Abstract
Background
Peyronie’s disease (PD) is a connective tissue disorder of the tunica albuginea (TA). Currently, no gold standard has been developed for the treatment of the disease in its active phase.
Objective
To test the effects of a local injection of adipose tissue–derived stem cells (ADSCs) in the active phase of a rat model of PD on the subsequent development of fibrosis and elastosis of the TA and underlying erectile tissue.
Design, setting, and participants
A total of 27 male 12-wk-old Sprague-Dawley rats were divided in three equal groups and underwent injection of vehicle (sham), 50-µg transforming growth factor (TGF)-β1 in a 50-µl vehicle in either a PD or a PD plus ADSC group in the dorsal aspect of the TA.
Intervention
The sham and PD groups were treated 1 d after TGF-β1 injection with intralesional treatment of vehicle, and the PD plus ADSC group received 1 million human-labeled ADSCs in the 50-µl vehicle. Five weeks after treatment, six rats per group underwent erectile function measurement. Following euthanasia, penises were harvested for histology and Western blot.
Outcome measurements and statistical analysis
The ratio of intracavernous pressure to mean arterial pressure (ICP/MAP) upon cavernous nerve stimulation, elastin, and collagen III protein expression and histomorphometric analysis of the penis. Statistical analysis was performed by analysis of variance followed by the Tukey-Kramer test for post hoc comparisons or the Mann-Whitney test when applicable.
Results and limitations
Erectile function significantly improved after ADSC treatment (ICP/MAP 0.37 in PD vs 0.59 in PD plus ADSC at 5-V stimulation; p = 0.03). PD animals developed areas of fibrosis and elastosis with a significant upregulation of collagen III and elastin protein expression. These fibrotic changes were prevented by ADSC treatment.
Conclusions
This study is the first to test stem cell therapy in an animal model of PD. Injection of ADSCs into the TA during the active phase of PD prevents the formation of fibrosis and elastosis in the TA and corpus cavernosum.
Keywords: Antifibrotic, Collagen III, Elastin, Immunohistochemistry, Mesenchymal stromal cells, Polymerase chain reaction, Stem cells, Tunica albuginea, Transforming growth factor beta, Western blot
1. Introduction
The tunica albuginea (TA) is composed mainly of collagen bundles and elastic fibers and encloses the corpora cavernosa of the penis. The elasticity and tensile strength of the TA is essential to achieve full rigidity during erection [1]. Peyronie’s disease (PD) is a localized connective tissue disorder characterized by changes in the collagen and elastin metabolism and composition of the TA and the underlying erectile tissue in the corpora cavernosa [2]. The development of PD consists of an active and a chronic phase. Impaired clearance of the inflammation during the active phase causes aberrant wound healing, which in the chronic phase of PD results in collagen-elastin deposits and calcifications. This may result in a permanent curvature or indurated plaque leading to intercourse-related pain and in some cases severe erectile dysfunction [3]. Taken together with the impact on self-perceived body image, the disease has a profound impact on relationships and the quality of life of patients [4].
Although various surgical options are currently being explored for the treatment of PD in the chronic phase, various attempts to diminish plaque and subsequent curvature formation during the active phase have been made to prevent the need for surgical correction of the disorder. Intralesional (verapamil, collagenase, interferon, steroids), topical (shock wave therapy, iontophoresis, traction devices), or systemic (pentoxifylline, colchicine, vitamin E) treatments have shown limited or variable efficacy, and no standard conservative treatment has been established [3].
Regenerative medicine is a novel branch of medicine, which in the field of PD has only been applied in the form of tissue-engineered neo-TA [5,6]. Preclinical results have been promising, but so far laboratory-fabricated live tissues may only serve as graft replacements for diseased tunica in the advanced stage of the disease. No information is available for PD, but based on findings on other fibrotic diseases, mesenchymal stem cell (MSC) therapy may be a promising approach to limit fibrosis formation in its early stages [7–14]. Among MSCs, adipose tissue-derived stem cells (ADSCs) provide many advantages: ease of harvesting, abundance of stem cells and source tissue, and low processing costs because extensive culturing is not needed to obtain sufficient numbers of stem cells for treatment [15]. In the current study, we investigated the effects of an early local injection of ADSCs on plaque formation and erectile function in a validated rat model for PD [16].
2. Materials and methods
2.1. Ethical approval
All experiments on animals and human tissues were approved by the ethics committees of San Raffaele University, Milan, Italy, and University Hospitals, Leuven, Belgium. Informed consent for adipose tissue processing was obtained.
2.2. Study design
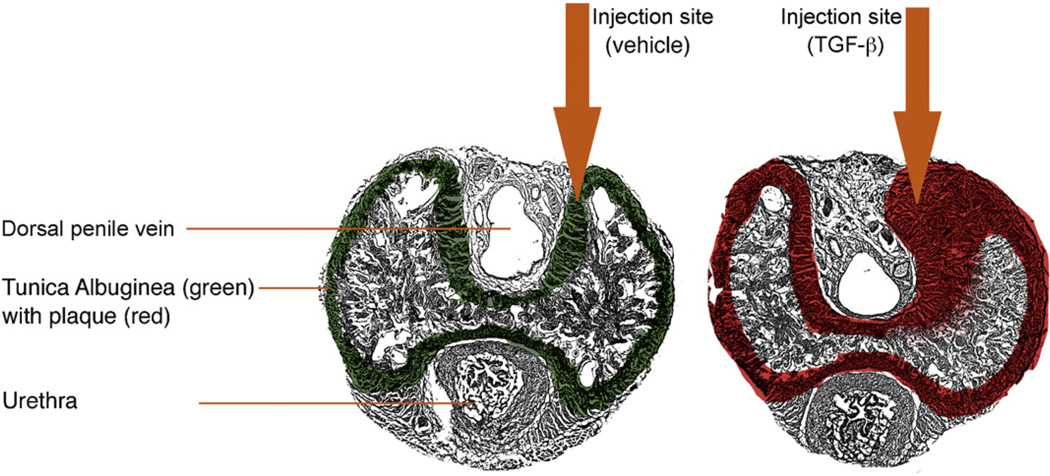
A total of 27 male Sprague-Dawley rats (12 wk old; 300–350 g) were obtained from Charles River Laboratories (Wilmington, MA, USA), and they were randomly divided into three equal groups. The sham group (n = 9) underwent injection of 50-µl vehicle (citrate buffer) in the right midshaft dorsomedial TA with a microliter syringe after opening the buck fascia (Fig. 1). The remaining 18 animals were injected with recombinant 50 µg of transforming growth factor (TGF)-β1 in 50-µl vehicle. After 1 d, rats received a second injection with either phosphate-buffered saline (PBS; sham and PD group) or 1 million human-labeled ADSCs in PBS (PD plus ADSC group). Injections sites were marked by a 5-0 suture placed on the contralateral side. Five weeks after treatment, six rats per group underwent erectile function evaluation after which the animals were killed and the penises harvested for histology. In the remaining three animals per group, the penises were directly harvested during anesthesia and snap frozen for protein extraction.
Fig. 1.
Establishment of the model. Schematic figure depicts the anatomy of a transversally transected penile midshaft specimen and injection site of 50-µl vehicle or 50-µg transforming growth factor (TGF)-β1 in 50-µl vehicle in the dorsal-dorsomedial tunica albuginea resulting in thickening of the tunica albuginea and plaque formation (red) after TGF-β1 injection.
2.3. Adipose tissue-derived stem cells isolation
Subcutaneous human adipose tissue was harvested from a female adult. ADSCs were isolated as previously described [17]. Cells were cultured up until passage 5 when cells were used for treatment. Before harvesting, cells were labeled with the thymidine analog 5-ethynyl-2-deoxyuridine (EdU; Invitrogen, Carlsbad, CA, USA) for 48 h. The cells were characterized by flow cytometry and tested for multiple lineage differentiation as required by the International Society for Cellular Therapy [18].
2.4. Erectile function measurement
Intracavernous pressure (ICP) response to electrostimulation of the cavernous nerve (CN) was used to evaluate erectile function [17]. Briefly, under ketamine (100 mg/kg) and midazolam (5 mg/kg) anesthesia, the right CN was exposed and the right crus of the corpus cavernosum was identified and cannulated with a heparinized (200 U/ml) 25-G needle connected to a pressure transducer. The CN was activated (2.5, 5, and 7.5 V) by platinum electrodes connected to a stimulator at 20 Hz for 50 s. The nerve was stimulated once per voltage, and a resting period of 3 min was allowed for nerve recovery between stimulations. Mean arterial pressure (MAP) was recorded by carotid artery cannulation.
2.5. Western blot
Buck fascia was stripped from the TA and the urethra, and the dorsal neurovascular bundle was removed. The corpus cavernosum was then divided into two halves at the midline. The noninjected side of the TA and corpus cavernosum served as an internal control. Primary antibodies were Rb Anti-Collagen III (1:1000; Abcam Inc., Cambridge, MA, USA), Ms Anti-Elastin (1:000, Abcam) and Rb controls against glyceraldehyde-3-phosphate dehydrogenase (GADPH) (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-Tubulin (1:1000, Santa Cruz). Blotting was performed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After incubation with secondary antibodies and detection reagent, immunoreactivebands were visualizedusing aKodak chemiluminescence detector. Densitometric analysis of the bands was performed using ImageJ (National Institutes of Health, Bethesda, MD, USA).
2.6. Histology
The penile midshaft at the level of the injection site was harvested, fixed, and further processed for immunofluorescence as previously described [16]. Primary antibodies were rabbit Anti-Collagen III (1:100, Abcam), rabbit Anti-Collagen I (1:100, Abcam), mouse anti-α smooth muscle actin (1:500, Sigma, St. Louis, MO, USA), and mouse Anti-Elastin (1:200, Abcam). The secondary antibodies used were anti-MS Alexa-488 and anti-Rb Alexa-594 conjugated antibodies (1:500; Invitrogen). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen). Hematoxylin and eosin (H and E) staining procedures were performed according to a standard protocol.
2.7. Statistical analysis
The results were analyzed using Prism v.4 (GraphPad Software, San Diego, CA, USA) and expressed as mean plus or minus standard error of the mean. Multiple groups were compared using one-way analysis of variance followed by the Student-Newman-Keuls test for post hoc comparisons. Statistical significance was set at p < 0.05.
3. Results
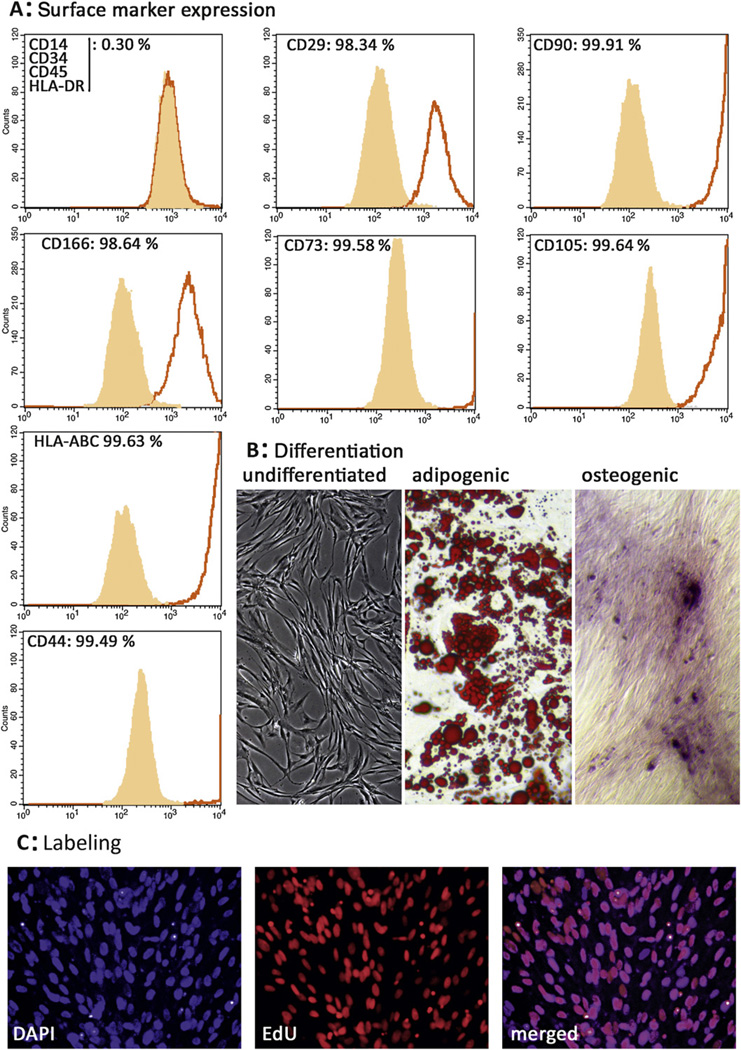
3.1. Characterization of adipose tissue-derived stem cells
ADSCs were successfully isolated from the harvested adipose tissue of the donor and formed a confluent monolayer in 8 d. Cells were strongly positive for MSC markers (Fig. 2A) [18]. In the initial passages, low positivity for CD34 was detected, whereas cells were negative for CD14, CD34, CD45, and HLA-DR after passage 3. Osteogenic differentiation was confirmed by alizarin red staining, and adipogenic differentiation was demonstrated by positive oil red O staining (Fig. 2B). EdU incubation labeled all cells (Fig. 2C).
Fig. 2.
Characterization, differentiation, and labeling efficacy of adipose tissue-derived stem cells (ADSCs). (A) Flow cytometry analysis on ADSCs isolated from a female human donor shows that the vast majority of cells possess a CD14−, CD34−, CD45−, HLA-DR−, and CD29+, CD90+, CD166+, CD73+, CD105+, CD44+, HLA-ABC+ phenotype, consistent with the typical mesenchymal multipotent stromal cell surface marker profile (light gray area: isotype control; black line: sample). (B) Cultured human ADSCs display a typical fibroblast-like morphology and are able to differentiate between adipogenic and osteogenic lineages in vitro, as demonstrated by positive oil red O staining for lipid droplet formation and alizarin red staining for calcium deposit formation. (C) ADSCs were incubated with the thymidine analog 5-ethynyl-2-deoxyuridine (EdU) for 48 h, resulting in complete labeling of attached ADSCs.
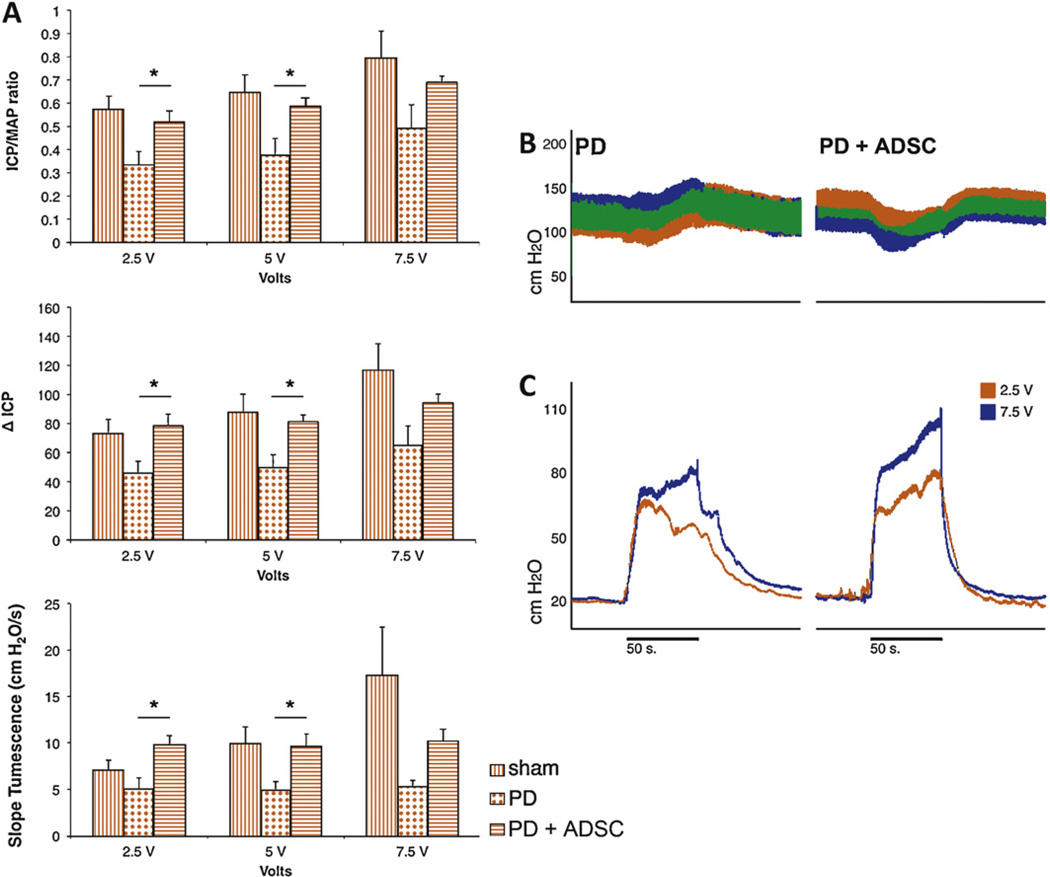
3.2. Erectile function
Rats injected with TGF-β1 displayed a diminished response to cavernous nerve electrostimulation (ΔICP: 49.3 cm H2O in PD vs 87.6 cm H2O in sham at 5-V stimulation; p = 0.03; ICP/MAP: 0.37 vs 0.65, respectively; p = 0.02; tumescence slopes: 4.9 cm H2O/s vs 9.8 cm H2O/s, respectively; p = 0.05). Treatment with ADSCs improved erectile function as displayed by increased ΔICP (49.3 cm H2O in PD vs 81.3 cm H2O in PD plus ADSC at 5-V stimulation; p = 0.01), ICP/MAP (0.37 vs 0.59, respectively; p = 0.03) and tumescence slopes (4.9 cm H2O/s vs 9.6 cm H2O/s, respectively; p = 0.01). Figure 3 shows the values for all voltage stimulations and representative ICP/MAP traces.
Fig. 3.
Erectile function measurement. (A) Summarized data comparing erectile function measurements in sham (black bars) PD rats (blocked bars) and rats treated with adipose tissue-derived stem cells (ADSCs) (open bars) at various voltages during cavernous nerve electrostimulation. Top: Intracavernous pressure (ICP) change from baseline to peak ICP (ΔICP); middle: ICP normalized over mean arterial pressure (MAP); bottom: steepness of slope of tumescence phase of erection. * p < 0.05 in analysis of variance with post hoc Student-Newman-Keuls analysis. (B) Representative example traces of MAP; (C) ICP recordings during 2.5- and 7.5-V cavernous nerve electrostimulation in PD and PD plus ADSC rats.
3.3. Fibrosis and elastosis
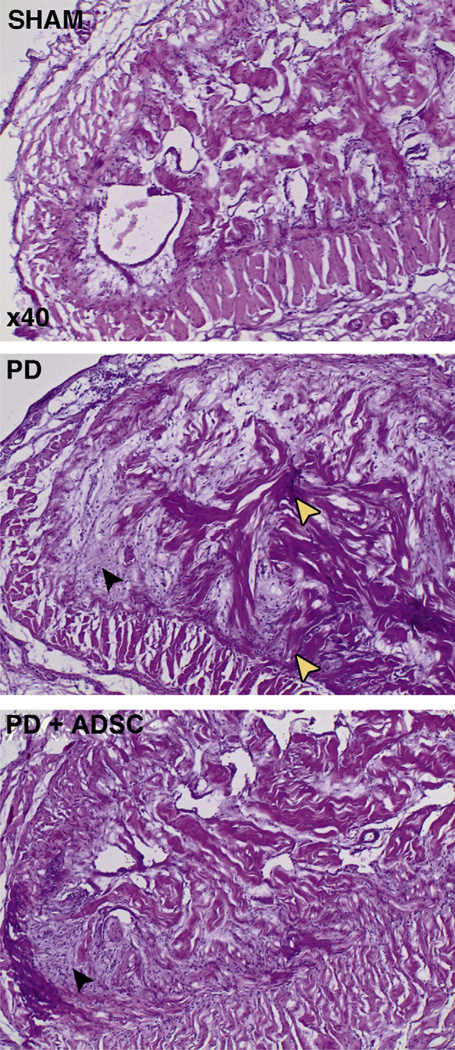
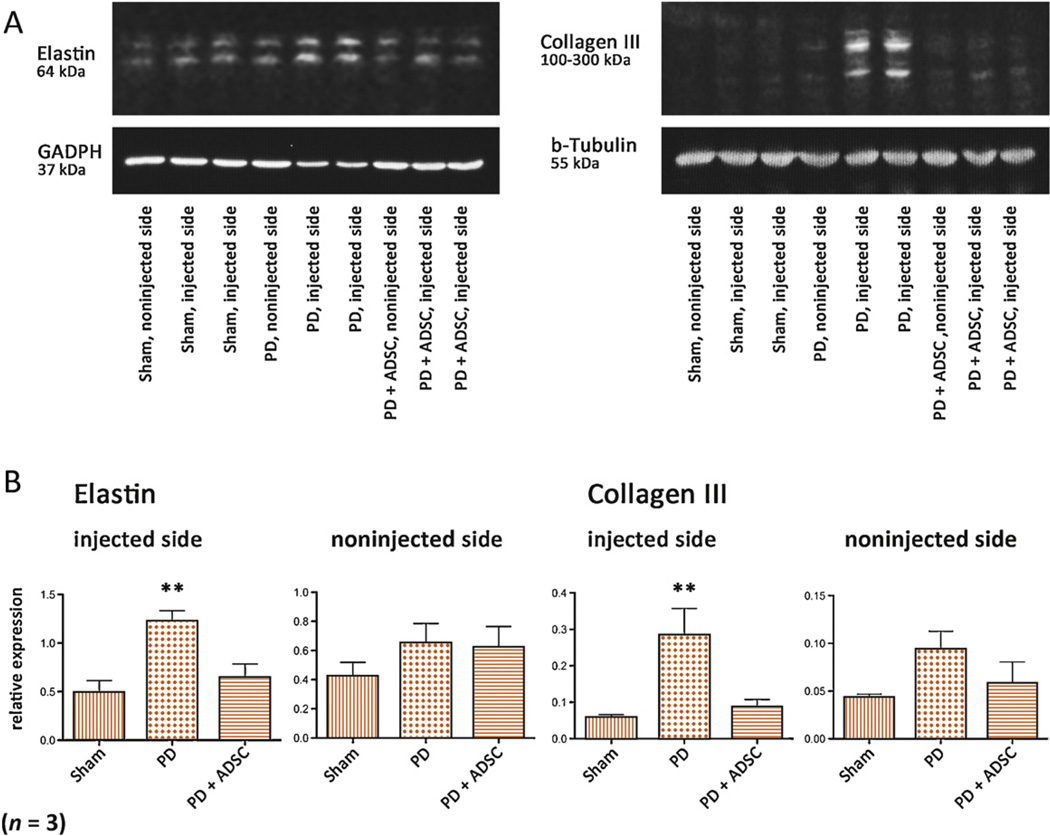
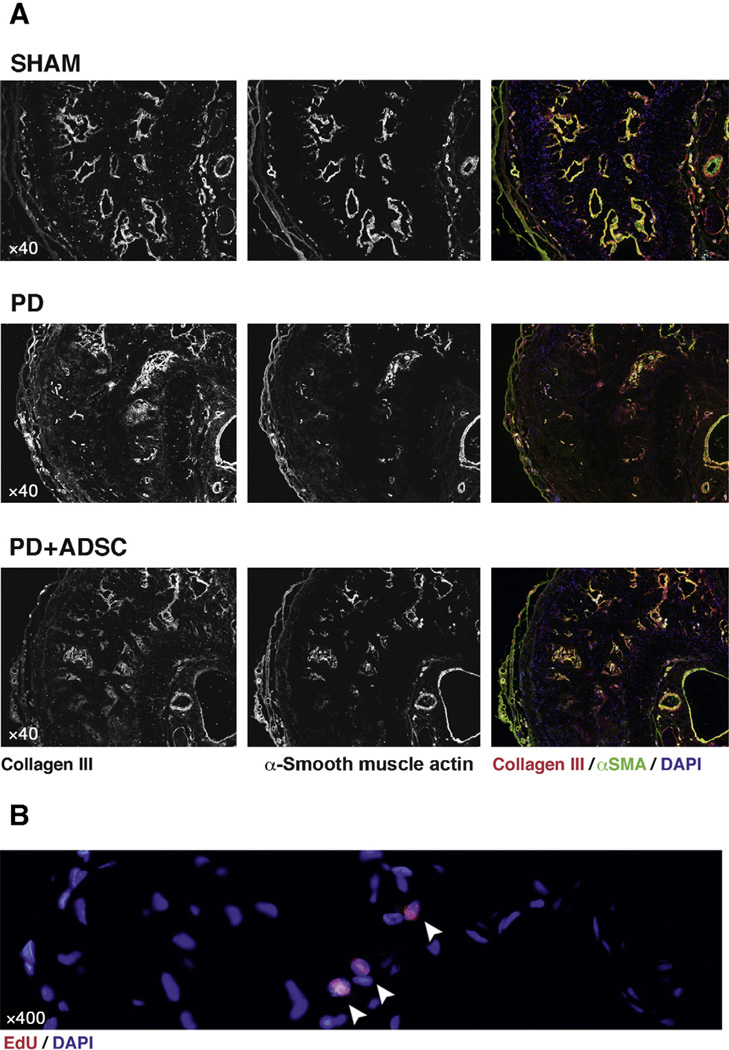
Rats injected with TGF-β1 (PD group) displayed extensive tunica-corporeal fibrosis and elastosis at the injection site (Figs. 4–7). The most consistent abnormality occurred in the orientation and haphazard organization of the collagen and elastin fibers, extending from the inner layer of the tunica into the erectile tissue. H and E staining revealed an increased number of cells in these plaques. Although not strictly tested, an assumption can be made that these cells may largely consist of fibroblasts based on their spindle-shaped morphology and their relation to extracellular matrix substance. Also, the sinusoid structure and bilayered smooth muscle organization was lost. These morphologic results were corroborated by quantitative Western blot analysis, which revealed increased protein content of both collagen III (5.0 times) and elastin (2.5 times) in the penises of rats in the PD group. After treatment with ADSCs (PD plus ADSC group), the development of these extensive plaques was largely prevented, and the overall structure and elastin and collagen III contents of the corpus cavernosum were preserved (Figs. 5–7). Quantitatively, the total content of elastin decreased by 47.7% at the treated side compared with untreated rats, and the protein content of collagen III was reduced by 69.9% after ADSC injection, as revealed by Western blot. Injected ADSC labeled with EdU were detectable but very scarce in the corpus cavernosum or tunica of the treated rats (Fig. 6B).
Fig. 4.
Hematoxylin and eosin staining in midshaft section of rat penis. Original magnification ×40. Photographs depict the dorsal part of the corpus cavernosum and tunica albuginea in sham, Peyronie’s disease (PD), and PD plus adipose tissue-derived stem cells (ADSC) rats. Note the open cavernous sinusoids in the sham rats and the surrounding normal bilayered structure of the tunica albuginea. In PD rats, there is deposition of amorphic extracellular matrix material with scattered high numbers of cells, which are expected to be fibroblasts based on their spindle-shaped morphology and relationship with the extracellular matrix (black arrowhead). Also note the haphazard organization of trabecular bundles arising from the dorsomedial tunica albuginea (white arrowheads). In the PD plus ADSC group, there is an increase in fibroblasts and extracellular matrix deposition; however, collagen fibers seem better organized and sinusoid structure is largely preserved.
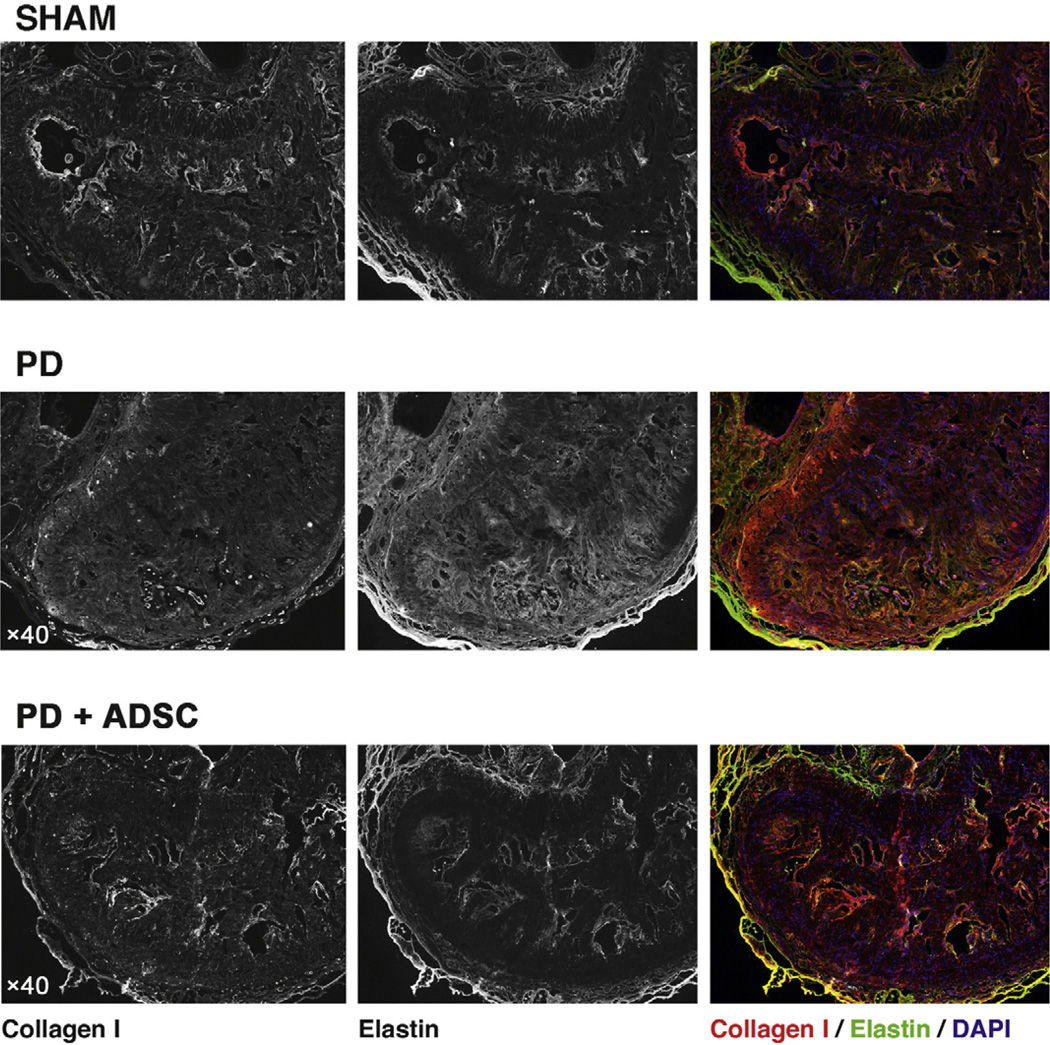
Fig. 7.
Collagen I and elastin in midshaft section of rat penis. Original magnification, ×40. (A) Top: In sham animals, collagen I (red) is localized around the sinusoids and mainly in the internal layer of the tunica albuginea. Elastin (green) displays a similar expression pattern and is highly present in the Buck fascia (outer layer of tissue), and by means of internal control it is visible in the internal and external elastic laminae of the dorsal penile vessels. Middle: A destruction of the normal anatomy of the dorsal tunica is observed in Peyronie’s disease (PD) animals, with collagen I present in all layers of the tunica and collagen fibers extending in a disorganized fashion into the erectile tissue. Also, elastin staining is very bright and fibers are haphazardly organized. A clear overgrowth of elastin fibers in the tunica and into the corpus cavernosum is observed. Bottom: When adipose tissue-derived stem cells (ADSCs) were administered to these animals in the acute phase of PD, the observed changes were prevented and the sinusoidal structure of the dorsal parts of the corpus cavernosum was observed.
Fig. 5.
Western blot analysis for collagen III and elastin. (A) Representative chemiluminescence images of blotted membranes containing protein extracts of all three groups, both injected/treated sides and noninjected sides serving as internal control for elastin and collagen III expression. Double bands are due to binding of antibodies to glycosylated and nonglycosylated forms of these molecules. (B) Summarized protein expression levels for elastin and collagen III. **p < 0.05 versus both sham and Peyronie’s disease (PD) plus adipose tissue-derived stem cells (ADSCs) in analysis of variance with post hoc Student-Newman-Keuls analysis.
Fig. 6.
Smooth muscle, collagen III, and adipose tissue-derived stem cells (ADSCs) in midshaft section of rat penis. Original magnification (a) ×40; (b) ×400. (A) Top: Note how in sham animals α-smooth muscle actin (αSMA; green) and collagen III (red) colocalize in the smooth muscle layers surrounding the cavernous sinusoids and the dorsal penile arteries. In normal tunica albuginea (TA), expression of these proteins is very low. Middle: In Peyronie’s disease (PD) animals, 5 wk following injection of transforming growth factor-β1, sinusoidal structure has largely disappeared near the injection site. αSMA is largely absent in the dorsal part of the corpus cavernosum (by means of internal antibody control, expression is preserved in the dorsal penile vessels). Note how collagen III is now deposited in an amorphic fashion in regions of the corpus cavernosum adjacent to the injection site in the dorsomedial TA. Bottom: The changes observed in PD rats are present in a mild or moderate degree after ADSC treatment. However, overall structure and sinusoids are largely preserved, and αSMA-positive sinusoids have undergone less dramatic reorganization than those in PD animals and collagen III. (B) High-magnification image of nuclear double staining of 4′,6-diamidino-2-phenylindole (DAPI) and 5-ethynyl-2-deoxyuridine (EdU) in a few cells in the corpus cavernosum of PD plus ADSC rats. These double-labeled cells were scarcely found.
4. Discussion
Regenerative medicine is a rapidly evolving field in urology and sexual medicine. For the treatment of PD, various authors have shown the applicability of tissue-engineered TA for the replacement of excised Peyronie’s plaques. Ferretti and colleagues showed the benefits of noncellular and cell-seeded synthetic grafts for TA replacement [5], and Imbeault et al. investigated the use of endothelialized multilayered skin fibroblast grafts in this setting [6]. Although the addition of living differentiated cells to these products has shown a preclinical benefit in TA reconstruction, these studies were aimed at replacing terminally and irreversibly damaged TA. The current study, however, targets the acute, or inflammatory, phase of PD and therefore a phase in which the disease process may be, at least partially, reversible [3]. For the first time we provide a preventive cellular strategy to limit the formation of fibrosis in the TA and the adjacent erectile tissue in the corpus cavernosum.
Various research groups have identified TGF-β as a major player in the development of PD [1,16,19,20]. TGF-β1 is the most strongly implicated isoform in penile fibrosis [1]. It has been shown that TGF-β1 is strongly upregulated in the TA of PD patients [21]. In the rat, injections of TGF-β1 or its synthetic analog cytomodulin produced chronic cellular infiltration, focal and diffuse elastosis and thickening, and disorganization and clumping of the collagen bundles in the tunica and subtunical erectile tissue [19]. Based on these findings, intratunical TGF-β1 injection has emerged as a valid rat model for mimicking PD [20]. As reported previously by Bivalacqua and colleagues, a single injection with TGF-β1 in the TA functionally results in diminished erections [16]. In the current study we have illustrated that the development of these Peyronie-like plaques is characterized by a diffuse and disorganized deposition of both elastin and collagen III. Because these findings resembled histopathologic findings in human PD, we verified that the TGF-β1 injection model is appropriate to study the effects of cellular treatment [2,22]. In addition to fibrosis, a decrease in corporal smooth muscle and the disappearance of cavernosal sinusoids was observed in the erectile tissue adjacent to the tunica. These changes resulted functionally in a moderate impairment of erectile function upon cavernous nerve electrostimulation. Local injection of ADSCs 1 d following induction of the inflammatory-fibrotic reaction prevented the formation of these plaque-like areas as illustrated by the preserved architecture of the corpus cavernosum in immunofluorescence staining and a near normalization of collagen III and elastin content in the penis in Western blot experiments. These findings were corroborated by preserved erectile function in the treated animals. Identification of ADSCs marked with EdU in the penis showed that at 5 wk following injury, ample stem cells could be found in the injection site, thus precluding engraftment of stem cells as a key mechanism of the observed beneficial effects on fibrosis formation [15].
MSCs have at various instances proven their preclinical benefit in the prevention of fibrosis. To illustrate, the early application of MSCs after kidney ischemia-reperfusion injury, chronic renal failure, or ureteral obstruction resulted in a decrease in parenchymatous collagen accumulation in the rat kidney [7–9]. Human MSC transplantation in bleomycin-injured mouse lungs nearly completely prevented collagen deposition [11], and human MSCs alleviated liver fibrosis in a rat model [10]. Furthermore, and more interesting as it pertains to the current study, local stem cell injections have proven efficacious in preventing corpus cavernosum fibrosis in rodent models with cavernous nerve injury in various instances [17,23,24]. The exact mechanisms of the antifibrotic effects of MSCs remain to be elucidated. Most preclinical studies hint at immunomodulation, thereby limiting the host response to injury and therefore preventing the onset of fibrosis [25]. Another proposed mechanism is the induction of phenotypical changes in resident fibroblast, illustrated by reduced collagen and increased hyaluronic acid production in fibroblasts cocultured with MSCs [12,13]. The direct interaction of MSCs with the extracellular matrix has been proposed, based on their ability to secrete high numbers of matrix metalloproteinases and other matrix-modulating enzymes [26]. However, definitive answers, particularly in penile fibrosis, have not been given, and further studies focusing on the mechanisms of action of penile MSC therapy are therefore ongoing.
The fate of MSCs injected into areas of fibrosis or scarring appears to resemble previous observations made in the cavernous nerve injury model. Falanga and colleagues reported that nearly all MSCs injected into mouse skin wounds have exited the affected area prior to the completion of wound healing [27]. In another study, bone marrow cells injected into the mouse myocardium scar following a simulated infarct resulted in reduced fibrosis and scar formation in the absence of cell incorporation [14]. In the previously mentioned study on lung fibrosis, very few engrafted cells were observed, in numbers insufficient to explain the observed effects [11]. In our study, ample EdU-labeled ADSCs were detectable in the penis after injection. These observations hint at a predominantly paracrine mechanism of action as detailed previously [15], and they are further supported by observations that conditioned media of MSCs or MSC lysates or extracts have been able to replicate the effects of live cells on tissue alterations in ischemic brain damage, acute limb ischemia, liver failure, myocardial infarction, and cavernous nerve injury [14,17,28–30]. The kidney, however, appears to be an exception to this rule because in all three previously kidney studies, MSC engraftment was reported.
Inherent to the use of animal experimentation, the model poses the bias that fibrosis extends into the corpus cavernosum and therefore does not perfectly resemble the disease in humans, which is most often limited to the TA. This effect has been shown in previous preclinical studies for PD, and to the best of our knowledge, this is the most reliable and most disease-resembling rat model currently available. Despite this even more extensive fibrotic reaction, however, stem cells still induced significant effects on plaque development. The current use of xenogenic stem cells can be regarded as a limitation but poses a translational advantage and was used with excellent results in rats previously [10,11]. We studied administration of stem cells only in the inflammatory phase of the disease. It is known that only a small portion of PD patients present in the early phases of the disease, and therefore this study should be regarded as a proof of principle that stem cell therapy is able to halt the natural development of PD plaques. Whether this cellular therapy is able to reduce established PD plaques remains to be elucidated. Therefore, further experimentation is now targeting the effects of ADSCs in the chronic disease state in this model.
5. Conclusions
This study is the first to test cellular therapy in PD. In the acute, or inflammatory, phase of the disease, injection of ADSCs into the affected area prevents formation of fibrosis and elastosis in the tunica and corpus cavernosum. Our current data, together with the availability of routine autograft stem cell isolation procedures and external location of the penis with convenient access for local therapy, present a promising translational potential of this treatment strategy to the bedside.
Acknowledgment statement
We would like to heartily thank Arianna Bettiga and Giorgia Colciago for their excellent technical contributions to the experiments in this study.
Funding/Support and role of the sponsor: This study was fully funded by the European Society for Sexual Medicine (ESSM) grant for basic medical research 2011 awarded to Fabio Castiglione and Maarten Albersen and the Urological Research Institute, Milan, Italy. Maarten Albersen is a fellow of the Federico Foundation and the Research Foundation–Flanders (FWO) and received an international mobility grant from FWO for this study.
Footnotes
Author contributions: Petter Hedlund had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Castiglione, Albersen.
Acquisition of data: Castiglione, Hedlund, Albersen.
Analysis and interpretation of data: Castiglione, Hedlund, Albersen.
Drafting of the manuscript: Albersen.
Critical revision of the manuscript for important intellectual content: Castiglione, Van Poppel, Bivalacqua, Van der Aa, Montorsi, Rigatti, De Ridder, Hedlund.
Statistical analysis: Hedlund, Albersen.
Obtaining funding: Albersen, Castiglione.
Administrative, technical, or material support: Bivalacqua, Van der Aa, Van Poppel, Montorsi, De Ridder, Rigatti.
Supervision: Hedlund.
Other (specify): None.
Financial disclosures: Petter Hedlund certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Lin CS, Lin G, Wang Z, Maddah SA, Lue TF. Upregulation of monocyte chemoattractant protein 1 and effects of transforming growth factor-beta 1 in Peyronie’s disease. Biochem Biophys Res Commun. 2002;295:1014–1019. doi: 10.1016/s0006-291x(02)00765-9. [DOI] [PubMed] [Google Scholar]
- 2.Brock G, Hsu GL, Nunes L, von Heyden B, Lue TF. The anatomy of the tunica albuginea in the normal penis and Peyronie’s disease. J Urol. 1997;157:276–281. [PubMed] [Google Scholar]
- 3.Hatzimouratidis K, Eardley I, Giuliano F, et al. Guidelines on penile curvature. Eur Urol. 2012;62:543–552. doi: 10.1016/j.eururo.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 4.Smith JF, Walsh TJ, Conti SL, Turek P, Lue T. Risk factors for emotional and relationship problems in Peyronie’s disease. J Sex Med. 2008;5:2179–2184. doi: 10.1111/j.1743-6109.2008.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferretti L, Giuliani M, Bessède T, et al. Tissue engineering for penile surgery: comparative study of noncellular and cell-seeded synthetic grafts for tunica albuginea replacement. J Sex Med. 2012;9:625–631. doi: 10.1111/j.1743-6109.2011.02561.x. [DOI] [PubMed] [Google Scholar]
- 6.Imbeault A, Bernard G, Ouellet G, Bouhout S, Carrier S, Bolduc S. Surgical option for the correction of Peyronie’s disease: an autologous tissue-engineered endothelialized graft. J Sex Med. 2011;8:3227–3235. doi: 10.1111/j.1743-6109.2011.02374.x. [DOI] [PubMed] [Google Scholar]
- 7.Alfarano C, Roubeix C, Chaaya R, et al. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant. doi: 10.3727/096368912X640448. In press. http://dx.doi.org/10.3727/096368912X640448. [DOI] [PubMed] [Google Scholar]
- 8.Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 9.Asanuma H, Vanderbrink BA, Campbell MT, et al. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J Surg Res. 2011;168:e51–e59. doi: 10.1016/j.jss.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao W, Li J-J, Cao D-Y, et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18:1048–1058. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moodley Y, Atienza D, Manuelpillai U, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumai Y, Kobler JB, Park H, Galindo M, Herrera VLM, Zeitels SM. Modulation of vocal fold scar fibroblasts by adipose-derived stem/stromal cells. Laryngoscope. 2010;120:330–337. doi: 10.1002/lary.20753. [DOI] [PubMed] [Google Scholar]
- 13.Kumai Y, Kobler JB, Park H, et al. Crosstalk between adipose-derived stem/stromal cells and vocal fold fibroblasts in vitro. Laryngoscope. 2009;119:799–805. doi: 10.1002/lary.20149. [DOI] [PubMed] [Google Scholar]
- 14.Yeghiazarians Y, Zhang Y, Prasad M, et al. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–1256. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albersen M, Kendirci M, Van der Aa F, Hellstrom WJG, Lue TF, Spees JL. Multipotent stromal cell therapy for cavernous nerve injury-induced erectile dysfunction. J Sex Med. 2012;9:385–403. doi: 10.1111/j.1743-6109.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 16.Bivalacqua TJ, Diner EK, Novak TE, et al. A rat model of Peyronie’s disease associated with a decrease in erectile activity and an increase in inducible nitric oxide synthase protein expression. J Urol. 2000;163:1992–1998. [PubMed] [Google Scholar]
- 17.Albersen M, Fandel TM, Lin G, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.El-Sakka AI, Hassoba HM, Chui RM, Bhatnagar RS, Dahiya R, Lue TF. An animal model of Peyronie’s-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. J Urol. 1997;158:2284–2290. doi: 10.1016/s0022-5347(01)68236-3. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Cadavid NF, Rajfer J. Experimental models of Peyronie’s disease. Implications for new therapies. J Sex Med. 2009;6:303–313. doi: 10.1111/j.1743-6109.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 21.Hassoba H, El-Sakka A, Lue T. Role of increased transforming growth factor beta protein expression in the pathogenesis of Peyronie’s disease. Egypt J Immunol. 2005;12:1–8. [PubMed] [Google Scholar]
- 22.Davis CJ. The microscopic pathology of Peyronie’s disease. J Urol. 1997;157:282–284. [PubMed] [Google Scholar]
- 23.Qiu X, Fandel TM, Ferretti L, et al. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;62:720–727. doi: 10.1016/j.eururo.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fandel TM, Albersen M, Lin G, et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61:201–210. doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 26.Huang W, Wang T, Zhang D, et al. Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev. 2012;21:778–789. doi: 10.1089/scd.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M, Suzuki E, Oba S, et al. Adipose tissue-derived stem cells inhibit neointimal formation in a paracrine fashion in rat femoral artery. Am J Physiol Heart Circ Physiol. 2010;298:H415–H423. doi: 10.1152/ajpheart.00391.2009. [DOI] [PubMed] [Google Scholar]
- 29.Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei X, Du Z, Zhao L, et al. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. 2009;27:478–488. doi: 10.1634/stemcells.2008-0333. [DOI] [PubMed] [Google Scholar]