Abstract
Muscle replacement for patients suffering from extensive tissue loss or dysfunction is a major objective of regenerative medicine. To achieve functional status, bioengineered muscle replacement constructs require innervation. Here we describe a method to bioengineer functionally innervated gut smooth muscle constructs using neuronal progenitor cells and smooth muscle cells isolated and cultured from intestinal tissues of adult human donors. These constructs expressed markers for contractile smooth muscle, glial cells, and mature neuronal populations. The constructs responded appropriately to physiologically relevant neurotransmitters, and neural network integration was demonstrated by responses to electrical field stimulation. The ability of enteric neuroprogenitor cells to differentiate into neuronal populations provides enormous potential for functional innervation of a variety of bioengineered muscle constructs in addition to gut. Functionally innervated muscle constructs offer a regenerative medicine-based therapeutic approach for neuromuscular replacement after trauma or degenerative disorders.
Introduction
The gastrointestinal (GI) tract is derived from cells of all three germ layers. The endoderm gives rise to the epithelial component of the GI tract, while the mesoderm and ectoderm, respectively, give rise to the smooth muscle and enteric nervous system (ENS) components. Following gastrulation, in human, a closed gut tube is formed by week 4 and a complete gut containing ganglion formation within the myenteric plexus can be visualized by week 10.1–6 The submucosal plexus is not observed until 2–3 weeks later.5–7 Neural crest stem cell migration into and along the gut supply the cells required for the formation of the ENS. In animal models during development, the gut is first colonized in an oral to anal direction by neural crest-derived cells from the vagal crest and then in the opposite direction from sacral neural crest-derived cells.8,9 Cells migrate along strands that intersect and divide during the migration, giving rise to the reticulated pattern of the ENS. These cells migrate to sequentially colonize the foregut, midgut, and hindgut. In mouse, when the midgut and hindgut are parallel and closely apposed, the most advanced enteric neural crest-derived cells (ENCCs) invade and colonize the hindgut, while the trailing ENCCs advance through the cecum.10 Additional migratory cells from the sacral crest help populate the hindgut.11 Due to ethical concerns, less is known of the cell populations involved and the dynamics of migration in human. However, it is clear that the human ENS is primarily derived from vagal neural crest cells, which are termed ENCCs when they enter the foregut at week 4. ENCCs migrate rostrocaudally and complete the migration to the terminal hindgut by week 7.4–6 During migration, the ENCCs proliferate and differentiation is initiated. The initiation of both glial and neuronal populations appears to occur simultaneously and precedes plexus formation.4 Differentiation occurs in both rostrocaudal and centripetal directions. Interstitial cells of Cajal (ICC) arise from the mesenchyme12,13 just before myenteric ganglia formation. After ICC formation, the differentiation of mesenchyme into smooth muscle is initiated. Alpha-smooth muscle actin (αSMA) (a marker of smooth muscle differentiation) is first seen at the basal aspect of the circular smooth muscle layer followed by its appearance in longitudinal smooth muscle and apical aspects of the circular smooth muscle.5,6 The coordinated migration, proliferation, differentiation, and association of different cell populations allow for the mature appearance of a human fetal gut by week 14. Functionally, the gut continues to mature throughout full-term development as determined by motor patterns in preterm and full-term infants14 and ENS maturation is not complete until at least 2 years after birth.2,15 The mature human ENS regulates secretion, absorption, motility, and blood flow in the gut and is composed of ∼0.5 billion enteric neurons16 as well as glial cells and a resident population of progenitor cells.
The culture of neuronal progenitor cells as spheroid bodies was first demonstrated in dissociated cells from embryonic mouse striatal tissue. When cultured in the presence of epidermal growth factor, isolated cells formed nestin-positive clusters. Following continued culture, these cells became immunopositive for neurotransmitter markers of the adult striatum and glial fibrillary acidic protein (GFAP), indicating the presence of both neuronal and glial cell lineages.17 Culture conditions were modified to allow growth of the cells as floating neurospheres.18 The culture of enteric neurosphere-like bodies from fetal and postnatal murine gut tissue was based on modifications of the methods first described for central nervous system neurosphere culture.19,20 The isolation and culture of ENS neuronal progenitor cells from embryonic, neonatal, and adult human gut followed.21,22
The bioengineering of smooth muscle replacements for treatment of human pathologies or tissue degeneration has great potential for providing a successful application in the developing field of organogenesis. Smooth muscle constructs have been bioengineered using several muscle types, including bladder,23 cardiac,24 vascular,25 internal anal sphincter (IAS),26 and longitudinal colonic smooth muscle.27 We have recently produced the first bioengineered smooth muscle constructs containing a neuronal component.28 Proper in vivo muscle function requires that the muscle tissue is innervated. In this study, we demonstrate a technique for bioengineering physiologically functional, intrinsically innervated human IAS tissues.
Materials and Methods
Isolation and culture of neuronal progenitor cells
Unless specified otherwise, all tissue culture reagents were purchase from Life Technologies Corporation. Tissues from human small intestine were obtained from organ donors through the Gift of Life Michigan (IRBMED No. HUM00023670). Tissues were isolated according to an approved institutional protocol and stripped of serosa. Tissues were washed extensively (5×) in a wash solution composed of the Hank's balanced salt solution, purchased from Thermo Scientific HyClone, Logan UT catalog # SH30015.03 (HBSS) containing 200 U/mL penicillin G, 200 μg/mL streptomycin, 20 μg/mL gentamicin, and 0.5 μg/mL amphotericine B (2× antibiotics/antimycotics). Tissues were then minced and washed an additional 5× before being subjected to digestion in a mixture containing 0.85 mg/mL collagenase type 2 (Worthington Biochemical Corporation), 0.85 mg/mL dispase II, and 30 μg/mL DNAse I (Roche Applied Science) at 37°C with shaking for 1 h. Cells were recovered from the supernatant following centrifugation at 200 g for 5 min, passed through a 70-μm cell strainer and recovered by centrifugation at 1000 g for 10 min. Recovered cells were suspended in the wash solution and stored on ice. The pellet was subjected to a second digest for 1 h and passed through a 70-μm cell strainer. Cell suspensions were combined and cells recovered by centrifugation at 1000 g for 10 min. Cells were washed an additional 5×, as described, suspended in a neuronal growth medium (Neurobasal medium containing 1% N2 supplement, 20 ng/mL recombinant human epidermal growth factor (Stemgent), 20 ng/mL recombinant basic fibroblast growth factor (Stemgent), 1 mM L-glutamine, and 1× antibiotics/antimycotics) before being filtered through a 40-μm cell strainer. Cells were counted using a hemocytometer and plated in the neuronal growth medium at a density of ≥250,000 cells/mL. Cultures were maintained in a humidified incubator at 37°C and 7% CO2.
Isolation and culture of IAS smooth muscle cells
Human IAS circular smooth muscle was obtained from organ donors through the Gift of Life Michigan (IRBMED No. HUM00023670). Tissues were collected following the procurement of all organs for transplantation. This typically occurred within 1 and 3 h following the cross-clamping of the aorta and infusion of the organ preservation solution. Smooth muscle cells were isolated from procured tissues essentially as previously described29 Briefly, specimens were washed extensively in an ice-cold wash solution (HBSS with 2× antibiotics/antimycotics), the striated muscle and connective tissue removed using sharp instruments, and the remaining IAS tissue finely minced. Following additional washes, the minced tissue was subjected to two serial 1-h digestions at 37°C in a mixture of 1 mg/mL type II collagenase and 30 μg/mL DNAse I. Following the second digestion, cells were recovered by centrifugation (600 g for 5 min), washed 3×, and plated in a growth medium consisting of the Dulbecco's modified Eagle's medium (DMEM) catalog # 12100 supplemented with 10% fetal bovine serum (FBS), 1× antibiotics/antimycotics, and 2.5 mM L-glutamine. Cultures were maintained in a humidified incubator at 37°C and 5% CO2.
Bioengineering intrinsically innervated IAS tissue constructs
Enteric neurosphere-like bodies were recovered by centrifugation at 400 g for 5 min, suspended in HBSS, and extensively triturated using a 2 mL pipette. The sample was split into two equal volume samples. One sample was dissociated with Accutase before cells were counted using a hemocytometer. The samples were combined and an estimated 200,000 cells/construct, consisting of small neurosphere-like bodies and single cells, were recovered by centrifugation at 1000 g for 10 min. The cells were suspended at a concentration of 200,000 cells/mL in a mixture containing a final concentration of 1× DMEM, 10% FBS, 0.4 mg/mL type I rat tail collagen, 10 μg/mL mouse laminin, physiological normal osmolarity and pH, and 1× antibiotics/antimycotics. One milliliter of the mixture was pipetted onto each Sylgard-coated 35-mm Petri dish with a central Sylgard post and allowed to gel at 37°C for 15 min. Dense monolayer smooth muscle cell cultures were trypsinized, the cells counted using a hemocytometer, and recovered by centrifugation at 600 g. Cells were suspended at a concentration of 500,000 cells/mL in the mixture described above except laminin was not included. Following gelation of the initial mixture, one milliliter the muscle cell mixture was overlaid and allowed to gel at 37°C for 15 min. Discs were released when needed from the edge of the culture dishes using a sterile 22 gauge 1.5′′ needle and overlaid with a medium consisting of the Neurobasal-A medium supplemented with the B-27 serum-free supplement, 1% fetal calf serum, and 1× antibiotics/antimycotics. Constructs were allowed to form in a humidified incubator at 37°C and 7% CO2.
RNA isolation and PCR analysis
Duplicate constructs were harvested at days 1, 3, 5, and 7 after gelation of the complex hydrogels. Constructs were washed with phosphate-buffered saline (PBS; catalog # 10010) before total RNA was isolated using a Quigen RNeasy kit according to the manufacturers' directions. cDNAs were generated from ∼1 μg samples of total RNA using oligo(dT) 12–18 primers and SuperScript II Reverse Transcriptase according to the manufacturers' directions. Previously published primer sequences shown to be specific for smooth muscle, progenitor cell, glial, and neuronal products were used.30–34 PCR products were generated using AccuPrime Pfx supermix from Life Technologies Corporation, USA. The specific primers used were smooth muscle actin forward 5′ACCCACAATGTCCCCATCTA3′, reverse 5′TGATCCACATCTGCTGGAAG3′, NM_001141945.1 (595 bp)32; CALD1 forward 5′AGATTGAAAGGCGAAGAGCA3′, reverse 5′TTCAAGCCAGCAGTTTCCTT3′, NM_033138.3 (397 bp)32; TUBB3 forward 5′CTCAGGGGCCTTTGGACATC3′, reverse 5′CAGGCAGTCGCAGTTTTCA3′, NM_001197181.1 (160 bp)30; GFAP forward 5′ACGCAGTATGAGGCAATGG3′, reverse 5′CGGTAGTCGTTGGCTTCG3′, NM_002055.4 (140 bp)34; NGFR forward 5′CGTATTCCGACGAGGCCAACC3′, reverse 5′CCACAAGGCCCACAACCACAGC3′ NM_002507.3 (345 bp)31; GAPDH forward 5′CAGGTGGTCTCCTCTGACTTCAAC3′, reverse 5′AGGGTCTCTCTCTTCCTCTTG3′ NM_002046.4 (223 bp).(33) To confirm the identity of the PCR products, DNAs were gel purified from the presumptive product bands and subjected to a second PCR amplification using the appropriate specific primers. The presence of single appropriately sized DNA bands from each set of primers confirmed the specificity of the reaction products.
Immunohistochemical analysis of constructs
Day 10–12 constructs were fixed overnight in a neutral buffered 3.7% formalin solution, dehydrated through graded ethanol, and embedded in paraffin. Sections (6–8 μm) were cut, deparaffinized, and rehydrated before being permeabilized with a solution containing 10% horse serum, 0.15% Triton X100 in PBS. Sections were incubated 1 h at room temperature with primary antibodies directed against neuronal, glial, or smooth muscle markers in a buffer containing 0.1% bovine serum albumin and 0.075% Triton X100 (Ab dilution buffer). Sections were washed with 1× PBS to remove the unbound antibody before incubation at room temperature for 1 h with the appropriate fluorescently tagged secondary antibody in the Ab dilution buffer. Sections were washed with 1× PBS to remove the unbound secondary antibody. Stained sections were mounted with the Prolong Gold antifade mounting medium containing 4′, 6-diamidino-2-phenylindole (DAPI; Invitrogen) to counterstain nuclei in blue. Fluorescence was visualized using a Nikon eclipse Ti inverted microscope. Negative controls for each secondary antibody were performed by replacing the primary antibody solution with just the Ab dilution buffer. Fluorescein isothiocyanate (FITC) - or rhodamine isothiocyanate (TRITC) -conjugated secondary antibodies were incubated for 1 h subsequently. Fluorescence was detected at similar exposure times and gain.
Antibodies directed against p75NTR (07–476; rabbit) and GFAP (04–0162; rabbit) were purchased from Millipore Corporation and were each used at titers of 1:200. Antibodies directed against β III tubulin (ab25770; mouse), vasoactive intestinal peptide (VIP) (ab78536; rabbit), and choline acetyltransferase (ChAT) (ab68779; rabbit) were purchased from abcam Biochemicals and were used at titers of 1:150, 1:100, and 1:100, respectively. Anti-smooth muscle Caldesmon (Cal D) (C4562; mouse) was purchased from Sigma-Aldrich and used at a titer of 1:150 and anti-neuronal nitric oxide synthase (nNOS) (51–9002027; mouse) was purchased from B.D. Transduction Laboratories and used at a titer of 1:100. FITC-conjugated sheep anti-mouse IgG (F6257) was purchased from Sigma-Aldrich and TRITC-conjugated goat anti-rabbit IgG (ab 6718) was purchased from abcam. Both were used at titers of 1:50.
Physiological testing of constructs
Day 10–12 constructs were removed from the central Sylgard posts and hooked on to an isometric force transducer (F10; Harvard Apparatus) housed in an organ bath apparatus. Constructs were placed between a stationary central pin and the measuring arm of the transducer. Tissues were immersed in 4 mL of basal DMEM buffered with 25 mM HEPES. The perfusion liquid was replaced every 30 min or at the end of an experiment, whichever was earlier. Perfusate was maintained at 37°C due to continuous water perfusion of the organ bath. Data were acquired at 50 samples per second using a Powerlab data acquisition system (Harvard Apparatus). Data were processed using GraphPad Prism 5.0 (www.graphpad.com) and second-order Savitsky-Golay smoothing was applied to raw data to generate force traces. During analysis, baselines were arbitrarily set to zero to analyze the magnitude of contraction or relaxation, which was termed Δ force. Some experiments were conducted in the presence of neurotoxin tetrodotoxin (TTX), to discern the neuronal component to smooth muscle contraction and relaxation. Constructs were allowed to generate spontaneous basal tone in the absence of any external stimulation over 30 min. An additional 20% stretch was applied to the constructs and they were allowed to equilibrate for 45 min, before treatment with 1 μM acetylcholine (Ach). Constructs were washed extensively and treated with 1 μM VIP. Neuronally evoked relaxation was achieved by electrical field stimulation (EFS) (5 Hz, 0.5 ms) between parallel platinum plate electrodes in the organ bath.
Statistical analysis
Raw data were acquired from the force transducer at 1000 samples/second. Second-order Savitsky-Golay smoothing was applied to data using GraphPad Prism 5.0 for Windows (GraphPad Software). One-way analysis of variance was used to compare means using GraphPad Prism. p<0.05 was considered significant. Physiological evaluation was carried out between 5–10 tissue-engineered rings within each experimental set; all values are expressed as mean±SEM.
Results
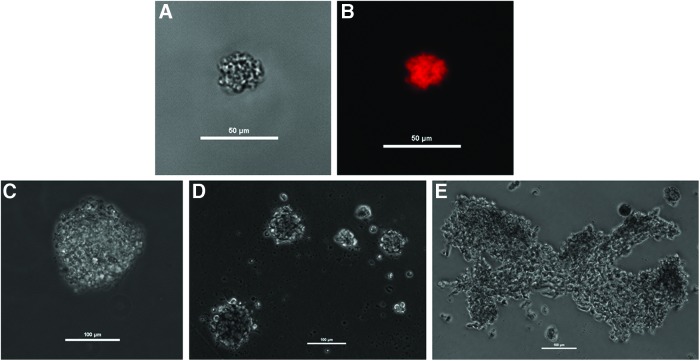
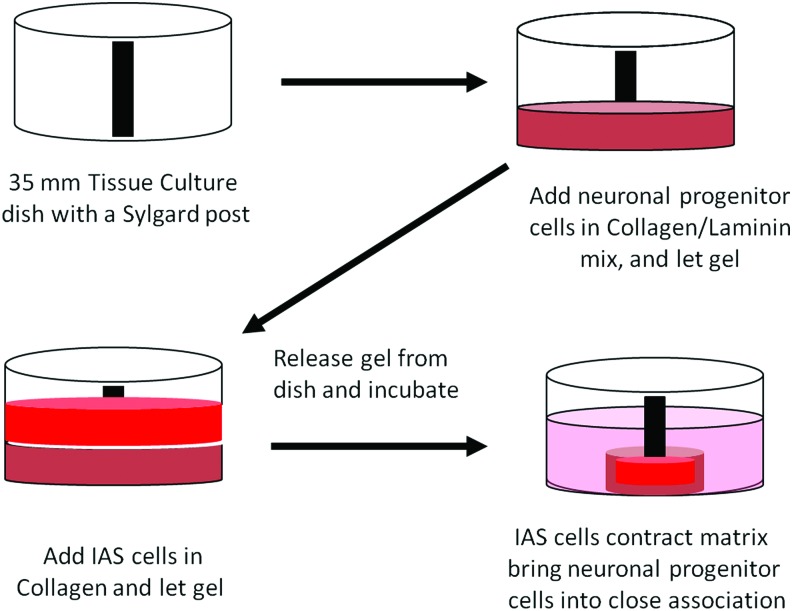
We used human neuronal progenitor cells from adult human intestinal tissues and adult human IAS smooth muscle cells to bioengineer functional intrinsically innervated human IAS tissues. We used a modification of cell isolation procedures previously reported to isolate these cells from both fetal and adult human tissues.21,22,35 Neuronal progenitor cells isolated from adult human intestinal tissues were cultured in nontissue culture-treated plastic Petri dishes in a medium designed to enhance cell proliferation and inhibit differentiation. Cells formed small clusters, replicated, and formed floating bodies resembling neurospheres. Cells of the neurosphere-like body tested positive for the neural crest-derived cell marker p75NTR by immunohistochemistry (Fig. 1A, B). Continued growth and/or aggregation allowed the formation of macroscopic cell clusters (Fig. 1C–E). Cultured neuronal progenitor cells along with primary smooth muscle cell cultures of adult human IAS were used to bioengineer constructs. A schematic diagram of the bioengineering process is depicted in Figure 2.
FIG. 1.
Human enteric neurosphere-like bodies. Brightfield (A) and fluorescence (B) microscopy of p75NTR-labeled enteric neurosphere-like body. Brightfield, live-cell microscopy was used to observe the formation of individual spheres (C, D) and large aggregates (E). Scale bars represent 50 μm for A, B and 100 μm for D–E. Color images available online at www.liebertpub.com/tea
FIG. 2.
Schematic representation of bioengineered innervated IAS. Neuronal progenitor cells were suspended in a type I collagen/laminin mixture and plated onto a Sylgard-coated Petri dish with a central post. The mixture was placed into a 37°C humidified incubator and allowed to gel. A second mixture containing IAS smooth muscle cells suspended in type I collagen was added. Following gelation of the second hydrogel, the construct was released from the dish, the neuronal differentiation medium was added, and the plate returned to the incubator. IAS, internal anal sphincter. Color images available online at www.liebertpub.com/tea
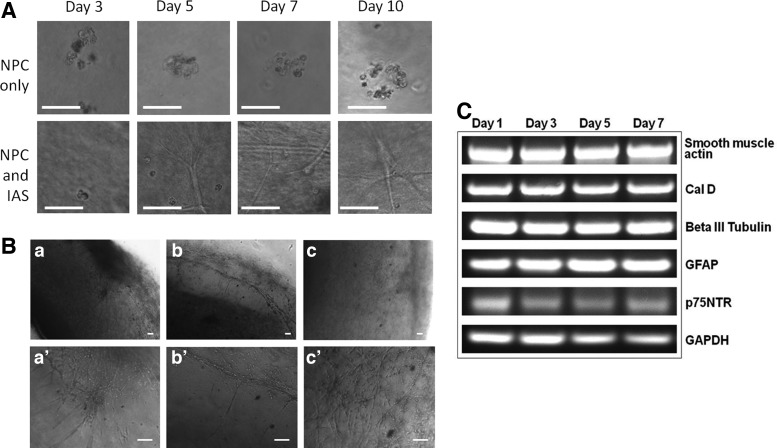
For control experiments, hydrogels containing neuronal progenitor cells, but lacking smooth muscle cells were produced. In the absence of smooth muscle cells, the gels did not retract and the neuronal progenitor cells demonstrated minimal differentiation (Fig. 3A, top panel). These observations are not completely consistent with those of Schafer et al.,19 who noted little growth or differentiation among single enteric neuronal precursor cells and both growth and differentiation from clusters of precursor cells when cultured in three-dimensional (3D) culture. The matrix material used by these researchers was the ECM gel (Sigma), a complex mixture of extracellular matrix components and growth factors secreted by Engelbreth-Holm-Swarm mouse sarcoma cells. The differences in matrix composition may explain our observed lack of cell cluster differentiation in the absence of smooth muscle. The smooth muscle containing hydrogels retracted into circular ring-like structures within 1–2 days. Contiguous smooth muscle formed immediately adjacent to the central post and single neuronal progenitor cells and small spheres were apparent in the surrounding hydrogel. The majority of the progenitor cells changed appearance by day 3 (Fig. 3A, bottom panel). The cells began to elongate and send off processes throughout the matrix. Whereas most cells elongated, some remained spherical in appearance. Spherical cells remained for the duration of the culture period. By day 5, extensive cell elongation/differentiation was easily observed and the cells appeared to be forming networks. Network formation appears to progress at the outer edge of the construct through day 10. A close examination of a maturing construct at day 8 (Fig. 3B) demonstrated the migration and differentiation of neuronal progenitor cells originating from a neurosphere-like body (Fig. 3B-a, a′) as well as a network formation with other developing neurons. Bundles of neural fibers were identified near the periphery of the construct with some associated neurons extending perpendicularly toward the smooth muscle component of the construct (Fig. 3B-b, b′). Additionally, a surface cellular network was observed (Fig. 3B-c, c′). Small, apparently nondifferentiating, cells from the neurosphere-like bodies remained throughout the course of construct maturation.
FIG. 3.
Construct development and neuronal outgrowth. (A) Top panel: Neuronal progenitor cells were cultured in the absence of smooth muscle and failed to morphologically differentiate through 10 days of culture. (A) Bottom panel: Neuronal progenitor cells cultured in the presence of smooth muscle cells demonstrated elongated morphologies by day 5. (B) By day 8, neuronal progenitor cells were observed to migrate from a sphere and to elongate and form networks (a, a′). The formation on neuronal bundles became evident (b, b′) and an extensive surface network was evident (c, c′). (C) The presence of markers for ENCC (p75NTR), contractile smooth muscle cells (Cal D), neurons (β III tubulin), and glial cells (GFAP) in bioengineered innervated constructs was demonstrated by PCR analysis. Scale bars represent 50 μm in A and 100 μm in B. Cal D, caldesmon; ENCC, neural crest-derived cell; GFAP, glial fibrillary acidic protein.
The constructs were tested for the presence of smooth muscle, ENCC, neuronal, and glial cell mRNA markers (Fig. 3C). GAPDH expression was used as a control. Duplicate constructs were harvested on days 1, 3, 5, and 7. Total RNA was isolated and cDNA synthesized before PCR analysis. Specific mature smooth muscle primers to αSMA and smooth muscle Cal D were used to generate PCR products. Both showed high mRNA expression levels from day 1–7, indicating the presence of mature, contractile smooth muscle for the duration of this study. Typically, PGP 9.5 or β III tubulin are used as generic neuronal markers, while GFAP or S 100 β indicate glial cells. We used the presence of PCR products for β III tubulin as a neuronal marker and GFAP to indicate the presence of glial cells. Both human and rodent enteric neuronal cells have been shown to express these generic neuronal and glial markers,20,36 so we were not surprised to see PCR products for each at day 1. We did not see any substantial change in expression in their PCR products by day 7 in a manner linked to the dramatic cellular morphological differentiation. No substantial increase in the PCR product for GFAP was observed and no change was observed for βIII tubulin. During ENS development in the mouse, glial cells strongly express both GFAP and p75NTR, while differentiating neuronal cells lose the ability to express p75NTR.37,38 Some p75NTR expression was maintained throughout the time course of these experiments.
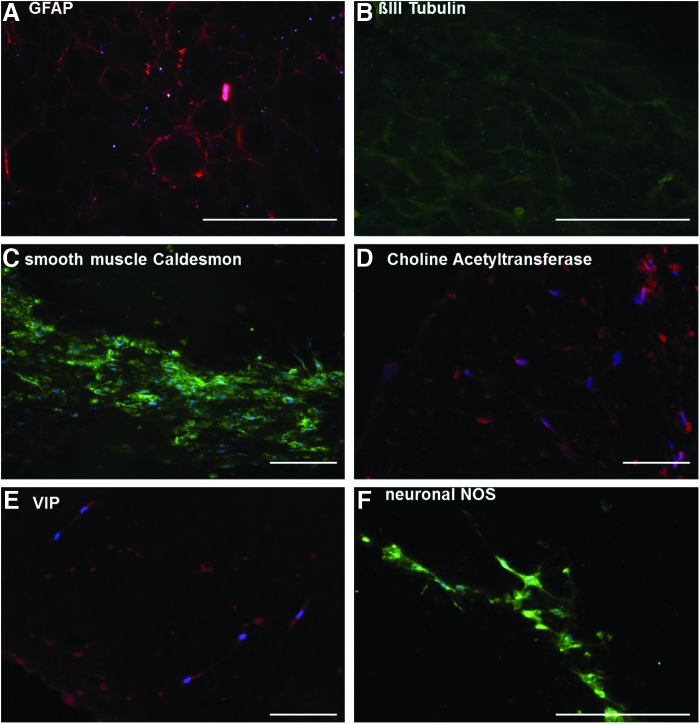
Day 10–12 constructs were fixed, embedded in paraffin, and sectioned for immunohistochemical analysis (Fig. 4). Immunohistochemical analysis of longitudinal sections demonstrated a mesh-like network near the surface, which stained positively for GFAP (Fig. 4A), indicating the presence of glial cells, and βIII tubulin (Fig. 4B) indicating the presence of neuronal cells. The staining patterns were reticulated in nature and appeared to be nearly identical, indicating the close associations of the two cell types. Mature, contractile smooth muscle cells within the constructs were visualized by the presence of smooth muscle (heavy) Cal D39,40 (Fig. 4C). In cross sections, smooth muscle Cal D appeared as a strong band near the inside edge of the constructs. These data indicate that the smooth muscle cell component of these constructs forms a contiguous multicellular structure near the post. Constructs also contained cell populations that stained positively for ChAT (Fig. 4D), VIP (Fig. 4E), and nNOS (Fig. 4F). ChAT is required for the production of Ach, the major physiologically relevant excitatory neurotransmitter in the gut. VIP and nNOS are the two major physiologically relevant inhibitory neurotransmitters in the gut. Positive stains for these markers were confirmed by negative controls, where staining with fluorophore-conjugated secondary antibodies alone was weak at similar exposure and amplifier gain settings. These data demonstrate the presence of mature neuronal cells able to produce excitatory or inhibitory neurotransmitters and demonstrate the potential of these constructs to contain intact neuronal networks, which could functionally innervate the smooth muscle. These observations led us to perform force transduction experiments to determine if the innervated muscle constructs were physiologically functional.
FIG. 4.
Immunohistochemical analysis of bioengineered constructs. Day 10–12 constructs were analyzed for the presence of contractile smooth muscle, glial, and neuronal markers. The presence of glial cells was demonstrated by positive staining for GFAP (A), while neuronal cells stained positive for BIII tubbulin (B). Mature, contractile smooth muscle was visualized by positive staining for smooth muscle Cal D (C). Differentiated neuronal cells capable of producing the excitatory neurotransmitter Ach were positive for ChAT (D). Evidence for two physiologically important inhibitory neurotransmitters was demonstrated by positive staining to VIP (E) and nNOS (F). Scale bar 200 μm. Ach, acetylcholine; ChAT, choline acetyltransferase; nNOS, neuronal nitric oxide synthase; VIP, vasoactive intestinal peptide. Color images available online at www.liebertpub.com/tea
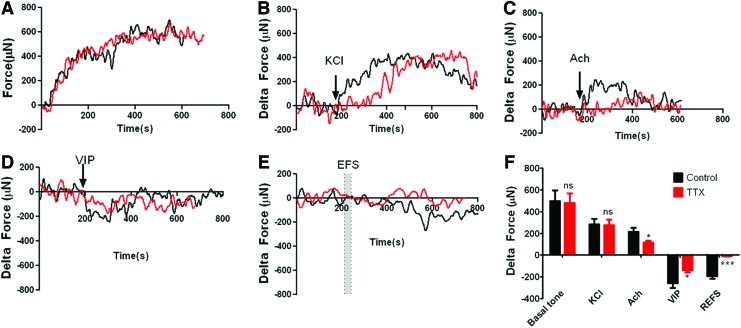
Day 10–12 constructs were tested for physiological functionality by measuring real-time force generation. Force generation was measured on an isometric force transducer as described. Without any external stimulation, the constructs were able to generate spontaneous basal tone (495±101 μN). Pretreatment of the construct with the potent neurotoxin, TTX41 had no effect on the generation of basal tone (480±90 μN) (Fig. 5A). Following determination of basal tone, baselines were set to zero to determine the magnitude of contraction or relaxation. KCl-induced smooth muscle contraction is primarily mediated by membrane depolarization, which regulates the amount of Ca2+ entering the cell through voltage-dependent Ca2+ channels. TTX pretreatment had no effect on KCl-induced contraction. Treatment with 30 mM KCl caused an increase in force generation of 282±53 μN, while constructs pretreated with TTX demonstrated an increase of 276±52 μN (Fig. 5B). These results demonstrated that the smooth muscle component of the constructs remained functionally intact. Constructs were next tested for the ability to respond to the major excitatory neurotransmitter in the gut, Ach. The effects of Ach are mediated by muscarinic receptors. M2R and M3R, the receptors responsible for mediating contraction,42 are abundant in longitudinal and circular smooth muscle, while M1R is most abundant in nerve cells.43 M1R stimulation results in enhanced neuronal Ach release.44,45 Treatment with 1 μM Ach caused an increase in force generation of 216±38 μN, while constructs pretreated with TTX demonstrated a significant attenuation in the increase to only 116±16 μN (Fig. 5C). These results demonstrated that both the smooth muscle component and the neuronal component of the constructs were capable of responding to the excitatory neurotransmitter, Ach, in a physiologically relevant manner. We next tested the response to the relaxant neuropeptide, VIP. Constructs treated with 1 μM VIP demonstrated a 256±46 μN reduction in force generation, which was significantly attenuated by TTX pretreatment (138±19 μN) (Fig. 5D). VIP is known to not only act directly on smooth muscle cells, but to also induce increased NO production from isolated ganglia of the IAS.46 These data indicate that the smooth muscle component of the bioengineered constructs responded appropriately to relaxant neurotransmitters, while the neuronal component mimicked in vivo IAS neurons in the ability to produce relaxant neurotransmitters in response to VIP. Finally, we directly tested the neuronal component of the constructs using EFS. Neuronally evoked relaxation was achieved by EFS (5 Hz, 0.5 ms) between parallel platinum plate electrodes in the organ bath. All constructs relaxed in response to EFS (−191±25 μN). Relaxation was completely abolished by TTX pretreatment (−8±4 μN) (Fig. 5E). These data demonstrate that EFS stimulation using parameters designed to induce relaxation in tissue, were able to induce relaxation in the bioengineered constructs, and that the relaxation was neuronally dependent. Data from the experiments are summarized in Figure 5F and Table 1.
FIG. 5.
Physiological analysis of bioengineered constructs. Day 10–12 constructs were tested for force generation in response to various stimuli in the presence or absence of the neurotoxin TTX (red line) (A) Constructs were observed to generate a spontaneous basal tone in the absence of any external stimulation over 30 min in a manner that was insensitive to TTX. (B) Constructs generated additional TTX-insensitive force in response to KCl treatment. Constructs contracted in response to Ach (C) and relaxed in response to VIP (D) in a TTX-sensitive manner. (E) Electrical field stimulation-evoked relaxation was completely abolished by TTX treatment. (F) A graphic representation of all force-generation experiments. *p<0.05; ***p<0.0001. ns, not significant; TTX, tetrodotoxin. Color images available online at www.liebertpub.com/tea
Table 1.
Summation of Physiological Data
| Control | TTX | |||||
|---|---|---|---|---|---|---|
| Mean | SEM | N | Mean | SEM | N | |
| Basal tone | 495.22 | 101.04 | 9 | 480.00 | 89.56 | 9 |
| KCl Δ Force | 282.23 | 52.72 | 8 | 276.26 | 52.08 | 8 |
| Ach Δ Force | 215.64 | 37.71 | 9 | 115.76 | 16.44 | 9 |
| VIP Δ Force | −255.61 | 45.77 | 9 | −138.38 | 19.06 | 8 |
| REFS Δ Force | −190.59 | 25.39 | 9 | −7.56 | 4.08 | 9 |
Ach, acetylcholine; REFS, relaxation electrical field stimulation; TTX, tetrodotoxin; VIP, vasoactive intestinal peptide.
Discussion
The cell–cell interactions, which result in the differentiation and development of the ENS primarily, occur between ENCCs and the developing mesenchyme. However, continued postnatal ENS development indicates that mature smooth muscle may be able to direct ENCC and ENS differentiation. To bioengineer a functionally innervated human gut smooth muscle construct of potential therapeutic use, mature smooth muscle cells isolated from postnatal tissue need to be used as a cell source.
The IAS is composed of tonic circular smooth muscle. It accounts for ∼70–85% of the resting anal canal pressure47 and plays a significant role in maintaining fecal continence. The IAS is characterized by its ability to maintain elevated basal tone; to relax to allow the passage of feces; and to contract following defecation, to reestablish closure. Establishment of basal tone is primarily due to the myogenic properties, while regulation of relaxation and contraction requires neuronal input.48,49
In this study, we report the bioengineering of physiologically functional, intrinsically innervated human IAS tissue constructs with human neurons and glial cells. Neuronal progenitor cells were cultured in a 3D matrix composed of type I collagen and laminin. The collagen component supplied mechanical strength, while the laminin component was included because of its importance in promoting neuronal development.19,50 A collagen matrix containing smooth muscle cells overlaid the 3D gel containing neuronal progenitor cells. The ability of smooth muscle cells from various tissue sources (including GI) to restructure collagen gels is well established51–53 as is the involvement of matrix metalloproteinase activity in this process.51,52 As the smooth muscle cells reformed the composite 3D hydrogel, the neuronal progenitor cells were brought into close association. The progenitor cells then differentiated to functionally innervate the smooth muscle. Our data indicate that development of mature neurons from ENCCs in this tissue culture model was completely dependent on smooth muscle input. It is likely that the development of a functional neural network in these constructs required signaling inputs from developing neural and glial as well as muscle cells. The bioengineered tissue constructs demonstrated characteristics of functional mature contractile IAS smooth muscle as well as functional excitatory and inhibitory motor neurons, and responded appropriately to physiologically relevant stimulatory and inhibitory neurotransmitters. In these experiments, both IAS smooth muscle cells and neuronal progenitor cells were isolated from tissues harvested from adult tissue donors. Whereas there is still lack of ICC and mucosa in these constructs, the ability to generate functionally innervated human smooth muscle tissue-like constructs from cells isolated from adult tissue represents an important step forward toward bioengineering gut replacement tissues. Adaptation of the techniques described here, which take advantage of enteric neuronal progenitor cell potential to differentiate into excitatory and inhibitory motor neurons and form bundles of neural fibers, could prove invaluable to the innervation/reinnervation of other tissues outside of the gut.
Acknowledgments
R.R.G. and S.R. contributed equally to this manuscript. We would like to thank Dr. D.H. Teitelbaum, Dr. E.A. Miyasaka, Dr. R.S. Herman, Dr. John E. Fortunato, and Dr. Giuseppe Orlando for supplying human tissues. This study was funded by the NIH Grants R01 DK071614 and 1RC1 DK087151. Corresponding author: Khalil N Bitar, PhD AGAF, kbitar@wakehealth.edu
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Okamoto E., and Ueda T.Embryogenesis of intramural ganglia of the gut and its relation to hirschsprung's disease. J Pediatr Surg 2,437, 1967 [Google Scholar]
- 2.Smith B.Pre- and postnatal development of the ganglion cells of the rectum and its surgical implications. J Pediatr Surg 3,386, 1968 [DOI] [PubMed] [Google Scholar]
- 3.Okamoto E., Satani M., and Kuwata K.Histologic and embryologic studies on the innervation of the pelvic viscera in patients with Hirschsprung's disease. Surg Gynecol Obstet 155,823, 1982 [PubMed] [Google Scholar]
- 4.Fu M., Lui V.C., Sham M.H., Cheung A.N., and Tam P.K.HOXB5 expression is spatially and temporarily regulated in human embryonic gut during neural crest cell colonization and differentiation of enteric neuroblasts. Dev Dyn 228,1, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Fu M., Tam P.K., Sham M.H., and Lui V.C.Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat Embryol (Berl) 208,33, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Wallace A.S., and Burns A.J.Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res 319,367, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Wester T., Eriksson L., Olsson Y., and Olsen L.Interstitial cells of Cajal in the human fetal small bowel as shown by c-kit immunohistochemistry. Gut 44,65, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young H.M., Bergner A.J., Anderson R.B., Enomoto H., Milbrandt J., Newgreen D.F., et al. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol 270,455, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Burns AJ.Migration of neural crest-derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int J Dev Biol 49,143, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama C., Uesaka T., Manabe T., Yonekura Y., Nagasawa T., Newgreen D.F., et al. Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat Neurosci 15,1211, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Burns A.J., and Douarin N.M.The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125,4335, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Lecoin L., Gabella G., and Le Douarin N.Origin of the c-kit-positive interstitial cells in the avian bowel. Development 122,725, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Young H.M., Ciampoli D., Southwell B.R., and Newgreen D.F.Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol 180,97, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Berseth C.L.Motor function in the stomach and small intestine in the neonate. NeoReviews 7,e28, 2006 [Google Scholar]
- 15.Wester T., O'Briain D.S., and Puri P.Notable postnatal alterations in the myenteric plexus of normal human bowel. Gut 44,666, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furness J.B.The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9,286, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Reynolds B.A., Tetzlaff W., and Weiss S.A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci 12,4565, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vescovi A.L., Reynolds B.A., Fraser D.D., and Weiss S.bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron 11,951, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Schafer K.H., Hagl C.I., and Rauch U.Differentiation of neurospheres from the enteric nervous system. Pediatr Surg Int 19,340, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Bondurand N., Natarajan D., Thapar N., Atkins C., and Pachnis V.Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 130,6387, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Rauch U., Hansgen A., Hagl C., Holland-Cunz S., and Schafer K.H.Isolation and cultivation of neuronal precursor cells from the developing human enteric nervous system as a tool for cell therapy in dysganglionosis. Int J Colorectal Dis 21,554, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Almond S., Lindley R.M., Kenny S.E., Connell M.G., and Edgar D.H.Characterisation and transplantation of enteric nervous system progenitor cells. Gut 56,489, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberpenning F., Meng J., Yoo J.J., and Atala A.De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 17,149, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Li R.K., Yau T.M., Weisel R.D., Mickle D.A., Sakai T., Choi A., et al. Construction of a bioengineered cardiac graft. J Thorac Cardiovasc Surg 119,368, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Matsubayashi K., Fedak P.W., Mickle D.A., Weisel R.D., Ozawa T., and Li R.K.Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation 108Suppl 1,II219, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Hecker L., Baar K., Dennis R.G., and Bitar K.N.Development of a three-dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289,G188, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Raghavan S., Lam M.T., Foster L.L., Gilmont R.R., Somara S., Takayama S., et al. Bioengineered three-dimensional physiological model of colonic longitudinal smooth muscle in vitro. Tissue Eng Part C Methods 16,999, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghavan S., Gilmont R.R., Miyasaka E.A., Somara S., Srinivasan S., Teitelbaum D.H., et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology 141 ,310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somara S., Gilmont R.R., Dennis R.G., and Bitar K.N.Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 137,53, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Joerger M., deJong D., Burylo A., Burgers J.A., Baas P., Huitema A.D., et al. Tubulin, BRCA1, ERCC1, Abraxas, RAP80 mRNA expression, p53/p21 immunohistochemistry and clinical outcome in patients with advanced non small-cell lung cancer receiving first-line platinum-gemcitabine chemotherapy. Lung Cancer 74,310, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Johnston A.L., Lun X., Rahn J.J., Liacini A., Wang L., Hamilton M.G., et al. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol 5,e212 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu S.H., Lin A.T., Chen K.K., Chiang H.S., and Chang L.S.Characterization of smooth muscle differentiation of purified human skeletal muscle-derived cells. J Cell Mol Med 15,587, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nourbakhsh N., Soleimani M., Taghipour Z., Karbalaie K., Mousavi S.B., Talebi A., et al. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int J Dev Biol 55,189, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Schiapparelli P., Enguita-German M., Balbuena J., Rey J.A., Lazcoz P., and Castresana J.S.Analysis of stemness gene expression and CD133 abnormal methylation in neuroblastoma cell lines. Oncol Rep 24,1355, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Lindley R.M., Hawcutt D.B., Connell M.G., Almond S.N., Vannucchi M.G., Faussone-Pellegrini M.S., et al. Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology 135,205, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Metzger M., Bareiss P.M., Danker T., Wagner S., Hennenlotter J., Guenther E., et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology 137,2063, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Young H.M., Ciampoli D., Hsuan J., and Canty A.J.Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev Dyn 216,137, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Young H.M., Bergner A.J., and Muller T.Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol 456,1, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Frid M.G., Shekhonin B.V., Koteliansky V.E., and Glukhova M.A.Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol 153,185, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Hall S.M., Hislop A.A., Pierce C.M., and Haworth S.G.Prenatal origins of human intrapulmonary arteries: formation and smooth muscle maturation. Am J Respir Cell Mol Biol 23,194, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Takata M., Moore J.W., Kao C.Y., and Fuhran F.A.Blokage of sodium conductance increase in lobster giant axon by tarichatoxin (tetrodotoxin). J Gen Physiol 49,977, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murthy K.S.Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68,345, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Harrington A.M., Peck C.J., Liu L., Burcher E., Hutson J.M., and Southwell B.R.Localization of muscarinic receptors M1R, M2R and M3R in the human colon. Neurogastroenterol Motil 22,999, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Ren J., and Harty R.F.Presynaptic muscarinic receptors modulate acetylcholine release from rat antral mucosal/submucosal nerves. Dig Dis Sci 39,1099, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Somogyi G.T., Tanowitz M., and de Groat WC.M1 muscarinic receptor-mediated facilitation of acetylcholine release in the rat urinary bladder. J Physiol 480 (Pt 1),81, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakder S., and Rattan S.Evidence for VIP-induced increase in NO production in myenteric neurons of opossum internal anal sphincter. Am J Physiol 270,G492, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Rao S.S.Pathophysiology of adult fecal incontinence. Gastroenterology 126,S14, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Freckner B.Function of anal sphincters in spinal man. Gut 16,638, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rattan S.The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil 17Suppl 1,50, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Chalazonitis A., Tennyson V.M., Kibbey M.C., Rothman T.P., and Gershon M.D.The alpha1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immunoselected from the fetal mouse gut. J Neurobiol 33,118, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Ceresa C.C., Knox A.J., and Johnson S.R.Use of a three-dimensional cell culture model to study airway smooth muscle-mast cell interactions in airway remodeling. Am J Physiol Lung Cell Mol Physiol 296,L1059, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Ivanov V., Roomi M.W., Kalinovsky T., Niedzwiecki A., and Rath M.Bioflavonoids effectively inhibit smooth muscle cell-mediated contraction of collagen matrix induced by angiotensin II. J Cardiovasc Pharmacol 46,570, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Oishi K., Itoh Y., Isshiki Y., Kai C., Takeda Y., Yamaura K., et al. Agonist-induced isometric contraction of smooth muscle cell-populated collagen gel fiber. Am J Physiol Cell Physiol 279,C1432, 2000 [DOI] [PubMed] [Google Scholar]