been engineered to create vectors for gene therapy. Both MLV in MLV-infected mice and the vectors administered to children can cause tumors by insertional mutagenesis— while the chromosomal integration site of the viral DNA is nearly random, it is occasionally near a gene involved in regulation of cell growth. It can then interrupt the gene or alter its expression. The resulting disruption of the signal-transduction pathways controlling cellular replication is a principal mechanism by which these viruses cause tumors
Subsequent work from the same group described the molecular cloning of full-length XMRV from cDNA isolated from the same prostate cancer samples used in the original study.5 Transfection of the LNCaP prostate cancer cell line with a molecular viral clone showed that XMRV was indeed a replication-competent xenotropic MLV (Box 2). One hallmark of retroviral infections is the integration of the DNA form of the viral genome into the host genome. Dong et al.5 also reported that DNA in which the end of the XMRV genome was joined to human sequences was present in the prostate cancer samples. This finding of viral integration sites was strong molecular evidence that authentic XMRV-infected cells were present in the tumors.
Box 2. Endogenous and xenotropic MLVs.
Integration of the viral DNA into the chromosomal DNA of the host cell is an essential step in the retroviral replication cycle. This DNA (the provirus) is then copied along with the rest of the cell’s DNA and is faithfully transmitted to the daughter cells at cell division. Infection sometimes occurs in cells of the germline of the infected organism, so that the provirus is also transmitted to the organism’s offspring. Such a provirus is referred to as an endogenous virus. The genomes of laboratory mice contain over 100 endogenous MLV genomes67
One of the factors that determines which cells can be infected by a given retrovirus is the interaction of the viral Env protein, which mediates entry into the cell via a receptor on the cell surface. MLV Env proteins are polymorphic and different MLV isolates use different cellular receptors. Some endogenous MLVs give rise to infectious MLVs with a surprising property: although these particles can infect cells of other species, they cannot infect mouse cells. These endogenous MLVs are termed xenotropic MLVs. Evidently, these viruses were able to utilize the cellular receptor when they infected mouse germline cells many generations ago. However, in the intervening years, natural selection has altered the receptor, rendering contemporary mice resistant to these viruses. The majority of endogenous MLV genomes are classified into groups called ‘polytropic’ and ‘modified polytropic’. To our knowledge, polytropic and modified polytropic endogenous MLV genomes do not give rise to infectious MLV
The results of these initial studies were intriguing for several reasons. Firstly, MLVs are not known to infect humans. Secondly, the results suggested that any causal association between XMRV and prostate cancer might occur predominantly in a subset of patients who carry the homozygous QQ variant of RNASEL. Finally, the detection of XMRV in rare stromal cells—as opposed to the cancer cells themselves—indicated that if XMRV does have a causal role in prostate cancer, it is indirect. Such a relationship has not been described before in retroviruses— gammaretroviruses do induce tumors in animals (and in children in genetherapy trials), but do so by insertional mutagenesis, giving rise to tumors in which every cell contains the viral genome (Box 1). Rare exceptions to this mode of transformation are found, but even in these cases the virus is present in all the tumor cells.6 A report by Pandhare-Dash et al.7 raises the possibility that XMRV infection alters the growth regulation of prostate cancer cells, and, therefore, might in theory contribute to malignant transformation. Although there is no precedent for this phenomenon among gammaretroviruses, a mechanism whereby a retrovirus can have such an effect cannot be excluded without further investigation.
Interest in the possible role of XMRV in prostate cancer was stimulated by the appearance of a second positive report in 2009.8 Here, samples from prostate cancer patients and benign controls were analyzed for XMRV using both quantitative PCR (qPCR) and IHC with anti-XMRV antiserum (Table 1). Using qPCR, XMRV was detected in 6% of prostate cancer samples and 2% of controls. The viral DNA was determined to be at a very low level in the tissues (1–10 copies per 660 diploid cells). Remarkably, IHC assays on tissues from the same patient cohort gave much higher frequencies of XMRV incidence (23% in cases and 4% in controls) than PCR. A correlation was found between the Gleason grade of the tumor and the probability of XMRV detection (by either PCR or IHC). Several aspects of the results of this study were quite unexpected. Firstly, XMRV was undetectable by PCR in most of the IHC-positive cases, despite the fact that the PCR techniques could detect 1–2 infected cells or slightly over five copies of XMRV DNA even in the presence of a >10,000-fold excess of uninfected human DNA. Secondly, there were several discrepancies between these results8 and the initial study published by Urisman et al.3 No association was observed between RNASEL genotype and the detection of XMRV in Schlaberg and colleagues’8 study. Furthermore, in contrast to the original report in which XMRV was only detected in rare stromal cells,3 here XMRV protein was detected predominantly in prostate cancer cells. However, the percentage of tumor cells expressing presumptive XMRV antigens was generally low and quite variable.8
Table 1.
XMRV studies in prostate cancer tissue and sera
| Study | XMRV? | Frequency | Methods |
|---|---|---|---|
| Urisman et al.3 | Yes | 8/19 (42%) using Virochip 9/86 (10%) by nested RT-PCR |
Virochip, nested RT-PCR, FISH, IHC |
| Schlaberg et al.8 | Yes | 14/233 (6%) cases, 2/101 (2%) controls by qPCR 54/233 (23%) cases, 4/101 (4%) controls by IHC |
qPCR, IHC |
| Arnold et al.9 | Yes | 11/40 (28%) with neutralizing antibody assay | Neutralizing antibody assay, nested PCR, FISH |
| Danielson et al.10 | Yes | 32/144 (22%) | Nested PCR |
| Sfanos et al.12 | No | 0/200 | Nested PCR |
| Fischer et al.13 | Rare, equal in controls | 1/105 cases (1%), 1/70 (1%) controls | Nested RT-PCR |
| D’Arcy et al.14 | No | 0/9 | RT-PCR |
| Hohn et al.15 | No | 0/589 with nested PCR/RT-PCR, 0/146 using ELISA | Nested PCR, nested RT-PCR, ELISA |
| Sakuma et al.16 | No* | 5/110 cases (5%), 1/40 (2.5%) controls by real-time PCR* 0/159 cases, 0/201 controls by neutralizing antibody assay |
Real-time PCR, IHC, neutralizing antibody assay |
| Switzer et al.17 | Rare/no | 3/162 (2%) with nested PCR 0/162 with Western blot |
Nested PCR, Western blot |
| Aloia et al.18 | No | 0/161 by real-time PCR 0/596 prostate cancer, 0/452 benign tissues by IHC |
Real-time PCR, IHC (2 antisera) |
| Martinez-Fierro et al.19 | Rare (control) | 0/55 cases, 1/75 (1%) controls | Nested PCR |
| Verhaegh et al.20 | Rare | 3/74 (4%) | Real-time PCR |
| Furuta et al.21 | Rare‡/no | 2/67 (3%) with immunoblot;‡ RT-PCR of immunoblot-positive plasma inconclusive‡ | Immunoblot, RT-PCR |
| Stieler et al.22 | Rare/no | 0/110 cases, 1/50 BPH (2%), 0/114 tumor and normal tissue TMA by IHC, 0/93 cases and 0/7 controls by nested PCR |
IHC, nested PCR, cell culture§ |
| Robinson et al.23 | No|| | 2/91 (2%)|| | Nested PCR |
Determined positive samples were due to laboratory contamination and nonspecific antibody binding. Positive PCR products were determined to be endogenous MLV sequences from mouse DNA contamination, not XMRV.
Positive for Gag, negative for Env viral protein, one of the Immunoblot-positive patient’s sera was positive by RT-PCR, however the sequence did not contain the “XMRV-specific” 24 nt deletion, and additional PCR tests were negative.
Cell culture results inconclusive.
Positive PCR products were determined to be due to mouse DNA contamination of samples. Abbreviation: TMA, tissue microarray.
Two additional studies have reported positive results in the detection of XMRV in patients with prostate cancer (Table 1).9,10 Arnold et al.9 reported that sera from a substantial fraction (27.5%) of men with prostate cancer seemed to contain neutralizing antibodies capable of inactivating XMRV. A higher incidence of neutralizing activity was found in patients carrying the homozygous QQ variant of RNASEL (40%) than in patients with either the RQ or RR genotype (15%). Within a subset of these patients, five were positive for XMRV according to FISH, nested PCR and XMRV neutralizing activity, whereas two were negative in all three assays. Although the concordance between the three assays was striking, the number of samples analyzed by all three methods was so small that the implications of the concordance are difficult to interpret. Furthermore, this study reported the detection of XMRV by FISH in rare stromal cells as opposed to malignant epithelium. In another positive study, XMRV was detected in 22% of prostate cancer samples by Danielson et al.10 using nested PCR. Here, positive results for XMRV did not correlate with presence of the homozygous QQ variant of RNASEL and XMRV was detected by nested PCR in DNA from both normal and tumor tissues.
Thus, to date, at least four independent studies have reported the detection of XMRV in prostate cancer patients at varying frequencies depending on the assay used. An apparent discordance exists regarding the association between the detection of XMRV and the QQ variant of RNASEL, as well as the cellular localization of the virus in FISH and IHC assays.
XMRV has also been investigated in nonhuman primates by intravenous injection into rhesus macaques. Interestingly, XMRV was found in the prostate as well as other organs of these infected animals, although no related pathology was observed.11
Negative reports in prostate cancer
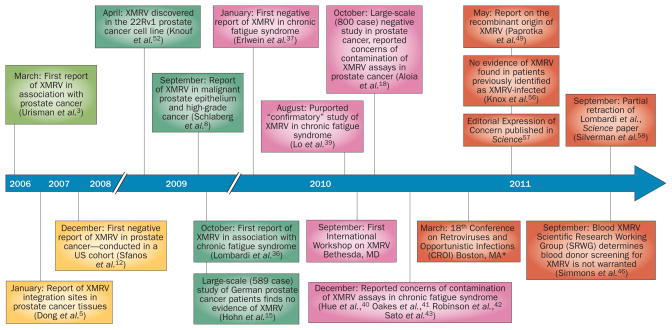
The first follow-up study that attempted to detect XMRV in prostate cancer samples was published online in December 2007 (Figure 1).12 In this study, DNA obtained from benign prostate and prostate cancer tissues from patients in the USA was tested for XMRV using nested PCR against XMRV gag sequences using the same primer sets reported in Urisman and colleagues’3 original paper (Table 1). No samples were found to be positive for XMRV. This particular study did not attempt to genotype the patients for RNASEL, and, therefore, the number of individuals carrying the QQ variant was unknown. However, multiple subsequent studies that included known numbers of homozygous QQ carriers also failed to detect XMRV at any significant frequency (Table 1).13–17 This included a large-scale study (589 cases) conducted in German patients with prostate cancer, which included samples from 76 QQ carriers and found no evidence of XMRV at either the DNA or RNA level in tumor tissue samples.15 Furthermore, sera from patients with prostate cancer were not found to contain XMRV-specific antibodies. 15 A number of additional reports either did not detect XMRV or detected XMRV at extremely low frequencies in prostate cancer samples (Table 1).18–23 As the first large-scale study of QQ carriers was conducted in Germany, reports in the literature have often suggested that there is a discrepancy between US and European studies regarding detection of XMRV in human prostate cancer. However, the first follow-up study—like the original report by Urisman et al.3—was conducted in a cohort from the United States,12 and multiple additional studies conducted in the United States also found very few or no XMRV-positive cases of prostate cancer.16–18 We have surveyed nearly 800 US cases using a combination of quantitative, duplex realtime PCR and well-controlled IHC using two monospecific antisera, each against a different MLV protein. In all cases, positive controls demonstrated the highly sensitive and specific nature of our assays for detecting XMRV, but no signs of XMRV were observed in clinical samples.18
Figure 1.
Timeline of key studies and events in the XMRV controversy. The timeline highlights an important subset of the studies and events involved in the rise and fall of XMRV. Online first publication dates given for articles if available. *A presentation on the finding of the recombinant origin of XMRV was presented by V. Pathak at the CROI conference.64
XMRV in other human disease
Since the initial discovery of XMRV, studies have been conducted to determine if this new virus could contribute to other human disorders, ranging from other malignancies to neurological disorders and immune and autoimmune diseases.24–35 Of particular note, a report of the detection of XMRV in blood cells from patients with chronic fatigue syndrome (CFS) appeared in the journal Science in October 2009.36 This paper reported the detection of both XMRV DNA and proteins in patient samples, and infectious XMRV could be isolated from these samples by exposure of permissive uninfected cells to plasma or peripheral blood mononuclear cells from patients with CFS. Of particular concern, the virus was reported not only in 67% of patients with CFS, but also in nearly 4% of healthy controls. The apparent presence of the virus in the blood of healthy individuals raised the possibility of a new, serious risk to the nation’s blood supply. Similar to the discovery of XMRV in prostate cancer, follow-up studies reporting no detection of XMRV in CFS patients were published, this time almost immediately after the initial report.37,38 Numerous additional negative data have been reported in CFS cohorts worldwide, and such studies continue to be published to date.
One additional report was originally described as confirming the presence of XMRV in CFS patients.39 Using nested PCR, Lo et al.39 amplified MLV sequences from peripheral blood mononuclear cells of CFS patients and healthy controls. In this study, nearly 90% of CFS samples were MLV-positive compared with 7% of the control samples. However, sequencing of the amplified fragments showed that they were not XMRV, but rather MLV sequences that are present in mouse DNA. These ‘polytropic endogenous’ MLVs are resident in the mouse genome as a result of infection of mouse germ cells in the past (Box 2). Furthermore, they contain genetic defects and cannot replicate. They are, therefore, completely distinct from XMRV and their presence strongly suggested the possibility that the samples were contaminated with murine DNA.
Addressing the contradictions
By 2010, the XMRV literature was filled with stark, inescapable contradictions and concerns were being raised regarding false-positive results in assays for XMRV caused by laboratory-based contamination. Detection of XMRV and related viruses in clinical samples often relied on PCR for detection of viral nucleic acids; however, mice contain endogenous viral sequences that can be amplified with “XMRV-specific” primers in PCR-based assays.40 Thus, PCR assays for XMRV are particularly susceptible to false-positive results owing to the presence of the MLV genomes in mouse DNA and the extraordinary sensitivity of the assays. Every mouse cell contains at least 100 MLV genomes. Therefore, a millionth of a microliter of mouse blood contains sequences that can potentially be amplified in “XMRV-specific” PCR. As mice are ubiquitous in biomedical research, it can be very difficult to obtain clinical samples without trace amounts of murine DNA. An important step that helped to clarify the role of contaminating mouse DNA in XMRV assays was the development of PCR assays for murine sequences, including mitochondrial cytochrome oxidase and intracisternal A particle (IAP) sequences.41 As there are approximately 1,000 copies of the IAP retrotransposon in the mouse genome, testing for IAP sequences is an extremely sensitive way of detecting the presence of mouse DNA. When these assays were applied to a series of clinical samples, every sample that had been scored as XMRV-positive was also found to contain IAP sequences.41,42 A number of commercial reagents used in assays for XMRV (such as Taq polymerases, PCR master mixes, RT-PCR kits and extraction columns) have also been found to contain trace amounts of murine nucleic acids and might, therefore, explain positive results.43–45
In some studies, XMRV is detected more frequently in samples from patients with a disease than control subjects. This apparent association of the virus with disease is, at first glance, difficult to reconcile with the idea that the positive results represent contamination. However, it must be considered that the disease samples might be collected or handled differently, or at different times, compared with the controls. As suggested by Weiss,2 it is also possible that disease samples might simply be handled more often than “job-lot” control samples, increasing the opportunities for potential contamination to occur. Given the extraordinary sensitivity of PCR, which can detect single molecules of template, it seems likely that it could even detect airborne nucleic acid molecules in the laboratory.
A very important development in reconciling the contradictions between different laboratories has emerged from a joint study organized as the Blood XMRV Scientific Research Working Group (SRWG).46 This group sent blinded samples (positive control samples spiked with XMRV, negative control samples with no XMRV, and clinical samples from patients previously reported to be positive for XMRV) to nine different laboratories for testing by nested PCR, virus culture and serology. Two laboratories that had collaborated in the original report on XMRV in CFS samples36 were the only laboratories to report detection of XMRV in clinical samples. However, these two laboratories also “detected” XMRV in negative control samples that did not contain the virus, providing direct, unequivocal evidence that the assays used in these laboratories suffer from some artifacts producing positive results in the absence of XMRV.46 Of particular note, in the SRWG study, the Lo et al.39 laboratory found no XMRV in any of five of their previously reported positive samples or in 10 samples previously reported as positive by Lombardi et al.,36 despite their ability to detect XMRV in all five of the spiked positive controls.46
IHC has also been used to detect XMRV in clinical samples. Surprisingly, this method seemed more sensitive than PCR in the work of Schlaberg et al.8 One concern regarding this work is the way in which the anti-XMRV antiserum was generated— whole viral particles produced in human cells were used as the immunogen. However, retrovirus particles contain cellular proteins in addition to viral proteins. 47,48 Thus, this immunization is likely to have generated antibodies against human proteins, as well as against the viral proteins. Stieler et al.22 have reported that the antiserum used by Schlaberg can recognize cellular proteins in noninfected human and mouse cell lines. Furthermore, Sakuma et al.16 compared the staining by an anti-MLV p30/gp70 antibody that can detect XMRV precursor Gag, CA, and Env proteins to that by the anti-XMRV antiserum used by Schlaberg and colleagues8 in IHC assays on prostate cancer tissues. Both antibodies reproducibly stained 293T cells transfected with an XMRV clone in positive control assays. However, only the anti-XMRV antiserum used by Schlaberg et al.8 stained areas of prostate tumor epithelium, whereas the anti-MLV p30/gp70 antibody did not show positive staining in any of the tissues. Sakuma et al.16 concluded that “we cannot detect XMRV in prostate cancer tissues and that the antibody described by Schlaberg, Singh and colleagues recognizes nonviral proteins in addition to XMRV”.16 In our own studies, we received prostate cancer tissue sections (kindly provided by Dr Ila Singh, University of Utah) from a number of the same patients tested by Schlaberg et al.8 The samples were predicted to be XMRV-positive based on the previous IHC results with the anti-XMRV antiserum.8 However, the sections did not stain with two different broadly reactive MLV antisera that had previously detected XMRV in positive control assays.18 Taken together, the evidence suggests that it is highly unlikely that the IHC staining observed by Schlaberg et al.8 represents the true presence of XMRV.
Finally, one surprising feature of all XMRV sequences reported in clinical samples is their uniformity. Five sequences purportedly isolated from patient samples were found to be, on average, >99.9% identical to each other at the nucleotide level.49 This near-identity of XMRV sequences is difficult to reconcile with the error-prone nature of retroviral replication. In fact, different HIV-1 genomes isolated from a single infected individual show far more divergence than is seen in the entire set of XMRV sequences published to date. However, it should be noted that the sequence diversity of different isolates of human T-cell leukemia virus type 1 (HTLV) (a member of the deltaretrovirus genus, distinct from both MLVs and lentiretroviruses such as HIV-1) is also far lower than that of HIV-1, although not as low as that in XMRV.50 As sequence diversity is generated during the viral replication cycle, it is likely that there is much less ongoing viral replication in an HTLV-infected individual than in an individual infected with HIV-1.51 The level of replication in an MLV-infected, viremic mouse is not known.
A recombinant origin of XMRV
Many mistaken claims of viral involvement in cancer have taken years to resolve. By contrast, the status of XMRV was clarified virtually overnight by a single study.49 The establishment of prostate cancer cell lines is somewhat unique in that it often requires long-term passage through mice as xenografts. One cell line developed in this way, CWR22Rv1 (also known as 22Rv1), was reported in 2009 to produce high levels of XMRV.52 This finding was consistent with a role for XMRV in prostate cancer, as it was possible that the cultured cells produced XMRV because they originated from an XMRV-infected tumor. On the other hand, the cells might have become infected during passage, as human cells are permissive for xenotropic MLVs, and it has been known for decades that they can be infected with these viruses when they are passaged in mice.53,54
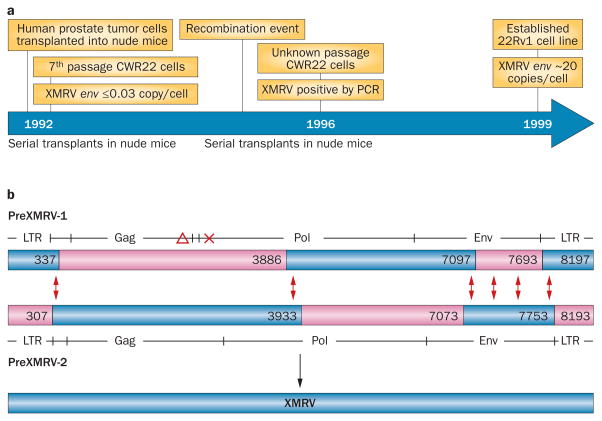
The CWR22 xenograft was serially transplanted (passaged) through mice for at least 7 years before the CWR22Rv1 cell line was established in culture. To determine whether XMRV was present in the original tumor or appeared during passage, Paprotka et al.49 performed PCR using primers that would detect XMRV (or viruses with similar sequences in their env coding regions) on samples taken from several intermediate points during the serial passage of the CWR22 tumor cells in the nude mice.49 They found that, at least for the first seven passages, the tumor cells contained no more than three copies of the XMRV-related sequence per 100 cells. This low level, which remained constant for several passages, probably represents the contamination of the xenograft samples with the surrounding mouse tissue. By contrast, established CWR22Rv1 cells contain ~2,000 copies per 100 cells,49,52 strongly suggesting that XMRV was not present in the original tumor, but arose during the passage of the tumor cells in the mice (Figure 2a).
Figure 2.
The recombinant origin of XMRV. a | Timeline of XMRV appearance in samples collected during the preparation of the 22Rv1 cell line. CWR22 cells from early nude mouse transplants do not contain XMRV, confirming that the human prostate tumor cells used to prepare the 22Rv1 cell line did not contain XMRV. After serial transplants of CWR22 cells in nude mice, XMRV was first detected in samples from a 1996 preparation of cells, suggesting that XMRV had been acquired during serial transplantation of the cells in nude mice. Nude mice do not harbor XMRV and, therefore, a recombination event between endogenous MLVs from the nude mouse genome is the predicted source of XMRV. b | Recombination between two endogenous MLVs—PreXMRV-1 and PreXMRV-2—in nude mice generates XMRV. PreXMRV-1 contains a deletion (red triangle) in Gag and a +1 frameshift (red cross) in Pol, rendering it replication-defective. PreXMRV-2 contains complete open reading frames for gag, pol and env. PreXMRV-1 and PreXMRV-2 regions of ≥99% sequence similarity to XMRV are shown in blue, and the predicted template switching events that generate XMRV from PreXMRV-1 and PreXMRV-2 are shown by the red double arrows. Numbers indicate the nucleotide positions flanking these switching events. The GenBank Accession numbers for the sequences denoted for PreXMRV-1 and PreXMRV-2 are FR871849 and FR871850, respectively. Abbreviation: LTR, long terminal repeat (found at ends of retroviral DNA copies).
Paprotka et al.49 then cloned and sequenced an MLV genome from the early xenografts and from the nude mice used to establish the cell line. This genome, which they termed PreXMRV-1, is a ~8.3 kb, replication-defective, endogenous MLV genome containing a deletion and a +1 frameshift in its coding regions that render it unable to direct the production of infectious progeny viruses (Figure 2b). Of greatest interest, a 3.2 kb stretch of the PreXMRV-1 genome is almost completely identical in sequence to XMRV, including portions of the coding regions for the reverse transcriptase and integrase enzymes and part of the Env protein, which is involved in the entry of MLV particles into susceptible host cells. Stretches at both ends of PreXMRV-1 are also virtually identical to XMRV, although the remainder of PreXMRV-1 has only ~90% identity to XMRV.
Using a similar strategy, Paprotka et al.49 isolated a second endogenous MLV genome from the host mice, which they named PreXMRV-2. This virus has the exactly complementary relationship to XMRV as PreXMRV-1 (Figure 2b)—it is essentially identical in sequence to XMRV in regions where PreXMRV-1 and XMRV diverge, but only ~90% identical where PreXMRV-1 and XMRV are the same. Thus, the XMRV sequence can be derived by a combination of the PreXMRV-1 and PreXMRV-2 sequences.
Retroviruses frequently undergo genetic recombination. Every virus particle contains two genomic RNA molecules, and reverse transcriptase can ‘jump’ between them while copying the RNA into DNA during infection of a new host cell. A detailed analysis showed that the entire XMRV genome can be accounted for by six such jumps between PreXMRV-1 and PreXMRV-2 RNA. This level of recombination is well within the normal range, as the average number of jumps per replication is about four55 (Figure 2b). Thus, XMRV seems to have arisen in a prostate cancer cell by recombination between two endogenous MLVs. Recombination occurred after the cell was infected with an MLV particle that was produced in a nude mouse and contained the two genomic RNAs. It is crucial to understand that this is an extremely lowprobability event. In other words, the odds of generating XMRV more than once are vanishingly small. Firstly, the two parental MLVs are apparently not present in most mice, but are both found in the nude mice used to passage the CWR22 tumor. Secondly, they could conceivably recombine at many sites, generating genomes encoding the same amino acid sequences as XMRV but differing from it in nucleotide sequence. The fact that all XMRV sequences are practically identical at the nucleotide level indicates that they must have been derived from a single recombination event.
The “final” word on XMRV
A manuscript that cast extreme doubt on the plausibility of XMRV having a role in CFS was published alongside the Paprotka et al.49 study describing the recombinant origin of XMRV. In this report, Knox et al.56 analyzed blood samples from the patients that had previously been described as XMRV-positive in the initial Lombardi et al.36 study, and found no evidence of XMRV or other MLVs in these samples.56 Both the Paprotka et al.49 and the Knox et al.56 studies, which were published in Science, were accompanied by an Editorial Expression of Concern regarding the original Lombardi et al.36 report.57 Furthermore, a partial retraction of the Lombardi et al.36 manuscript, indicating that contamination from XMRV plasmid DNA might have been responsible for positive PCR results obtained from CFS samples, has been published. 58 In essence, all PCR data from the initial manuscript were retracted.
As previously noted, one strong indication that human cells in prostate cancer samples are infected with XMRV was the isolation of junction fragments of DNA in which XMRV sequences were joined to human sequences.5 However, two studies have since shown that even these observations are attributable to contamination as opposed to true infection.59,60 The same laboratory that reported on the junction fragments had previously introduced XMRV into cultured DU145 prostate cancer cells and had isolated junction fragments from these infected cultures. It was noted that in two cases, the integration sites were identical between the patient and DU145 samples.59 The probability of finding two integration events that independently occurred at the same location within the 3.5 billion bases of the human genome is extremely low. Thus, the authors concluded that the “patient-derived” integration sites must reflect PCR contamination. Another study confirms this conclusion— single nucleotide polymorphism analysis of patient integration sites showed that the XMRV integration sites did not originate from the patient samples but from XMRVinfected cell lines.60 Additional studies arguing against the possibility that XMRV is a human pathogen include evidence that XMRV replication is blocked by several antiviral restriction factors that are present in human cells, including APOBEC3 proteins and tetherin,61–63 and evidence that XMRV can be inactivated by sera from patients with CFS and healthy controls.56
Conclusions
XMRV arose in the laboratory as the result of a rare recombination event. We believe that all of the reported PCR-based evidence of XMRV in clinical specimens can be explained by contamination of the assays with mouse DNA, XMRV from cultured cells (CWR22Rv1 cells or cells infected by XMRV from CWR22Rv1 cells), or DNA from infected cells or XMRV plasmids. Positive IHC data probably reflect the lack of specificity of the antiserum used in these assays. There is no reason to believe that XMRV actively circulates in humans or is present in the environment outside the laboratory.
Acknowledgments
The authors would like to acknowledge Dr Saraswati Sukumar for helpful discussions. Research by A. Aloia and A. Rein is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors researched data for the article, made substantial contributions to discussions of content, wrote the article and reviewed and edited the manuscript before submission.
Contributor Information
Karen S. Sfanos, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD 21231, USA
Amanda L. Aloia, HIV Drug Resistance Program, National Cancer Institute, Building 535, Room 211, 1050 Boyles Street, Frederick, MD 21702-1201, USA
Angelo M. De Marzo, The Brady Urological Research Institute and The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21231, USA
Alan Rein, HIV Drug Resistance Program, National Cancer Institute, Building 535, Room 211, 1050 Boyles Street, Frederick, MD 21702-1201, USA.
References
- 1.Voisset C, Weiss RA, Griffiths DJ. Human RNA “rumor” viruses: the search for novel human retroviruses in chronic disease. Microbiol Mol Biol Rev. 2008;72:157–196. doi: 10.1128/MMBR.00033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss R. A cautionary tale of virus and disease. BMC Biol. 2010;8:124. doi: 10.1186/1741-7007-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urisman A, et al. Identification of a novel Gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2:e25. doi: 10.1371/journal.ppat.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Carpten J, et al. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat Genet. 2002;30:181–184. doi: 10.1038/ng823. [DOI] [PubMed] [Google Scholar]
- 5.Dong B, et al. An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc Natl Acad Sci USA. 2007;104:1655–1660. doi: 10.1073/pnas.0610291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruscetti SK. Deregulation of erythropoiesis by the Friend spleen focus-forming virus. Int J Biochem Cell Biol. 1999;31:1089–1109. doi: 10.1016/s1357-2725(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 7.Pandhare-Dash J, Mantri CK, Gong Y, Chen Z, Dash C. XMRV accelerates cellular proliferation, transformational activity, and invasiveness of prostate cancer cells by downregulating p27Kip1. Prostate. doi: 10.1002/pros.21491.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci USA. 2009;106:16351–16356. doi: 10.1073/pnas.0906922106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Arnold RS, et al. XMRV infection in patients with prostate cancer: novel serologic assay and correlation with PCR and FISH. Urology. 2010;75:755–761. doi: 10.1016/j.urology.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Danielson BP, Ayala GE, Kimata JT. Detection of xenotropic murine leukemia virus-related virus in normal and tumor tissue of patients from the Southern United States with prostate cancer is dependent on specific polymerase chain reaction conditions. J Infect Dis. 2010;202:1470–1477. doi: 10.1086/656146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onlamoon N, et al. Infection, viral dissemination, and antibody responses of rhesus macaques exposed to the human gammaretrovirus XMRV. J Virol. 2011;85:4547–4557. doi: 10.1128/JVI.02411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sfanos KS, et al. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate. 2008;68:306–320. doi: 10.1002/pros.20680. [DOI] [PubMed] [Google Scholar]
- 13.Fischer N, et al. Prevalence of human gammaretrovirus XMRV in sporadic prostate cancer. J Clin Virol. 2008;43:277–283. doi: 10.1016/j.jcv.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 14.D’Arcy F, et al. No evidence of XMRV in Irish prostate cancer patients with the R462Q mutation. Eur Urol Suppl. 2008;7:271. [Google Scholar]
- 15.Hohn O, et al. Lack of evidence for xenotropic murine leukemia virus-related virus (XMRV) in German prostate cancer patients. Retrovirology. 2009;6:92. doi: 10.1186/1742-4690-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakuma T, et al. No evidence of XMRV in prostate cancer cohorts in the Midwestern United States. Retrovirology. 2011;8:23. doi: 10.1186/1742-4690-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Switzer WM, Jia H, Zheng H, Tang S, Heneine W. No association of xenotropic murine leukemia virus-related viruses with prostate cancer. PLoS ONE. 2011;6:e19065. doi: 10.1371/journal.pone.0019065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aloia AL, et al. XMRV: a new virus in prostate cancer? Cancer Res. 2010;70:10028–10033. doi: 10.1158/0008-5472.CAN-10-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Fierro ML, et al. Identification of viral infections in the prostate and evaluation of their association with cancer. BMC Cancer. 2010;10:326. doi: 10.1186/1471-2407-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhaegh GW, et al. Prevalence of human xenotropic murine leukemia virus-related gammaretrovirus (XMRV) in Dutch prostate cancer patients. Prostate. 2011;71:415–420. doi: 10.1002/pros.21255. [DOI] [PubMed] [Google Scholar]
- 21.Furuta R, et al. No association of xenotropic murine leukemia virus-related virus with prostate cancer or chronic fatigue syndrome in Japan. Retrovirology. 2011;8:20. doi: 10.1186/1742-4690-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stieler K, et al. No detection of XMRV in blood samples and tissue sections from prostate cancer patients in Northern Europe. PLoS ONE. 2011;6:e25592. doi: 10.1371/journal.pone.0025592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson MJ, et al. No evidence of XMRV or MuLV sequences in prostate cancer, diffuse large B-Cell lymphoma, or the UK blood donor population. Adv Virol. doi: 10.1155/2012/782353.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waugh EM, et al. The retrovirus XMRV is not directly involved in the pathogenesis of common types of lymphoid malignancy. Cancer Epidemiol Biomarkers Prev. 2011;20:2232–2236. doi: 10.1158/1055-9965.EPI-11-0561. [DOI] [PubMed] [Google Scholar]
- 25.Balada E, Castro-Marrero J, Felip L, Vilardell-Tarres M, Ordi-Ros J. Xenotropic murine leukemia virus-related virus (XMRV) in patients with systemic lupus erythematosus. J Clin Immunol. 2011;31:584–587. doi: 10.1007/s10875-011-9535-5. [DOI] [PubMed] [Google Scholar]
- 26.Lintas C, et al. Lack of infection with XMRV or other MLV-related viruses in blood, post-mortem brains and paternal gametes of autistic individuals. PLoS ONE. 2011;6:e16609. doi: 10.1371/journal.pone.0016609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray ER, et al. No evidence of XMRV or related retroviruses in a London HIV-1-positive patient cohort. PLoS ONE. 2011;6:e18096. doi: 10.1371/journal.pone.0018096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes E, et al. Failure to detect xenotropic murine leukemia virus-related virus in blood of individuals at high risk of blood-borne viral infections. J Infect Dis. 2011;202:1482–1485. doi: 10.1086/657167. [DOI] [PubMed] [Google Scholar]
- 29.Tang S, et al. Absence of detectable xenotropic murine leukemia virus-related virus in plasma or peripheral blood mononuclear cells of human immunodeficiency virus Type 1-infected blood donors or individuals in Africa. Transfusion. 2011;51:463–468. doi: 10.1111/j.1537-2995.2010.02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggi F, et al. Xenotropic murine leukaemia virus-related virus is not found in peripheral blood cells from treatment-naive human immunodeficiency virus-positive patients. Clin Microbiol Infect. doi: 10.1111/j.1469-0691.2011.03580.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt M, Hofler D, Koleganova N, Pawlita M. Human polyomaviruses and other human viruses in neuroendocrine tumors. Cancer Epidemiol Biomarkers Prev. 2011;20:1558–1561. doi: 10.1158/1055-9965.EPI-11-0424. [DOI] [PubMed] [Google Scholar]
- 32.Cornelissen M, et al. Lack of detection of XMRV in seminal plasma from HIV-1 infected men in The Netherlands. PLoS ONE. 2010;5:e12040. doi: 10.1371/journal.pone.0012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeziorski E, et al. No evidence for XMRV association in pediatric idiopathic diseases in France. Retrovirology. 2010;7:63. doi: 10.1186/1742-4690-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterfield BC, Garcia RA, Gurrieri F, Schwartz CE. PCR and serology find no association between xenotropic murine leukemia virus-related virus (XMRV) and autism. Mol Autism. 2010;1:14. doi: 10.1186/2040-2392-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick AL, Brown RH, Cudkowicz ME, Al-Chalabi A, Garson JA. Quantification of reverse transcriptase in ALS and elimination of a novel retroviral candidate. Neurology. 2008;70:278–283. doi: 10.1212/01.wnl.0000297552.13219.b4. [DOI] [PubMed] [Google Scholar]
- 36.Lombardi VC, et al. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326:585–589. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- 37.Erlwein O, et al. Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS ONE. 2011;5:e8519. doi: 10.1371/journal.pone.0008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groom H, et al. Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology. 2010;7:10. doi: 10.1186/1742-4690-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo SC, et al. Detection of MLV-related virus gene sequences in blood of patients with chronic fatigue syndrome and healthy blood donors. Proc Natl Acad Sci USA. 2010;107:15874–15879. doi: 10.1073/pnas.1006901107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Hue S, et al. Disease-associated XMRV sequences are consistent with laboratory contamination. Retrovirology. 2010;7:111. doi: 10.1186/1742-4690-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakes B, et al. Contamination of human DNA samples with mouse DNA can lead to false detection of XMRV-like sequences. Retrovirology. 2010;7:109. doi: 10.1186/1742-4690-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson MJ, et al. Mouse DNA contamination in human tissue tested for XMRV. Retrovirology. 2010;7:108. doi: 10.1186/1742-4690-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato E, Furuta RA, Miyazawa T. An endogenous murine leukemia viral genome contaminant in a commercial RT-PCR kit is amplified using standard primers for XMRV. Retrovirology. 2010;7:110. doi: 10.1186/1742-4690-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuke PW, Tettmar KI, Tamuri A, Stoye JP, Tedder RS. PCR master mixes harbour murine DNA sequences. Caveat emptor! PLoS ONE. 2011;6:e19953. doi: 10.1371/journal.pone.0019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erlwein O, et al. DNA extraction columns contaminated with murine sequences. PLoS ONE. 2011;6:e23484. doi: 10.1371/journal.pone.0023484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmons G, et al. Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: A multi-laboratory study. Science. 2011;334:814–817. doi: 10.1126/science.1213841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bubbers JE, Lilly F. Selective incorporation of H-2 antigenic determinants into Friend virus particles. Nature. 1977;266:458–459. doi: 10.1038/266458a0. [DOI] [PubMed] [Google Scholar]
- 48.Ott DE. Cellular proteins detected in HIV-1. Rev Med Virol. 2008;18:159–175. doi: 10.1002/rmv.570. [DOI] [PubMed] [Google Scholar]
- 49.Paprotka T, et al. Recombinant origin of the retrovirus XMRV. Science. 2011;333:97–101. doi: 10.1126/science.1205292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paine E, Garcia J, Philpott TC, Shaw G, Ratner L. Limited sequence variation in human T-lymphotropic virus type 1 isolates from North American and African patients. Virology. 1991;182:111–123. doi: 10.1016/0042-6822(91)90654-t. [DOI] [PubMed] [Google Scholar]
- 51.Overbaugh J, Bangham CR. Selection forces and constraints on retroviral sequence variation. Science. 2001;292:1106–1109. doi: 10.1126/science.1059128. [DOI] [PubMed] [Google Scholar]
- 52.Knouf EC, et al. Multiple integrated copies and high-level production of the human retrovirus XMRV (xenotropic murine leukemia virus-related virus) from 22Rv1 prostate carcinoma cells. J Virol. 2009;83:7353–7356. doi: 10.1128/JVI.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Todaro GJ, Arnstein P, Parks WP, Lennette EH, Huebner RJ. A Type-C virus in human rhabdomyosarcoma cells after inoculation into NIH swiss mice treated with antithymocyte serum. Proc Natl Acad Sci USA. 1973;70:859–862. doi: 10.1073/pnas.70.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sfanos KS, et al. Identification of replication competent murine gammaretroviruses in commonly used prostate cancer cell lines. PLoS ONE. 2011;6:e20874. doi: 10.1371/journal.pone.0020874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhuang J, Mukherjee S, Ron Y, Dougherty JP. High rate of genetic recombination in murine leukemia virus: Implications for influencing proviral ploidy. J Virol. 2006;80:6706–6711. doi: 10.1128/JVI.00273-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knox K, et al. No evidence of murine-like gammaretroviruses in CFS patients previously identified as XMRV-infected. Science. 2011;333:94–97. doi: 10.1126/science.1204963. [DOI] [PubMed] [Google Scholar]
- 57.Alberts B. Editorial expression of concern. Science. 2011;333:35. doi: 10.1126/science.1208542. [DOI] [PubMed] [Google Scholar]
- 58.Silverman RH, et al. Partial retraction. Science. 2011;334:176. doi: 10.1126/science.1212182. [DOI] [PubMed] [Google Scholar]
- 59.Garson JA, Kellam P, Towers GJ. Analysis of XMRV integration sites from human prostate cancer tissues suggests PCR contamination rather than genuine human infection. Retrovirology. 2011;8:13. doi: 10.1186/1742-4690-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rusmevichientong A, Das Gupta J, Elias PS, Silverman RH, Chow SA. Analysis of single nucleotide polymorphisms in XMRV patient-derived integration sites reveals contamination from cell lines acutely infected by XMRV. J Virol. 2011;85:12830–12834. doi: 10.1128/JVI.05624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groom HCT, Yap MW, Galao RP, Neil SJD, Bishop KN. Susceptibility of xenotropic murine leukemia virus-related virus (XMRV) to retroviral restriction factors. Proc Natl Acad Sci USA. 2010;107:5166–5171. doi: 10.1073/pnas.0913650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stieler K, Fischer N. Apobec 3G efficiently reduces infectivity of the human exogenous gammaretrovirus XMRV. PLoS ONE. 2010;5:e11738. doi: 10.1371/journal.pone.0011738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paprotka T, et al. Inhibition of xenotropic murine leukemia virus-related virus by APOBEC3 proteins and antiviral drugs. J Virol. 2010;84:5719–5729. doi: 10.1128/JVI.00134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen J. More negative data for link between mouse virus and human disease. Science. 2011;331:1253–1254. doi: 10.1126/science.331.6022.1253. [DOI] [PubMed] [Google Scholar]
- 65.Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor Laboratory Press; Plainview: 1997. [PubMed] [Google Scholar]
- 66.Rein A. Murine leukemia viruses: objects and organisms. Adv Virol. doi: 10.1155/2011/403419.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cingöz O, Coffin JM. Endogenous murine leukemia viruses: relationship to XMRV and related sequences detected in human DNA samples. Adv Virol. doi: 10.1155/2011/940210. [DOI] [PMC free article] [PubMed] [Google Scholar]