Abstract
Objective
We investigated the viability of psychometrically robust executive function measures as markers for premanifest Huntington’s disease (HD).
Methods
Fifteen premanifest HD subjects and 42 controls were compared on the NIH EXAMINER executive function battery. This battery yields an overall Executive Composite score, plus Working Memory, Cognitive Control, and Fluency Scores that are measured on psychometrically matched scales. The scores were correlated with two disease markers, disease burden and striatal volumes, in the premanifest HD subjects.
Results
The premanifest HD subjects scored significantly lower on the Working Memory Score. The Executive Composite positively correlated with striatal volumes, and Working Memory Score negatively correlated with disease burden. The Cognitive Control and Fluency Scores did not differ between the groups or correlate significantly with the disease markers.
Conclusions
The NIH EXAMINER Executive Composite and Working Memory Score are sensitive markers of cognitive dysfunction, striatal volume, and disease burden in premanifest HD.
Keywords: Huntington’s disease, Executive function, Working memory, Striatal volume, Disease burden
Introduction
Huntington disease (HD) is an autosomal dominant neurodegenerative disease characterized by progressive motor, cognitive, and psychiatric symptoms. It is caused by the expansion of the trinucleotide cytosine-adenine-guanine (CAG).[1] Clinical diagnosis typically depends on motor abnormalities; however, an insidious decline in cognition can be evident 15 years prior to motor symptom onset.[2,3]
Although premanifest HD (pmHD) individuals perform lower than neurologically healthy controls (NC) on a range of cognitive skills,[4-9] targeting executive functions is compelling given their association with functional decline including difficulties with employment, financial management and driving.[10-12] Also, individuals with HD often lack awareness of their executive dysfunction,[13] which may lead to unreliable judgments regarding themselves and their healthcare needs.[14] Furthermore, executive functions are vulnerable to early brain changes in pmHD,[13,15] including striatal atrophy,[8,16] a reliable disease marker.[17] Deficits in working memory, a key component of executive function, are common.[18-20]
Given that cognitive dysfunction often precedes motor symptoms, sensitive cognitive markers are needed to guide when to initiate early interventions and measure treatment efficacy. There is no consensus, however, on what the best cognitive markers might be. Single measure scores can cause biases in estimated rates of change over time due to curvilinear scaling.[21] Composite scores encompassing several measures increase measurement precision over individual test scores,[22,23] but this approach may not make full use of the available information. Application of item response theory can improve sensitivity and increase statistical power, two important considerations for clinical trials. Item response theory takes item difficulty into account, resulting in linear scaling properties that measure with similar precision differences across the ability spectrum.[23,24] The scores are on the same scale and can be meaningfully contrasted.
We investigated the viability of the NIH EXAMINER as a disease marker in pmHD. The NIH EXAMINER battery produces four scores using item response theory: overall Executive Composite, Working Memory Score, Cognitive Control Score, and Fluency Score. We predicted that the Executive Composite and Working Memory Score would be lower in pmHD than NC, and would decline with increasing disease burden and striatal atrophy.
Methods
Subjects
This study was approved by the UCSF Committee on Human Research. Written informed consent was obtained from each subject. The NIH EXAMINER battery was administered to all 15 pmHD subjects, recruited through clinic or participation in other research studies, who completed a 3T MRI at the UCSF Memory and Aging Center between August 2011 and August 2013. The 42 NC were selected from the NIH EXAMINER validation study at UCSF to match the pmHD subjects on age and gender.
The pmHD individuals tested positive for the HD mutation with at least 40 CAG repeats and did not meet criteria for manifest motor HD according to previous methods.[3] Motor symptoms were evaluated by a neurologist using the Unified Huntington’s Disease Rating Scale (UHDRS) Motor subscale, M=8.0(6.9). Global cognition was evaluated using the Montreal Cognitive Assessment (MoCA), M=27.2(2.1). Disease burden was calculated using the CAG-Age Product Scaled (CAPS), formulated by the PREDICT-HD study.[25] The CAPS is classified into Low (>0 and ≤.67), Medium (>.67 and ≤.85), and High groups (>.85), denoting cumulative disease burden, with a higher score indicating closer proximity to diagnosis. The pmHD cohort sample included three Low, three Medium, and nine High burden individuals, with a mean CAPS of .9(.2) and CAG repeats of 42.5(2.2). NC status was determined based on neurological history and examination. The groups were closely matched in age and gender, but the NC had higher education, p=0.04.
Executive Function Assessment
Participants were administered 11 tests from the NIH EXAMINER battery (see Supplementary Table) using a 15.4” Dell Latitude D830 laptop. Derivation of the executive scores and their reliability is detailed in Kramer et al.[26] and http://examiner.ucsf.edu.
Neuroimaging Data Acquisition and Image Processing
High-resolution 3D T1-weighted imaging (IR-SPGR) was performed on a 3T MR scanner (GE Healthcare, Milwaukee, WI) on the 15 subjects with pmHD using an 8-channel head coil. Images were acquired in the sagittal plane using the following parameters: TE/TE=7.0/2.0 ms, 15° flip angle, 23 cm FOV, NEX=1, 1 mm slice thickness, 6:18 min duration. Each individual’s imaging data was assessed for problems of movement artifact and adequacy of scan coverage. Segmentation and volumetric measurements of the total intracranial volume, caudate and putamen were obtained using FreeSurfer version 5.1.0 (http://surfer.nmr.mgh.harvard.edu/). Right and left caudate and putamen volumes were summed to generate total striatal volumes.
Data Analysis
Group differences in NIH EXAMINER scores were evaluated using analysis of covariance, controlling for education. In the pmHD sample we correlated the scores with striatal volumes, controlling for intracranial volume, and with CAPS (disease burden). P-values < .05 were considered significant, whereas p-values <.10 were considered trends for all analyses. Cohen’s d effect sizes are reported for group differences.
Results
The pmHD subjects scored lower than NC on the Working Memory Score, F(1, 53) = 2.49, p = .03, d = .66 and there was a trend on the Executive Composite, F(1, 53) = .99, p = .09, d = .52. The groups did not differ on the Cognitive Control Score, F(1, 53) = .49, p = .31, d = .34 or the Fluency Score, F(1, 53) = .11, p = .57, d = .17 (Table 1).
Table 1.
Demographic and Examiner Scores of pmHD and Controls*
| N | Age | Gender | Education | Executive | Working Memory | Cognitive Control | Fluency | |
|---|---|---|---|---|---|---|---|---|
| pmHD | 15 | 45.6 (12.0) | 46.7% male | 15.1 (2.4) | .66 (.65) | .24 (.84) | .68 (.62) | .74 (.65) |
| NC | 42 | 47.0 (14.2) | 45.2% male | 16.4 (2.1) | 1.00 (.54) | .76 (.65) | .94 (.69) | .82 (.54) |
Scores represent mean (standard deviation).
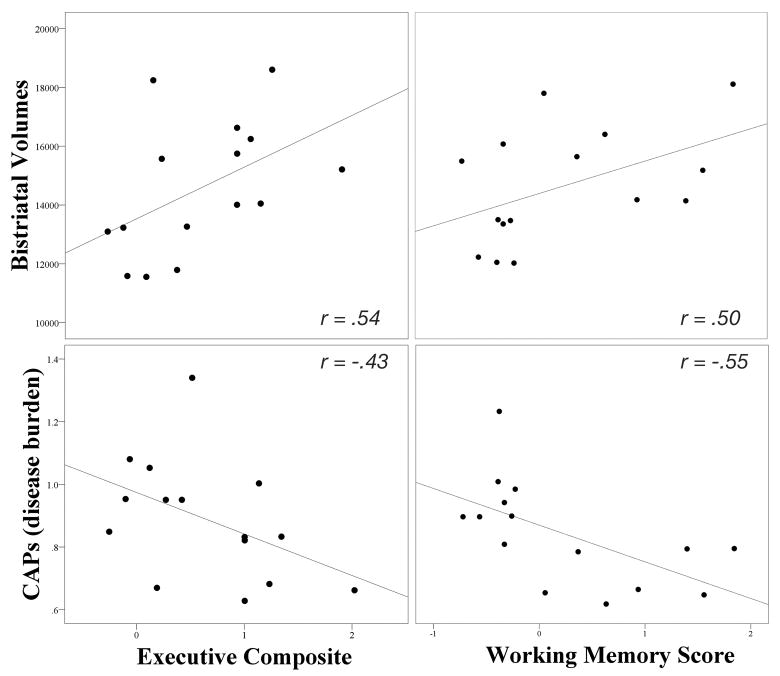
Striatal volumes positively correlated with the Executive Composite, p = .04, and trended with the Working Memory Score, p = .07; both were in the large effect size range (Figure 1).[27] Disease burden negatively correlated with the Working Memory Score, p = .03. The Cognitive Control and Fluency Scores did not correlate significantly with either striatal volumes (p = .20; p = .19, respectively) or disease burden (p = .40; p = .32, respectively), and the Executive Composite did not correlate significantly with disease burden (p = .11).
Figure 1.
Scatterplots of Executive Composite and Working Memory Score to Striatal Volumes and CAPS
Discussion
PmHD subjects scored lower than NC on the NIH EXAMINER Working Memory Score with a trend on the Executive Composite. Striatal volumes correlated with the Executive Composite and trended with the Working Memory Score, which correlated with lower disease burden. Cognitive Control and Fluency Scores did not differ between the groups or correlate with striatal volumes or disease burden. The lower Executive Composite in pmHD may be driven in part by the Working Memory Score due to overlapping measures; however, the Executive Composite was the most robust correlate of striatal volumes.
Our findings suggest that a decline in executive function, particularly working memory, precedes clinical (motor) diagnosis of HD and dysfunction is associated with early anatomical changes. Consistent with these findings, striatal volumes have been shown to predict the onset of HD[17] and correlate with executive dysfunction.[8,16] Additionally, executive functions, including working memory, have been shown to be impaired in pmHD.[18-20] In Harrington et al., for example, pmHD patients with medium to high disease burden were impaired on a factor score composed of verbal working memory and letter fluency tasks. A recent study by Georgiou-Karistianis et al.[28] did not find significant differences in working memory between pmHD and controls, however. This discrepancy may be related to methodological differences. The NIH EXAMINER’s spatial N-back uses 15 locations, whereas their spatial N-back used 4 locations. Our pmHD patients had a higher disease burden. In addition, our study used IRT generated composite scores, which can be more precise and sensitive to cognitive decline than individual test or factor scores.[21,22] A limitation of our study, however, is the small sample size; although the effect sizes were robust, replication is needed.
Our study does not support fluency and cognitive control as disease markers for pmHD. Verbal fluency deficits have been reported in pmHD,[7,19,30] but are inconsistent across studies and often small in magnitude.[31] Prior studies on cognitive control have also been inconsistent with impaired set-shifting[7] and 2-choice response time,[19] but intact flanker,[32] and similar levels of impairment on Stroop Interference relative to Color Naming or Word Reading.[19]
A critical step in validating a cognitive measure as a disease marker is to document its sensitivity to longitudinal decline. Future research will address this important issue using a larger sample size with subjects at different levels of disease burden and manifest HD. The Executive Composite has been shown to correlate with real world executive behavior in patients with a variety of neurological disorders,[33] and this question of ecological validity should be investigated in HD.
Clinical trials for neurodegenerative diseases are extraordinarily expensive, and rely heavily on clinical measures of efficacy.[34] Clinical measures sensitive to the effects of HD across animal models and human patients may have predictive value for guiding which therapeutics to move forward into clinical trials. Rodent behavioral assays requiring working memory include the radial arm maze[35,36] and complex operant conditioning paradigms including delayed alternation[37] and touch screen tasks that can use the same types of stimulus materials used in human subjects.[38,39] Incorporating species-appropriate tests of working memory into preclinical work could elucidate the extent to which different animal models of HD recapitulate this behavioral feature, and may help predict the impact of new therapies on HD-related premotor cognitive decline.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Neurological Disorders and Stroke (Kramer: HHSN271200623661C), the UC Discovery Research and Development Grant (ITLBIO 178688), the National Institute on Aging (Possin: K23AG037566; Kramer: R01AG032289; Miller: P50AG023501), the Larry L. Hillblom Foundation (Miller, 2007/2I), the Hellman Family Foundation, the Michael J. Homer Family Fund, the Taube/Koret Center, and the Huntington’s Disease Society of America (made possible with a gift from the James E. Bashaw Family). We are grateful to our research participants for their generous time and efforts.
Footnotes
Author Roles and Financial Disclosures
You: Led the design and execution of analyses and writing of the manuscript. Ms. You has no disclosures to report.
Geschwind: Organization and execution of research project and manuscript review and critique. Dr. Geschwind receives support from R01AG031189, K23AG021989, AG031220, P50AG023501, UL1RR024131, AG021601, N01NS02328, and the Michael J. Homer Family Fund. He also serves as a consultant for Lundbeck Inc, MedaCorp, The Council of Advisors, and Neurophage.
Sha: Organization and execution of research project and manuscript review and critique. Dr. Sha reports no disclosures.
Apple: Research project execution and contributed to writing of the first draft. Ms. Apple reports no disclosures.
Gooblar: Research project organization and execution and manuscript review and critique. Mr. Gooblar reports no financial disclosures.
Satris: Research project organization and execution and manuscript review and critique. Ms. Satris reports no disclosures.
Wood: Execution of research project and manuscript review and critique. Ms. Wood reports no disclosures.
Johnson: Execution of research project and manuscript review and critique. Ms. Johnson reports no disclosures.
Feuerstein: Execution of analyses and manuscript review and critique. Ms Feuerstein reports no financial disclosures.
Finkbeiner: Conception of research project, study design, and manuscript review and critique. Dr. Finkbeiner receives support from the Taube/Koret Center (SF), the Hellman Program for Alzheimer’s Disease Research (SF) and the Huntington’s Disease Society of America (made possible with a gift from the James E. Bashaw Family).
Kang: Organization and execution of research project and manuscript review and critique. Dr. Kang reports no disclosures.
Miller: Conception and organization of research project. Dr. Miller receives grant support from the National Institute on Aging and serves as a consultant for TauRx, Allon Therapeutics, Lilly USA LLC, and Seimens Medical Solutions. He is on the Board of Directors for the John Douglas French Foundation for Alzheimer’s Research and for The Larry L. Hillblom Foundation.
Hess: Research project execution and writing of the first draft. Dr. Hess receives support from R01HD072074, R01EB009756, R01FD004103, U01NS058634, U01NS053998 and has provided expert testimony (medical-legal).
Kramer: Oversaw all aspects of this project including study design, data interpretation, and manuscript review and critique. Dr. Kramer receives research support from P50AG023501, R01AG022983, and R01AG032289. He receives honoraria for serving on the University of Indiana Alzheimer Disease Center external advisory board and royalties from the California Verbal Learning Test. He previously received support from Novartis Pharmaceuticals.
Possin: Oversaw all aspects of this project including study design, data interpretation, and manuscript review and critique. Dr. Possin has received support from K23AG037566, the Hellman Family Foundation, and the Michael J. Fox Foundation.
References
- 1.Tabrizi SJ, Langbehn DR, Leavitt BR, et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2011;8:791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC. Psychiatric symptoms in Huntington’s disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007;62:1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemiere J, Decruyenaere M, Evers-Kiebooms G, Vandenbussche E, Dom R. Cognitive changes in patients with Huntington’s disease (HD) and asymptomatic carriers of the HD mutation--a longitudinal follow-up study. J Neurol. 2004;251:935–942. doi: 10.1007/s00415-004-0461-9. [DOI] [PubMed] [Google Scholar]
- 5.Solomon AC, Stout JC, Johnson SA, et al. Verbal episodic memory declines prior to diagnosis in Huntington’s disease. Neuropsychologia. 2007;45:1767–1776. doi: 10.1016/j.neuropsychologia.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson SA, Stout JC, Solomon AC, et al. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington’s disease. Brain. 2007;130:1732–1744. doi: 10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence AD, Hodges JR, Rosser AE, et al. Evidence for specific cognitive deficits in preclinical Huntington’s disease. Brain. 1998;121:1329–1341. doi: 10.1093/brain/121.7.1329. [DOI] [PubMed] [Google Scholar]
- 8.Papp KV, Snyder PJ, Mills JA, et al. Measuring executive dysfunction longitudinally and in relation to genetic burden, brain volumetrics, and depression in prodromal Huntington disease. Arch Clin Neuropsychol. 2013;28:156–168. doi: 10.1093/arclin/acs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snowden JS, Craufurd D, Thompson J, Neary D. Psychomotor, executive, and memory function in preclinical Huntington’s disease. J Clin Exp Neuropsychol. 2002;24:133–145. doi: 10.1076/jcen.24.2.133.998. [DOI] [PubMed] [Google Scholar]
- 10.Beglinger LJ, O’Rourke JJ, Wang C, Langbehn DR, Duff K, Paulsen JS. Earliest functional declines in Huntington disease. Psychiatry Res. 2010;178:414–418. doi: 10.1016/j.psychres.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 12.Nehl C, Paulsen JS. Cognitive and psychiatric aspects of Huntington disease contribute to functional capacity. J Nerv Ment Dis. 2004;192:72–74. doi: 10.1097/01.nmd.0000106004.67587.57. [DOI] [PubMed] [Google Scholar]
- 13.Duff K, Paulsen JS, Beglinger LJ, et al. “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci. 2010;22:196–207. doi: 10.1176/appi.neuropsych.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prigatano GP. Anosognosia: clinical and ethical considerations. Curr Opin Neurol. 2009;22:606–611. doi: 10.1097/WCO.0b013e328332a1e7. [DOI] [PubMed] [Google Scholar]
- 15.Hart E, Middelkoop H, Jurgens CK, Witjes-Ané MN, Roos RA. Seven-year clinical follow-up of premanifest carriers of Huntington’s disease. PLoS Curr. 2011;3:RRN1288. doi: 10.1371/currents.RRN1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J. Executive dysfunction in early stages of Huntington’s disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J Neurol Sci. 2005;239:11–19. doi: 10.1016/j.jns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Aylward EH, Liu D, Nopoulos PC, et al. Striatal volume contributes to the prediction of onset of Huntington disease in incident cases. Biol Psychiatry. 2012;71:822–828. doi: 10.1016/j.biopsych.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirogovsky E, Goldstein J, Peavy G, Jacobson MW, Corey-Bloom J, Gilbert PE. Temporal order memory deficits prior to clinical diagnosis in Huntington’s disease. J Int Neuropsychol Soc. 2009;15:662–670. doi: 10.1017/S1355617709990427. [DOI] [PubMed] [Google Scholar]
- 19.Stout JC, Paulsen JS, Queller S, et al. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25:1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumas EM, Say MJ, Jones R, et al. Visual working memory impairment in premanifest gene-carriers and early Huntington’s disease. J of Huntingtons Dis. 2012;1:97–106. doi: 10.3233/JHD-2012-120010. [DOI] [PubMed] [Google Scholar]
- 21.Crane PK, Narasimhalu K, Gibbons LE, et al. Item response theory facilitated cocalibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol. 2008;61:1018–1027.e9. doi: 10.1016/j.jclinepi.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington DL, Smith MM, Zhang Y, Carlozzi NE, Paulsen JS. Cognitive domains that predict time to diagnosis in prodromal Huntington disease. J Neurol Neurosurg Psychiatry. 2012;83:612–619. doi: 10.1136/jnnp-2011-301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbons LE, Carle AC, Mackin RS, et al. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS. Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:751–763. doi: 10.1002/ajmg.b.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer JH, Mungas D, Possin KL, et al. NIH EXAMINER: Conceptualization and Development of an Executive Function Battery. J Int Neuropsychol Soc. doi: 10.1017/S1355617713001094. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 28.Georgiou-Karistianis N, Stout JC, Domínguez DJF, et al. Functional magnetic resonance imaging of working memory in Huntington’s disease: Cross-sectional data from the IMAGE-HD study. Hum Brain Mapp. 2013;34 doi: 10.1002/hbm.22296. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 30.Larsson MU, Almkvist O, Luszcz MA, Wahlin TB. Phonemic fluency deficits in asymptomatic gene carriers for Huntington’s disease. Neuropsychology. 2008;22:596–605. doi: 10.1037/0894-4105.22.5.596. [DOI] [PubMed] [Google Scholar]
- 31.Henry JD, Crawford JR, Phillips LH. A meta-analytic review of verbal fluency deficits in Huntington’s disease. Neuropsychology. 2005;19:243–252. doi: 10.1037/0894-4105.19.2.243. [DOI] [PubMed] [Google Scholar]
- 32.Beste C, Saft C, Yordanova J, et al. Functional compensation or pathology in corticosubcortical interactions in preclinical Huntington’s disease? Neuropsychologia. 2007;45:2922–2930. doi: 10.1016/j.neuropsychologia.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Possin KL, Lamarre AK, Wood KA, Mungas DM, Kramer JH. Ecological Validity and Neuroanatomical Correlates of the NIH EXAMINER Executive Composite Score. J Int Neuropsychol Soc. 2013;14:1–9. doi: 10.1017/S1355617713000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkbeiner S. Bridging the Valley of Death of therapeutics for neurodegeneration. Nat Med. 2010;16:1227–32. doi: 10.1038/nm.2222. [DOI] [PubMed] [Google Scholar]
- 35.Haik KL, Shear DA, Schroeder U, Sabel BA, Dunbar GL. Quinolinic acid released from polymeric brain implants causes behavioral and neuroanatomical alterations in a rodent model of Huntington’s disease. Exp Neurol. 2000;163:430–439. doi: 10.1006/exnr.2000.7384. [DOI] [PubMed] [Google Scholar]
- 36.Kolata W, Kolata S. A model of working memory capacity in the radial-arm maze task. J Math Psychol. 2009;53:242–252. [Google Scholar]
- 37.Trueman RC, Jones L, Dunnett SB, Brooks SP. Early onset deficits on the delayed alternation task in the Hdh(Q92) knock-in mouse model of Huntington’s disease. Brain Res Bull. 2012;88:156–162. doi: 10.1016/j.brainresbull.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Romberg C, Horner AE, Bussey TJ, Saksida LM. A touchscreen-automated cognitive test battery reveals impaired attention, memory abnormalities and increased response inhibition in the TgCRND8 mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:731–744. doi: 10.1016/j.neurobiolaging.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bizon JL, Foster TC, Alexander GE, Glisky EL. Characterizing cognitive aging of working memory and executive function in animal models. Front Aging Neurosci. 2012;4:19. doi: 10.3389/fnagi.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.