Abstract
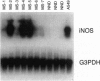
Nitric oxide (NO) has been implicated as a pathogenic mediator in a variety of central nervous system (CNS) disease states, including the animal model of multiple sclerosis (MS) and experimental allergic encephalomyelitis. We have examined post-mortem brain tissues collected from patients previously diagnosed with MS, as well as tissues collected from the brains of patients dying without neuropathies. Both Northern blot analysis and reverse transcriptase (RT)-driven in situ PCR (RT-in situ PCR) studies demonstrated that inducible NO synthase (iNOS) mRNA was present in the brain tissues from MS patients but was absent in equivalent tissues from normal controls. We have also performed experiments identifying the cell type responsible for iNOS expression by RT-in situ PCR in combination with immunohistochemistry. Concomitantly, we analyzed the tissues for the presence of the NO reaction product nitrotyrosine to demonstrate the presence of a protein nitrosylation adduct. We report here that iNOS mRNA was detectable in the brains of 100% of the CNS tissues from seven MS patients examined but in none of the three normal brains. RT-in situ PCR experiments also demonstrated the presence of iNOS mRNA in the cytoplasm of cells that also expressed the ligand recognized by the Ricinus communis agglutinin 1 (RCA-1), a monocyte/macrophage lineage marker. Additionally, specific labeling of cells was observed when brain tissues from MS patients were exposed to antisera reactive with nitrotyrosine residues but was significantly less plentiful in brain tissue from patients without CNS disease. These results demonstrate that iNOS, one of the enzymes responsible for the production of NO, is expressed at significant levels in the brains of patients with MS and may contribute to the pathology associated with the disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano K., Chee C. B., Gaston B., Lilly C. M., Gerard C., Drazen J. M., Stamler J. S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Hauptman S. P., Lischner H. W., Sachs M., Pomerantz R. J. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992 May 21;326(21):1385–1391. doi: 10.1056/NEJM199205213262103. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Bolaños J. P., Heales S. J., Land J. M., Clark J. B. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem. 1995 May;64(5):1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M. I., Nottet H. S., Schmidtmayerova H., Dubrovsky L., Flanagan C. R., Mullins M. E., Lipton S. A., Gendelman H. E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995 Feb 1;181(2):735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bö L., Dawson T. M., Wesselingh S., Mörk S., Choi S., Kong P. A., Hanley D., Trapp B. D. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol. 1994 Nov;36(5):778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cross A. H., Misko T. P., Lin R. F., Hickey W. F., Trotter J. L., Tilton R. G. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994 Jun;93(6):2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria R., Cifone M. G., Trotta R., Rippo M. R., Festuccia C., Santoni A., Testi R. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med. 1994 Nov 1;180(5):1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D. A., Lowenstein C. J., Shapiro R. A., Nussler A. K., Di Silvio M., Wang S. C., Nakayama D. K., Simmons R. L., Snyder S. H., Billiar T. R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad I. Y., Pataki G., Hu P., Galliani C., Beckman J. S., Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994 Dec;94(6):2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Kaur H., Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994 Aug 15;350(1):9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- Kooy N. W., Royall J. A., Ye Y. Z., Kelly D. R., Beckman J. S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med. 1995 Apr;151(4):1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Zheng Y. M., Heber-Katz E., Fraser N., Rorke L., Fu Z. F., Hanlon C., Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Dickson D. W., Liu W., Brosnan C. F. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993 Jul;46(1-2):19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- Lin R. F., Lin T. S., Tilton R. G., Cross A. H. Nitric oxide localized to spinal cords of mice with experimental allergic encephalomyelitis: an electron paramagnetic resonance study. J Exp Med. 1993 Aug 1;178(2):643–648. doi: 10.1084/jem.178.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannoji H., Yeger H., Becker L. E. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. 1986;71(3-4):341–343. doi: 10.1007/BF00688060. [DOI] [PubMed] [Google Scholar]
- Padgett E. L., Pruett S. B. Evaluation of nitrite production by human monocyte-derived macrophages. Biochem Biophys Res Commun. 1992 Jul 31;186(2):775–781. doi: 10.1016/0006-291x(92)90813-z. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cannella B., Brosnan C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991 Mar;87(3):949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M. L., Murphy S. Induction of nitric oxide synthase in glial cells. J Neurochem. 1992 Sep;59(3):897–905. doi: 10.1111/j.1471-4159.1992.tb08328.x. [DOI] [PubMed] [Google Scholar]
- Simmons M. L., Murphy S. Roles for protein kinases in the induction of nitric oxide synthase in astrocytes. Glia. 1994 Jul;11(3):227–234. doi: 10.1002/glia.440110303. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Franz H., Yamamoto T., Iwasaki Y., Konno H. Identification of the normal microglial population in human and rodent nervous tissue using lectin-histochemistry. Neuropathol Appl Neurobiol. 1988 May-Jun;14(3):221–227. doi: 10.1111/j.1365-2990.1988.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Tracey W. R., Nakane M., Pollock J. S., Förstermann U. Nitric oxide synthases in neuronal cells, macrophages and endothelium are NADPH diaphorases, but represent only a fraction of total cellular NADPH diaphorase activity. Biochem Biophys Res Commun. 1993 Sep 15;195(2):1035–1040. doi: 10.1006/bbrc.1993.2148. [DOI] [PubMed] [Google Scholar]
- Traugott U., Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann Neurol. 1988 Aug;24(2):243–251. doi: 10.1002/ana.410240211. [DOI] [PubMed] [Google Scholar]
- Zheng Y. M., Schäfer M. K., Weihe E., Sheng H., Corisdeo S., Fu Z. F., Koprowski H., Dietzschold B. Severity of neurological signs and degree of inflammatory lesions in the brains of rats with Borna disease correlate with the induction of nitric oxide synthase. J Virol. 1993 Oct;67(10):5786–5791. doi: 10.1128/jvi.67.10.5786-5791.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]