Abstract
It is an axiom that blood cellular immunity is provided by leukocytes. As to erythrocytes, it is generally accepted that their main function is respiration. Our research provides objective video and photo evidence regarding erythrocyte bactericidal function.
Phase-contrast immersion vital microscopy of the blood of patients with bacteremia was performed, and the process of bacteria entrapping and killing by erythrocytes was shot by means of video camera.
Video evidence demonstrates that human erythrocytes take active part in blood bactericidal action and can repeatedly engulf and kill bacteria of different species and size.
Erythrocytes are extremely important integral part of human blood cellular immunity.
Compared with phagocytic leukocytes, the erythrocytes: a) are more numerous; b) are able to entrap and kill microorganisms repeatedly without being injured; c) are more resistant to infection and better withstand the attacks of pathogens; d) have longer life span and are produced faster; e) are inauspicious media for proliferation of microbes and do not support replication of chlamidiae, mycoplasmas, rickettsiae, viruses, etc.; and f) are more effective and uncompromised bacterial killers.
Blood cellular immunity theory and traditional view regarding the function of erythrocytes in human blood should be revised.
Keywords: erythrocyte, immunity, phagocytosis, sepsis
Introduction
After discovery of phagocytosis [1], it is axiomatic that blood cellular immunity is provided by leukocytes. As to erythrocytes, it is a general view that erythrocytes have no role in immune response and their main function is respiration.
Sixty years ago, Nelson [2] supposed erythrocytes as directly participating in the immune complex reaction (bacteria, complement, and antibody). This interaction of erythrocytes with the immune complex has been repeatedly shown [3].
When erythrocytes are lysed by bacteria, their hemoglobin releases free radicals which break down the pathogen’s cell wall and membrane, killing it [4]. Human erythrocytes also may play a role in modulating T cell proliferation and survival by enhancing cytokine secretion and induction of the interleukin 2 receptors (IL2R), thus, modulating CD4+/8+ ratios [5–7].
Erythrocyte functional responses include amongst others glycophorin A-mediated pathogen binding [8], endothelial nitric oxide synthase (eNOS)-like protein activity [9], specific human immunodeficiency virus type 1 (HIV-1) binding [10], interferon-α mRNA induction [11], hormone binding [12], and complement receptor (CR1)-dependent immune complex clearance [3].
Erythrocytes form rosettes to facilitate the clearance of pathogens by macrophages [13] and could produce cytokines or specific signaling molecules in response to binding [14]. A relationship was hypothesized between erythrocytes, hemoglobin, and the immune system [15]. Hemoglobin is a source of bioactive peptides that participate in the innate immune response [16]. The antimicrobial activity of the respiratory globins is likely one of the most ancient antimicrobial mechanisms [17]. These respiratory protein-derived peptides exhibit antimicrobial activity against Gram-positive and Gram-negative bacteria, and yeast [18, 19]. Thus, it would appear that diverse ranges of nonrespiratory biological processes in erythrocytes are observed [20].
Materials and methods
One of the most frequent kinds of bacteremia in humans is oral cavity multibacterial contamination of peripheral blood [21], so blood samples were taken from patients with acute and chronic dental problems during tooth extraction and other invasive dental procedures (gingivectomy, deep gum pocket curettage, etc.). Eight samples of blood (15 ml each) were taken from the cubital vein of two patients with severe periodontitis after 5, 15, 30, and 60 min of tooth extraction (four samples from a patient). Blood samples (15 ml each) were also taken from two patients suffering from: 1) low grade fever after gingivectomy, gum pocket curettage, and plaque removal; 2) gum abscess with low grade fever. 1.8 mg K2EDTA per milliliter of blood was used as anticoagulant. Every blood sample was distributed to three test tubes, 5 ml of blood each (the first, second, and third test tube).
After gentle centrifugation of the first test tube and sedimentation of the blood cells, the plasma was incubated for 24 h (37 °C) while the blood cells were kept in refrigerator for 24 h (4 °C); then (after 24 h in the incubator), 1 ml of plasma was taken for centrifugation of bacteria, and the pellet vital phase-contrast and Gram stain preparation microscopy were performed; the rest of the plasma was remixed with the blood cells and put into incubator (37 °C), and after 30 min, an hour, 6 h, and 12 h phase-contrast microscopy was performed.
The second test tube was kept in incubator (37 °C) for 24 h, and phase-contrast microscopy was performed after 30 min, 1, 6, 12, and 24 h.
The third test-tube blood was used for mixing with the suspension (in 0.9% NaCL) of subgingival dental plaque taken from the patients. 2.5 ml of blood from the third test tube was mixed with 0.25 ml of dental plaque suspension and placed into incubator (37 °C), and after 30 min, 1 h, 6 h, and 12 h phase-contrast microscopy was performed. The plasma of the remaining 2.5 ml blood (after centrifugation and separation from blood cells) was mixed with 0.2 ml of the dental plaque suspension and placed into incubator (37 °C) for 24 h and then remixed with the blood cells kept after centrifugation in refrigerator (4 °C). The mixture was placed to incubator (37 °C), and after 15 min, 30 min, 1 h, 6 h, and 12 h phase-contrast microscopy was performed.
Phase-contrast microscopy was performed by means of phase-contrast condenser and objective 90 × 1.25 Ph Carl Zeiss; video was shot by means of digital camera, Nikon with 10.34 megapixel, image size 640 × 480, and 30 frames per second.
Results
Phase-contrast microscopy of blood samples taken from patients with transitory bacteremia for detection (after appropriate processing) of viable bacteria in the bloodstream has revealed that the microorganisms are cleared from the blood predominantly by erythrocytes.
Erythrocytes directly entrap and kill bacteria, proliferated in plasma (Figs 1–4, Videos 1–8).
Fig. 1.
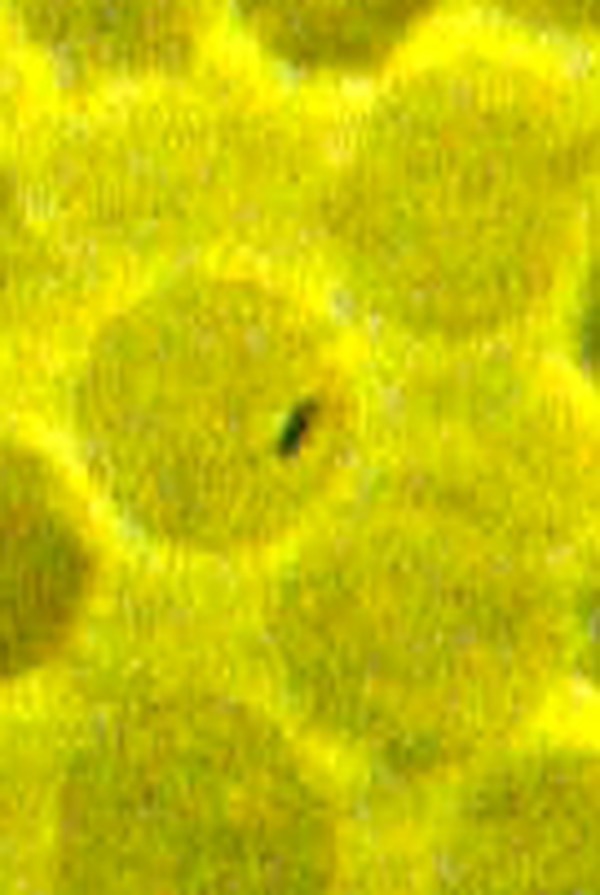
A small bacterium is entrapped inside the erythrocyte. Phase-contrast microscopy, magnification 900×
Fig. 4.

An amphitrichous bacterium is entrapped inside the erythrocyte. Phase-contrast microscopy, magnification 1350×
Fig. 2.
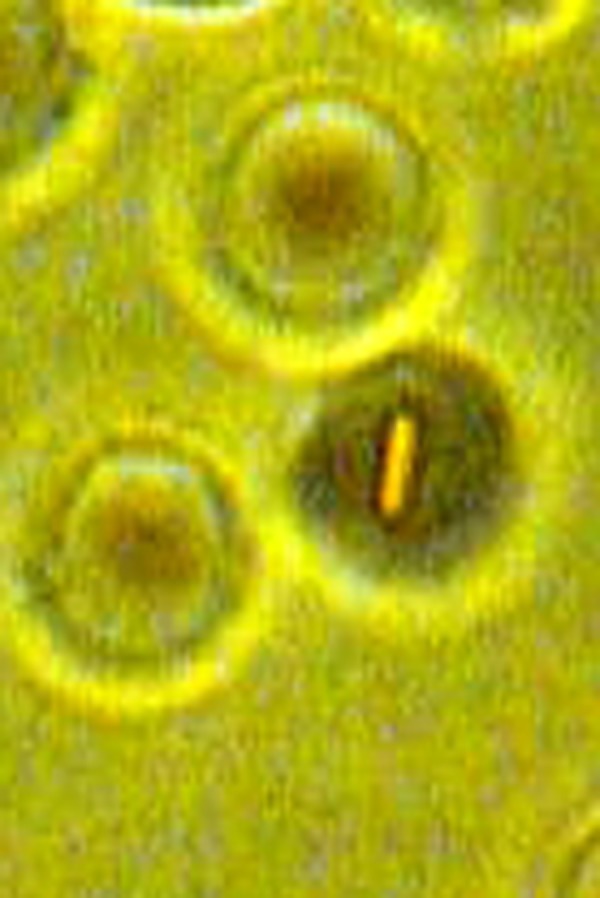
A medium size bacterium is entrapped inside the erythrocyte. Phase-contrast microscopy, magnification 900×
Fig. 3.
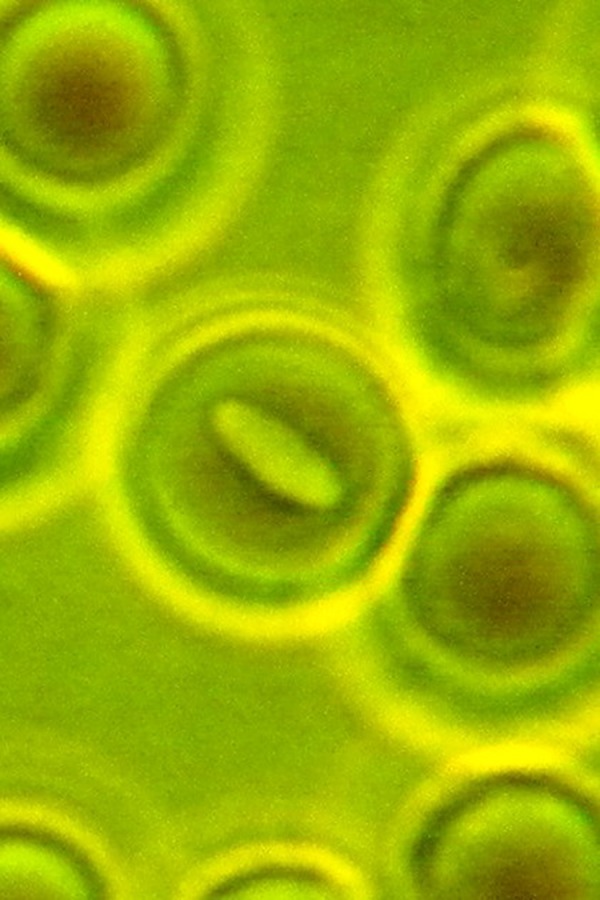
A big bacterium is entrapped inside the erythrocyte. Phase-contrast microscopy, magnification 900×
Bacteria entrapping does not make a hole in erythrocyte membrane, and erythrocyte’s inner contents do not pour out: bacteria remain entrapped in uninjured erythrocyte. Entrapped bacteria try to penetrate erythrocyte membrane from inside for escaping (Videos 1–6). Тhe bacteria cannot penetrate the membrane of even ghost erythrocytes (Figs 5–8). Entrapped bacteria finally exhaust (Videos 7 and 8) and die (Videos 9 and 11). Erythrocyte ghost membrane and cytoskeleton components tightly grip bacteria and hold them immobile for a while (Fig. 6, Video 10). After erythrocyte ghost membrane destruction, killed bacteria are released and slowly drift in blood plasma (Video 12).
Fig. 5.

A bacterium is entrapped in erythrocyte with injured membrane (hemoglobin is pouring out with erythrocyte ghost formation). Phase-contrast microscopy, magnification 1350×
Fig. 8.

Dead bacterium is drifting in the blood plasma. Phase-contrast microscopy, magnification 1350×
Fig. 6.
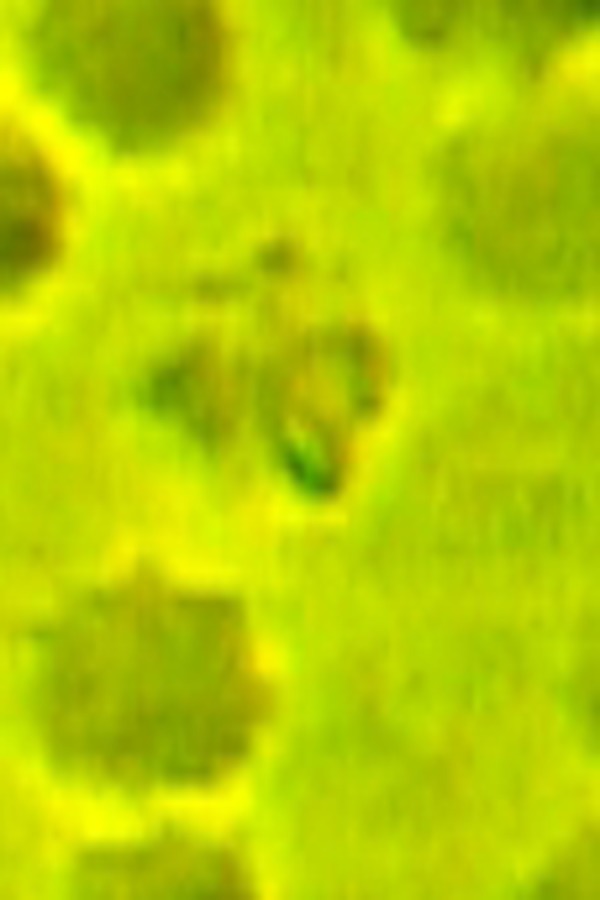
Bacterium is squeezed by erythrocyte ghost membrane. Phase-contrast microscopy, magnification 1350×
Fig. 7.

The release of the dead bacterium to the blood plasma. Phase-contrast microscopy, magnification 1350×
The activity of bacteria after entrapping increases, and bacteria try to penetrate erythrocyte membrane and escape. These efforts usually are futile, and the motility of bacteria gradually decreases and finally stops. The parallel process of erythrocyte color darkening occurs, which indicates hemoglobin deoxygenation and oxygen binding with bacterial structures (Videos 5 and 9).
Bacteria move in erythrocyte ghost longer than in normal erythrocytes. High motility of bacterium inside erythrocyte decreases erythrocyte’s cytoplasm density: bacterium acts as a mixer and makes the cytoplasm more liquid. Big membrane hole and more liquid cytoplasm both may cause cytoplasm pouring out and ghost formation (happens in 10% of especially big and active bacteria engulfing). Erythrocyte repeatedly entraps, kills, and releases small and average size bacteria without loss of cytoplasm and ghost formation.
Discussion
Video materials demonstrate that erythrocyte has active bactericidal function in blood. Before entrapping, a bacterium is attached to erythrocyte membrane. Video 13 demonstrates a bacterium gliding on the surface of the erythrocyte and nothing locally “glues” the bacterium to the erythrocyte, so electric charge is probably the main attracting force. Electrochemical zeta potential [ζ]) on the surface of erythrocyte membrane is –15.7 millivolts (mV) [22]. Erythrocyte discriminates dead and alive microbes by means of its electric charge that automatically attracts and keeps living (charged) microbes.
Very few species of bacteria can enter and survive in the erythrocyte, and so, the diseases (caused by bacteria able to survive in erythrocyte) are very rare. Regarding adaptation and survival inside erythrocyte, only some kinds of protozoa should be mentioned: malaria [23], babesiosis [24], and theileriosis [25]. As to microbes that penetrate erythrocyte and continue their life cycle inside them, only the genus Bartonella is partially able to do that [26].
Erythrocyte is the cell with maximal amount of oxygen inside. Oxygen is fatal for microorganisms. Oxygen is used for bacteria killing also by leukocytes [27]. Leukocytes are vulnerable regarding the attacks of bacteria, viruses, and other microorganisms. Incomplete (imperfect) phagocytosis is often, and for some microorganisms (Mycobacterium tuberculosis, Neisseria meningitidis, Neisseria gonorrhoeae), phagocytosis is indispensable for their life cycle, proliferation, and dissemination [28–30]. Prevention of phagolysosome formation after phagocytosis is an effective way to evade the attack of hydrolytic enzymes. It is characteristic for Legionella pneumophila [31], Chlamydia psittaci [32], Toxoplasma gondii [33], and M. tuberculosis [32]. Viruses can inhibit phagolysosome formation and provide survival of microbes in phagosome [34]. Toxoplasma gondii being engulfed by phagocyte does not cause “respiratory burst” in phagosome and so remains uninjured [35]. Coxiella burnetii resides in phagolysosome because of its own acidophilic biochemistry [36]. Nocardia asteroides produces high levels of superoxide dismutase and catalase that protect them from the effects of O2− and H2O2 [37]. L. pneumophila [38], M. tuberculosis [39], and Leishmania [40] cover themselves with C3 fragments and then enter the macrophage via C1 receptors.
Producing some toxins, microorganisms kill the phagocytic cells. Streptolysins O and S are responsible for cytolytic activity of Streptococcus pyogenes [41]. Staphylococcal leukocidin, anthrax toxin, and exotoxin A from Pseudomonas aeruginosa are toxic for phagocytes [42]. Listeria monocytogenes produce hemolysin that degrades phagolysosomial membrane; the microorganism enters to cytoplasm for proliferation there and kills the phagocyte [43]. Yersinia pestis survives and produces F1 and V antigens while it is residing within monocytes [44]. Ehrlichiosis infects either the neutrophil and the monocyte [45]. Francisella tularensis virulence is related to its ability to invade and replicate itself within phagocytes [46]. Thus, bacteria grow more readily and replicate more in leukocytes than in erythrocytes.
Many viruses replicate in leukocytes. HIV infects helper T cells (specifically CD4+ T cells), macrophages, and dendritic cells [47]; primary target of human T-cell lymphotropic virus (HTLV) (all types) is also CD4+ T cells [48]; Epstein–Barr virus infects B cells [49]; hepatitis C virus (HCV) may replicate in peripheral blood mononuclear cells and can infect B lymphocytes [50]; cytomegalovirus attacks lymphocytes and monocytes [51]; and enteroviruses (polio-, coxsackie-, echo-, and others) replicate in human mononuclear cells [52]. On the contrary, erythrocytes do not support viral replication [53]. Erythrocytes lack the nuclei and organelles required to replicate nucleic acids and elaborate proteins. Because viruses depend on the use of the host cell machinery to replicate, erythrocytes are invulnerable to viral infection. Leukocytes engulf not only germs but also have scavenger function and phagocytes may be overloaded by engulfed cell fragments and particles [54].
In humans, there are 1000 times more erythrocytes than leucocytes. Adult humans have 2–3 × 1013 (20–30 trillion) erythrocytes (approximately a quarter of the cells in the human body) while there are only 20–30 million leucocytes in peripheral blood [55]. Approximately 2.4 million new erythrocytes are produced per second [56]. For 10 s, 24 million new erythrocytes are produced (the same number of leucocytes are in all peripheral blood circulation). Neutrophils and monocytes are the most phagocytic of the white blood cells, so no more than 68% (60% neutrophils and 8% monocytes) of peripheral blood leucocytes are “professional” phagocytic cells. Neutrophils are not able to renew their lysosomes (used in digesting microbes) and die after having phagocytosed a few pathogens [57]. The life span of a circulating human neutrophil is about 5.4 days [58]. Erythrocytes circulate for 100–120 days, and so, they live 18–22 times longer than neutrophils [59]. According to our data, erythrocytes are able to trap and kill bacteria again and again without being injured. Erythrocyte membrane consists of three basic components: a lipid bilayer, transmembrane (integral) proteins, and a cytoskeletal network [60]. Thickness, tensile strength, elasticity, and firmness provide the ability of erythrocyte to entrap and hold even big bacteria long enough for killing the latter by means of oxidation and bacterial energy exhaustion.
Erythrocyte volume is about 90 fl with a surface of about 136 μm2, and it can swell up to a sphere shape containing 150 fl without membrane distension [61]. The volume of an average bacteria is 2 fl or 2 × 10−15 l [62], and so, the erythrocyte has enough inner volume for entrapping numerous bacteria.
There is huge number of bacteria in little amount of fluid in local inflammation regions (abscesses, phlegmonas, purulent wounds, etc.), infected organs, and cavities (pneumonia, cholangitis, haimoritis, frontitis, osteomyelitis, etc.). Even body’s ordinary liquids contain lots of germs: 1 ml of saliva may contain 750 million bacteria, and 1 gram of subgingival plaque may contain 200 billion bacteria [63]. Trauma to oral mucosal surfaces releases these microbial species transiently into the bloodstream [64]. The frequency of bacteremia after tooth extraction is 39–100% [21]. It is transient since the microorganisms are cleared from the bloodstream within a few minutes – 1 hour after the procedure [65]. Transient bacteremia occurs also during eating, chewing gum, brushing the teeth, or using toothpicks [66]. Rates for bacteremia in adults range from 23% to 57% for toothbrushing, and billions of bacteria enter the bloodstream [66, 67]. Comparing such amount of bacteria with peripheral blood “leukocyte army” (20–30 million cells with 60–70% of active phagocytes), it becomes clear that leukocytes are unable to withstand the attack of massive infection.
Thus, the erythrocytes are indispensable integrative part of human blood cellular bactericidal immunity. Taking into account all the data above, erythrocytes compared with phagocytic leukocytes are: a) more numerous; b) entrap and kill microorganisms repeatedly without being injured; and c) resistant to infection and better withstand the attacks of pathogens. Erythrocytes have longer life span and are produced faster, and their inner space is an inauspicious media for survival and proliferation of germs and does not support replication of smaller intracellular parasitic organisms (chlamidiae, mycoplasmas, rickettsiae, viruses, etc.); erythrocytes are more effective and unconditional, bacterial killers.
Bactericidal effect of erythrocytes is especially critical in the cases of: 1) blood massive microbial infection; 2) impossibility to recruit immediately enough amount of phagocytes; 3) fast proliferation and dissemination of microorganisms; and 4) functional ineffectiveness of fagocytes and/or incomplete phagocytosis. In such cases, erythrocytes become the first line of blood cellular bactericidal defense and the phagocytes become an auxiliary force.
Presented video materials should become an impetus for revision of both cellular immunity theory and traditional view regarding the function of erythrocytes in human blood and its cellular immunity.
The supplemental material contains the following videos:
A bacterium entrapped inside the erythrocyte is trying to escape.
A bacterium entrapped inside the erythrocyte is desperately trying to escape.
An amphitrichous bacterium inside the erythrocyte.
Two bacteria inside the erythrocytes.
A bacterium inside the erythrocyte. The erythrocyte has become darker because of oxygen consumption for the bacterium oxidation.
The entrapped bacterium is gradually losing energy and stamina.
Last efforts of exhausted bacterium to escape from the erythrocyte.
The bacterium is exhausted and is dying.
Dead (killed by means of oxidation) bacterium inside the erythrocyte.
The bacterium inside the erythrocyte is killing by squeezing and immobilization.
The bacterium inside the erythrocyte is in agony.
The bacterium after killing is released by erythrocyte to plasma and is drifting with the flow.
The bacterium is gliding on the surface of erythrocyte and then escapes.
References
- 1.Metschnikoff E. Lecture on Phagocytosis and Immunity. Br Med J. 1891 Jan 31;1(1570):213–217. doi: 10.1136/bmj.1.1570.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson RA., Jr. The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953 Dec 18;118(3077):733–737. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- 3.Hess C, Schifferli JA. Immune adherence revisited: novel players in an old game. News Physiol Sci. 2003 Jun;18:104–108. doi: 10.1152/nips.01425.2002. [DOI] [PubMed] [Google Scholar]
- 4.Jiang N, Tan NS, Ho B, Ding JL. Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat Immunol. 2007 Oct;8(10):1114–1122. doi: 10.1038/ni1501. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca AM, Pereira CF, Porto G, Arosa FA. Red blood cells promote survival and cell cycle progression of human peripheral blood T cells independently of CD58/LFA-3 and heme compounds. Cell Immunol. 2003 Jul;224(1):17–28. doi: 10.1016/s0008-8749(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 6.Porto B, Fonseca AM, Godinho I, Arosa FA, Porto G. Human red blood cells have an enhancing effect on the relative expansion of CD8+ T lymphocytes in vitro. Cell Prolif. 2001 Dec;34(6):359–367. doi: 10.1046/j.1365-2184.2001.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalechman Y, Herman S, Gafter U, Sredni B. Enhancing effects of autologous erythrocytes on human or mouse cytokine secretion and IL-2R expression. Cell Immunol. 1993 Apr 15;148(1):114–129. doi: 10.1006/cimm.1993.1095. [DOI] [PubMed] [Google Scholar]
- 8.Baum J, Ward RH, Conway DJ. Natural selection on the erythrocyte surface. Mol Biol Evol. 2002 Mar;19(3):223–229. doi: 10.1093/oxfordjournals.molbev.a004075. [DOI] [PubMed] [Google Scholar]
- 9.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozüyaman B, Schnürch HG, Gödecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rösen P, Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006 Apr 1;107(7):2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 10.Beck Z, Brown BK, Wieczorek L, Peachman KK, Matyas GR, Polonis VR, Rao M, Alving CR. Human erythrocytes selectively bind and enrich infectious HIV-1 virions. PLoS One. 2009 Dec 14;4(12):e8297. doi: 10.1371/journal.pone.0008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workenhe ST, Kibenge MJ, Wright GM, Wadowska DW, Groman DB, Kibenge FS. Infectious salmon anaemia virus replication and induction of alpha interferon in Atlantic salmon erythrocytes. Virol J. 2008 Feb 28;5:36. doi: 10.1186/1743-422X-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pottinger T, Brierley I. A putative cortisol receptor in the rainbow trout erythrocyte: stress prevents starvation-induced increases in specific binding of cortisol. J Exp Biol. 1997;200(Pt 14):2035–2043. doi: 10.1242/jeb.200.14.2035. [DOI] [PubMed] [Google Scholar]
- 13.Passantino L, Altamura M, Cianciotta A, Patruno R, Tafaro A, Jirillo E, Passantino GF. Fish immunology. I. Binding and engulfment of Candida albicans by erythrocytes of rainbow trout (Salmo gairdneri Richardson) Immunopharmacol Immunotoxicol. 2002 Nov;24(4):665–678. doi: 10.1081/iph-120016050. [DOI] [PubMed] [Google Scholar]
- 14.Passantino L, Massaro MA, Jirillo F, Di Modugno D, Ribaud MR, Modugno GD, Passantino GF, Jirillo E. Antigenically activated avian erythrocytes release cytokine-like factors: a conserved phylogenetic function discovered in fish. Immunopharmacol Immunotoxicol. 2007;29(1):141–152. doi: 10.1080/08923970701284664. [DOI] [PubMed] [Google Scholar]
- 15.Bishlawy IM. Red blood cells, hemoglobin and the immune system. Med Hypotheses. 1999 Oct;53(4):345–346. doi: 10.1054/mehy.1997.0778. [DOI] [PubMed] [Google Scholar]
- 16.Liepke C, Baxmann S, Heine C, Breithaupt N, Ständker L, Forssmann WG. Human hemoglobin-derived peptides exhibit antimicrobial activity: a class of host defense peptides. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 5;791(1-2):345–356. doi: 10.1016/s1570-0232(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 17.Iwanaga S. The molecular basis of innate immunity in the horseshoe crab. Curr Opin Immunol. 2002 Feb;14(1):87–95. doi: 10.1016/s0952-7915(01)00302-8. [DOI] [PubMed] [Google Scholar]
- 18.Fogaça AC, da Silva PI Jr, Miranda MT, Bianchi AG, Miranda A, Ribolla PE, Daffre S. Antimicrobial activity of a bovine hemoglobin fragment in the tick Boophilus microplus. J Biol Chem. 1999 Sep 3;274(36):25330–25334. doi: 10.1074/jbc.274.36.25330. [DOI] [PubMed] [Google Scholar]
- 19.Mourant AE, Kopec AC. Blood Groups and Diseases. Oxford: Oxford University Press; 1978. pp. 60–67. [Google Scholar]
- 20.Morera D, MacKenzie SA. Is there a direct role for erythrocytes in the immune response? Vet Res. 2011 Jul 29;42:89. doi: 10.1186/1297-9716-42-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomás I, Alvarez M, Limeres J, Potel C, Medina J, Diz P. Prevalence, duration and aetiology of bacteraemia following dental extractions. Oral Dis. 2007 Jan;13(1):56–62. doi: 10.1111/j.1601-0825.2006.01247.x. [DOI] [PubMed] [Google Scholar]
- 22.Tokumasu F, Ostera GR, Amaratunga C, Fairhurst RM. Modifications in erythrocyte membrane zeta potential by Plasmodium falciparum infection. Exp Parasitol. 2012 Jun;131(2):245–251. doi: 10.1016/j.exppara.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975 Feb 28;187(4178):748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- 24.Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008 Sep;38(11):1219–1237. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Florin-Christensen M, Schnittger L. Piroplasmids and ticks: a long-lasting intimate relationship. Front Biosci (Landmark Ed) 2009 Jan 1;14:3064–3073. doi: 10.2741/3435. [DOI] [PubMed] [Google Scholar]
- 26.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997 Jul;16(7):487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 27.Ernst JD, Stendahl O. Phagocytosis of Bacteria and Bacterial Pathogenicity. New York: Cambridge University Press; 2006. p. 286. [Google Scholar]
- 28.Stohl EA, Seifert HS. Neisseria gonorrhoeae DNA recombination and repair enzymes protect against oxidative damage caused by hydrogen peroxide. J Bacteriol. 2006 Nov;188(21):7645–7651. doi: 10.1128/JB.00801-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 30.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horwitz MA. Phagocytosis and Intracellular Biology of Legionella pneumophila. In: Horwitz MA, editor. Bacteria–Host Cell Interaction. New York: 1988. pp. 173–179. [Google Scholar]
- 32.Moulder JW. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones TC, Hirsch JG. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramson JS, Lewis JC, Lyles DS, Heller KA, Mills EL, Bass DA. Inhibition of neutrophil lysosome-phagosome fusion associated with influenza virus infection in vitro. Role in depressed bactericidal activity. J Clin Invest. 1982 Jun;69(6):1393–1397. doi: 10.1172/JCI110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson CB, Tsai V, Remington JS. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baca OG, Paretsky D. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol Rev. 1983 Jun;47(2):127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaman BL, Black CM, Doughty F, Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellinger-Kawahara CG, Horwitz MA. The major outer membrane protein is a prominent acceptor molecule for complement component C3 on Legionella pneumophila. Clin Res. 1987;35:468–470. [Google Scholar]
- 39.Payne NR, Bellinger-Kawahara CG, Honvitz MA. Phagocytosis of Mycobacterium tuberculosis by human monocytes is mediated by receptors for the third component of complement. Clin Res. 1987;35:617–711. [Google Scholar]
- 40.Da Silva RP, Hall BF, Joiner KA, Sacks DL. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989 Jul 15;143(2):617–622. [PubMed] [Google Scholar]
- 41.Hirsch JG, Bernteimer AW, Weissman G. Motion picture study of the toxic actions of streptolysins on leukocytes. J Exp Med. 1963 Aug 1;118:223–228. doi: 10.1084/jem.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Densen P, Mandell GL. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- 43.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins FM. Medical Microbiology. 4. University of Texas Medical Branch; 1996. p. 536. [Google Scholar]
- 45.Schutze GE, Buckingham SC, Marshall GS, Woods CR, Jackson MA, Patterson LE, Jacobs RF. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J. 2007 Jun;26(6):475–479. doi: 10.1097/INF.0b013e318042b66c. [DOI] [PubMed] [Google Scholar]
- 46.Horzempa J, O'Dee DM, Stolz DB, Franks JM, Clay D, Nau GJ. Invasion of erythrocytes by Francisella tularensis. J Infect Dis. 2011 Jul 1;204(1):51–59. doi: 10.1093/infdis/jir221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy JA. HIV and the pathogenesis of AIDS. USA: American Society for Microbiology; 2007. p. 752. [Google Scholar]
- 48.Murphy EL, Biswas HH. Human T-cell lymphotropic virus types I and II. In: Mandell G, Bennett J, Dolin R, editors. Bennett’s Principles and Practice of Infectious Diseases. 7. Philadelphia, PA: Churchill Livingston/Elsevier; 2010. pp. 168–177. [Google Scholar]
- 49.Armitage JO. Early-stage Hodgkin's lymphoma. N Engl J Med. 2010 Aug 12;363(7):653–662. doi: 10.1056/NEJMra1003733. [DOI] [PubMed] [Google Scholar]
- 50.Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol. 2000 Jul;81(Pt 7):1631–1648. doi: 10.1099/0022-1317-81-7-1631. [DOI] [PubMed] [Google Scholar]
- 51.Yamanishi K, Arvin A, Campadelli-Fiume G, Mocarski E, Moore P, Roizman B, Whitley R. Human Herpes Viruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, UK: Cambridge University Press; 2007. p. 308. [PubMed] [Google Scholar]
- 52.Dagan R, Menegus MA. Replication of enteroviruses in human mononuclear cells. Isr J Med Sci. 1992 Jun;28(6):369–372. [PubMed] [Google Scholar]
- 53.Asher DR, Cerny AM, Finberg RW. The erythrocyte viral trap: transgenic expression of viral receptor on erythrocytes attenuates coxsackievirus B infection. Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12897–12902. doi: 10.1073/pnas.0506211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jersmann HP, Ross KA, Vivers S, Brown SB, Haslett C, Dransfield I. Phagocytosis of apoptotic cells by human macrophages: analysis by multiparameter flow cytometry. Cytometry A. 2003 Jan;51(1):7–15. doi: 10.1002/cyto.a.10005. [DOI] [PubMed] [Google Scholar]
- 55.Pierigè F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008 Jan 14;60(2):286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 56.Sackmann E. Biological Membranes Architecture and Function. In: Lipowsky R, Sackmann E, editors. Handbook of Biological Physics, vol. 1. Elsevier; 1995. pp. 403–425. [Google Scholar]
- 57.Wheater PL, Stevens A. Wheater’s basic histopathology: a color atlas and text. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- 58.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010 Jul 29;116(4):625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 59.Harrison KL. Fetal erythrocyte lifespan. Aust Paediatr J. 1979 Jun;15(2):96–97. doi: 10.1111/j.1440-1754.1979.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 60.Discher DE. New insights into erythrocyte membrane organization and microelasticity. Curr Opin Hematol. 2000 Mar;7(2):117–122. doi: 10.1097/00062752-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 61.McLaren CE, Brittenham GM, Hasselblad V. Statistical and graphical evaluation of erythrocyte volume distributions. Am J Physiol. 1987 Apr;252(4 Pt 2):H857–H866. doi: 10.1152/ajpheart.1987.252.4.H857. [DOI] [PubMed] [Google Scholar]
- 62.Mengin-Lecreulx D, Flouret B, van Heijenoort J. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1109–1117. doi: 10.1128/jb.151.3.1109-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacFarlane TW, Samaranayake LP. Clinical Oral Microbiology. London: Wright; 1989. p. 223. [Google Scholar]
- 64.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008 Jun 17;117(24):3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heimdahl A, Hall G, Hedberg M, Sandberg H, Söder PO, Tunér K, Nord CE. Detection and quantitation by lysis-filtration of bacteremia after different oral surgical procedures. J Clin Microbiol. 1990 Oct;28(10):2205–2209. doi: 10.1128/jcm.28.10.2205-2209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sconyers JR, Crawford JJ, Moriarty JD. Relationship of bacteremia to toothbrushing in patients with periodontitis. J Am Dent Assoc. 1973 Sep;87(3):616–622. doi: 10.14219/jada.archive.1973.0453. [DOI] [PubMed] [Google Scholar]
- 67.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006 Jun;33(6):401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A bacterium entrapped inside the erythrocyte is trying to escape.
A bacterium entrapped inside the erythrocyte is desperately trying to escape.
An amphitrichous bacterium inside the erythrocyte.
Two bacteria inside the erythrocytes.
A bacterium inside the erythrocyte. The erythrocyte has become darker because of oxygen consumption for the bacterium oxidation.
The entrapped bacterium is gradually losing energy and stamina.
Last efforts of exhausted bacterium to escape from the erythrocyte.
The bacterium is exhausted and is dying.
Dead (killed by means of oxidation) bacterium inside the erythrocyte.
The bacterium inside the erythrocyte is killing by squeezing and immobilization.
The bacterium inside the erythrocyte is in agony.
The bacterium after killing is released by erythrocyte to plasma and is drifting with the flow.
The bacterium is gliding on the surface of erythrocyte and then escapes.


