Abstract
Background: This study was performed to evaluate the safety and efficacy of a fully automated artificial pancreas using zone-model predictive control (zone-MPC) with the health monitoring system (HMS) during unannounced meals and overnight and exercise periods.
Subjects and Methods: A fully automated closed-loop artificial pancreas was evaluated in 12 subjects (eight women, four men) with type 1 diabetes (mean±SD age, 49.4±10.4 years; diabetes duration, 32.7±16.0 years; glycosylated hemoglobin, 7.3±1.2%). The zone-MPC controller used an a priori model that was initialized using the subject's total daily insulin. The controller was designed to keep glucose levels between 80 and 140 mg/dL. A hypoglycemia prediction algorithm, a module of the HMS, was used in conjunction with the zone controller to alert the user to consume carbohydrates if the glucose level was predicted to fall below 70 mg/dL in the next 15 min.
Results: The average time spent in the 70–180 mg/dL range, measured by the YSI glucose and lactate analyzer (Yellow Springs Instruments, Yellow Springs, OH), was 80% for the entire session, 92% overnight from 12 a.m. to 7 a.m., and 69% and 61% for the 5-h period after dinner and breakfast, respectively. The time spent <60 mg/dL for the entire session by YSI was 0%, with no safety events. The HMS sent appropriate warnings to prevent hypoglycemia via short and multimedia message services, at an average of 3.8 treatments per subject.
Conclusions: The combination of the zone-MPC controller and the HMS hypoglycemia prevention algorithm was able to safely regulate glucose in a tight range with no adverse events despite the challenges of unannounced meals and moderate exercise.
Background
People with type 1 diabetes lack the ability to make insulin and therefore are unable to regulate their blood glucose endogenously. They thus require exogenous insulin in order to avoid hyperglycemia, which over the long term can lead to a variety of micro- and macrovascular complications. Intensive glucose control with multiple daily injections or insulin pump therapy and self-monitoring of blood glucose, also known as standard care, has been shown to reduce the risk for long-term complications, but overly aggressive control can increase the risk of severe hypoglycemia, which can quickly lead to coma or death.1,2 Maintaining optimal glucose control in type 1 diabetes is therefore important for both short- and long-term health. Intensive control by the patient can be extremely difficult because of intra-individual variability in food intake, insulin sensitivity, and life events that affect glucose control (e.g., exercise, sickness, stress).
The rapid improvement of technology has enabled more precise glucose control. Continuous subcutaneous (SC) insulin infusion (CSII) pumps allow more flexible and more physiological insulin administration than multiple daily injections therapy. Continuous glucose monitoring (CGM) provides a richer set of information about glucose levels than fingerstick blood glucose monitoring, with values reported every 5 min, as opposed to three to four times daily with the fingerstick method. However, using these technologies effectively and safely continues to require a great deal of time and effort.
The difficulty of controlling blood glucose for subjects is evident in the lack of good control experienced by most people with type 1 diabetes: in a study of 25,833 people with type 1 diabetes by the T1D Exchange Clinic Registry, it was shown that the average glycosylated hemoglobin (A1C) was 8.3%,3 above the recommended limit of 7% set by the American Diabetes Association.4 In addition, 7% of those studied had experienced severe hypoglycemia with seizure or coma, and 8% had experienced diabetic ketoacidosis within the last year. To achieve better control and quality of life for people with type 1 diabetes, there has been a worldwide effort to develop an automated (closed-loop) system, or artificial pancreas (AP), for diabetes management using CGM, CSII, and intelligent algorithms to modulate CSII therapy based on CGM data. AP systems using SC continuous glucose monitors and CSII, called SC-SC APs, are widely studied but present a variety of design and control challenges that AP systems must take into account. These challenges include the time lag of insulin absorption and peak activity, delays between blood glucose concentration and interstitial glucose concentration, inaccuracy and imprecision of CGM sensors, and reliability issues with the hardware components or the communication among them.
Several promising AP designs that differ by the control algorithm and/or the level of automation (e.g., meal announcement to fully automated designs) have been presented in recent literature.5–22 Several groups have used the classical proportional-integral-derivative form of control with good results.9,21,22 For instance, Weinzimer et al.21 explored using proportional-integral-derivative control in a fully closed-loop formulation with higher set points at night. With three meals and an overnight period after a 12-h excluded run-in period, the mean glucose level was 141 mg/dL, with a mean of 112 mg/dL overnight for eight subjects.
Another approach that uses a set of rules to artificially mimic how an expert would control diabetes, known as Fuzzy Logic, has been tested and has also showed good results.10,20,23 For example, Phillip et al.24 conducted an overnight study in adolescents using MD-Logic, based on fuzzy logic, in which meals were announced and insulin boluses were given, hypoglycemia events were reduced by 68% over open-loop control, and the mean glucose level was reduced by 14 mg/dL. Mean time in the 70–140 mg/dL range was 55%, with 0% under 60 mg/dL.
One of the most promising approaches in AP algorithm design is model predictive control (MPC), as it predicts future glucose values and the effect of insulin delivery on the trajectory, optimizing delivery to achieve the desired control. It is also extremely flexible and can include system constraints and other features in its controller objective function, used to calculate the insulin dose. Since the initial publication on MPC for closed-loop insulin infusion in 1996 by Parker et al.,25,26 many researchers have successfully tested various MPC-controlled AP systems in controlled research settings.5,11,12,14–18,27 For example, Hovorka et al.20 used an MPC approach with a set point that varies between 104 and 131 mg/dL, depending on glycemia, prediction accuracy, and proximity to meals. For one segment of the study with 12 subjects (mean A1C of 7.8%) in a 13-h closed-loop session, meals were announced, and boluses were given; with meals containing 60 g of carbohydrates (CHO), 80% of the time was spent in the 70–144 mg/dL range overall, with 92% overnight.
Control to a specific glucose target or to a predefined glycemic “zone” or “range” is still an open question among the AP researchers, and different implementations use either concept. However, the latter approach more closely resembles clinical diabetes management recommendations in that it reverts to basal when predicted to remain in the zone,8,28 requires less frequent communication with the pump (i.e., less power consumption), and has been proposed as an important intermediate step toward tight, fully closed-loop glucose control. Recently, Grosman et al.29 and van Heusden et al.30 have described an approach called zone-MPC, which is explicitly designed to keep the glucose concentration within a particular zone instead of a set point to create a more quiescent controller that does not overreact to small changes in glucose (i.e., insulin dosage is not modified unless an excursion outside the zone is predicted). Breton et al.14 presented pilot study results of a multicenter clinical evaluation of a partially closed-loop system using a “control-to-range” MPC approach, which was designed to safely maximize time in the desired range by targeting a specific glucose concentration. In addition, the hypoglycemia–hyperglycemia minimizer system demonstrates zone control using a semiautomated control design with a manual preprandial bolus.31,32
Here we present results from the first clinical evaluation and proof of concept of a diabetes management system controlled by zone-MPC.33 The system was designed to be fully automated closed-loop, with no manual boluses or meal announcements necessary. In addition, this study represents the first clinical evaluation of the health monitoring system (HMS), an independent software layer designed to monitor safety risks (e.g., impending hypoglycemia) and accordingly send alerts to the user and/or remote caregivers.34 The HMS is used in parallel to the zone-MPC controller to act as a safety layer that does not inhibit the controller by “clipping” the output.
Research Design and Methods
Twelve subjects with type 1 diabetes were recruited for the study, which was approved by the Food and Drug Administration and the Santa Barbara Cottage Health System's Institutional Review Board. All subjects signed the Institutional Review Board–approved informed consent form. Inclusion criteria included age between 21 and 65 years, type 1 diabetes duration of at least 1 year, and use of an insulin pump with rapid-acting insulin for at least 6 months. Exclusion criteria included pregnancy, diabetic ketoacidosis within the past 6 months, A1C >9.0%, severe hypoglycemia within the past year, and concomitant disease or medication use affecting metabolic control. Subjects were screened with a comprehensive metabolic panel, complete blood count, and thyroid tests, height and weight were measured, and the subject's insulin pump information was downloaded and confirmed [basal rates, average total daily dose, insulin-to-CHO ratio(s), and correction factor(s)].
Closed-loop system
The objective of this study was to evaluate the ability of a closed-loop AP to regulate blood glucose during a 24-h trial with unannounced meals and an unannounced exercise challenge. The control algorithm of the system was the zone-MPC algorithm described by Grosman et al.29 with an a priori model tuned only by the subject's daily insulin requirements, described by van Heusden et al.30 The HMS, described by Harvey et al.,34 operated in parallel to the zone-MPC algorithm as an additional safety feature to alarm for imminent hypoglycemia. The AP system was programmed to adjust insulin doses only when glucose concentration was, or was predicted to be, outside the specified zone (80–140 mg/dL).
In this study, measurement of interstitial glucose was performed with the Dexcom® SEVEN® PLUS (Dexcom Corp., San Diego, CA) CGM system, and rapid-acting insulin was administered with the OneTouch® Ping® glucose management system (Animas Corp., West Chester, PA) insulin pump. Communication among the system's components occurred via APS©35 version 3.0 run within a MATLAB© (MathWorks, Natick, MA) environment on a laptop computer. The Dexcom SEVEN PLUS and OneTouch Ping insulin pump are both approved devices and have not been modified.
The system functioned autonomously, unless intervention was requested by the HMS. Specifically, if the HMS predicted that hypoglycemia (<70 mg/dL) was imminent in the next 15 min, the system issued a local audiovisual alert and sent redundant short message service and multimedia message service alerts to the attending physician. Alerts contained the time, glucose level, predicted glucose levels, and a recommendation to consume 16 g of rescue CHO, which was consumed immediately by the subject per protocol.
In-clinic schedule
The study consisted of a single 24-h closed-loop admission to Sansum Diabetes Research Institute (Santa Barbara, CA). Subjects were admitted to the clinic at 4:00 p.m., and the closed-loop sessions started between 4:30 and 6:00 p.m. that day and ended at approximately the same time the next day. No adjustments to the subjects' basal rates, correction factors, or insulin-to-CHO ratio were made prior to admission. Subjects were asked to aim for a glucose concentration around 100 mg/dL at admission. During the 24-h study subjects consumed two unannounced mixed meals: dinner (50 g of CHO) and breakfast (40 g of CHO). Two optional snacks containing 16 g of CHO were given just before and 3 h after the exercise if the glucose level was below 120 mg/dL. All meals and snacks were unannounced to the AP system. All subjects exercised for 30 min on a recumbent bicycle (15 min on, 5 min rest, 15 min on) at 50% of their predicted heart rate reserve based on the formula of Karvonen et al.36 Exercise was also unannounced to the AP device. Each subject was monitored during the exercise using a Polar© (Polar Electro, Kempele, Finland) RS400 heart rate monitor, along with a continuous electrocardiogram if the individual's type 1 diabetes duration was >20 years. Subjects were monitored for approximately 4 h following exercise before completing the closed-loop session.
At 2–3 days prior to each subject's admission, two CGM sensors were inserted with receivers set in blinded mode. On the afternoon of admission both CGM devices were unblinded, and the investigator determined a single sensor to be used as the primary sensor for closed-loop control based on communication quality and accuracy. The secondary sensor was only used as a backup in case of primary sensor failure. During the closed-loop visit, CGM sensors were calibrated 30 min prior to meals and at bedtime in addition to the default manufacturer's recommended calibrations.
Reference laboratory plasma blood glucose measurements were obtained from venous samples every 30 min and analyzed using the YSI 2300 STAT Plus™ glucose and lactate analyzer (Yellow Springs Instruments, Yellow Springs, OH). Both the CGM and the YSI results obtained during the sessions were used in the analysis of the AP device performance. Plasma glucose values were obtained every 15 min during exercise and any hypo- or hyperglycemic excursion and every 30 min otherwise. Increased sample frequency for hypoglycemia occurred when a plasma glucose sample was <70 mg/dL by YSI or after an HMS alert and continued every 15 min until the plasma glucose level was >80 mg/dL. The goal of the AP device was to operate without outside intervention when challenged by unannounced meals and exercise unless the outside intervention was requested by the HMS. Ideally, the HMS would not be invoked; it was designed as a safety instrumented system to be used when levels were predicted to go out of control.
Results
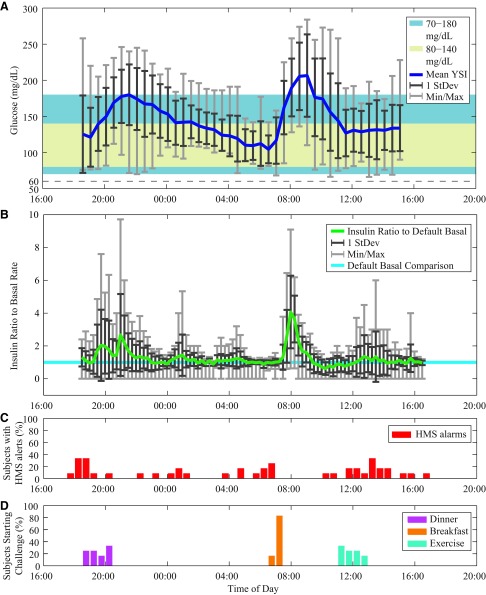
Twelve subjects (eight women, four men) with type 1 diabetes participated in single 24-h closed-loop sessions. Demographics and closed-loop details are shown in Table 1. A summary of results is shown in Figure 1, with data from each session aligned by time and averaged for YSI blood glucose (Fig. 1A) and insulin delivered (Fig. 1B). The majority of time (80% on average) was spent in the 70–180 mg/dL range, with deviations into the higher range after mealtimes. The average amount of insulin given during the trial was around basal overnight and during exercise, with the major deviations above basal after meals, up to six times the basal level. The total insulin delivered was an average of 65% of the usual total daily insulin requirement for each subject, which is expected with the absence of a lunch meal. The percentage of subjects experiencing HMS alerts per 30 min is shown in Figure 1C, with the starting time of each of the three unannounced challenges (dinner, breakfast, and exercise), shown as the percentage of subjects who have commenced the challenge (Fig. 1D). For the dinner challenge, the start time varied by 75 min among subjects; for breakfast, the start time varied by 30 min; and for exercise, the start time varied by 90 min. All the results are available in Supplementary Figures 1-12 (Supplementary Data are available online at www.liebertonline.com/dia) with full disclosure.
Table 1.
Results Summary as Provided by Continuous Glucose Monitoring and Blood Glucose Reference (YSI) of 12 Fully Automated Closed-Loop Studies with Demographic and Clinical Parameters of the Individual Subjects
| Study number | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Total or mean±SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Sex | 4 M/8 F | F | M | M | M | F | F | F | F | M | F | F | F |
| Height (cm) | 170.4±11.3 | 157 | 168 | 193 | 173 | 158 | 165 | 165 | 175 | 175 | 189 | 165 | 162 |
| Weight (kg) | 78.1±22.8 | 61 | 96 | 98 | 74 | 132 | 66 | 60 | 67 | 95 | 76 | 61 | 53 |
| Age (years) | 49.4±10.4 | 39 | 41 | 56 | 58 | 61 | 42 | 52 | 28 | 62 | 45 | 56 | 53 |
| TDD (U) | 37.8±14.4 | 30 | 73 | 45 | 27 | 55 | 35 | 23 | 34 | 43 | 32 | 32 | 26 |
| CF (mg/dL/U) | 52.7±23.9 | 70 | 25 | 33 | 30 | 32 | 30 | 100 | 50 | 53 | 70 | 60 | 80 |
| C:I (g of CHO/U) | 10.8±2.7 | 15 | 8 | 10 | 8 | 6 | 12 | 10 | 9 | 14 | 15 | 12 | 11 |
| Duration of diabetes (years) | 32.7±16.0 | 23 | 21 | 43 | 47 | 60 | 14 | 43 | 7 | 38 | 34 | 17 | 45 |
| Duration of CL (h) | 23.5±0.7 | 24.0 | 23.3 | 24.2 | 23.9 | 22.2 | 23.8 | 24.0 | 23.3 | 23.0 | 23.9 | 24.0 | 22.5 |
| HMS treatments (g of CHO) | 58±31 | 32 | 64 | 80 | 88 | 80 | 16 | 48 | 32 | 16 | 80 | 112 | 48 |
| Sensor glucose (CGM) | |||||||||||||
| BG (mg/dL) at | |||||||||||||
| Start of CL | 126±55.9 | 263 | 204 | 116 | 108 | 87.0 | 85.0 | 175 | 91.0 | 93.0 | 79.0 | 132 | 81.0 |
| End of CL | 128±41.4 | 162 | 115 | 112 | 72.0 | 241 | 108 | 127 | 86.0 | 130 | 150 | 131 | 107 |
| Start of exercise | 166±41.1 | 183 | 91.0 | 147 | 256 | 108 | 170 | 149 | 177 | 187 | 144 | 176 | 199 |
| 3 h after exercise | 140±38.3 | 141 | 154 | 135 | 106 | 249 | 118 | 162 | 111 | 159 | 108 | 112 | 122 |
| LBGI | 0.7±0.4 | 0.2 | 0.7 | 0.6 | 1.1 | 1.3 | 0.1 | 0.6 | 0.5 | 0.3 | 0.9 | 1.2 | 0.5 |
| HBGI | 6.2±2.5 | 4.1 | 5.6 | 5.9 | 6.1 | 5.0 | 12.9 | 8.3 | 5.6 | 8.4 | 4.3 | 4.6 | 3.3 |
| BG (mg/dL) | |||||||||||||
| Maximum | 295±37.2 | 263 | 312 | 254 | 297 | 287 | 320 | 378 | 287 | 313 | 307 | 296 | 221 |
| Minimum | 62.1±11.2 | 73.0 | 64.0 | 64.0 | 64.0 | 43.0 | 84.0 | 60.0 | 64.0 | 70.0 | 42.0 | 54.0 | 63.0 |
| Mean | 153±16.4 | 149 | 150 | 154 | 142 | 142 | 198 | 164 | 150 | 170 | 142 | 139 | 139 |
| SD | 55±10 | 36 | 54 | 50 | 70 | 56 | 57 | 72 | 55 | 59 | 51 | 57 | 38 |
| Time (%) at glucose level (mg/dL) | |||||||||||||
| <60 | 0.4±0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 2.9 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 0.7 | 0.0 |
| <70 | 1.8±1.4 | 0.0 | 2.6 | 1.1 | 1.1 | 3.3 | 0.0 | 2.3 | 1.7 | 0.0 | 3.0 | 4.5 | 2.0 |
| <80 | 4.8±3.0 | 1.9 | 5.9 | 5.0 | 8.0 | 7.1 | 0.0 | 5.1 | 3.5 | 2.4 | 5.2 | 11.2 | 2.8 |
| 80–140 | 45.7±12.4 | 39.3 | 45.4 | 39.5 | 64.9 | 54.4 | 14.9 | 41.0 | 50.6 | 41.5 | 48.3 | 54.9 | 53.8 |
| 140–180 | 20.7±9.1 | 38.9 | 19.7 | 22.8 | 4.6 | 13.7 | 23.7 | 22.7 | 21.2 | 12.3 | 30.5 | 13.1 | 25.1 |
| 180–250 | 20.5±10.4 | 18.3 | 21.9 | 30.6 | 4.2 | 17.8 | 41.2 | 18.8 | 16.5 | 34.8 | 11.2 | 12.7 | 18.3 |
| >250 | 8.3±6.2 | 1.6 | 7.1 | 2.1 | 18.3 | 7.1 | 20.2 | 12.5 | 8.2 | 9.1 | 4.8 | 8.2 | 0.0 |
| Plasma glucose (YSI) | |||||||||||||
| BG (mg/dL) at | |||||||||||||
| Start of CL | 131±58.5 | 244 | 258 | 133 | 106 | 105 | 98.7 | 150 | 129 | 89.7 | 63.9 | 114 | 76.0 |
| End of CL | 119±38.3 | 84.5 | 116 | 112 | 88.6 | 226 | 113 | 94.8 | 120 | 93.8 | 157 | 139 | 88.8 |
| Start of exercise | 140±29.8 | 128 | 74.6 | 133 | 189 | 128 | 159 | 120 | 182 | 120 | 141 | 167 | 141 |
| 3 h after exercise | 127±33.9 | 83.3 | 131 | 133 | 123 | 228 | 113 | 120 | 134 | 93.8 | 127 | 123 | 112 |
| LBGI | 0.6±0.5 | 1.3 | 1.0 | 0.4 | 0.4 | 0.2 | 0.0 | 1.1 | 0.0 | 0.2 | 1.0 | 0.3 | 1.6 |
| HBGI | 3.9±1.8 | 1.4 | 4.1 | 5.1 | 4.9 | 5.1 | 8.4 | 3.3 | 3.1 | 3.3 | 2.6 | 4.5 | 1.4 |
| BG (mg/dL) | |||||||||||||
| Maximum | 245±27.6 | 244 | 258 | 246 | 273 | 284 | 274 | 246 | 219 | 238 | 210 | 261 | 185 |
| Minimum | 78.8±12.1 | 69.2 | 66.1 | 80.6 | 75.9 | 88 | 97.1 | 68.1 | 101 | 83.2 | 63.9 | 86.7 | 66.3 |
| Mean | 140±15.7 | 115 | 137 | 150 | 146 | 149 | 177 | 131 | 142 | 141 | 133 | 145 | 116 |
| SD | 43±6.9 | 35 | 50 | 47 | 51 | 50 | 43 | 46 | 33 | 36 | 36 | 49 | 34 |
| Time (%) at glucose level (mg/dL) | |||||||||||||
| <60 | 0.0±0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| <70 | 1.2±1.7 | 1.9 | 4.3 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | 1.9 | 0.0 | 4.3 |
| <80 | 5.1±5.9 | 13.5 | 8.7 | 0.0 | 4.1 | 0.0 | 0.0 | 8.0 | 0.0 | 0.0 | 11.5 | 0.0 | 14.9 |
| 80–140 | 51.8±13.0 | 67.3 | 52.2 | 47.1 | 49.0 | 55.8 | 18.4 | 54.0 | 61.2 | 58.7 | 38.5 | 60.0 | 59.6 |
| 140–180 | 24.5±8.6 | 15.4 | 17.4 | 29.4 | 24.5 | 18.6 | 36.7 | 18.0 | 24.5 | 30.4 | 42.3 | 18.0 | 19.1 |
| 180–250 | 16.5±9.4 | 3.8 | 19.6 | 23.5 | 16.3 | 20.9 | 38.8 | 20.0 | 14.3 | 10.9 | 7.7 | 16.0 | 6.4 |
| >250 | 2.1±2.8 | 0.0 | 2.2 | 0.0 | 6.1 | 4.7 | 6.1 | 0.0 | 0.0 | 0.0 | 0.0 | 6.0 | 0.0 |
BG, blood glucose; CF, correction factor; CGM, continuous glucose monitoring; C:I, carbohydrate:insulin; CHO, carbohydrates; CL, closed-loop; F, female; HBGI, High Blood Glucose Index; HMS, health monitoring system; LBGI, Low Blood Glucose Index; TDD, total daily dose.
FIG. 1.
(A) Glucose results for all trials, as mean YSI (YSI glucose and lactate analyzer) values, 1 SD (StDev), and the minimum/maximum (Min/Max) values. The control zone (80–140 mg/dL) is shown as the cream-colored band, and the safe zone shown as the light blue band (70–180 mg/dL). (B) Mean insulin delivered for all trials, along with 1 StDev and the Min/Max values of the insulin delivered as a ratio to the default basal pattern for each subject. (C) The percentage of subjects experiencing health monitoring system (HMS) alarms each 30 min. (D) The time of starting the challenges, shown as the percentage of subjects who had commenced each task of dinner (purple), breakfast (orange), and exercise (blue). (Color graphics available online at www.liebertonline.com/dia)
As can be seen in the glucose tracing in Figure 1A, both unannounced meals resulted in an overall moderate postprandial rise in glucose level. Some subjects had a higher postprandial peak, generally when they had started at a higher value at mealtime. After dinner, the average peak was 230±38 mg/dL at 124±51 min by CGM and 196±26 mg/dL at 128±43 min by YSI. After breakfast, the average peak was 275±59 mg/dL at 109±26 min by CGM and 220±51 mg/dL at 106±32 min by YSI. Average baseline premeal glucose values for all meals were 111±29 mg/dL by YSI and 114±40 mg/dL by CGM for dinner and 115±34 mg/dL by YSI and 116±26 mg/dL by CGM for breakfast. Mild activity was undertaken for all subjects for 30 min beginning between 11:10 a.m. and 12:40 p.m. The average YSI values were relatively steady during exercise and for the rest of the study, with average values of 140±30 mg/dL at the start of exercise and 127±34 mg/dL at 3 h after exercise. Five subjects consumed 16-g CHO snacks before exercise, and three subjects consumed 16-g CHO snacks after exercise, as per protocol.
The HMS alerts in Figure 1C show that the majority of hypoglycemia alerts occurred within 2 h of startup (23%), overnight or in the early morning (30%), or after exercise (27%). On average, four alerts were activated per subject (44 total alerts), at average CGM and YSI values of 82 mg/dL and 96 mg/dL, respectively. The “rescue” CHO (16 g) given for impending hypoglycemia resulted in an average increase of 11 mg/dL within 30 min. Some of the scenarios necessitated a second alarm within 1 h of the first treatment (25%). Only 1.2% of time was spent below 70 mg/dL, and no time was spent under 60 mg/dL by YSI.
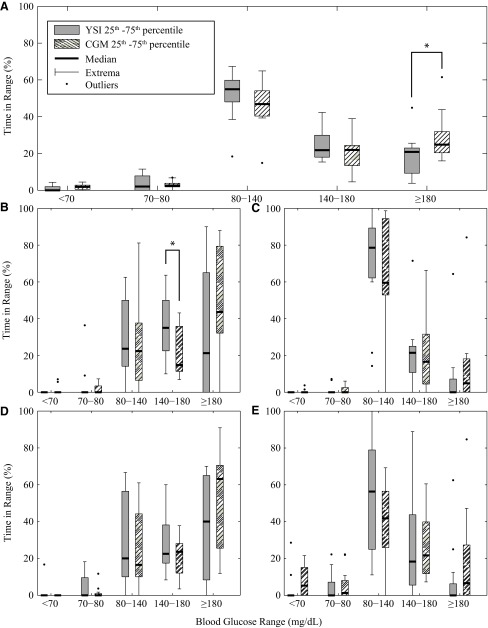
Time in zone values for both CGM and YSI samples are shown in Figure 2 for the trial duration (Fig. 2A), 0–5 h after dinner (Fig. 2B), overnight from midnight to 7 a.m. (Fig. 2C), 0–5 h after breakfast (Fig. 2D), and 0–3 h after exercise (Fig. 2E). The time in each glycemic zone is represented as a box plot to show both which glucose range was dominant during each period as well as the difference between YSI and CGM data. The target zone of 80–140 mg/dL was dominant for the overall closed-loop, overnight (63% by CGM, 70% by YSI), and exercise, with notable deviations into the 140–180 mg/dL and >180 mg/dL range after meals, as can be expected because of the delays in the system and unannounced disturbance of meals. Time in mild hyperglycemia (180–250 mg/dL) was limited to 17% despite the challenge of postprandial rises in glucose level from unannounced meals, with only 2.1% between 250 and 300 mg/dL. The unannounced meals resulted in an average rise of 95 mg/dL from baseline by YSI (range, 39–197 mg/dL) that peaked approximately 2 h after consumption.
FIG. 2.
Percentage of time in different glycemic ranges based on continuous glucose monitoring (CGM) (striped columns) and YSI (YSI glucose and lactate analyzer) (gray columns) for (A) the whole study, (B) start of dinner to 5 h after, (C) overnight from midnight to 7 a.m., (D) start of breakfast to 5 h after, and (E) start of exercise to 3 h after end. Statistically significant differences between CGM and YSI results (P<0.05) are denoted by asterisks above the box-and-whisker plots.
A direct comparison between CGM and YSI is shown in Figure 3, with cumulative time in range shown in Figure 3A and the Clarke Error Grid37 shown in Figure 3B. The YSI data align more closely to the 70–180 mg/dL region (80% and 19%, respectively, of values were in the 70–180 mg/dL and >180 mg/dL regions), whereas the CGM values were skewed higher (69% in the 70–180 mg/dL region and 29% in the >180 mg/dL region, respectively). This is confirmed in the Clarke Error Grid, with 98% of points in the combined Zones A and B but 32% in Zone B, generally the upper Zone B region.
FIG. 3.
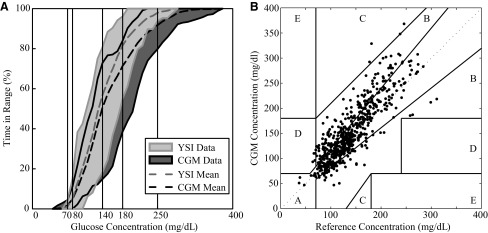
Percentage of time in different glycemic ranges over the study day for all subjects based on (A) continuous glucose monitoring (CGM) (dark gray) and YSI (YSI glucose and lactate analyzer) (light gray) and (B) Clarke Error Grid37 for YSI versus CGM. No subjects experienced hypoglycemia, and on average 80% of the time was in the 70–180 mg/dL range, with 17% of the time in the mild hyperglycemia range (180–250 mg/dL) and 2% of the time in the hyperglycemia range (>250 mg/dL), as measured by YSI. As seen in the Clarke Error Grid, 98% of measurements were in Zone A or B (clinically accurate or benign, respectively).37
Discussion
The fully automated system with unannounced meals and exercise using advanced design of zone-MPC with the HMS successfully regulated glycemia with no safety events. This study suggests that a fully automated AP using current glucose sensor technology is feasible but may be limited to small- to medium-sized meals with currently available insulin delivery methods. The ability to provide safe and effective control to people with type 1 diabetes without the constant need to estimate meals or to modulate insulin delivery due to exercise is an achievable goal that will be beneficial to people with type 1 diabetes and their families.
Becasue of the major differences in protocols, comparison with other studies is difficult. Overnight control is less affected by the protocol, meals, etc., and the results of the current study compare favorably to the studies mentioned earlier. In the current study, 65% of time was spent in the 70–140 mg/dL range by CGM (71% by YSI), and 86% of time was spent in the 70–180 mg/dL range (92% by YSI). In the studies of Hovorka et al.20 and Phillip et al.,24 overnight control in that range was 92% (by plasma) and 55%, respectively. In the study of Breton et al.,14 overnight control in the 70–180 mg/dL range was 98%. All of those trials included announced meals, whereas, during the study discussed here, unannounced meals sometimes resulted in hyperglycemia that lasted into the night. In fact, 21% of time was spent in the 140–180 mg/dL range by CGM, indicating that the high glucose levels after unannounced meals were still affecting overnight glycemia.
For the entire closed-loop duration, excluding meals (from start to 5 h after), excluding exercise (from start to 3 h after), and excluding both meals and exercise, 64%, 49%, and 70%, respectively, of time was spent in the 70–140 mg/dL range by CGM and 72%, 55%, and 76%, respectively, by YSI. In the study of Hovorka et al.,20 80% of time was spent in the 70–140 mg/dL range by plasma. In the 70–180 mg/dL range, 85%, 69%, and 88% of time was spent measured by CGM and 91%, 79%, and 92% measured by YSI by excluding meals, exercise, and both, respectively. In the study of Breton et al.,14 closed-loop control resulted in 90% of time in the 70–180 mg/dL range by CGM. Both of those studies included announced meals and, excluding unannounced portions of the study presented here, had comparable results.
The study had some limitations: no control group was tested; meal sizes were small to medium sized, although unannounced; some subjects overdosed insulin before admission (not per protocol), resulting in HMS alarms very soon after startup; several HMS treatments were given to many of the subjects, dramatically increasing the total amount of CHO consumed and sometimes resulting in rebound hyperglycemia when near meals; many HMS alarms occurred overnight, which may be ignored in an outpatient setting; and subjects remained mostly sedentary during the 24-h study except for the 30-min exercise session. This feasibility study was useful in updating future protocols to avoid some of these problems, as well as providing information in tuning of future algorithms (including alternative zones for different subjects or time-dependent zones). Assessing the AP in conditions more closely resembling real life will yield valuable results. Long-term and/or outpatient studies are needed to fully assess the performance of the AP device and its components and to optimize the controller algorithms. Rigorous testing during various meals, varied exercise intensity levels, and stress conditions need to be examined in the future.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grant RO1-DK-085628). Product support was received from Dexcom Inc., LifeScan, Inc., and Animas Corporation. The authors would like to thank the clinical staff at the Sansum Diabetes Research Institute who spent long overnight hours during the trials, including Kristin Castorino, DO, Kateryna Markova, MD, Erin Beveridge, Adam Castorino, Maia Bradley, Nicolas Santibanez, Jacqueline Wiley, Jennifer Lane, and Joseph Shivers.
Author Disclosure Statement
E.D. received honoraria for scientific lectures from Animas and is a board member of Artificial Pancreas Technologies. L.J. received honoraria for scientific lectures and travel reimbursement from Animas, Eli Lilly, Insulet, MannKind, Novo Nordisk, and Roche and research grants and product support from Animas, Abbott, Dexcom, Eli Lilly, Insulet, LifeScan, Medtronic, Novo Nordisk, Roche, and Sanofi. F.J.D. III received honoraria for scientific lectures from Animas and is a board member of Artificial Pancreas Technologies. H.C.Z. received honoraria for scientific lectures and travel reimbursement from Animas, Cellnovo, Insulet, MannKind, and Roche and research grants and product support from Animas, Abbott, Dexcom, Eli Lilly, GluMetrics, Insulet, LifeScan, Medtronic, Novo Nordisk, Roche, and Sanofi and is a board member of Artificial Pancreas Technologies. R.A.H., W.B., and D.S. declare no competing financial interests exist.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996;45:1289–1298 [PubMed] [Google Scholar]
- 3.Bellazzi R, Arcelloni M, Bensa G, Blankenfeld H, Brugues E, Carson E, Cobelli C, Cramp D, D'Annunzio G, De Cata P, De Leiva A, Deutsch T, Fratino P, Gazzaruso C, Garcia A, Gergely T, Gomez E, Harvey F, Ferrari P, Hernando E, Boulos MK, Larizza C, Ludekke H, Maran A, Nucci G, Pennati C, Ramat S, Roudsari A, Rigla M, Stefanelli M: Design, methods, and evaluation directions of a multi-access service for the management of diabetes mellitus patients. Diabetes Technol Ther 2003;5:621–629 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association: Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER: A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010;2(27):27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Khatib FH, Jiang J, Damiano ER: A feasibility study of bihormonal closed-loop blood glucose control using dual subcutaneous infusion of insulin and glucagon in ambulatory diabetic swine. J Diabetes Sci Technol 2009;3:789–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castle JR, Engle JM, Youssef JE, Massoud RG, Yuen KC, Kagan R, Ward WK: Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haidar A, Legault L, Dallaire M, Alkhateeb A, Coriati A, Messier V, Cheng P, Millette M, Boulet B, Rabasa-Lhoret R: Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 2013;185:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steil GM, Palerm CC, Kurtz N, Voskanyan G, Roy A, Paz S, Kandeel FR: The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M: MD-Logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care 2010;33:1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruttomesso D, Farret A, Costa S, Marescotti MC, Vettore M, Avogaro A, Tiengo A, Dalla Man C, Place J, Facchinetti A, Guerra S, Magni L, De Nicolao G, Cobelli C, Renard E, Maran A: Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Technol 2009;3:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovorka R, Kumareswaran K, Harris J, Allen JM, Elleri D, Xing D, Kollman C, Nodale M, Murphy HR, Dunger DB, Amiel SA, Heller SR, Wilinska ME, Evans ML: Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patek SD, Magni L, Dassau E, Karvetski C, Toffanin C, De Nicolao G, Del Favero S, Breton M, Man CD, Renard E, Zisser H, Doyle FJ, III, Cobelli C, Kovatchev BP; International Artificial Pancreas (iAP) Study Group: Modular closed-loop control of diabetes. IEEE Trans Biomed Eng 2012;59:2986–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Toffanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle FJ, III, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B; International Artificial Pancreas Study Group: Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes 2012;61:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B: Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Technol 2009;3:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dassau E, Zisser H, Harvey RA, Percival MW, Grosman B, Bevier W, Atlas E, Miller S, Nimri R, Jovanovic L, Doyle FJ, III: Clinical evaluation of a personalized artificial pancreas. Diabetes Care 2013;36:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB: Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 18.Cobelli C, Renard E, Kovatchev BP, Keith-Hynes P, Ben Brahim N, Place J, Del Favero S, Breton M, Farret A, Bruttomesso D, Dassau E, Zisser H, Doyle FJ, III, Patek SD, Avogaro A: Pilot studies of wearable outpatient artificial pancreas in type 1 diabetes. Diabetes Care 2012;35:e65–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dauber A, Corcia L, Safer J, Agus MS, Einis S, Steil GM: Closed-loop insulin therapy improves glycemic control in children aged <7 years: a randomized controlled trial. Diabetes Care 2013;36:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovorka R, Kumareswaran K, Harris J, Allen JM, Elleri D, Xing D, Kollman C, Nodale M, Murphy HR, Dunger DB, Amiel SA, Heller SR, Wilinska ME, Evans ML: Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ Apr 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV: Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 22.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF: Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 23.Mauseth R, Wang Y, Dassau E, Kircher R, Jr, Matheson D, Zisser H, Jovanovic L, Doyle FJ, III: Proposed clinical application for tuning fuzzy logic controller of artificial pancreas utilizing a personalization factor. J Diabetes Sci Technol 2010;4:913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, Biester T, Stefanija MA, Muller I, Nimri R, Danne T: Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 25.Parker RS, Doyle FJ, III, Harting JE, Peppas NA: Model predictive control for infusion pump insulin delivery. In: Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vol. 5: Bridging Disciplines for Biomedicine. Piscataway, NJ: IEEE, 1996:1822–1823 [Google Scholar]
- 26.Parker RS, Doyle FJ, III, Peppas NA: A model-based algorithm for blood glucose control in type 1 diabetic patients. IEEE Trans Biomed Eng 1999;46:148–157 [DOI] [PubMed] [Google Scholar]
- 27.Kovatchev B, Cobelli C, Renard E, Anderson S, Breton M, Patek S, Clarke W, Bruttomesso D, Maran A, Costa S, Avogaro A, Dalla Man C, Facchinetti A, Magni L, De Nicolao G, Place J, Farret A: Multinational study of subcutaneous model-predictive closed-loop control in type 1 diabetes mellitus: summary of the results. J Diabetes Sci Technol 2010;4:1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda O, Garber AJ, Hirsch IB, Horton ES, Ismail-Beigi F, Jellinger PS, Jones KL, Jovanovic L, Lebovitz H, Levy P, Moghissi ES, Orzeck EA, Vinik AI, Wyne KL: American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract 2011;17(Suppl 2):1–53 [DOI] [PubMed] [Google Scholar]
- 29.Grosman B, Dassau E, Zisser HC, Jovanovic L, Doyle FJ, III: Zone model predictive control: a strategy to minimize hyper- and hypoglycemic events. J Diabetes Sci Technol 2010;4:961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Heusden K, Dassau E, Zisser HC, Seborg DE, Doyle FJ, III: Control-relevant models for glucose control using a priori patient characteristics. IEEE Trans Biomed Eng 2012;59:1839–1849 [DOI] [PubMed] [Google Scholar]
- 31.Mackowiak L, Finan DA, McCann TW, Jr, Venugopalan R, Zisser H, Anhalt H: Feasibility study assessing hypoglycemia-hyperglycemia minimizer (HHM) system in patients with type 1 diabetes (T1DM) in a clinical research center [abstract]. Diabetes 2012;61(Suppl 1):A233 [Google Scholar]
- 32.Venugopalan R, Finan DA, McCann TW, Jr, Mackowiak L, Dassau E, Patek SD, Anhalt H: Performance metrics of a hypoglycemia-hyperglycemia minimizer (HHM) system in a closed-loop feasibility study [abstract]. Diabetes 2012;61(Suppl 1):A235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zisser CH, Dassau E, Bevier W, Harvey R, Jovanovič L, Doyle FJ, III: Clinical evaluation of a fully-automated artificial pancreas using zone-model predictive control with health monitoring system [abstract]. Diabetes 2012;61(Suppl 1):LB8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey RA, Dassau E, Zisser H, Seborg DE, Jovanovič L, Doyle FJ, III: Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol 2012;6:1345–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dassau E, Zisser H, Palerm CC, Buckingham BA, Jovanovič L, Doyle FJ, III: Modular artificial β-cell system: a prototype for clinical research. J Diabetes Sci Technol 2008;2:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karvonen MJ, Kentala E, Mustala O: The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn 1957;35:307–315 [PubMed] [Google Scholar]
- 37.Clarke WL: The original Clarke Error Grid Analysis (EGA). Diabetes Technol Ther 2005;7:776–779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.