Abstract
Intervertebral discs are biologically regulated by the maintenance of a balance between the anabolic and catabolic activities of disc cells. Therapeutic agents, initially evaluated using in vitro studies on disc cells and explants, have been used as intradiscal injections in preclinical settings to test in vivo efficacy. These include anabolic growth factors and other biostimulatory agents as well as antagonistic agents against matrix-degrading enzymes and cytokines. Additional work is needed to identify suitable patient populations, using methods such as MRI, and to better understand the mechanism of healing. Clinical trials are currently underway for a few of these agents, while many other promising candidates are on the horizon.
Keywords: back pain, animal model, disc injection, growth factor, cytokine, MRI
Intervertebral Disc Degeneration and Homeostasis of the Extracellular Matrix
Intervertebral disc (IVD) degeneration is one of the major causes of low back pain and lumbar disc herniation. Biologically, the cells in the disc actively regulate the homeostasis of the IVD extracellular matrix by maintaining a balance between anabolism and catabolism. The modulation of disc cell metabolism involves a variety of molecules (e.g., cytokines, enzymes, enzyme inhibitors, and growth factors) that act in a paracrine and/or autocrine fashion. The degeneration of an IVD may result from the loss of steady state metabolism, maintained in the normal discs, due to an imbalance between the anabolic and catabolic processes. The proteoglycan (PG) content of the extracellular matrix (ECM) and the rate of PG synthesis decreased markedly with age and degeneration, similar to the case in articular cartilage.1–9 The anabolic regulators include polypeptide growth factors, such as insulin-like growth factor (IGF-1), transforming growth factor-β (TGF-β), and the bone morphogenetic proteins (BMPs).10,11 Catabolic regulators include many cytokines, notably interleukin-1 (IL-1)12,13 and tumor necrosis factor (TNF-α),12–14 which influenced the synthesis of matrix-degrading enzymes, such as the matrix metalloproteinases (MMPs).15–20 Alterations in both anabolic and catabolic processes are thought to play key roles in the onset and progression of IVD degeneration; the biochemical processes that regulate these changes are important to understand for the development of effective treatments of disc degeneration.
Catabolic Mediators and Enzymes in Disc Degeneration
Under pathological conditions, including IVD degeneration,21 physical injury (e.g., puncture or stab wounds)15,22 and abnormal mechanical loading,23,24 the IVD expresses cytokines and proteinases. While macrophages that infiltrate herniated tissue or granulation tissue seem to be the major source of these cytokines,25–27 recent studies clearly indicate that IVD cells may also synthesize these molecules in an autocrine fashion.28 In particular, increases in both protein and mRNA levels of IL-1 and its major regulator, TNF-α, have been observed in degenerated or herniated IVD tissues and both are spontaneously expressed by these tissues in culture.29–36
The catabolic processes induced by cytokines are mediated by various enzymes, such as the MMPs13,31,37–39 or the aggrecanases.40 IL-1 has been shown to stimulate matrix degradation as well as to inhibit the synthesis of ECM macromolecules41–44 with minimum effect on cell proliferation.41 IL-1 induced the production of collagenase,45 cycloxygenase-2 (COX-246), prostaglandin E2,13,47 nitric oxide,45,44 MMP-1,48 total46 and active43 MMP-3, MMP-13,20,44 the aggrecanase, a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4),21,44 IL-6,46 and monocyte chemoattractant protein-1 (MCP-1).49,50 Recently, IL-1β was shown to increase the sensitivity of IVD cells to shear stress,51 suggesting that this cytokine is involved in the acceleration of degradation processes in IVDs subjected to biomechanical stress. Although IL-1 affects both synthetic and degradative processes, it is worth noting that, at lower concentrations, IL-1 was much more effective in inhibiting the synthesis of aggrecan than in stimulating its degradation in articular cartilage.52–54 An epidemiological study has shown that IL-1 gene cluster polymorphisms significantly increased the risk of disc degeneration; this has shed more light on the possible involvement of IL-1 in IVD degeneration.55
The effects of the inhibition of catabolic cytokines have been evaluated in a number of studies. IL-1 receptor antagonist (IL-1ra), applied in vitro to degenerated56 and herniated57 human disc tissues, reduced the expression of MMP-3.56,57 IL-1ra pretreatment of nucleus pulposus (NP) cells from moderately degenerate human discs reduced the expression of ADAMTS-4 and MMP-3 in subsequent treatment with IL-1.58 In another human cell culture study, the addition of IL-1ra and soluble TNF receptor significantly upregulated PG synthesis, suggesting that IL-1 and TNF suppress PG de novo synthesis.59 IL-1ra, and other agents, may benefit from administration of slow-release agents, such as IL-1ra mixed with thermally responsive elastin-like polypeptide (ELP)58 or with gelatin hydrogel.60 In addition to IL-1, TNF-α has gained attention in association with disc herniation.33,34,36,61 TNF-α inhibition, using a monoclonal antibody in herniated human disc explants, showed suppression of MMP-3 levels57; other anti-TNF agents are beginning to be applied to reduce pain in patients with sciatica62–64 and discogenic pain.65 Other anti-cytokine therapeutics include the p38 mitogen-activated protein kinase (MAPK) inhibitor, which hindered the catabolic effects of IL-166 as well as nuclear factor-kappaB (NF-κB) decoy, which reduced pain in a rat lumbar disc herniation model.67
Anabolic Effects of Cytokines and Growth Factors on IVD Cells
A variety of growth factors and cytokines (Table 1) can alter IVD homeostasis and stimulate ECM synthesis by shifting cellular metabolism to a more anabolic state.68 The effects of TGF-β on PG synthesis10,69 and cell proliferation69 were noted early in the literature. Similar effects of IGF-1 have also been reported.11 IGF-1 and platelet-derived growth factor (PDGF) were also shown to reduce the percentage of apoptotic anulus fibrosus (AF) cells induced by serum depletion in culture.70
Table 1.
The in vitro effects of therapeutic agents.
| Agent | Target | Dose | Effect | Author |
|---|---|---|---|---|
| TGF-β | mature canine IVD | 1 ng/ml | PG synthesis increased up to 5×; higher in NP than AF. | Thompson 199110 |
| IGF-1 | mature canine IVD | 20 ng/ml | PG synthesis marginally increased in NP. | Thompson 199110 |
| TGF-β | human anulus cells, 3D culture | 0.25 to 5 ng/ml | Cell proliferation and PG synthesis increased; reduced apoptosis with serum depletion. | Gruber 199769 |
| IGF-1 | young and old bovine NP cells | 0 to 1000 ng/ml | Cell proliferation and matrix synthesis stimulated; more IGF-1 receptors. | Osada 199611 |
| OP-1 | young rabbit NP and AF cells; alginate beads | 0, 100, 200 ng/ml | Increased PG and collagen production and content. | Masuda 200371 |
| BMP-2 | rat IVD cells monolayer | 0 to 1000 ng/ml | Increased cell number, GAG, expression for collagen, aggrecan at higher doses. | Yoon 2003129 |
| OP-1 | human NP and AF cells, alginate beads | 0, 100, 200 ng/ml | Maintained cell density, increased PG synthesis and accumulation. | Imai 200772 |
| OP-1 | rabbit IVD cells; alginate beads: IL-1a pre-exposure | 200 ng/ml | IL-1 decreased PG and collagen; reversed and exceeded with OP-1. | Takegami 200242 |
| OP-1 | rabbit IVD cells; alginate bead: C-ABC pre-exposure | 200 ng/ml | OP-1 upregulated PG synthesis. Greater effect on C-ABC pre-exposure than control. | Takegami 200573 |
| BMP-2 | human IVD cells | 300, 1500 ng/ml | Increased PG synthesis, expression of aggrecan, types I and II collagen; no bone formation. | Kim 2003130 |
| rhBMP-2 & 12 | human IVD cells in monolayer | 25 to 300 ng/ml | PG, collagen synthesis increased in NP cells; minimal effect on AF cells. | Gilbertson 2008131 |
| GDF-5 | bovine IVD cells; alginate bead | 100, 200 ng/ml | Increased DNA and PG content; at higher dose, PG and collagen synthesis increased. | Chujo 200676 |
| PRP | porcine IVD cells; alginate bead | 10% PRP | Mild increase in cell proliferation; marked increase in PG and collagen synthesis and PG accumulation. | Akeda 200678 |
| TGF-β1 and PRP | human NP cells | 1 ng/mL TGF-β1 in PRP | NP cell proliferation and aggregation; increase in mRNA of SOX-9, type II collagen, aggrecan. | Chen 200679 |
| Ad-TIMP-1, Ad-BMP-2 | IVD cells from human degenerated IVD | 50–150 MOI | 2000 pg/ml production of TIMP w/100 MOI at day-4. PG synthesis increased with both Ad-TIMP-1 and Ad-BMP-2. | Wallach 2003132 |
| Dexa-methasone | human disc herniation tissue explants | 0.01 mM | Decreased MMP-1 and -3 levels. | Genevay 200957 |
| IL-1ra | human disc herniation tissue explants | 100 ng/ml | Decreased MMP-3 levels. | Genevay 200957 |
| IL-1ra | human normal and degenerated disc tissues in situ with IL-1 treatment | 100 ng/ml | IL-1ra reduced cytokine levels (MMP-3, -7, -13) and matrix degradation in all tissue types. | Le Maitre 200756 |
| IL-1ra/ELP | human IVD cells (grade 2–3); alginate beads: IL-1ra pre-Tx then IL-1β insult | 4–8000 pM | Reduced ADAMTS-4, MMP-3 transcription. | Shamji 200758 |
| p38 MAPK inhibitor (SB 202190) | rabbit NP cells pre-treated with IL-1 | 1 µM | Decreased message for collagen, aggrecan, IGF-1. Increased message for iNOS, COX-2, MMP-3, IL-6. | Studer 200866 |
| TNF inhibitor mAb | human IVD herniation tissue explants | 10 µg/ml | Decreased MMP-3 levels | Fujita 199338 |
TGF-β: transforming growth factor-β; PG: proteoglycan; NP: nucleus pulposus: AF: annulus fibrosus; IGF-1 insulin-like growth factor-1; 3D: three-dimensional; OP-1 osteogenic protein-1; BMP-2: bone morphogenetic protein-2; IVD: intervertebral disc; GAG: glycosaminoglycan; C-ABC: chondroitinase-ABC; GDF-5 growth differentiation factor-5; PRP: platelet-rich plasma; Ad-TIMP-1: adenoviral vector delivering cDNA of tissue inhibitor of matrix metalloproteinases-1; Ad-BMP-2: adenoviral vector delivering cDNA of BMP-2; MOI: multiplicity of infection; IL-1ra: IL-1 receptor antagonist; ELP: elastin-like polypeptide; Tx: treatment; ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; MAPK: mitogen activated protein kinase; NP: nucleus pulposus; iNOS: inducible nitric oxide synthase; COX-2: cyclooxygenase-2; TNF: tumor necrosis factor; mAb monoclonal antibody.
Osteogenic protein-1 (OP-1),71 which is a member of the BMP family, upregulates the PG metabolism of IVD cells. OP-1 strongly stimulated the production and formation of the ECM by rabbit IVD cells;71 similar effects have been noted using human IVD cells.72 OP-1 also replenished PGs and collagens after depletion of the ECM following exposure of IVD cells to IL-142 or chondroitinase ABC (C-ABC).73
Growth and differentiation factor-5 (GDF-5), originally found to be a factor responsible for skeletal alterations in brachypodism mice,74 is another member of the BMP family that has anabolic effects on IVD cells. GDF-5 stimulated PG and type II collagen expression by mouse IVD cells.75 Recombinant human GDF-5 (rhGDF-5) enhanced cell proliferation and matrix synthesis and accumulation by cells from bovine NP, and to lesser extent, AF cells.76
Because biological molecules, prepared in an autologous fashion, avoid certain regulatory complications, they may be useful clinically. Autologous IL-1ra has been used, along with IGF- 1 and PDGF proteins, to reduce apoptosis of disc cells and their production of IL-1 and IL-6.77 Platelet-rich plasma (PRP) can be produced by centrifugal separation of a patient’s own blood in the operating room; it contains multiple growth factors concentrated at high levels. In vitro, PRP stimulated cell proliferation and matrix synthesis, as shown using porcine disc cells.78 PRP induced cell proliferation and differentiation, and facilitated NP-like tissue formation by human disc cells.79
Intradiscal Therapeutics: Animal Studies
To study the ability of growth factors and other therapeutic agents to stimulate repair in vivo (Table 2), small animal models, in which the degree of degeneration can be controlled, are useful. Rabbit lumbar76,80–83 and rat caudal discs84–87 have been used extensively, although other animal models exist.85,86 Both physical injury, such as stab wound6,88 or controlled needle puncture,89,90 and chemical degradation83–87 approaches have been used.
Table 2.
The in vivo effects of intradiscal injection treatments.
| Agent | Species | Site | Model | Dose per Disc | Effect | Author |
|---|---|---|---|---|---|---|
| IGF-1 | Rat | Tail | Static compression | 8 ng/8 µl | Clustering of inner anulus cells after single injection | Walsh 200499 |
| GDF-5 | Rat | Tail | Static compression | 8 ng/8 µl | Clustering of cells, increase in disc height (single injection) | Walsh 200499 |
| TFG-β | Rat | Tail | Static compression | 1.6 ng/8 µl | Proliferation of cells (multiple injections) | Walsh 200499 |
| bFGF | Rat | Tail | Static compression | 8 ng/8 µl | No response | Walsh 200499 |
| OP-1 | Rabbit | Lumbar | None (normal) | 2 µg/10 µl | Increased disc height and PG content in NP | An 2005133 |
| OP-1 | Rabbit | Lumbar | C-ABC: Co-injection | 100 µg/10 µl | Increased disc height and PG content in NP | Imai 2003134 |
| OP-1 | Rabbit | Lumbar | Needle puncture: Tx 4 wks later | 100 µg/10 µl | Increased disc height and PG content in NP and AF, improvement of MRI and histology grades | Masuda 200680 |
| OP-1 | Rabbit | Lumbar | Needle puncture | 100 µg/10 µl | Increased disc height and viscoelastic properties | Miyamoto 200681 |
| OP-1 | Rabbit | Lumbar | C-ABC: Tx 4 wks later | 100 µg/10 µl | Increased disc height, PG content in NP and AF | Imai 200782 |
| GDF-5 | Rabbit | Lumbar | Needle puncture: Tx 4 wks later | 1, 100 ng/10 µl; 1, 100 µg/10 µl | Increased disc height, improvement of MRI and histology grades | Chujo 200676 |
| GDF-5 | Rabbit | Lumbar | Thrombin-degraded: Tx 4 wks later | 10 µg/10 µl | Increased disc height, improved T1rho and T2 values. Decreased ADAMTS-4, -5, COX9 expression | Bae 200983 |
| BMP-2 | Rabbit | Lumbar | Anular stab (5×7 mm) | 100 µg/100 µl | More degeneration, vascularity and fibroblast | Hwang 2007135 |
| PRP | Rabbit | Lumbar | Nucleotomy, immediate Tx | PRP+GHM(20 µl) or PRP(5µl) +PBS(15µl) | PRP+GHM group had less degeneration and increased PG; PRP+PBS group showed no differences | Nagae 2007108 |
| PRP | Rabbit | Lumbar | Nucleotomy, immediate Tx | 20 µl of PRP+GHM, PBS+GHM, PRP+PBS; puncture-only | PRP+GHM had greater disc height, water content, mRNA for PG core protein and type II collagen; fewer apoptotic cells in NP | Sawamura 200960 |
| BMP-17 | Sheep | Lumbar | Anular stab (3×6 mm), immediate Tx | 300 µg/70 µl | BMP-17 maintained disc height, MRI and histology scores, NP cell density; increased PG and collagen synthesis. | Wei 2009136 |
| ADAMTS -5 siRNA | Rabbit | Lumbar | Needle puncture: Tx 4 wks later | 10 µg/10 µl | Improved MRI and histology scores | Seki 2009111 |
IGF-1: insulin-like growth factor-1; GDF-5 growth differentiation factor-5; TGF-β: transforming growth factor-β; bFGF: basic fibroblast growth factor; OP-1: osteogenic protein-1; PG: proteoglycan; NP: nucleus pulposus; C-ABC: chondroitinase-ABC; Tx: treatment; MRI: magnetic resonance imaging; AF: annulus fibrosus; ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; COX: cyclooxygenase; BMP-2: bone morphogenetic protein-2; PRP: platelet-rich plasma; GHM: gelatin hydrogel microspheres; PBS: phosphate-buffered saline; siRNA: small interference RNA (siRNA).
The in vivo efficacy of OP-1 injection has been evaluated in a number of animal studies. In adolescent rabbits, an injection of recombinant human OP-1, but not the lactose vehicle, reversed the reduction in disc height and worsened the magnetic resonance imaging (MRI) grade caused by an anular needle-puncture.80 In another study with the same experimental design, the injection of OP-1 restored dynamic viscoelastic biomechanical properties, such as elastic and viscous moduli, of puncture-degenerated IVDs.81 OP-1 treatment was also effective in restoring discs that have been chemically degraded. C-ABC has been used as an alternative to chymopapain for chemonucleolysis,91–98 as well as an animal model of disc degeneration in the rat tail84–87 and goat85,86 disc. When OP-1 or vehicle was injected into rabbit discs degraded with C-ABC for 4 weeks, the disc height initially decreased (~34%), then recovered and gradually approached the level of the normal control.82
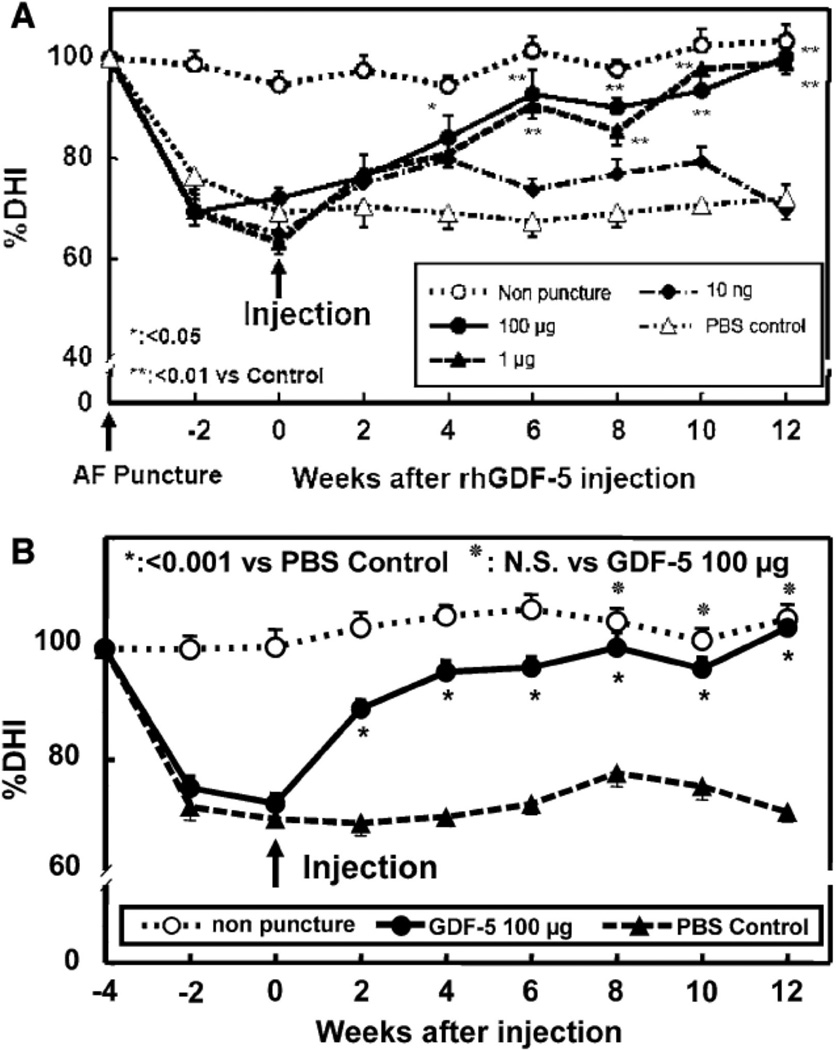
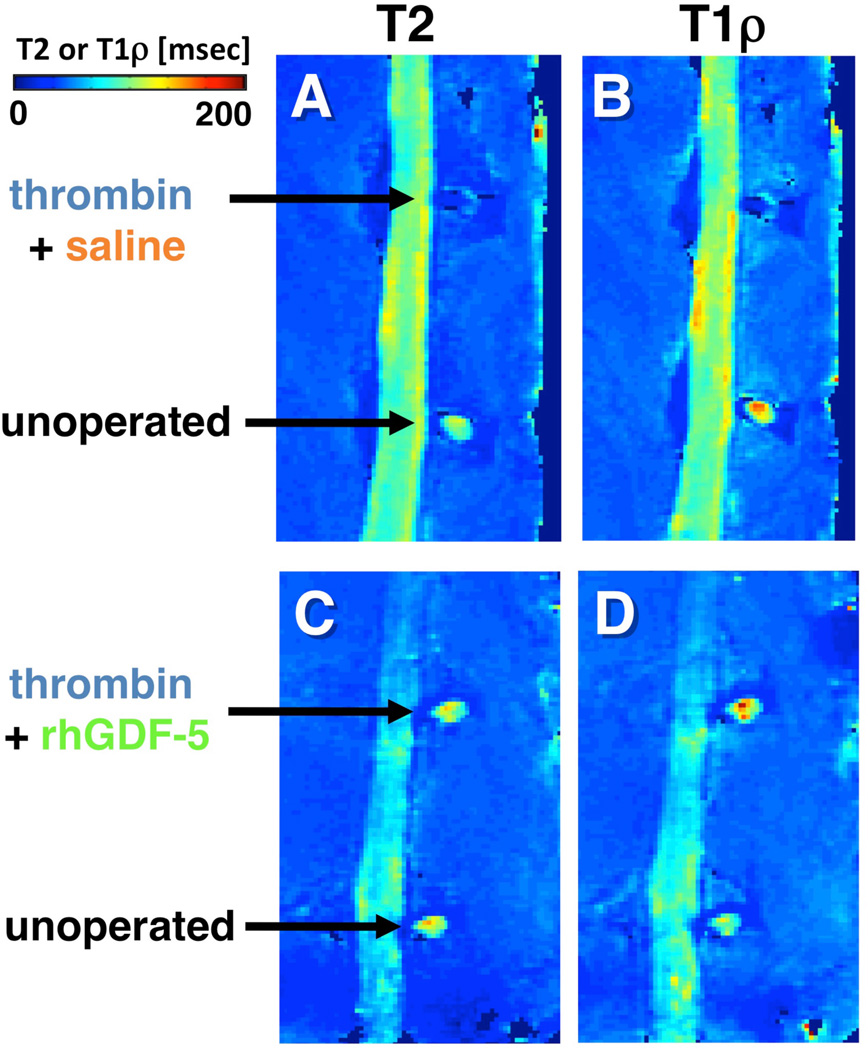
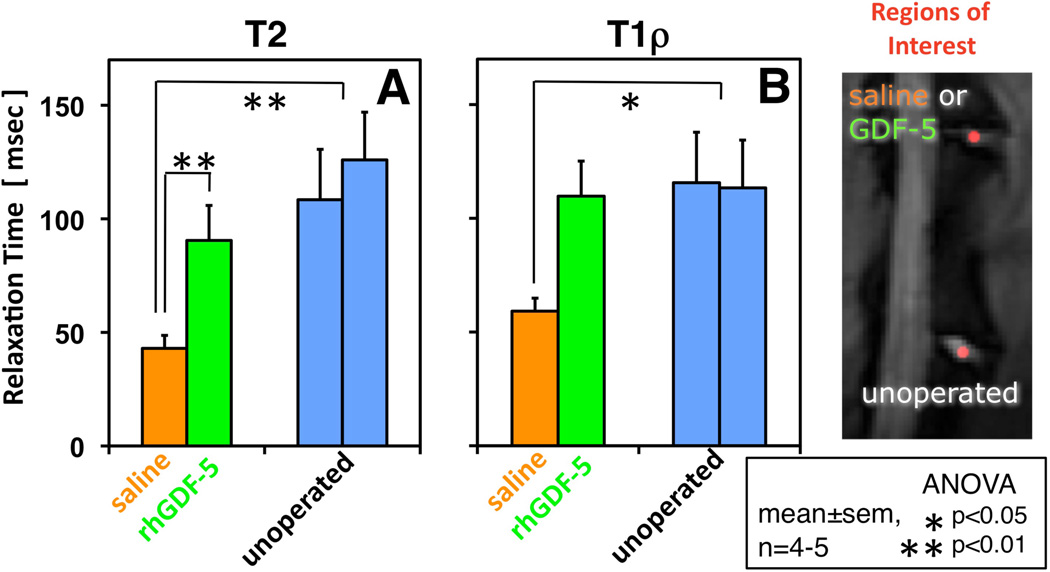
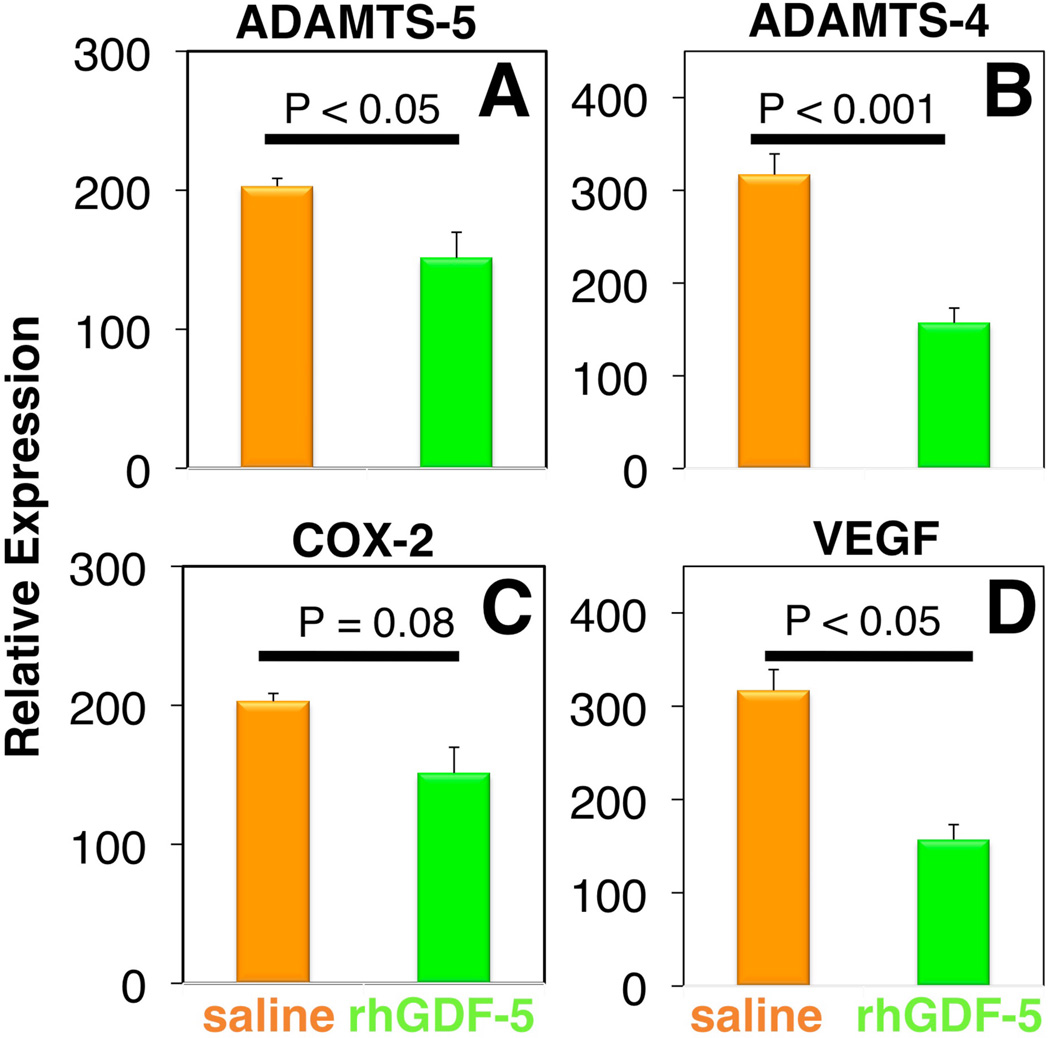
rhGDF-5 is another promising growth factor whose efficacy has been evaluated in animal models. The efficacy of injecting a single dose of GDF-5 has been reported in the mouse caudal disc with degeneration induced by static compression.99 In that study, GDF-5 induced an increase in disc height and the expansion of the inner AF fibrochondrocytic population into the NP. In a needle-puncture model of disc degeneration (4 weeks) in adolescent (5–6 months-old) rabbits, a single injection of rhGDF-5 restored disc height (Figure 1A) and improved MRI and histological grading scores within approximately 6 weeks.76 There are concerns that aged or degenerated discs might have lower cellularity, especially in the number of notochordal cells often found in normal adolescent rabbit discs,100,101 and might respond poorly to growth factor treatment. Despite this, a study using 2-year old rabbits showed that rhGDF-5 effectively recovered disc height (Figure 1B) as well as MRI and histologic grades after a 12-week observation period.102 Biomechanical analyses indicated that the viscous and elastic moduli of the IVDs in the rhGDF-5-injected discs were significantly higher than those in the PBS-injected discs. Additionally, rhGDF-5 has shown efficacy in rabbit discs that have been degraded using thrombin,83 a serine proteinase that results in cleavage of PGs and decreased disc height.103,104 In this study, discs of adolescent rabbits were injected with thrombin (100 U/10 µl) and the rabbits were maintained for 4 weeks. A single injection of rhGDF-5 (10 µg/10 µl) or saline was then given, and endpoint measures (MRI and gene expression) were determined 12 weeks later. Quantitative MRI was used to evaluate T2 and T1rho105,106 MR properties. T2 (Figure 2AC) and T1rho (Figure 2BD) MRI maps showed maintenance of NP morphology and MR values in rhGDF-5-treated discs. Both T2 (Figure 3A) and T1rho (Figure 3B) values of the NP were higher in the rhGDF-5 group, approaching those of the unoperated control. Treatment with rhGDF-5 also reduced the level of expression for ADAMTS-5 (Figure 4A), ADAMTS-4 (Figure 4B), COX-2 (Figure 4C), and vascular endothelial growth factor (VEGF) (Figure 4D), molecules related to PG degradation, pain, and neovascularization seen in degenerated IVDs.107
Figure 1. Changes in the intervertebral disc (IVD) height index (DHI) after anular puncture and recombinant human growth and differentiation factor-5 (rhGDF-5) injection.
In a needle-puncture model of disc degeneration (4 weeks) in adolescent (5–6 months-old) rabbits, a single injection of rhGDF-5 restored disc height (A). In a study using 2-year old rabbits, rhGDF-5 disc height was also effectively recovered (B). (Modified from Chujo T, et al. Effects of growth differentiation factor-5 on the intervertebral disc- in vitro bovine study and in vivo rabbit disc degeneration model study. Spine 2006;31(25):2909-17 and Chujo T, et al. In vivo effects of recombinant human growth and differentiation factor-5 on the repair of the mature rabbit intervertebral disc. Spine J, 6(5):23S–24S, 2006 (North American Spine Society, 21st Annual Meeting Proceeding, abstract). AF: annulus fibrosus; PBS: phosphate-buffered saline.
Figure 2. Quantitative magnetic resonance (MR) parameter maps of thrombin-induced degraded rabbit lumbar spines after injection with the growth factor, recombinant human growth and differentiation factor-5 (rhGDF-5).
Four weeks after thrombin injection (100 U/10 µl) into adolescent rabbit discs, a single injection of saline (10 µl) (A, B) or rhGDF-5 (10 µg/10 µl) (C, D) was given. T2 (A, C) and T1rho (B, D) MRI maps were obtained 12 weeks later. High MR values in the nucleus pulposus regions are apparent in the samples treated with the growth factor (C, D). (Modified from Bae WC, et al. Effect of rhGDF-5 on the thrombin model of rabbit intervertebral disc degeneration: T1rho quantification using 3T MRI. Rad Soc North Am 2009;95:SSE14-02, abstract)
Figure 3. Treatment with recombinant human growth and differentiation factor-5 (rhGDF-5) restored T2 and T1rho values after thrombin-induced degeneration in adolescent rabbit discs.
Four weeks after thrombin injection (100 U/10 µl) into adolescent rabbit discs, a single injection of saline (10 µl) or rhGDF-5 (10 µg/10 µl) was given. T2 and T1rho MRI maps were obtained 12 weeks later. Regions of interest were selected to determine T2 and T1rho values in the nucleus pulposus (NP). Both T2 (A) and T1rho (B) values of the NP were higher in the rhGDF-5-treated group, approaching those of the unoperated group. The saline group had significantly lower values compared to the unoperated control. (Modified from Bae WC, et al. Effect of rhGDF-5 on the thrombin model of rabbit intervertebral disc degeneration: T1rho quantification using 3T MRI. Rad Soc North Am 2009;95:SSE14-02, abstract)
Figure 4. Treatment with recombinant human growth and differentiation factor-5 (rhGDF-5) inhibits the expression of molecules related to disc degradation, pain, and neovascularization in thrombin-degenerated adolescent rabbit discs.
Four weeks after thrombin injection (100 U/10 µl) into adolescent rabbit discs, a single injection of saline (10 µl) or rhGDF-5 (10 µg/10 µl) was given. Twelve weeks later, mRNA expression levels for ADAMTS-5 (A), ADAMTS-4 (B), COX-2 (C) and VEGF (D), in saline-and rhGDF-5-treated discs showed a trend or significantly lower levels in the rhGDF-5-treated samples. (Modified from Masuda K, et al. Intradiscal injection of recombinant human growth and differentiation factor-5 significantly suppressed the expression of cytokines, catabolic enzymes and pain markers in the rabbit anular puncture model. Paper presented at: The 35th Annual Meeting of the International Society for the Study of the Lumbar Spine 2009:47, abstract) ADAMTS: a disintegrin and metalloproteinase with a thrombospondin type 1 motif; COX-2: cyclooxygenase-2; VEGF: vascular endothelial growth factor.
PRP is another agent that has been evaluated in animal models. Using a nucleotomy rabbit model, the effects of allograft PRP with or without gelatin hydrogel microspheres (to provide slow release and mechanical support) and phosphate buffered-saline (PBS)-only injectates were evaluated.108 The PRP+microspheres group had markedly suppressed degeneration, compared to the PBS- and PRP-only groups. A recent study by the same group reported additional findings that microspheres alone without PRP did not have therapeutic value, and that the PRP+microsphere group benefited from increased disc height, water content, expression of PG core protein and collagen II, and fewer apoptotic cells in the NP.60 While the use of a sustained delivery system has been effective in nucleotomy models in a less severe anular puncture model, an injection of PRP alone was sufficient to induce restoration of disc height and T2 MRI values.109 Interestingly, the use of fibrin glue alone had a positive effect in a porcine nucleotomy model, where the fibrin glue resulted in the suppression of IL-6 and TNF expression, while restoring mechanical properties and glycosaminoglycan (GAG) content.110
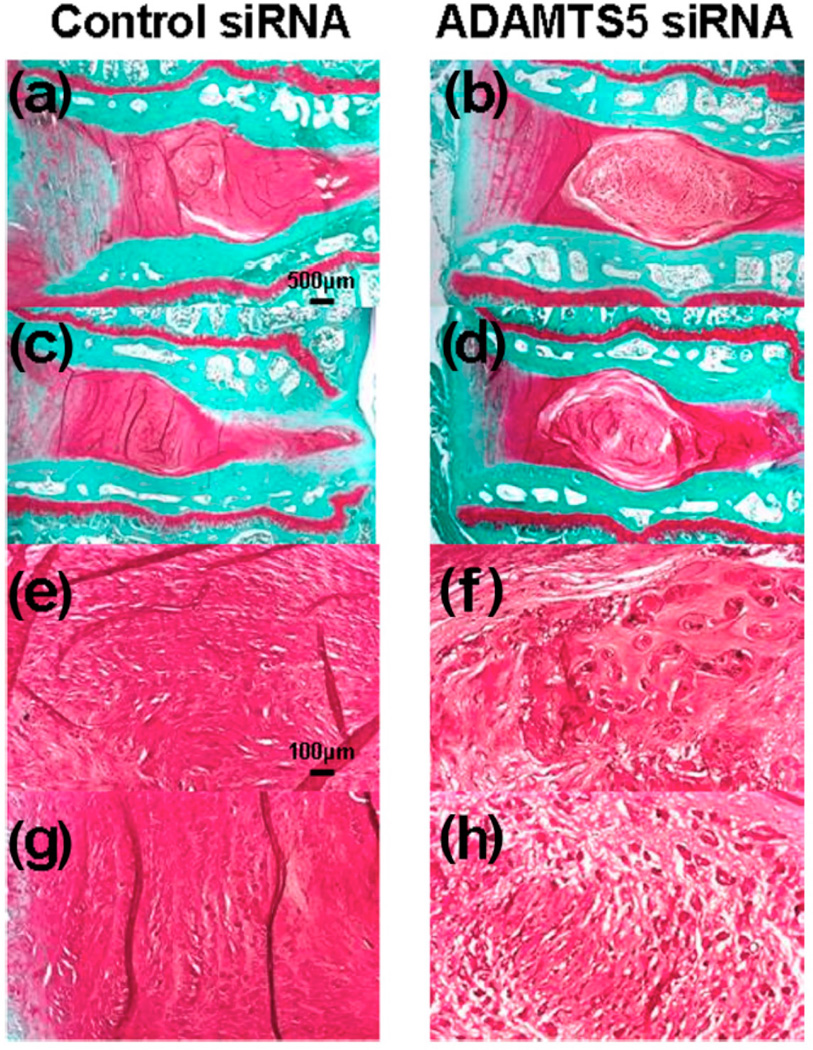
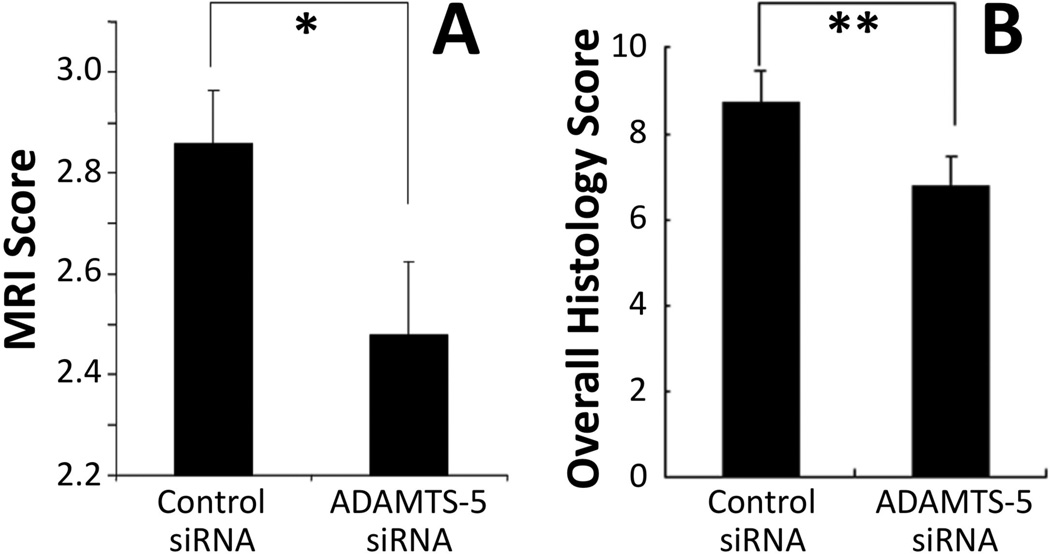
A recent study has developed and evaluated the efficacy of an inhibitor for ADAMTS-5 expression using small interference RNA (siRNA).111 First, the ability of siRNA to reduce ADAMTS-5 expression was determined using rabbit NP cells in vitro. Adolescent rabbit discs, punctured in the anulus to induce degeneration, were injected with either ADAMTS-5 or control siRNA. After 8 weeks, the discs receiving ADAMTS-5 siRNA had markedly better MRI and histology results (Figure 5). The control group exhibited complete loss of NP tissues (Figure 5ac) that had been replaced by a fibrocartilaginous tissue (Figure 5eg). In contrast, the ADAMTS siRNA group had maintained disc structure (Figure 5bd), including a clearly distinguishable NP. Both MRI grade (Figure 6A) and overall histology grade (Figure 6B) were significantly better for the ADAMTS siRNA group. The inhibition of degradative enzymes and catabolic cytokines provides a complimentary approach to treat degenerated discs at different levels of the modulation mechanism.
Figure 5. Histology of anular puncture-induced degenerated adolescent rabbit discs injected with control small interference RNA (siRNA) or ADAMTS-5 siRNA.
Representative safranin-O-stained sections after injection of ADAMTS-5 siRNA or control siRNA in the rabbit anular puncture model of disc degeneration. Eight weeks after the siRNA injections, the control siRNA group displayed a complete loss of nucleus pulposus (NP) tissues that had been replaced by a fibrocartilaginous tissue (a, c). The severely degenerated discs that had received the control siRNA showed a loss of proteoglycans and the collapsed, wavy fibrocartilage lamellae typical of the anulus fibrosus (AF), with associated fibrochondrocytes (e, g). In the ADAMTS5 siRNA-injected discs, safranin-O staining demonstrated the maintenance of intervertebral disc structure with a lightly stained matrix and large cells (b, d); the NP was rounded and bloated in appearance, and consisted of numerous large, vacuolated cells and smaller chondrocyte-like cells (f, h). A clear demarcation was seen between the NP and inner anulus in the ADAMTS5 siRNA-injected discs. (Magnification is 20× (a–d) or 100× (e–h)). The level in a, b, e, and f is L2/3, and in c, d, g, and h is L4/5. (Reproduced from Seki S, et al. Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Research & Therapy 2009;11:R166). ADAMTS: a disintegrin and metalloproteinase with a thrombospondin type 1 motif.
Figure 6. Magnetic resonance imaging (MRI) and histology grading scores of anular puncture-induced degenerated adolescent rabbit discs injected with control small interference RNA (siRNA) or ADAMTS-5 siRNA.
(A) MRI assessment 8 weeks after siRNA injection, using a modified Thompson scale (1–4). The MRI grade in the ADAMTS-5 siRNA-treated discs show significantly better (lower) MRI grade compared to control siRNA-treated discs. (B) Histological assessment for structure, cellularity and matrix staining showed significantly better (lower) overall score (range 0–12) for ADAMTS-5 siRNA-treated samples. Mean ± S.E.M., n=6. * p<0.05, ** p<0.01 Mann-Whitney U test. (Modified from Seki S, et al. Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Research & Therapy 2009;11:R166).
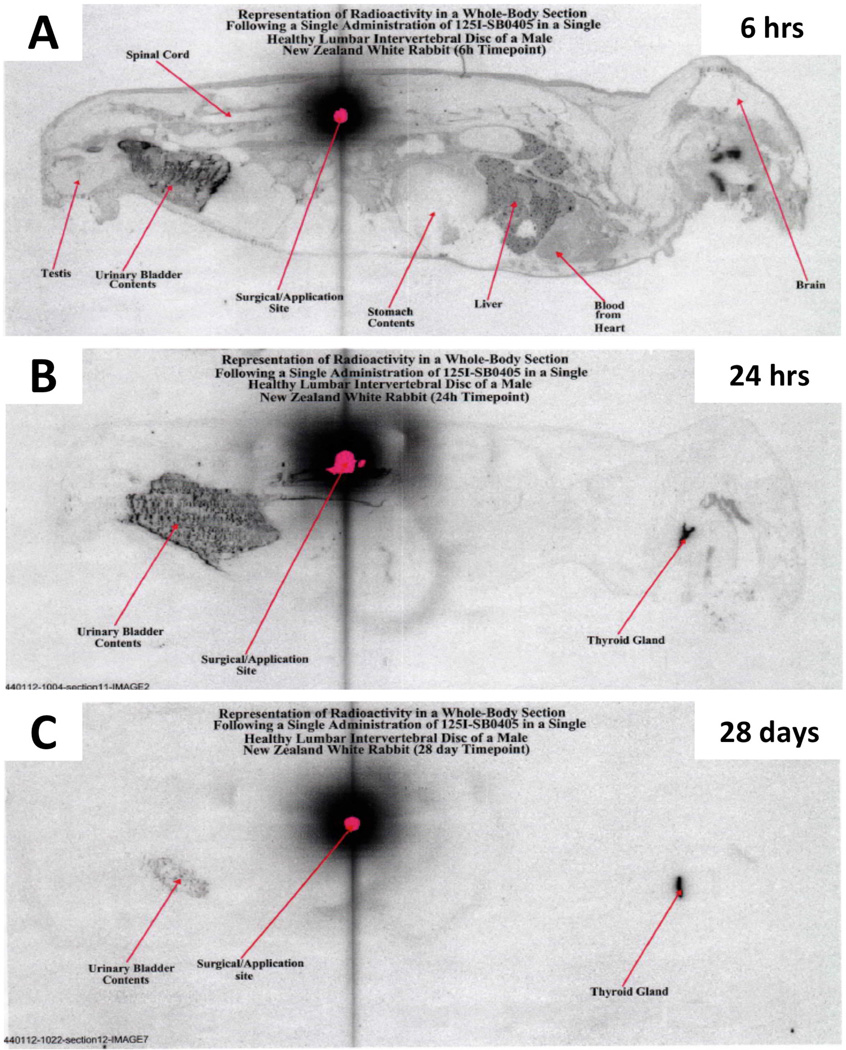
Several possible mechanisms governing the long-term effects of growth factor injection warrant further discussion. First, the residence time of an injected protein in the disc has not been solidly established. Some authors have suggested a short half-life in the order of minutes,112 while others, using radiolabeling, have noted much greater times, likely over 1 month (Figure 7).113 Both the structural integrity of the disc and injection location may affect the movement of injected materials in the disc. Second, it has been suggested that OP-1 binds to collagen molecules, which can explain its long acting effects.114 Finally, the duration of the anabolic effect resulting from a single exposure to a growth factor needs to determined.
Figure 7. Retention of radiolabeled bone morphogenetic-7 (BMP-7) in a rabbit disc.
Normal rabbits that received a single intradiscal injection of 125I-labeled BMP-7 (otherwise known as osteogenic protein-1) were imaged using autoradiography (A) 6 hours, (B) 24 hours or (C) 28 days after the injection. The signal from the radiolabeled BMP-7 is prominent even after 14 days. (Modified from Pierce, A. et al. Distribution, pharmacokinetics and excretion of 125-Iodine labeled BMP-7 (OP-1) following a single dose administration in lumbar IVD or knee joint of NZW rabbits. Paper presented at: The sixth International conference on bone morphogenetic protein 2006; Cavtat, Croatia.)
Injection Therapeutics: Effect on Pain
In addition to the structural-modifying effects of injection therapy seen in many preclinical animal studies, there is growing evidence that suggests its pain-relieving effects. In a rat model of pain in which degenerated disc tissue was applied to lumbar nerve roots, mechanical hyperalgesia was observed in the sham- and saline-injected groups, but not in the OP-1-treated group.115 The pain relief may have been due to the ability of OP-1 treatment to result in a reduction of immunohistologic staining for aggrecanase, MMP-13, substance P, TNF-α and IL-1β.116 These changes are consistent with the suppression by OP-1 treatment of the expression of IL-1β, TNF-α, IL-6, MMP-3 and aggrecanse-1 in IL-1 insulted human disc cells.117 These results suggest that OP-1, in addition to being an anabolic mediator, is a catabolic regulator of the metabolism of IVD cells. OP-1 suppression of pro-inflammatory factors can also suppress a variety of pain markers, such as nerve growth factor (NGF), and can lead to pain reduction.
Considerations for Injectable Therapeutics and their Limitations
While the clinical application of injectable therapeutics is being actively pursued, several important limitations need to be considered. First, the target population of this therapeutic approach is mainly an aged population, which has fewer cells in the IVD than the normal population. IVD cell density is also lower in discs with advanced degeneration.118 Because injection therapy seeks to influence existing cells in the disc, the appropriate stage of disc degeneration and age of the patients may need to be defined. For those with very low cellularity, it may be possible to utilize a tissue-engineering approach. For example, a small number of functional cells recovered from herniated tissues or mesenchymal stem cells may be expanded for later use.119–123
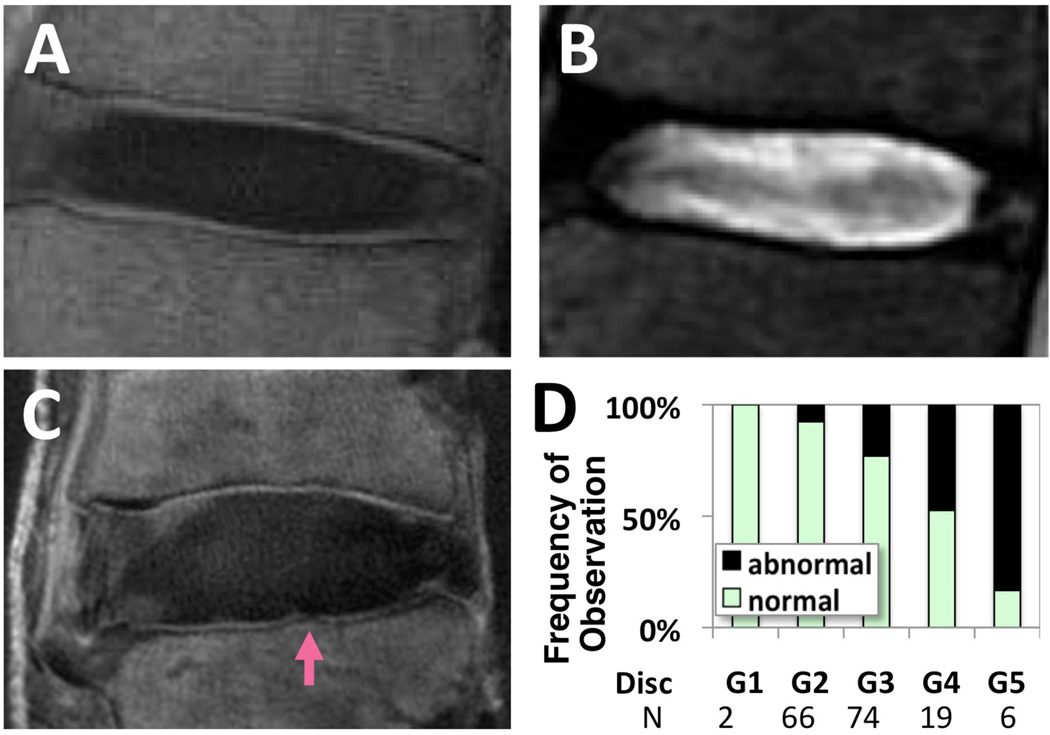
In addition to cellular changes, nutritional supply and removal of metabolic wastes may be compromised by changes in the endplate, such as calcification seen in sclerosis. Hindrance of the nutritional pathway may render an injection therapy ineffective because the lack of nutrition may impair the synthesis of the disc matrix needed for repair. Similarly, when tissue-engineered cells are added to discs, they may not survive without a proper supply of nutrients to meet the demands of increased energy consumption. Thus, a non-invasive means to assess environmental conditions (e.g., changes in cartilage endplate) in the IVD is essential. Using a contrast-enhanced MRI method, the diffusion function of the endplate has been assessed indirectly in human subjects.124,125 Recent advances in MRI, such as the ultrashort time-to-echo (UTE) technique,125–127 allow for direct visualization (Figure 8A) of regions of the cartilage endplate with high contrast, unlike conventional MRI sequences (Figure 8B). Abnormalities (Figure 8C) of the cartilage endplate in UTE MRI have been significantly associated with disc degeneration (p<0.01 chi-square test), as evaluated by Pfirrman grading,128 in approximately 30 cadaveric human lumbar spines. It would be useful to determine if the appearance of the cartilage endplate by MRI provides information on the time course of subsequent disc degeneration, as well as the outcome of therapeutic treatments or the proper selection of patients.
Figure 8. Evaluation of region of cartilage endplate using ultrashort time-to-echo (UTE) magnetic resonance imaging (MRI).
A normal lumbar disc imaged using UTE MRI (A) and conventional T2-weighted spin echo MRI (B). Note the characteristic high-intensity lines (A) near the regions of cartilage endplate. The same regions appear dark in conventional MRI (B). Abnormalities such as focal signal loss have been observed (arrow, C) and correlated with the grade of the adjacent disc (D). (Modified from Bae WC, et al. Ultrashort time-to-echo MRI of human intervertebral disc endplate: association with disc degeneration. Proc Int'l Soc Magn Reson Med 2010;18:534)
Although much evidence of structural modifications in animal disc degeneration models has been demonstrated in this review, there are significant limitations to applying these results to human patients. Studies using large animals with discs of a size similar to those of the human that do not contain notochordal cells may be needed to answer the remaining issues of nutritional supply and cellular composition. However, the cost of studies using large animals and the ethical problems associated with these animals are some of the issues that delay their use. In addition, pain measures have not been well-established for large animals, making pain research difficult. However, once a therapeutic agent is proven safe, a clinical trial on carefully selected patients (e.g., those patients who would otherwise undergo spinal fusion) may be a good approach for testing therapeutic efficacy.
Injection Therapeutics in Clinical Trials
Several clinical trials involving intradiscal injection therapeutics are underway. These studies typically select patients exhibiting chronic moderate-to-severe discogenic pain, without involvement of nerve compression or facet joints, for which non-surgical therapy has not been effective. Sponsored by Advanced Technologies and Regenerative Medicine and DePuy Spine, multi-center studies in the United States (ClinicalTrials.gov identifier: NCT01124006) and in Korea and Australia (NCT01158924 and NCT01182337) are currently (as of Nov 2010) recruiting patients to evaluate safety, tolerability and effectiveness of single injection of rhGDF-5 vs. placebo, with outcome measures including safety outcomes, pain and MRI at 12 mo.
Another study sponsored by Spinal Restoration (NCT01011816) will be evaluating the effects of injecting Biostat fibrin sealant on the time course of pain reduction (visual analog scale) and function restoration (Roland-Morris Disability score) through 78 weeks, along with safety measures, on 260 patients. This study seeks to achieve a 30% decrease in pain and 30% improvement in function. Once safety concerns are addressed in the current studies, it is hoped that later stage studies with additional measures and a greater number of patients will reveal the mechanism of treatment as well as suitable patient selection.
Conclusion
Abundant evidence for the efficacy of injectable therapeutics, including many growth factors, for the treatment of IVD degeneration has been presented in studies using IVD cells in vitro as well as in preclinical in vivo animal studies. For animal studies, outcomes focused mainly on structural modification, and the effects of injection therapy on pain generation are not well known. Recent data obtained from small animal studies suggest that injection therapy can lead to modification of pain behaviors, as well as changes in cytokine expression. Such results offer great potential for patients with chronic discogenic low back pain. Multiple clinical trials for injection therapy are now underway, and their results will be useful for establishing safety and efficacy injection treatments. In addition, for discs with advanced stages of degeneration, the prophylactic use of growth factor injection therapy, such as its application to discs adjacent to a fusion level, may be an alternative approach. Quantitative studies on the effects of a growth factor injection on pain reduction and on long-term cell survival using large animals are desirable. Diagnostic techniques to evaluate the disc environment, such as the MRI evaluation of the cartilage endplate, may also become a desirable tool for patient selection. Furthermore, comprehensive studies looking at the muscles, facet joints and vasculature that comprise the whole spinal structure are important to seek new innovative diagnostic and therapeutic approaches, encompassing surgical and conservative approaches.
Acknowledgments
This work was supported in part by Grant No. K01AR059764 and Grant No. P01AR48152 from the National Institutes of Health.
Footnotes
Disclosures: Koichi Masuda, MD—research/institutional support: DePuy Spine, Inc; Stryker Biotech.
References
- 1.Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine. 2000;25:166. doi: 10.1097/00007632-200001150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Kuettner KE, Thonar EJ. Age-related changes in articular cartilage proteoglycans: electron microscopic studies. J Orthop Res. 1985;3:251. doi: 10.1002/jor.1100030301. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Pedrini MA, Pedrini V, et al. Proteoglycans of human infant intervertebral disc. Electron microscopic and biochemical studies. J Bone Joint Surg [Am] 1985;67:284. [PubMed] [Google Scholar]
- 5.Cole TC, Ghosh P, Taylor TK. Variations of the proteoglycans of the canine intervertebral disc with ageing. Biochim Biophys Acta. 1986;880:209. doi: 10.1016/0304-4165(86)90082-6. [DOI] [PubMed] [Google Scholar]
- 6.Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24:12. doi: 10.1002/art.1780240103. [DOI] [PubMed] [Google Scholar]
- 7.Melrose J, Ghosh P, Taylor TK, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992;10:665. doi: 10.1002/jor.1100100509. [DOI] [PubMed] [Google Scholar]
- 8.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 9.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine. 1997;22:2781. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JP, Oegema TJ, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Osada R, Ohshima H, Ishihara H, et al. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 12.Ahn S-H, Teng P-N, Niyibizi C, et al. The effects of BMP-12 and BMP-2 on proteoglycan and collagen synthesis in nucleus pulposus cells from human degenerated discs. The International Society for the Study of the Lumbar Spine, 29th Annual Meeting Proceeding. 49 [Google Scholar]
- 13.Takahashi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto H, Saura R, Harada T, et al. The role of cyclooxygenase-2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J Med Sci. 2000;46:13. [PubMed] [Google Scholar]
- 15.Anderson DG, Izzo MW, Hall DJ, et al. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27:1291. doi: 10.1097/00007632-200206150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Doita M, Kanatani T, Ozaki T, et al. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine. 2001;26:1522. doi: 10.1097/00007632-200107150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Roberts S, Caterson B, Menage J, et al. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Tsuru M, Nagata K, Ueno T, et al. Electron microscopic observation of established chondrocytes derived from human intervertebral disc hernia (KTN-1) and role of macrophages in spontaneous regression of degenerated tissues. Spine J. 2001;1:422. doi: 10.1016/s1529-9430(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 19.Weiler C, Nerlich AG, Zipperer J, et al. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 21.Patel KP, Sandy JD, Akeda K, et al. Aggrecanases and Aggrecanase-generated Fragments in the Human Intervertebral Disc at Early and Advanced Stages of Disc Degeneration. Spine. 2007;32:2596. doi: 10.1097/BRS.0b013e318158cb85. [DOI] [PubMed] [Google Scholar]
- 22.Sobajima S, Shimer AL, Chadderdon RC, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5:14. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 23.Yurube T, Nishida K, Suzuki T, et al. Matrix metalloproteinase (MMP)-3 gene upregulation in a rat tail compression loading-induced disc degeneration model. J Orthop Res. 2010;28:1026. doi: 10.1002/jor.21116. [DOI] [PubMed] [Google Scholar]
- 24.Iatridis JC, Godburn K, Wuertz K, et al. Region-Dependent Aggrecan Degradation Patterns in the Rat Intervertebral Disc Are Affected by Mechanical Loading In Vivo. Spine (Phila Pa 1976) 2010 doi: 10.1097/BRS.0b013e3181cec247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doita M, Kanatani T, Harada T, et al. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine. 1996;21:235. doi: 10.1097/00007632-199601150-00015. [DOI] [PubMed] [Google Scholar]
- 26.Gronblad M, Virri J, Tolonen J, et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine. 1994;19:2744. doi: 10.1097/00007632-199412150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Baba H, Maezawa Y, Furusawa N, et al. Herniated cervical intervertebral discs: histological and immunohistochemical characteristics. Eur J Histochem. 1997;41:261. [PubMed] [Google Scholar]
- 28.Rand N, Reichert F, Floman Y, et al. Murine nucleus pulposus-derived cells secrete interleukins-1-beta, -6, and -10 and granulocyte-macrophage colony-stimulating factor in cell culture. Spine. 1997;22:2598. doi: 10.1097/00007632-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 29.Ahn SH, Cho YW, Ahn MW, et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Burke JG, Watson RW, Conhyea D, et al. Human nucleus pulposus can respond to a pro-inflammatory stimulus. Spine. 2003;28:2685. doi: 10.1097/01.BRS.0000103341.45133.F3. [DOI] [PubMed] [Google Scholar]
- 31.Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Weiler C, Nerlich AG, Bachmeier BE, et al. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30:44. doi: 10.1097/01.brs.0000149186.63457.20. [DOI] [PubMed] [Google Scholar]
- 33.Igarashi T, Kikuchi S, Shubayev V, et al. 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 34.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 35.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Roughley PJ, Mort JS. Identification of human intervertebral disc stromelysin and its involvement in matrix degradation. J Orthop Res. 1991;9:568. doi: 10.1002/jor.1100090413. [DOI] [PubMed] [Google Scholar]
- 38.Fujita K, Nakagawa T, Hirabayashi K, et al. Neutral proteinases in human intervertebral disc. Role in degeneration and probable origin. Spine. 1993;18:1766. doi: 10.1097/00007632-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1995;20:2373. doi: 10.1097/00007632-199511001-00001. [DOI] [PubMed] [Google Scholar]
- 40.Sztrolovics R, Alini M, Roughley PJ, et al. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326:235. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinmei M, Kikuchi T, Yamagishi M, et al. The role of interleukin-1 on proteoglycan metabolism of rabbit annulus fibrosus cells cultured in vitro. Spine. 1988;13:1284. doi: 10.1097/00007632-198811000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Takegami K, Thonar EJ, An HS, et al. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27:1318. doi: 10.1097/00007632-200206150-00014. [DOI] [PubMed] [Google Scholar]
- 43.Shen B, Melrose J, Ghosh P, et al. Induction of matrix metalloproteinase-2 and-3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1beta: a potential pathway of disc degeneration. Eur Spine J. 2003;12:66. doi: 10.1007/s00586-002-0454-2. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto K, Pichika R, An H, et al. Tumor Necrosis Factor-a exhibits potent effects on the metabolism of human intervertebral disc cells. Trans Orthop Res Soc. 2005:188. [Google Scholar]
- 45.Sakuma M, Fujii N, Takahashi T, et al. Effect of chondroitinase ABC on matrix metalloproteinases and inflammatory mediators produced by intervertebral disc of rabbit in vitro. Spine. 2002;27:576. doi: 10.1097/00007632-200203150-00004. [DOI] [PubMed] [Google Scholar]
- 46.Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 47.Rannou F, Corvol MT, Hudry C, et al. Sensitivity of anulus fibrosus cells to interleukin 1 beta. Comparison with articular chondrocytes. Spine. 2000;25:17. doi: 10.1097/00007632-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 48.Akeda K, An H, Gemba T, et al. A new gene therapy approach: in vivo transfection of naked NFkB decoy oligonucleotide restored disc degeneration in the rabbit annular needle puncture model. Trans Orthop Res Soc. 2005:45. [Google Scholar]
- 49.Yoshida M, Nakamura T, Kikuchi T, et al. Expression of monocyte chemoattractant protein-1 in primary cultures of rabbit intervertebral disc cells. J Orthop Res. 2002;20:1298. doi: 10.1016/S0736-0266(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 50.Jimbo K, Park JS, Yokosuka K, et al. Positive feedback loop of interleukin-1beta upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J Neurosurg Spine. 2005;2:589. doi: 10.3171/spi.2005.2.5.0589. [DOI] [PubMed] [Google Scholar]
- 51.Elfervig MK, Minchew JT, Francke E, et al. IL-1beta sensitizes intervertebral disc annulus cells to fluid-induced shear stress. J Cell Biochem. 2001;82:290. doi: 10.1002/jcb.1153. [DOI] [PubMed] [Google Scholar]
- 52.Arner EC, Pratta MA. Independent effects of interleukin-1 on proteoglycan breakdown, proteoglycan synthesis, and prostaglandin E2 release from cartilage in organ culture. Arthritis Rheum. 1989;32:288. doi: 10.1002/anr.1780320310. [DOI] [PubMed] [Google Scholar]
- 53.Benton HP, Tyler JA. Inhibition of cartilage proteoglycan synthesis by interleukin I. Biochem Biophys Res Commun. 1988;154:421. doi: 10.1016/0006-291x(88)90703-6. [DOI] [PubMed] [Google Scholar]
- 54.Dingle JT, Horner A, Shield M. The sensitivity of synthesis of human cartilage matrix to inhibition by IL-1 suggests a mechanism for the development of osteoarthritis. Cell Biochem Funct. 1991;9:99. doi: 10.1002/cbf.290090206. [DOI] [PubMed] [Google Scholar]
- 55.Solovieva S, Kouhia S, Leino-Arjas P, et al. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology. 2004;15:626. doi: 10.1097/01.ede.0000135179.04563.35. [DOI] [PubMed] [Google Scholar]
- 56.Le Maitre CL, Hoyland JA, Freemont AJ. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res Ther. 2007;9:R83. doi: 10.1186/ar2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Genevay S, Finckh A, Mezin F, et al. Influence of cytokine inhibitors on concentration and activity of MMP-1 and MMP-3 in disc herniation. Arthritis Res Ther. 2009;11:R169. doi: 10.1186/ar2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shamji MF, Betre H, Kraus VB, et al. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: Sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56:3650. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 59.Kakutani K, Kanaji A, Asanuma K, et al. Effect of IL-1 receptor antagonist and soluble TNF receptor on the anabolism of human intervertebral disc cells. 54th Annual Meeting of Orthopedic Research Society. 33:442. [Google Scholar]
- 60.Sawamura K, Ikeda T, Nagae M, et al. Characterization of in vivo effects of platelet-rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng Part A. 2009;15:3719. doi: 10.1089/ten.TEA.2008.0697. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida M, Nakamura T, Sei A, et al. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin-1beta, and monocyte chemoattractant protein-1 immediately after herniation: an experimental study using a new hernia model. Spine. 2005;30:55. doi: 10.1097/01.brs.0000149194.17891.bf. [DOI] [PubMed] [Google Scholar]
- 62.Karppinen J, Korhonen T, Malmivaara A, et al. Tumor necrosis factor-alpha monoclonal antibody, infliximab, used to manage severe sciatica. Spine. 2003;28:750. [PubMed] [Google Scholar]
- 63.Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis. 2004;63:1120. doi: 10.1136/ard.2003.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okoro T, Tafazal SI, Longworth S, et al. Tumor necrosis alpha-blocking agent (etanercept): a triple blind randomized controlled trial of its use in treatment of sciatica. J Spinal Disord Tech. 2010;23:74. doi: 10.1097/BSD.0b013e31819afdc4. [DOI] [PubMed] [Google Scholar]
- 65.Tobinick EL, Britschgi-Davoodifar S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med Wkly. 2003;133:170. doi: 10.4414/smw.2003.10163. [DOI] [PubMed] [Google Scholar]
- 66.Studer RK, Gilbertson LG, Georgescu H, et al. p38 MAPK inhibition modulates rabbit nucleus pulposus cell response to IL-1. J Orthop Res. 2008 doi: 10.1002/jor.20604. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki M, Inoue G, Gemba T, et al. Nuclear factor-kappa B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur Spine J. 2009 doi: 10.1007/s00586-009-0940-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 69.Gruber HE, Fisher EC, Jr, Desai B, et al. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 70.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine. 2000;25:2153. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 71.Masuda K, Takegami K, An H, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 72.Imai Y, Miyamoto K, An HS, et al. Recombinant human osteogenic protein-1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells. Spine. 2007;32:1303. doi: 10.1097/BRS.0b013e3180593238. [DOI] [PubMed] [Google Scholar]
- 73.Takegami K, An HS, Kumano F, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Storm EE, Huynh TV, Copeland NG, et al. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Leo BM, Beck G, et al. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine. 2004;29:2229. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 76.Chujo T, An HS, Akeda K, et al. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 77.Wehling P. Antiapoptotic and antidegenerative effect of an autologous IL-1ra/IGF-1/PDGF combination on human intervertebral disc cells in vivo. The International Society for the Study of the Lumbar Spine. 29th Annual Meeting Proceeding. 24 [Google Scholar]
- 78.Akeda K, An HS, Pichika R, et al. Platelet-Rich Plasma (PRP) Stimulates the Extracellular Matrix Metabolism of Porcine Nucleus Pulposus and Anulus Fibrosus Cells Cultured in Alginate Beads. Spine. 2006;31:959. doi: 10.1097/01.brs.0000214942.78119.24. [DOI] [PubMed] [Google Scholar]
- 79.Chen WH, Lo WC, Lee JJ, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209:744. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 80.Masuda K, Imai Y, Okuma M, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31:742. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto K, Masuda K, Kim JG, et al. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6:692. doi: 10.1016/j.spinee.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 82.Imai Y, Okuma M, An H, et al. Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine. 2007;32:1197. doi: 10.1097/BRS.0b013e3180574d26. [DOI] [PubMed] [Google Scholar]
- 83.Bae WC, Yoshikawa T, Kakutani K, et al. Effect of rhGDF-5 on the thrombin model of rabbit intervertebral disc degeneration: T1ρ quantification using 3T MRI. Radiol Soc North Am. 2009;95:SSE14. [Google Scholar]
- 84.Norcross JP, Lester GE, Weinhold P, et al. An in vivo model of degenerative disc disease. J Orthop Res. 2003;21:183. doi: 10.1016/S0736-0266(02)00098-0. [DOI] [PubMed] [Google Scholar]
- 85.Hoogendoorn RJ, Helder MN, Kroeze RJ, et al. Reproducible long-term disc degeneration in a large animal model. Spine. 2008;33:949. doi: 10.1097/BRS.0b013e31816c90f0. [DOI] [PubMed] [Google Scholar]
- 86.Hoogendoorn RJ, Wuisman PI, Smit TH, et al. Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine. 2007;32:1816. doi: 10.1097/BRS.0b013e31811ebac5. [DOI] [PubMed] [Google Scholar]
- 87.Boxberger JI, Auerbach JD, Sen S, et al. An in vivo model of reduced nucleus pulposus glycosaminoglycan content in the rat lumbar intervertebral disc. Spine. 2008;33:146. doi: 10.1097/BRS.0b013e31816054f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lipson SJ, Muir H. 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981;6:194. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 90.Sobajima S, Kompel JF, Kim JS, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine. 2005;30:15. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 91.Eurell JA, Brown MD, Ramos M. The effects of chondroitinase ABC on the rabbit intervertebral disc. A roentgenographic and histologic study. Clin Orthop. 1990;256:238. [PubMed] [Google Scholar]
- 92.Fry TR, Eurell JC, Johnson AL, et al. Radiographic and histologic effects of chondroitinase ABC on normal canine lumbar intervertebral disc. Spine. 1991;16:816. doi: 10.1097/00007632-199107000-00022. [DOI] [PubMed] [Google Scholar]
- 93.Henderson N, Stanescu V, Cauchoix J. Nucleolysis of the rabbit intervertebral disc using chondroitinase ABC. Spine. 1991;16:203. [PubMed] [Google Scholar]
- 94.Kato F, Mimatsu K, Kawakami N, et al. Serial changes observed by magnetic resonance imaging in the intervertebral disc after chemonucleolysis. A consideration of the mechanism of chemonucleolysis. Spine. 1992;17:934. doi: 10.1097/00007632-199208000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Ando T, Kato F, Mimatsu K, et al. Effects of chondroitinase ABC on degenerative intervertebral discs. Clin Orthop. 1995;318:214. [PubMed] [Google Scholar]
- 96.Sugimura T, Kato F, Mimatsu K, et al. Experimental chemonucleolysis with chondroitinase ABC in monkeys. Spine. 1996;21:161. doi: 10.1097/00007632-199601150-00001. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi T, Kurihara H, Nakajima S, et al. Chemonucleolytic effects of chondroitinase ABC on normal rabbit intervertebral discs. Course of action up to 10 days postinjection and minimum effective dose. Spine. 1996;21:2405. doi: 10.1097/00007632-199611010-00001. [DOI] [PubMed] [Google Scholar]
- 98.Yamada K, Tanabe S, Ueno H, et al. Investigation of the short-term effect of chemonucleolysis with chondroitinase ABC. J Vet Med Sci. 2001;63:521. doi: 10.1292/jvms.63.521. [DOI] [PubMed] [Google Scholar]
- 99.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 100.Kim KW, Lim TH, Kim JG, et al. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 101.Scott NA, Harris PF, Bagnall KM. A morphological and histological study of the postnatal development of intervertebral discs in the lumbar spine of the rabbit. J Anat. 1980;130:75. [PMC free article] [PubMed] [Google Scholar]
- 102.Chujo T, An H, Takatori R, et al. In Vivo effects of recombinant human growth and differentiation factor-5 on the repair of the mature rabbit intervertebral disc. Spine J. 2006;6:23S. [Google Scholar]
- 103.Asanuma K, Abe Y, Pichika R, et al. Direct cleavage by thrombin at the Arg375-Gly376 bond in the interglobular domain is involved in accelerated degradation of aggrecan by intervertebral disc cells. 54th Anual Meeting of Orthopedic Research Society. 33:1420. [Google Scholar]
- 104.Asanuma K, Abe Y, Muehleman C, et al. A Thrombin-injection Model of Disc Degeneration in the Rabbit. 54th Anual Meeting of Orthopedic Research Society. 33:1394. [Google Scholar]
- 105.Duvvuri U, Reddy R, Patel SD, et al. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 106.Han ET, Busse RF, Li X. 3D segmented elliptic-centric spoiled gradient echo imaging for the in vivo quantification of cartilage T1rho. ISMRM. 13:473. [Google Scholar]
- 107.Masuda K, Pichika R, Kakutani K, et al. Intradiscal injection of recombinant human growth and differentiation factor-5 significantly suppressed the expression of cytokines, catabolic enzymes and pain markers in the rabbit anular puncture model; The 35th Annual Meeting of the International Society for the Study of the Lumbar Spine; p. 47. [Google Scholar]
- 108.Nagae M, Ikeda T, Mikami Y, et al. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13:147. doi: 10.1089/ten.2006.0042. [DOI] [PubMed] [Google Scholar]
- 109.Obata K, Akeda K, Morimoto R, et al. Intradiscal injection of autologous platelet-rich plasma-serum induces the restoration of disc height in the rabbit anular needle puncture model. Int'l Soc Study Lumbar Spine. :12. [Google Scholar]
- 110.Buser Z, Kuelling F, Jane L, et al. Fibrin injection stimulates early disc healing in the porcine model. Spine J. 2009;9:105S. [Google Scholar]
- 111.Seki S, Asanuma-Abe Y, Masuda K, et al. Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Res Ther. 2009;11:R166. doi: 10.1186/ar2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Larson JW, 3rd, Levicoff EA, Gilbertson LG, et al. Biologic modification of animal models of intervertebral disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):83. doi: 10.2106/JBJS.F.00043. [DOI] [PubMed] [Google Scholar]
- 113.Pierce A, Feng M, Masuda K, et al. Distribution, pharmacokinetics and excretion of 125-Iodine labeled BMP-7 (OP-1) following a single dose administration in lumbar IVD or knee joint of NZW rabbits; The sixth International conference on bone morphogenetic protein. [Google Scholar]
- 114.Reddi AH. Morphogenetic messages are in the extracellular matrix: biotechnology from bench to bedside. Biochem Soc Trans. 2000;28:345. [PubMed] [Google Scholar]
- 115.Kawakami M, Matsumoto T, Hashizume H, et al. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine. 2005;30:1933. doi: 10.1097/01.brs.0000176319.78887.64. [DOI] [PubMed] [Google Scholar]
- 116.Chubinskaya S, Kawakami M, Rappoport L, et al. Anti-catabolic effect of OP-1 in chronically compressed intervertebral discs. J Orthop Res. 2007;25:517. doi: 10.1002/jor.20339. [DOI] [PubMed] [Google Scholar]
- 117.Pichika R, An H, Miyamoto K, et al. Suppressive effect of bone morphogenetic protein-7 on interleukin-1β mediated gene expression of cytokines and catabolic enzymes in human intervertebral disc cells. Ortho Res Soc Trans. 2007;32:243. [Google Scholar]
- 118.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23:751. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 119.Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine. 1998;23:1531. doi: 10.1097/00007632-199807150-00006. [DOI] [PubMed] [Google Scholar]
- 120.Okuma M, Mochida J, Nishimura K, et al. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18:988. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 121.Anderson DG, Albert TJ, Fraser JK, et al. Cellular therapy for disc degeneration. Spine. 2005;30:S14. doi: 10.1097/01.brs.0000175174.50235.ba. [DOI] [PubMed] [Google Scholar]
- 122.Gruber HE, Johnson TL, Leslie K, et al. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine. 2002;27:1626. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 123.Ganey T, Libera J, Moos V, et al. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 124.Rajasekaran S, Venkatadass K, Naresh Babu J, et al. Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs : Results from in-vivo serial post-contrast MRI studies in 365 human lumbar discs. Eur Spine J. 2008;17:626. doi: 10.1007/s00586-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rajasekaran S, Babu JN, Arun R, et al. ISSLS prize winner: A study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29:2654. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 126.Bydder GM. New approaches to magnetic resonance imaging of intervertebral discs, tendons, ligaments, and menisci. Spine (Phila Pa 1976) 2002;27:1264. doi: 10.1097/00007632-200206150-00005. [DOI] [PubMed] [Google Scholar]
- 127.Gatehouse PD, Bydder GM. Magnetic resonance imaging of short T2 components in tissue. Clin Radiol. 2003;58:1. doi: 10.1053/crad.2003.1157. [DOI] [PubMed] [Google Scholar]
- 128.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 129.Yoon TS, Su Kim K, Li J, et al. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28:1773. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 130.Kim DJ, Moon SH, Kim H, et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28:2679. doi: 10.1097/01.BRS.0000101445.46487.16. [DOI] [PubMed] [Google Scholar]
- 131.Gilbertson L, Ahn SH, Teng PN, et al. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 132.Wallach CJ, Sobajima S, Watanabe Y, et al. Gene Transfer of the Catabolic Inhibitor TIMP-1 Increases Measured Proteoglycans in Cells from Degenerated Human Intervertebral Discs. Spine. 2003;28:2331. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 133.An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 134.Imai Y, An H, Thonar E, et al. Co-injected recombinant human osteogenic protein-1 minimizes chondroitinase ABC-induced intervertebral disc degeneration: An in vivo study using a rabbit model. Trans Orthop Res Soc. 2003:1143. [Google Scholar]
- 135.Huang KY, Yan JJ, Hsieh CC, et al. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine. 2007;32:1174. doi: 10.1097/01.brs.0000263369.95182.19. [DOI] [PubMed] [Google Scholar]
- 136.Wei A, Williams LA, Bhargav D, et al. BMP13 prevents the effects of annular injury in an ovine model. Int J Biol Sci. 2009;5:388. doi: 10.7150/ijbs.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]