Abstract
Background
Coumarins are an important class of widely distributed heterocyclic natural products exhibiting a broad pharmacological profile. In this work, a new series of coumarins bearing substituted 3,4-dihydro-2H-benzothiazines were described as potential analgesic agents. The clinical use of NSAIDs as traditional analgesics is associated with side effects such as gastrointestinal lesions and nephrotoxicity. Therefore, the discovery of new safer drugs represents a challenging goal for such a research area.
Results
The target compounds 3-(3-methyl-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-2H-chromen-2-ones 2a-u were synthesized and characterized by spectral data. The antinociceptive properties of target compounds were determined by formalin-induced test and acetic acid-induced writhing test in mice. Among the tested compounds, compound 2u bearing 2-(4-(methylsulfonyl)benzoyl)- moiety on benzothiazine ring and 4-(methylsulfonyl)phenacyloxy- group on the 7 position of coumarin nucleus showed better profile of antinocecieption in both models. It was more effective than mefenamic acid during the late phase of formalin-induced test as well as in the acetic acid-induced writhing test.
Conclusion
Considering the significant antinoceciptive action of phenacyloxycoumarin derivatives, compound 2u prototype might be further used as model to obtain new more potent analgesic drugs.
Keywords: Analgesic activity, Antinociception, Coumarin, Benzothiazine, Formalin test, Writhing test
Introduction
Pain is an uncomfortable sensation that alerts the human organs about a current or potential damage to tissues [1]. It has been accepted that pain can widely affect human life quality, and its management is considered as a main challenge in pharmacotherapy [2]. NSAIDs are one of major classes of traditional analgesics for treatment of pain. The clinical use of NSAIDs is associated with side effects such as gastrointestinal lesions and nephrotoxicity [3]. Therefore, the discovery of new safer drugs represents a challenging goal for such a research area.
Coumarins are an important class of widely distributed heterocyclic natural products exhibiting a broad pharmacological profile [4]. Several coumarin derivatives have been synthesized with diverse biological activities [5-9] especially analgesic/anti-inflammatory activity [10-13]. Recently, the synthesis and anti-inflammatory/analgesic activities of several coumarin derivatives with various substitutions on 3-position of coumarin nucleus have been reported [14-16]. On the other hand, benzothiazine derivatives are also important heterocyclic compounds with wide spectrum of biological activities [17,18]. In view of the above facts and in continuation of our research program on the synthesis of biologically active heterocyclic compounds [19,20], we introduced herein the new coumarin derivatives bearing substituted 3,4-dihydro-2H-benzothiazines as analgesic agents. The antinociceptive properties of target compounds were determined by formalin-induced paw licking test and acetic acid-induced writhing test in mice. Indeed, the formalin-induced paw licking method is used to investigate both peripheral and central mechanisms whereas the acetic acid test is believed to demonstrate the involvement of peripheral mechanisms in the control of pain [21,22].
Materials and methods
Chemistry
The target compounds 3-(3-methyl-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-2H-chromen-2-ones 2a-r (Additional file 1: Table S1) were synthesized according to the pathway outlined in Scheme 1[23]. All reagents and chemicals were commercially available and used as received. Alumina-supported potassium fluoride (KF/Al2O3) was prepared by literature method [24]. The dihydrobenzothiazole derivatives 1 were prepared as reported method by us [19,20]. The synthesis of compounds 2a-d, 2f-i and 2p-r was described in our previous paper [23]. Column chromatography was carried out on silica gel (70–230 mesh). TLC was conducted on silica gel 250 micron, F254 plates. Melting points were measured on a Kofler hot stage apparatus and are uncorrected. The IR spectra were taken using Nicolet FT-IR Magna 550 spectrographs (KBr disks). 1H NMR spectra were recorded on a Bruker 400 or 500 MHz NMR instruments. The chemical shifts (δ) and coupling constants (J) are expressed in parts per million and Hertz, respectively. Mass spectra of the products were obtained with an HP (Agilent technologies) 5937 Mass Selective Detector. Elemental analyses were carried out by a CHN-Rapid Heraeus elemental analyzer. The results of elemental analyses (C, H, N) were within ± 0.4% of the calculated values.
Scheme 1.
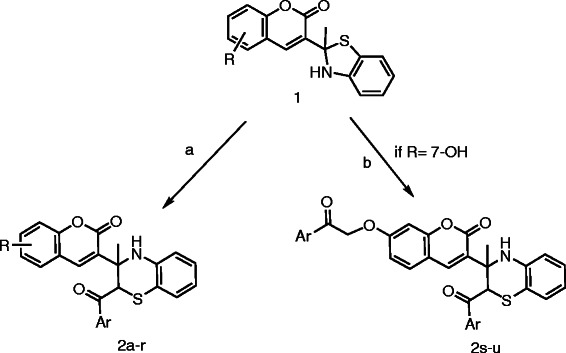
Synthesis of coumarin based dihydrobenzothiazines 2a-u. Reagents and conditions: (a) phenacyl halide (1.2 mmol), KF/Al2O3 (0.7 g), quinine hydrochloride (10 mol%), EtOH (3 mL), r.t. (b) phenacyl halide (2.5 mmol), KF/Al2O3 (1.5 g), quinine hydrochloride (10 mol%), EtOH (3 mL), r.t.
General procedure for the synthesis of compounds 2
A suspension of dihydrobenzothiazole derivatives 1 (1.0 mmol), KF/Al2O3 (0.7 g), and quinine hydrochloride (10 mol%) in ethanol (3.0 mL) was stirred at room temperature for 5 min. Then, appropriate phenacyl halide (1.2 mmol) was added to the mixture and stirring was continued. After completion of the reaction (3–5 h), the solvent was removed under reduced pressure. The residue was mixed with ethyl acetate (5 mL) and the catalyst was filtered and washed with ethyl acetate (3 × 5 mL). After evaporation of the solvent at reduced pressure, the crude product was purified by column chromatography (n-hexane/ethyl acetate, 9:1) and crystallized from ethanol for further purification.
3-(2-(3,4-Dichlorobenzoyl)-3-methyl-3,4-dihydro-2H-benzo[b] [1,4]thiazin-3-yl)-2H-chromen-2-one (2e)
Yellow solid (361 mg, 75%); syn-isomer; mp 91–93°C; IR (KBr, cm-1) 3382 (NH), 1708 (C=O); 1H NMR (500 MHz, CDCl3) δ 1H NMR (500 MHz, CDCl3) δ 1.94 (s, 3H, CH3 benzothiazine), 4.49 (s, 1H, NH), 5.77 (s, 1H, C-H benzothiazine), 6.75 (dt, J = 7.2 and 1.2 Hz, 1H, H7 benzothiazine), 6.95 (m, 2H, H5,8 benzothiazine), 7.14 (dt, J = 7.2 and 1.2 Hz, 1H, H6 benzothiazine), 7.22 (t, J = 8.0 Hz, 1H, H6 chromene), 7.28 (dd, J = 8.0 and 1.9 Hz, 1H, H5 benzoyl), 7.33 (d, J = 8.0 Hz, 1H, H6 benzoyl), 7.40 (m, 2H, H5,8 chromene), 7.43 (d, J = 1.9, 1H, H3 benzoyl), 7.49 (dt, J = 8.0 and 1.2 Hz, 1H, H7 chromene), 7.77 (s, 1H, H4 chromene); 13C NMR (125 MHz, CDCl3) δ 24.3, 42.9, 58.0, 110.9, 116.1, 117.1, 119.1, 119.3, 124.3, 127.0, 127.1, 128.4, 128.7, 130.1, 130.8, 131.2, 131.4, 132.0, 136.9, 137.1, 139.8, 141.0, 153.2, 160.9, 192.6; Anal. calcd for C25H17Cl2NO3S: C, 62.25; H, 3.55; N, 2.90. Found: C, 62.41; H, 3.67; N, 3.15.
3-(2-(4-Fluorobenzoyl)-3-methyl-3,4-dihydro-2H-benzo[b] [1,4]thiazin-3-yl)-2H-chromen-2-one (2j)
Yellow solid (336 mg, 78%); syn-isomer; mp 161–163°C; IR (KBr, cm-1) 3413 (NH), 1708 (C=O); 1H NMR (500 MHz, CDCl3) δ 1.76 (s, 3H, CH3 benzothiazine), 4.50 (s, 1H, NH), 5.97 (s, 1H, C-H benzothiazine), 6.73 (t, J = 7.4 Hz, 1H, H7 benzothiazine), 6.93 (m, 2H, H5,6 benzothiazine), 7.14 (m, 3H, H8 benzothiazine and H3,5 benzoyl), 7.28 (t, J = 7.4 Hz, 1H, H6 chromene), 7.35 (d, J = 7.4 Hz, 1H, H8 chromene), 7.40 (d, J = 7.4 Hz, 1H, H5 chromene), 7.50 (t, J = 7.4 Hz, 1H, H7 chromene), 7.79 (s, 1H, H4 chromene), 8.05 (m, 2H, H2,6 benzoyl); 13C NMR (125 MHz, CDCl3) δ 24.6, 37.5, 57.6, 111.9, 115.7, 115.9, 116.1, 119.1, 119.4, 124.4, 126.7, 128.4, 128.5, 131.2, 131.3, 131.5, 133.2, 139.5, 141.1, 153.3, 161.3, 164.7, 166.7, 191.3; Anal. calcd for C25H18FNO3S: C, 69.59; H, 4.20; N, 3.25. Found: C, 69.42; H, 4.03; N, 3.47.
3-(3-Methyl-2-(thiophene-2-carbonyl)-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-2H-chromen-2-one (2k)
Yellow solid (356 mg, 85%); as mixture of diastereomers (anti/syn: 15/85); IR (KBr, cm-1) 3389 (NH), 1707 (C=O); 1H NMR (500 MHz, CDCl3) δ 1.77syn (s, CH3 benzothiazine), 1.87anti (s, CH3 benzothiazine), 4.48syn (s, NH), 4.53anti (s, NH), 5.50anti (s, C-H benzothiazine), 5.87syn (s, C-H benzothiazine), 6.77syn (t, J = 8.0, H7 benzothiazine), 6.81anti (t, J = 8.0, H7 benzothiazine), 6.92anti (d, J = 8.0 Hz, H5 benzothiazine), 6.95syn (d, J = 8.0 Hz, H5 benzothiazine), 7.07-7.10anti (m, H6,8 benzothiazine), 7.10-7.13syn (m, H6,8 benzothiazine), 7.20syn (t, J = 7.6 Hz, H7 chromene), 7.25syn (t, J = 7.6 Hz, H6 chromene), 7.25-7.29anti (m, H4 thiophene and H7 chromene), 7.38syn (d, J = 7.6 Hz, H5 chromene), 7.42syn (d, J = 7.6 Hz, H8 chromene), 7.48-7.54 (m, H5,7,8 chromene (anti) and H4 thiophene (syn)), 7.56anti (d, J = 4.0, H3 thiophene), 7.74syn (d, J = 4.0, H3 thiophene), 7.79anti (d, J = 4.0, H5 thiophene), 7.80syn (s, H4 chromene), 7.97syn (d, J = 4.0, H5 thiophene), 8.15anti (s, H4 chromene); 13C NMR (syn-isomer, 125 MHz, CDCl3) δ 27.5, 45.3, 54.2, 116.1, 117.0, 118.6, 119.0, 120.1, 124.5, 126.2, 127.1, 128.2, 128.4, 131.4, 131.7, 132.2, 134.4, 140.2, 140.9, 143.5, 153.1, 160.1, 186.8; Anal. calcd for C23H17NO3S2: C, 65.85; H, 4.08; N, 3.34. Found: C, 65.92; H, 3.91; N, 3.29.
3-(2-(5-Bromothiophene-2-carbonyl)-3-methyl-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-2H-chromen-2-one (2l)
Yellow solid (378 mg, 77%); as mixture of diastereomers (anti/syn: 26/74); IR (KBr, cm-1) 3390 (NH), 1712 (C=O); 1H NMR (500 MHz, CDCl3) δ 1.75syn (s, CH3 benzothiazine), 1.87anti (s, CH3 benzothiazine), 4.45syn (s, NH), 4.55anti (s, NH), 5.48anti (s, C-H benzothiazine), 5.78syn (s, C-H benzothiazine), 6.76syn (t, J = 8.0, H7 benzothiazine), 6.81anti (t, J = 8.0, H7 benzothiazine), 6.92syn (d, J = 8.0 Hz, H5 benzothiazine), 6.94anti (d, J = 8.0 Hz, H5 benzothiazine), 6.92anti (d, J = 8.0 Hz, H8 benzothiazine), 6.96syn (d, J = 8.0 Hz, H8 benzothiazine), 7.09anti (t, J = 8.0 Hz, H6 benzothiazine), 7.12syn (t, J = 8.0 Hz, H6 benzothiazine), 7.16syn (d, J = 4.0 Hz, H4 thiophene), 7.23anti (d, J = 4.0 Hz, H4 thiophene), 7.24syn (t, J = 7.5, H6 chromene), 7.27-7.29anti (m, H6,8 chromene), 7.36syn (d, J = 7.5 Hz, H8 chromene), 7.40syn (d, J = 7.5 Hz, H5 chromene), 7.50 syn (t, J = 7.5 Hz, H7 chromene), 7.51-7.53anti (m, H5,7 chromene), 7.56anti (d, J = 4.0 Hz, H3 thiophene), 7.69syn (d, J = 4.0 Hz, H3 thiophene), 7.77syn (s, H4 chromene), 8.15anti (s, H4 chromene); 13C NMR (syn-isomer, 125 MHz, CDCl3) δ 24.8, 38.7, 57.4, 112.3, 116.1, 116.9, 119.1, 119.5, 123.6, 124.4, 126.6, 128.1, 128.4, 130.8, 131.5, 131.6, 132.4, 139.2, 141.0, 145.9, 153.3, 161.2, 185.6; MS, m/z (%) 499 ([M + 2]+, 40%), 497 (M+, 37), 375 (47), 373 (44), 308 (100), 294 (51), 280 (84); Anal. calcd for C23H16BrNO3S2: C, 55.43; H, 3.24; N, 2.81 Found: C, 55.22; H, 3.47; N, 2.73.
3-(3-Methyl-2-(thiophene-3-carbonyl)-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-2H-chromen-2-one (2m)
Yellow solid (335 mg, 80%); as mixture of diastereomers (anti/syn: 32/68); IR (KBr, cm-1) 3374 (NH), 1708 (C=O); 1H NMR (500 MHz, CDCl3) δ 1.77syn (s, CH3 benzothiazine), 1.87anti (s, CH3 benzothiazine), 4.50anti (s, NH), 4.55syn (s, NH), 5.48anti (s, C-H benzothiazine), 5.81syn (s, C-H benzothiazine), 6.74syn (t, J = 7.5, H7 benzothiazine), 6.80anti (t, J = 7.5, H7 benzothiazine), 6.93anti (d, J = 7.5 Hz, H5 benzothiazine), 6.97syn (d, J = 7.5 Hz, H5 benzothiazine), 7.08anti (d, J = 7.5 Hz, H8 benzothiazine), 7.12syn (t, J = 7.5 Hz, H6 benzothiazine), 7.13syn (t, J = 7.2 Hz, H6 chromene), 7.24-7.25syn (m, H8 benzothiazine and H8 chromene), 7.26-7.29anti (m, H6 benzothiazine and H6,8 chromene), 7.35-7.37syn (m, H4 thiophene and H5,7 chromene), 7.37-7.40anti (m, H6 chromene and H4 thiophene), 7.50-7.53anti (m, H7 chromene and H5 thiophene), 7.58syn (d, J = 5.0 Hz, H5 thiophene), 7.79anti (s, H2 thiophene), 8.04syn (s, H2 thiophene), 8.14anti (s, H4 chromene), 8.25syn (s, H4 chromene); 13C NMR (syn-isomer, 125 MHz, CDCl3) δ 27.8, 45.7, 54.4, 116.1, 116.8, 119.1, 119.9, 124.6, 126.3, 126.5, 127.3, 128.2, 128.4, 130.6, 131.5, 131.7, 132.6, 140.0, 140.2, 141.2, 153.1, 160.2, 187.7; MS, m/z (%) 419 (M+, 68%), 404 (12), 386 (12), 308 (97), 295 (100), 280 (64), 111 (63); Anal. calcd for C23H17NO3S2: C, 65.85; H, 4.08; N, 3.34. Found: C, 65.98; H, 3.82; N, 3.60.
8-Methoxy-3-(3-methyl-2-(4-methylbenzoyl)-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-2H-chromen-2-one (2n)
Yellow solid (343 mg, 75%); syn-isomer; mp 145–147°C; IR (KBr, cm-1) 3360 (NH), 1700 (C=O); 1H NMR (500 MHz, CDCl3) δ 1.77 (s, 3H, CH3 benzothiazine), 2.43 (s, 3H, CH3 benzoyl), 3.90 (s, 3H, O-CH3 chromene), 4.52 (s, 1H, NH), 6.00 (s, 1H, C-H benzothiazine), 6.78 (dt, J = 7.5 and 1.3 Hz, 1H, H7 benzothiazine), 6.93 (dd, J = 8.0 and 1.3 Hz, 1H, H7 chromene), 7.02-7.11 (m, 4H, H5,6,8 benzothiazine and H6 chromene), 7.18 (m, 3H, H5 chromene and H3,5 benzoyl), 7.74 (d, J = 8.3 Hz, 2H, H2,6 benzoyl), 8.13 (s, 1H, H4 chromene); 13C NMR (125 MHz, CDCl3) δ 21.6, 27.9, 43.6, 54.6, 56.1, 113.3, 114.8, 118.9, 119.4, 119.7, 119.9, 124.3, 126.3, 127.4, 128.5, 129.3, 131.1, 133.7, 140.0, 140.2, 142.8, 143.8, 146.7, 159.5, 192.9 cm-1; Anal. calcd for C27H23NO4S: C, 70.88; H, 5.07; N, 3.06. Found: C, 70.64; H, 5.23; N, 3.22.
3-(2-(4-Fluorobenzoyl)-3-methyl-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-8-methoxy-2H-chromen-2-one (2o)
Yellow solid (323 mg, 70%); syn-isomer; mp 236–238°C; IR (KBr, cm-1) 3398 (NH), 1690 (C=O); 1H NMR (500 MHz, CDCl3) δ 1.78 (s, 3H, CH3 benzothiazine), 3.98 (s, 3H, O-CH3 chromene), 4.51 (s, 1H, NH), 5.98 (s, 1H, C-H benzothiazine), 6.74 (t, J = 7.4 Hz, 1H, H7 benzothiazine), 6.93 (m, 2H, H5,6 benzothiazine), 6.98 (d, J = 8.0 Hz, 1H, H7 chromene), 7.05 (m, 2H, H5,6 chromene), 7.16 (m, 3H, H8 benzothiazine and H3,5 benzoyl), 7.77 (s, 1H, H4 chromene), 8.05 (m, 2H, H2,6 benzoyl); 13C NMR (125 MHz, CDCl3) δ 24.5, 37.4, 56.3, 57.6, 111.9, 113.3, 115.7, 115.9, 116.9, 119.3, 119.8, 124.2, 126.7, 128.4, 131.2, 131.3, 131.4, 133.1, 139.5, 141.2, 146.8, 160.7, 164.7, 166.7, 191.2; Anal. calcd for C26H20FNO4S: C, 67.67; H, 4.37; N, 3.04. Found: C, 67.43; H, 4.18; N, 3.25.
3-(2-(4-Bromobenzoyl)-3-methyl-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-7-(2-(4-bromophenyl)-2-oxoethoxy)-2H-chromen-2-one (2s)
Yellow solid (507 mg, 72%); as mixture of diasteromers (anti/syn: 18/82); IR (KBr, cm-1) 3382 (NH), 1697 (C=O); 1H NMR (500 MHz, CDCl3) δ 1.74syn (s, CH3 benzothiazine), 1.85anti (s, CH3 benzothiazine), 4.48anti (s, NH), 4.51syn (s, NH), 5.29anti (s, O-CH2), 5.31syn (s, O-CH2), 5.60anti (s, C-H benzothiazine), 5.91syn (s, C-H benzothiazine), 6.71anti (t, J = 7.5, H7 benzothiazine), 6.73syn (t, J = 7.5, H7 benzothiazine), 6.80anti (s, H8 chromene), 6.82syn (s, H8 chromene), 6.87syn (d, J = 8.5 Hz, H6 chromene), 6.90anti (d, J = 8.5 Hz, H6 chromene), 6.93syn (d, J = 8.0 Hz, H3,5 phenyl-2-oxoethoxy), 7.05anti (d, J = 8.0 Hz, H3,5 phenyl-2-oxoethoxy), 7.07anti (t, J = 7.5 Hz, H6 benzothiazine), 7.12syn (t, J = 7.5 Hz, H6 benzothiazine), 7.33syn (d, J = 8.5, H5 chromene), 7.44anti (d, J = 8.5, H5 chromene), 7.52anti (d, J = 8.5, H3,5 benzoyl), 7.63syn (d, J = 8.0 Hz, H3,5 benzoyl), 7.64-7.67anti (m, H5,8 benzothiazine), 7.68syn (m, H5,8 benzothiazine), 7.72syn (s, H4 chromene), 7.82-7.85anti (m, H2,6 benzoyl and H2,6 phenyl-2-oxoethoxy), 7.85-7.89syn (m, H2,6 benzoyl and H2,6 phenyl-2-oxoethoxy), 8.06anti (s, H4 chromene); 13C NMR (syn-isomer, 125 MHz, CDCl3) δ 24.5, 37.6, 57.4, 70.6, 101.1, 111.8, 112.9, 116.9, 118.7, 119.3, 126.7, 128.3, 128.4, 129.5, 129.6, 129.9, 130.0, 131.9, 132.0, 132.3, 132.8, 135.5, 139.6, 140.9, 154.7, 160.6, 161.3, 191.6, 192.4; Anal. calcd for C33H23Br2NO5S: C, 56.19; H, 3.29; N, 1.99. Found: C, 56.21; H, 4.31; N, 2.09.
3-(3-Methyl-2-(4-methylbenzoyl)-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-7-(2-oxo-2-p-tolylethoxy)-2H-chromen-2-one (2t)
Yellow solid (397 mg, 70%); as mixture of diastereomers (anti/syn: 30/70); IR (KBr, cm-1) 3397 (NH), 1702 (C=O); 1H NMR (500 MHz, CDCl3) δ 1.74syn (s, CH3 benzothiazine), 1.85anti (s, CH3 benzothiazine), 2.36anti (s, CH3 phenyl-2-oxoethoxy), 2.42syn (s, CH3 phenyl-2-oxoethoxy), 2.44anti (s, CH3 benzoyl), 2.45syn (s, CH3 benzoyl), 4.50syn (s, NH), 4.61anti (s, NH), 5.31anti (s, O-CH2), 5.35syn (s, O-CH2), 5.61anti (s, C-H benzothiazine), 5.96syn (s, C-H benzothiazine), 6.69anti (d, J = 2.1 Hz, H8 chromene), 6.72syn (t, J = 7.3 Hz, H7 benzothiazine), 6.75anti (t, J = 7.3 Hz, H7 benzothiazine), 6.81syn (d, J = 2.1 Hz, H8 chromene), 6.87syn (dd, J = 8.0 and 2.1 Hz, H6 chromene), 6.90-6.93syn/anti (m, H5,8 benzothiazine), 7.05anti (t, J = 7.3 Hz, H6 benzothiazine), 7.11syn (t, J = 7.3 Hz, H6 benzothiazine), 7.17anti (d, J = 8.0 Hz, H3,5 phenyl-2-oxoethoxy), 7.28-7.33 (m, H5 chromene (syn/anti), H3,5 benzoyl (syn/anti) and H3,5 phenyl-2-oxoethoxy (syn)), 7.34anti (d, J = 8.0 Hz, H2,6 phenyl-2-oxoethoxy), 7.73syn (s, H4 chromene), 7.79anti (d, J = 8.0 Hz, H2,6 benzoyl), 7.87syn (d, J = 8.0 Hz, H2,6 phenyl-2-oxoethoxy), 7.94syn (d, J = 8.0 Hz, H2,6 benzoyl), 8.06anti (s, H4 chromene); 13C NMR (syn-isomer, 125 MHz, CDCl3) δ 24.0, 27.7, 32.6, 43.3, 56.0, 80.2, 110.4, 110.6, 113.6, 116.1, 118.7, 118.9, 119.9, 122.4, 124.5, 126.2, 127.2, 128.4, 129.2, 131.6, 140.1, 154.2, 161.2, 161.4, 192.1, 192.6; MS, m/z (%) 575 (M+, 8%), 557 (64), 542 (43), 410 (35), 264 (44), 239 (29), 119 (100); Anal. calcd for C35H29NO5S: C, 73.02 ; H, 5.08; N, 2.43. Found: C, 73.21; H, 5.12; N, 2.54.
3-(3-Methyl-2-(4-(methylsulfonyl)benzoyl)-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-7-(2-(4-(methylsulfonyl)phenyl)-2-oxoethoxy)-2H-chromen-2-one (2u)
Yellow solid (576 mg, 82%); as mixture of diastereomers (anti/syn: 28/72); IR (KBr, cm-1) 3394 (NH), 1688 (C=O), 1320 (SO2), 1153 (SO2); 1H NMR (500 MHz, CDCl3) δ 1.78syn (s, CH3 benzothiazine), 1.89anti (s, CH3 benzothiazine), 3.02anti (s, SO2-CH3 phenyl-2-oxoethoxy), 3.04anti (s, SO2-CH3 benzoyl), 3.09syn (s, SO2-CH3 phenyl-2-oxoethoxy), 3.12syn (s, SO2-CH3 benzoyl), 4.50anti (s, NH), 4.69syn (s, NH), 5.37syn (s, O-CH2), 5.41anti(s, O-CH2), 5.61anti (s, C-H benzothiazine), 5.95syn (s, C-H benzothiazine), 6.69anti (s, H8 chromene), 6.74syn (t, J = 7.5, H7 benzothiazine), 6.80anti (t, J = 7.5, H7 benzothiazine), 6.83syn (s, H8 chromene), 6.88-6.95syn (m, H6 chromene and H5,8 benzothiazine), 7.08anti (d, J = 8.0, H6 chromene), 7.11anti (t, J = 7.5, H6 benzothiazine), 7.13syn (t, J = 7.5 Hz, H6 benzothiazine), 7.36syn (d, J = 8.0, H5 chromene), 7.50anti (d, J = 8.0, H5 chromene), 7.59anti (d, J = 7.5 Hz, H8 benzothiazine), 7.74syn (s, H4 chromene), 7.82anti (d, J = 7.5 Hz, H5 benzothiazine), 7.94anti (s, H4 chromene), 8.04-8.19syn/anti (m, H2,3,5,6 phenyl-2-oxoethoxy and H2,3,5,6 benzoyl); 13C NMR (syn-isomer, 125 MHz, CDCl3) δ 24.5, 44.2, 44.3, 57.5, 65.5, 70.9, 101.0, 110.9, 113.0, 116.9, 119.4, 126.9, 127.4, 127.9, 128.1, 128.5, 129.1, 129.3, 129.8, 138.0, 139.6, 140.8, 141.0, 143.9, 145.1, 154.7, 160.4, 161.2, 190.6, 192.6; Anal. calcd for C35H29NO9S3: C, 59.73 ; H, 4.15; N, 1.99. Found: C, 59.59; H, 4.31; N, 2.30.
Pharmacology
Animals
Male NMRI mice weighing 20–30 g were used for studying in vivo antinociceptive activities of target compounds. Animals were maintained under standard conditions (24 ± 2°C, 60-70% humidity) and allowed food and water ad libitum. They were housed in appropriate cages with 12 h light/dark cycle. Before each experiment animals randomly selected and allocated into groups. The whole protocol was approved by the Ethics Committee of the Faculty of Pharmacy at Tehran University of Medical Sciences.
Formalin-induced pain test
All target compounds 2a-u were subjected for testing their analgesic activity using formalin paw test [25]. The compounds or standard drug mefenamic acid were administered i.p. (30 mg/kg, 0.2 mL/20 g body weight) as a suspension in saline and tween 80 (4% w/v). Each group of mice (n = 6 animals per group) were pretreated by test compounds, mefenamic acid or vehicle, 30 minutes before injection of formalin (20 μL, 0.5%, s.c.) into the planar surface of the right hind paw. The amount of time that the animal spent licking injected paw was measured during the first 10 minutes (phase 1, neurogenic) and 10–30 minutes (phase 2, inflammatory) after formalin injection.
Acetic acid-induced writhing test
The analgesic activity was also determined in vivo by the abdominal constriction test induced by acetic acid (0.6%; 0.1 mL/10 g) in mice [21]. An acetic acid solution was administered i.p. 30 minutes after administration of compounds or mefenamic acid. After the treatment, pairs of mice were placed in separate boxes and the numbers of constrictions of the abdominal muscles, together with stretching, were counted cumulatively over a period of 60 minutes. Antinociceptive activity was expressed as the percentage of inhibition of constrictions when compared with the vehicle control group.
Statistical analysis
The nociception data are expressed as means ± SEM. Variance analysis (ANOVA) followed by Bonferroni’s test was used to compare means. P-values less than 0.05 were considered to be statistically significant.
Results and discussion
Chemistry
The dihydrobenzothiazole derivatives 1 were quantitatively obtained by reaction of 3-acetylcoumarins with 2-aminothiophenol derivatives in the presence of acetic acid under reflux condition or microwave irradiation [19,20]. The intramolecular Mannich-type reaction of compounds 1 with different phenacyl halides in the present of KF/Al2O3 and catalyzing by quinine hydrochloride in ethanol afforded 3,4-dihydro-2H-benzothiazine derivatives 2a-r via a ring expansion. When 7-hydroxy-3-(benzothiazol-2-yl) coumarin derivative 1e was treated with 2.5 equivalents of phenacyl halides, without protection of hydroxyl group, O-phenacyl derivatives 2s-u was obtained in excellent yields (Scheme 1). The physicochemical and spectral data of new compounds 2e, 2j-o, and 2s-u are described in experimental section.
Biological activity
Formalin-induced nociception test
All target compounds 2a-u were tested using formalin-induced pain test in mice [25]. The obtained results were reported as mean ± SEM of licking time and as percent of inhibition in Table 1. In general, the results showed that most of compounds were significantly able to reduce the licking time with percent of inhibition in the range of 25% to 60% at the first phase. The standard drug mefenamic acid showed 89% reduction of the licking time during the first phase. Amongst the tested compounds, 2a, 2c, 2f, 2h, 2i, 2l-n and 2r-u significantly reduced the formalin induced licking time in the range of 39-98% as compared to mefenamic acid with 85% of inhibition during the second phase. Compounds 2m and 2r-u showed more effective antinociceptive activity in the second phase rather than first phase, indicating their ability to inhibit nociception associated with inflammatory response. Indeed, 7-hydroxy- and 7-phenacyloxy-coumarin derivatives (2r and 2s-u, respectively) were more effective than mefenamic acid. Compounds 2s and 2t were the most effective compounds at the dose of 30 mg/kg.
Table 1.
Antinociception activity of target compounds 2a-u and mefenamic acid (30 mg/kg, i.p.) assessed by formalin test in mice
| Compounds |
Phase 1 |
Phase 2 |
||||
|---|---|---|---|---|---|---|
| Licking time a | Inhibition b (%) | Relative activity c | Licking time | Inhibition (%) | Relative activity | |
|
2a |
58 ± 3.46 |
48.10** |
0.54 |
37.33 ± 3.93 |
44.28** |
0.52 |
|
2b |
51.33 ± 2.96 |
54.06*** |
0.61 |
50.33 ± 4.91 |
24.88 |
0.29 |
|
2c |
68.33 ± 4.05 |
38.85** |
0.44 |
38 ± 1.73 |
43.28** |
0.51 |
|
2d |
55 ± 2.74 |
50.78*** |
0.57 |
57.33 ± 6.35 |
14.43 |
0.17 |
|
2e |
44 ± 2.89 |
60.63*** |
0.68 |
54 ± 4.93 |
19.40 |
0.23 |
|
2f |
60.33 ± 3.76 |
46.01** |
0.52 |
38.33 ± 4.63 |
42.79** |
0.50 |
|
2g |
56.66 ± 8.74 |
49.29** |
0.55 |
55.66 ± 3.92 |
16.92 |
0.20 |
|
2h |
70.25 ± 2.95 |
37.14** |
0.42 |
38 ± 1 |
43.28** |
0.51 |
|
2i |
51.33 ± 2.40 |
54.06*** |
0.61 |
37 ± 1.15 |
44.78** |
0.53 |
|
2j |
46.25 ± 2.56 |
58.61*** |
0.66 |
50.33 ± 0.33 |
24.88 |
0.29 |
|
2k |
51.66 ± 2.18 |
53.77*** |
0.60 |
51.66 ± 5.54 |
22.89 |
0.27 |
|
2l |
70 ± 11.13 |
37.36** |
0.42 |
37 ± 4.35 |
44.78** |
0.53 |
|
2m |
63.33 ± 8.21 |
43.33** |
0.49 |
18.33 ± 0.33 |
72.64*** |
0.85 |
|
2n |
69 ± 9.16 |
38.26** |
0.43 |
40.66 ± 1.20 |
39.30** |
0.46 |
|
2o |
53.8 ± 3.21 |
51.85** |
0.58 |
65 ± 6.41 |
2.98 |
0.03 |
|
2p |
53.9 ± 3.18 |
51.76** |
0.58 |
64.8 ± 4.19 |
3.28 |
0.04 |
|
2q |
61.33 ± 5.78 |
45.12** |
0.51 |
49.5 ± 2.02 |
26.12 |
0.31 |
|
2r |
94.4 ± 4.89 |
25.86** |
0.29 |
11 ± 1.7 |
93.64*** |
1.1 |
|
2s |
50 ± 3.22 |
33.33** |
0.37 |
5.2 ± 2.78 |
96.99*** |
1.14 |
|
2t |
46 ± 2.4 |
38.86** |
0.43 |
3 ± 1.04 |
98.26*** |
1.15 |
|
2u |
34.8 ± 2.65 |
53.6*** |
0.61 |
14.8 ± 1.92 |
91.44*** |
1.07 |
| Control |
111.75 ± 6.94 |
- |
- |
67 ± 3.14 |
- |
- |
| Mefenamic acid | 12.33 ± 3.93 | 88.96*** | 1 | 10 ± 2.52 | 85.07*** | 1 |
aData are expressed as mean ± S.E.M (number of animals in each group, n = 6).
bThe percentage inhibition was determined by using the following formula: Inhibition % = 100 × (control – experiment)/control. The asterisks denote the levels of significance in comparison with control groups (*P <0.05, **P <0.01 and ***P <0.001).
cActivity relative to mefenamic acid was determined by using the following formula: Relative Activity = Inhibition % of compound/Inhibition % of mefenamic acid.
Acetic acid-induced writhing test
The analgesic activity of compounds 2b-d, 2g-i, 2k, 2o and 2r-s was also evaluated in vivo by using abdominal constriction test induced by acetic acid in mice [21]. The abdominal constriction response induced by acetic acid is sensitive procedure to establish efficacy of peripherally acting analgesics. The analgesic activity was expressed as the percentage of inhibition of constrictions when compared with the control group. The results are summarized in Table 2.
Table 2.
Antinociception activity of selected compounds in comparison with mefenamic acid (30 mg/kg, i.p.) assessed by acetic acid-induced writhing test in mice
| Compound | Nociception (Mean ± SEM) | Inhibition (%) a | Relative activity b |
|---|---|---|---|
|
2b |
0.6 ± 0.24*** |
99 |
1.4 |
|
2c |
38 ± 4.04*** |
49 |
0.7 |
|
2d |
9.6 ± 2.54*** |
87 |
1.3 |
|
2g |
3.5 ± 1.09*** |
96 |
1.37 |
|
2h |
3 ± 1.84*** |
97 |
1.38 |
|
2i |
4.6 ± 2*** |
94 |
1.34 |
|
2k |
20 ± 2.48*** |
73 |
1.04 |
|
2o |
6 ± 3.2*** |
92 |
1.31 |
|
2r |
29 ± 2.12*** |
63 |
0.9 |
|
2s |
14 ± 2.28*** |
80 |
1.14 |
|
2t |
30 ± 7.6*** |
60 |
0.85 |
|
2u |
2 ± 1.3*** |
98 |
1.4 |
| Controlc |
75 ± 3.2 |
|
|
| Mefnamic acid | 23 ± 1.3*** | 70 |
aThe percentage inhibition was determined by using the following formula: Inhibition% = 100 × (control – experiment)/control.
bActivity relative to mefenamic acid was determined by using the following formula: Relative Activity = Inhibition % of compound/Inhibition % of mefenamic acid.
cTween 80 in saline (4% w/v).
***P <0.001 vs. control.
Significant protection against writhing was observed in animals treated with all test compounds where the mean numbers of writhes after 1 h were less than 38 compared to 75 in the control group. The percent of inhibition was in the range of 49-99%. All tested compounds were more effective than standard drug mefenamic acid with the exception of 2c, 2r and 2t. Compounds 2b and 2u with percent of inhibition ≥98% were the most effective compounds in acetic acid-induced writhing test. Moreover, compounds 2g-i and 2o exhibited high protection against writhing (percent of inhibition > 90%).
Structure–activity relationships
From the structure–activity relationships of unsubstituted coumarin series (compound 2a-m) based on the late stage of formalin-induced test, it was inferred that 3-thienylcarbonyl group is more favorable for activity. By comparing the activity of 7-substituted coumarin compounds 2r-u with those of other compounds it is appeared that the 7-hydroxy or 7-phenacyloxy groups dramatically increase the effectiveness of compounds and their ability to inhibit nociception associated with inflammatory response. On contrary, compounds 2r-u showed low level of inhibition at early phase of formalin test.
By comparing the percent of inhibition of 4-(methylsulfonyl)benzoyl derivatives 2d, 2r and 2u, it is revealed that the introduction of hydroxyl group on 7-position of coumarin ring diminished the antinociception activity, while the introduction of 4-(methylsulfonyl)phenacyloxy- group increased the activity as resulted from writhing test. In the 7-phenacyloxy-coumarin derivatives 2s-u, methylsulfonyl substituent was more favorable than bromo and methyl groups. The observed results of unsubstituted coumarin derivatives in Table 2 demonstrate that electron donating or bulky groups (for example, methoxy or phenyl, respectively) can increase antinociceptive activity in writhing test.
Conclusion
In summary, a series of 3-(3-methyl-3,4-dihydro-2H-benzo[b][1,4]thiazin-3-yl)-2H-chromen-2-one derivatives 2a-u bearing different aroyl group on the 2-position of benzothiazine ring were described as potential analgesic agents. The antinociceptive properties of target compounds were determined by formalin-induced test and acetic acid-induced writhing test in mice. The effect of substituent on aroyl moiety was explored by introduction of various electron withdrawing, electron donating or bulky groups. Surprisingly, compound 2u bearing 2-[4-(methylsulfonyl)benzoyl]- moiety on benzothiazine ring and 4-(methylsulfonyl)phenacyloxy- group on the 7 position of coumarin nucleus showed better profile of antinoceciption in both models. It was more effective than mefenamic acid during the late phase of formalin-induced test as well as in the acetic acid-induced writhing test. However, unsubstituted coumarin derivative 2b containing 4-methylbenzoyl moiety on benzothiazine ring, fully protected animals against writhing and was moderately able to inhibit the both phases of the formalin test. Considering the significant antinoceciptive action of phenacyloxycoumarin derivatives, compound 2u prototype might be further used as model to obtain new more potent analgesic drugs.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MA: Synthesis of target compounds. MK: Synthesis of target compounds. SE: Collaboration in identifying of the structures of target compounds, manuscript preparation. SF: Collaboration in determination of antinociceptive properties. SFG: Collaboration in determination of antinociceptive properties. MA: Supervision of the pharmacological part, AF: Collaboration in identifying of the structures of target compounds. AS: Design of target compounds and supervision of the synthetic and pharmacological parts. All authors read and approved the final manuscript.
Supplementary Material
Chemical structure of coumarin compounds 2a-u.
Contributor Information
Masoumeh Alipour, Email: masoumeh_alipour_k@yahoo.com.
Mehdi Khoobi, Email: m-khoobi@tums.ac.ir.
Saeed Emami, Email: sd_emami@yahoo.com.
Saeed Fallah-Benakohal, Email: saeedfallah.bnk1425@yahoo.com.
Seyedeh Farnaz Ghasemi-Niri, Email: ghasemi_farnaz@yahoo.com.
Mohammad Abdollahi, Email: Mohammad.Abdollahi@UToronto.Ca.
Alireza Foroumadi, Email: aforoumadi@yahoo.com.
Abbas Shafiee, Email: ashafiee@ams.ac.ir.
Acknowledgments
This work was financially supported by grants from Research Council of Tehran University of Medical Sciences and INSF (Iran National Science Foundation).
References
- Ruoff G, Lema M. Strategies in pain management: new and potential indications for COX-2 specific inhibitors. J Pain Symptom Manage. 2003;25:S21–S31. doi: 10.1016/S0885-3924(02)00628-0. [DOI] [PubMed] [Google Scholar]
- Giovannoni MP, Cesari N, Graziano A, Vergelli C, Biancalani C, Biagini P, Dal Piaz V. Synthesis of pyrrolo[2,3-d]pyridazinones as potent, subtype selective PDE4 inhibitors. J Enzyme Inhib Med Chem. 2007;22:309–318. doi: 10.1080/14756360601114700. [DOI] [PubMed] [Google Scholar]
- Cesari N, Biancalani C, Vergelli C, Dal Piaz V, Graziano A, Biagini P, Ghelardini C, Galeotti N, Giovannoni MP. Arylpiperazinylalkylpyridazinones and analogues as potent and orally active antinociceptive agents: synthesis and studies on mechanism of action. J Med Chem. 2006;49:7826–7835. doi: 10.1021/jm060743g. [DOI] [PubMed] [Google Scholar]
- Magiatis P, Melliou E, Skaltsounis AL, Mitaku S, Léonce S, Renard P, Pierré A, Atassi G. Synthesis and cytotoxic activity of pyranocoumarins of the seselin and xanthyletin series. J Nat Prod. 1998;61:982–986. doi: 10.1021/np9800295. [DOI] [PubMed] [Google Scholar]
- Beillerot A, Domínguez JCR, Kirsch G, Bagrel D. Synthesis and protective effects of coumarin derivatives against oxidative stress induced by doxorubicin. Bioorg Med Chem Lett. 2008;18:1102–1105. doi: 10.1016/j.bmcl.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wang XB, Wang T, Kong LY. Design, synthesis, and acetylcholinesterase inhibitory activity of novel coumarin analogues. Bioorg Med Chem. 2008;16:8011–8021. doi: 10.1016/j.bmc.2008.07.068. [DOI] [PubMed] [Google Scholar]
- Sashidhara KV, Kumar A, Kumar M, Sarkar J, Sinha S. Synthesis and in vitro evaluation of novel coumarin–chalcone hybrids as potential anticancer agents. Bioorg Med Chem Lett. 2010;20:7205–7211. doi: 10.1016/j.bmcl.2010.10.116. [DOI] [PubMed] [Google Scholar]
- Sashidhara KV, Kumar A, Kumar M, Srivastava A, Puri A. Synthesis and antihyperlipidemic activity of novel coumarin bisindole derivatives. Bioorg Med Chem Lett. 2010;20:6504–6507. doi: 10.1016/j.bmcl.2010.09.055. [DOI] [PubMed] [Google Scholar]
- Lee S, Sivakumar K, Shin WS, Xie F, Wang Q. Synthesis and anti-angiogenesis activity of coumarin derivatives. Bioorg Med Chem Lett. 2006;16:4596–4599. doi: 10.1016/j.bmcl.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Leal LKAM, Ferreira AAG, Bezerra GA, Matos FJA, Viana GSB. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: a comparative study. J Ethnopharmacol. 2000;70:151–159. doi: 10.1016/S0378-8741(99)00165-8. [DOI] [PubMed] [Google Scholar]
- Keri RS, Hosamani KM, Shingalapur RV, Hugar MH. Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Eur J Med Chem. 2010;45:2597–2605. doi: 10.1016/j.ejmech.2010.02.048. [DOI] [PubMed] [Google Scholar]
- Kalkhambkar RG, Kulkarni GM, Kamanavalli CM, Premkumar N, Asdaq SMB, Sun CM. Synthesis and biological activities of some new fluorinated coumarins and 1-aza coumarins. Eur J Med Chem. 2008;43:2178–2188. doi: 10.1016/j.ejmech.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Ghate M, Kusanur RA, Kulkarni MV. Synthesis and in vivo analgesic and anti-inflammatory activity of some bi heterocyclic coumarin derivatives. Eur J Med Chem. 2005;40:882–887. doi: 10.1016/j.ejmech.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Bolakatti GS, Maddi VS, Mamledesai SN, Ronad PM, Palkar MB, Swamy S. Synthesis and evaluation of anti-inflammatory and analgesic activities of a novel series of coumarin mannich bases. Arzneim-Forsch/Drug Res. 2008;58:515–520. doi: 10.1055/s-0031-1296551. [DOI] [PubMed] [Google Scholar]
- Khode S, Maddi V, Aragade P, Palkar M, Ronad PK, Mamledesai S, Thippeswamy AHM, Satyanarayana D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted)aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur J Med Chem. 2009;44:1682–1688. doi: 10.1016/j.ejmech.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Melagraki G, Afantitis A, Igglessi-Markopoulou O, Detsi A, Koufaki M, Kontogiorgis C, Hadjipavlou-Litina DJ. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur J Med Chem. 2009;44:3020–3026. doi: 10.1016/j.ejmech.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Rathore BS, Kumar M. Synthesis of 7-chloro-5-trifluoromethyl/7-fluoro/7-trifluoromethyl-4H-1,4-benzothiazines as antimicrobial agents. Bioorg Med Chem. 2006;14:5678–5682. doi: 10.1016/j.bmc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Trapani G, Reho A, Morlacchi F, Latrofa A, Marchini P, Venturi F, Cantalamessa F. Synthesis and antiinflammatory activity of various 1,4-benzothiazine derivatives. Farmaco Sci. 1985;40:369–376. [PubMed] [Google Scholar]
- Khoobi M, Emami S, Dehghan G, Foroumadi A, Ramazani A, Shafiee A. Synthesis and free radical scavenging activity of coumarin derivatives containing a 2-methylbenzothiazoline motif. Arch Pharm. 2011;344:588–594. doi: 10.1002/ardp.201000271. [DOI] [PubMed] [Google Scholar]
- Khoobi M, Ramazani A, Foroumadi A, Hamadi H, Hojjati Z, Shafiee A. Efficient microwave-assisted synthesis of 3-benzothiazolo and 3-benzothiazolino coumarin derivatives catalyzed by heteropoly acids. J Iran Chem Soc. 2011;8:1036–1042. doi: 10.1007/BF03246560. [DOI] [Google Scholar]
- Collier HDJ, Dinnin LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Khoobi M, Ramazani A, Foroumadi A, Emami S, Jafarpour F, Mahyari A, Ślepokura K, Lis T, Shafiee A. Highly cis-diastereoselective synthesis of coumarin-based 2,3-disubstituted dihydrobenzothiazines by organocatalysis. Helv Chim Acta. 2012;95:660–671. doi: 10.1002/hlca.201100357. [DOI] [Google Scholar]
- Victoria FN, Radatz CS, Sachini M, Jacob RG, Perin G, da Silva WP, Lenard EJ. KF/Al2O3 and PEG-400 as a recyclable medium for the selective α-selenation of aldehydes and ketones. Preparation of potential antimicrobial agents. Tetrahedron Lett. 2009;50:6761–6763. doi: 10.1016/j.tetlet.2009.09.093. [DOI] [Google Scholar]
- Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemical structure of coumarin compounds 2a-u.


