Abstract
Secondary organic aerosol (SOA) formation from 2-methyl-3-buten-2-ol (MBO) photooxidation has recently been observed in both field and laboratory studies. Similar to the level of isoprene, the level of MBO-derived SOA increases with elevated aerosol acidity in the absence of nitric oxide; therefore, an epoxide intermediate, (3,3-dimethyloxiran-2-yl)methanol (MBO epoxide), was synthesized and tentatively proposed to explain this enhancement. In this study, the potential of the synthetic MBO epoxide to form SOA via reactive uptake was systematically examined. SOA was observed only in the presence of acidic aerosol. Major SOA constituents, 2,3-dihydroxyisopentanol and MBO-derived organosulfate isomers, were chemically characterized in both laboratory-generated SOA and in ambient fine aerosol collected from the BEACHON-RoMBAS field campaign during the summer of 2011, where MBO emissions are substantial. Our results support the idea that epoxides are potential products of MBO photooxidation leading to the formation of atmospheric SOA and suggest that reactive uptake of epoxides may explain acid enhancement of SOA observed from other biogenic hydrocarbons.
1. Introduction
Secondary organic aerosol (SOA) formed from biogenic volatile organic compounds (BVOCs) is a major component of atmospheric fine particulate matter (PM2.5).1,2 The hydroxyl radical (OH)-initiated oxidation of MBO, which is a locally abundant BVOC species in certain regions (e.g., western United States), has recently been demonstrated to have the potential to form SOA.3−5 Prior chamber studies have revealed SOA formation from MBO photooxidation only in the absence of nitric oxide (NO). Jaoui et al.4 and Zhang et al.5 have demonstrated that SOA formation from MBO is enhanced with increasing aerosol acidity, as is the case for other biogenic SOA precursor gases such as isoprene,6−9 α-pinene,8,10 and β-caryophyllene.8 The major MBO-derived SOA constituents, 2,3-dihydroxyisopentanol (DHIP, C5H12O3) and MBO-derived organosulfates (C5H12O6S), have been observed in both laboratory-generated and ambient aerosol.4,5
By analogy to major SOA constituents (2-methyltetrols and organosulfates) derived from isoprene epoxydiols (IEPOX) under the low-NO limit,7,9,11 both the enhancing effect of acidic seed aerosol on SOA formation and the structures of the major MBO-derived SOA constituents can be explained by the reactive uptake of MBO-derived epoxides. It is therefore plausible that MBO photooxidation results in the formation of epoxides, which subsequently produce SOA in the presence of acidified sulfate aerosol. In this work, we have used a published procedure to synthesize an MBO epoxide,12 (3,3-dimethyloxiran-2-yl)methanol, for use in controlled dark environmental chamber experiments. The reactive uptake chemistry of this synthetic MBO epoxide was then systematically examined in the presence of either neutral or acidified sulfate seed aerosol to investigate the potential role of MBO-derived epoxides in forming SOA enhanced by increasing aerosol acidity. SOA formed in these experiments was collected, analyzed, and compared to aerosol collected in laboratory chamber MBO photooxidation experiments as well as in field studies.
2. Experimental Section
2.1. Synthesis of the MBO Epoxide
(3,3-Dimethyloxiran-2-yl)methanol was synthesized from 3-methylbut-2-en-1-ol by modification of a procedure recently reported for synthesis of the cis- and trans-IEPOX isomers.12 MBO (1.5 g, 17.4 mmol) was dissolved in acetonitrile (ACN) (5 mL) and cooled in an ice/water bath. A solution of m-chloroperoxybenzoic acid (m-CPBA; 3.91 g, 77%, 22.0 mmol) in ACN (15 mL) was added dropwise, and the resulting clear solution stirred in an ice/water bath for 2 h and then at room temperature until the starting material had been completely transformed, as monitored by thin-layer chromatography. The reaction mixture was cooled to −20 °C, and the resulting precipitate was separated by filtration to remove the bulk of the reacted oxidant (m-chlorobenzoic acid). The filtrate was concentrated under reduced pressure and the residue purified by column chromatography (SiO2, 2:1 hexane/ethyl ether mixture) to give MBO epoxide (795.4 mg, 45% yield). The proton (1H) and carbon (13C) nuclear magnetic resonance (NMR) spectra of the product were identical to the spectra previously reported:131H NMR (400 MHz, CDCl3) δ 3.83 (dd, 1H, J = 12.2, 4.3 Hz, CH2), 3.67 (dd, 1H, J = 12.2, 6.7 Hz, CH2), 2.96 (dd, 1H, J = 6.7, 4.3 Hz, oxiranyl-H), 2.0 (bs, 1H, OH), 1.33 (s, 3H, CH3), 1.30 (s, 3H, CH3) (Figure S1A of the Supporting Information); 13C NMR (100 MHz, CDCl3) δ 64.0, 61.7, 59.1, 25.0, 19.0 (Figure S1B of the Supporting Information).
2.2. Reactive Uptake Chamber Experiments
Reactive uptake experiments using the synthetic MBO epoxide were conducted in an 10 m3 indoor Teflon chamber at The University of North Carolina at Chapel Hill (UNC-CH)6,7 (Table 1). The chamber was flushed continuously with clean house air for at least 24 h prior to each experiment. All experiments were performed under dark conditions at 20–25 °C and a relative humidity (RH) of <5%. A differential mobility analyzer [DMA (BMI, Inc.)] coupled to a mixing condensation particle counter [MCPC (BMI, Inc.)] was used to measure aerosol size distributions and volume concentrations. Either neutral or acidic seed aerosol were injected into the chamber by atomizing 0.06 M (NH4)2SO4 or 0.06 M MgSO4/0.06 M H2SO4 aqueous solutions, respectively. Aerosol acidity measurements were not available in this study, but the pH of the acidic seed aerosol solution was estimated to be ∼0.53 using ISORROPIA,14 assuming a temperature of 298 K and 5% RH. Although the level of acidity in our experiments is higher than that commonly observed under ambient conditions, our pH value falls within what has been previously observed for ambient fine aerosol.15,16 After the seed aerosol concentrations had stabilized in the chamber, different amounts of MBO epoxide were injected into the chamber through a 10 mL glass manifold heated at 50 °C and flushed with heated N2 (50 °C) at a rate of 3 L min−1 for 30 min. In selected experiments, aerosol samples were collected onto Teflon filters (Pall Life Sciences, Teflon membrane, 47 mm diameter, 1.0 μm pore size) at a sampling flow rate of ∼20 L min–1 after the SOA mass had been maximized.
Table 1. Experimental Conditions for and Results of MBO Epoxide Reactive Uptake Experiments.
| experiment | seed aerosol type | initial seed (μm3 cm–3) | initial epoxide (ppb) | SOA formeda (μg m–3) | SOA yieldb (%) |
|---|---|---|---|---|---|
| 1 | no seed | <0.1 | 300 | <0.01 | <0.01 |
| 2 | neutral | 30 | 300 | <2 | <0.01 |
| 3 | acidic | 28 | 300 | 30.8 | 2.5 |
| 4 | acidic | 36 | 200 | 32.9 | 3.9 |
| 5 | acidic | 35 | 100 | 16.5 | 4.0 |
| 6 | acidic | 42 | 50 | 14.6 | 7.0 |
| 7 | acidic | 55 | 300 | 58.4 | 4.7 |
The SOA mass concentration was calculated by assuming that the density of the SOA is 1.25 g cm–3. Wall loss of particles was corrected for this calculation.
The SOA yield was defined as the ratio of the mass concentration of SOA formed to the mass concentration of reacted MBO epoxide. Reacted MBO epoxide was estimated by assuming all the injected compounds reacted. Thus, this is only a conservative lower-bound estimate.
2.3. Aerosol Samples from MBO Photooxidation and Ambient Air
Details of the MBO photooxidation experiments performed at UNC-CH and the BEACHON-RoMBAS campaign were described by Zhang et al.5 Briefly, the UNC-CH MBO photooxidation chamber experiments were performed under initially high-NO conditions (∼200 ppb) with acid or neutral seed aerosol. Aerosol samples were collected at a rate of ∼17 L min–1. During the BEACHON-RoMBAS campaign in a Colorado Rocky Mountain pine forest,17 samples were collected on quartz filters (Pall Life Sciences, Pallflex Tissuquartz, 20.3 cm × 25.4 cm) using a 72 h integrated aerosol filter sampling approach with a high-volume PM2.5 sampler at a flow rate of 1 m3 min–1.
2.4. Aerosol-Phase Chemical Analysis
Filters from both the laboratory experiments and the field campaign were stored at −20 °C prior to extraction in individual aluminum packets that had been prebaked at 550 °C to drive off all organic compounds. The filters were then extracted following the procedures described by Zhang et al.5 For analysis by gas chromatography - electron ionization mass spectrometry (GC/EI-MS), dried residues were trimethylsilylated by addition of 100 μL of BSTFA and trimethylchlorosilane [99:1 (v/v), Supelco] and 50 μL of pyridine (Sigma-Aldrich, 98%, anhydrous), followed by heating for 1 h at 70 °C. For analysis by ultra performance liquid chromatography - negative electrospray ionization high-resolution quadrupole time-of-flight mass spectrometry [UPLC/(−)ESI-HR-Q-TOFMS], dried residues from filter extracts were reconstituted with 150 μL of a 50:50 (v/v) mixture of 0.1% acetic acid in methanol (LC-MS ChromaSolv-Grade, Sigma-Aldrich) and 0.1% acetic acid in water (LC-MS ChromaSolv-Grade, Sigma-Aldrich). Detailed descriptions of the GC/EI-MS and UPLC/(−)ESI-HR-Q-TOFMS operating conditions were described by Zhang et al.5
3. Results and Discussion
3.1. SOA Formation from Reactive Uptake Experiments
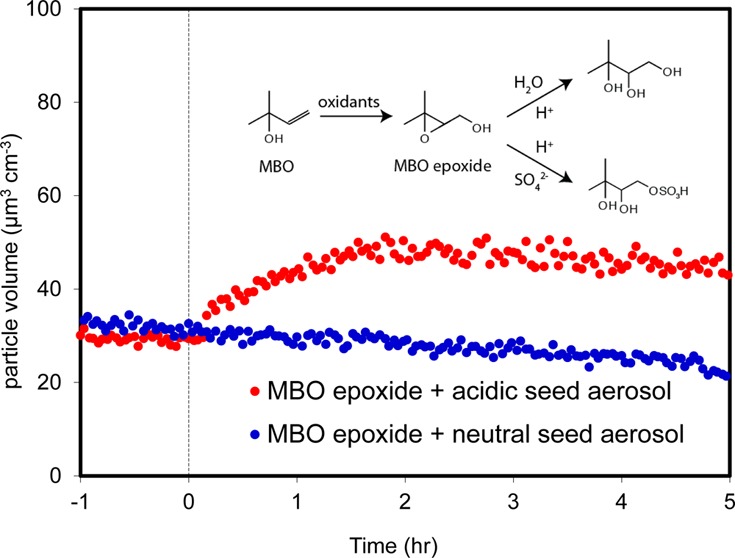
Particle volume concentrations from the 300 ppb MBO epoxide reactive uptake experiments with neutral and acidic seed aerosol are shown in Figure 1 and mass concentrations in Table 1, for experiments 2 and 3, respectively. These results were obtained from size-resolved DMA-MCPC results and have not been corrected for wall losses. SOA growth was observed only when acidic seed aerosol was present. Assuming all the MBO epoxide was successfully injected and reacted (a procedure for direct measurement of MBO epoxides is not available), the calculated SOA yield is 2.5% for experiment 3 with particle wall loss correction.7 However, with similar initial acidic seed aerosol concentrations, when the initial MBO epoxide concentrations were decreased (experiments 4–6), calculated SOA yields increased monotonically to 7%. This result suggests that reactive uptake is more efficient when the concentration of the gas-phase species is decreased with respect to the volume/surface concentration of seed aerosol. In experiment 7, the initial MBO epoxide concentration was identical to that in experiment 3, but the initial seed aerosol concentration was doubled. The rate of SOA growth also doubled, supporting the conclusion that aerosol growth in experiment 3 was limited by saturation of the seed aerosol surface or bulk depending on the mixing state within the particles. These results suggest a more general conclusion that reactive uptake is a particle surface or bulk-limited process for other epoxides, such as IEPOX, in addition to MBO epoxide. A consequence of this conclusion is that SOA yields from reactive uptake of epoxides may be underestimated, especially in chamber studies in which reactive gas-phase species are much more concentrated than under ambient field conditions. The SOA yields measured in this study serve only to provide an initial estimate of the atmospheric burden of MBO-derived SOA. Further investigation of the reactive uptake of MBO epoxide is required to elucidate the complex gas–particle interactions. Understanding the kinetics of the MBO epoxide reactive uptake under varying atmospheric conditions (e.g., aerosol liquid water content, acidity, phase, and composition) is essential for inclusion of MBO as an SOA source in atmospheric chemistry models such as the Weather Research and Forecasting model coupled with Chemistry (WRF-Chem).
Figure 1.
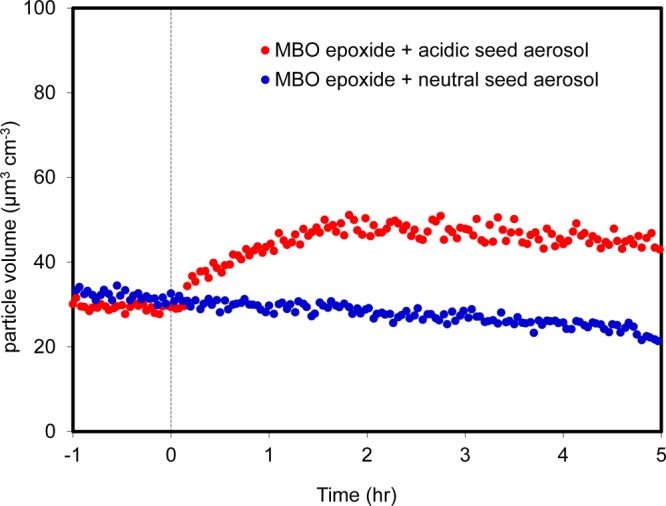
Time dependence of wall loss-uncorrected DMA-MCPC particle volume concentration of the reactive uptake of an MBO epoxide in a chamber experiment. Either neutral seed aerosol (blue) or acidic seed aerosol (red) and 300 ppb synthetic MBO epoxide were injected into the chamber at time zero. SOA growth was observed only in the acidic experiment.
3.2. Chemical Characterization of SOA Constituents
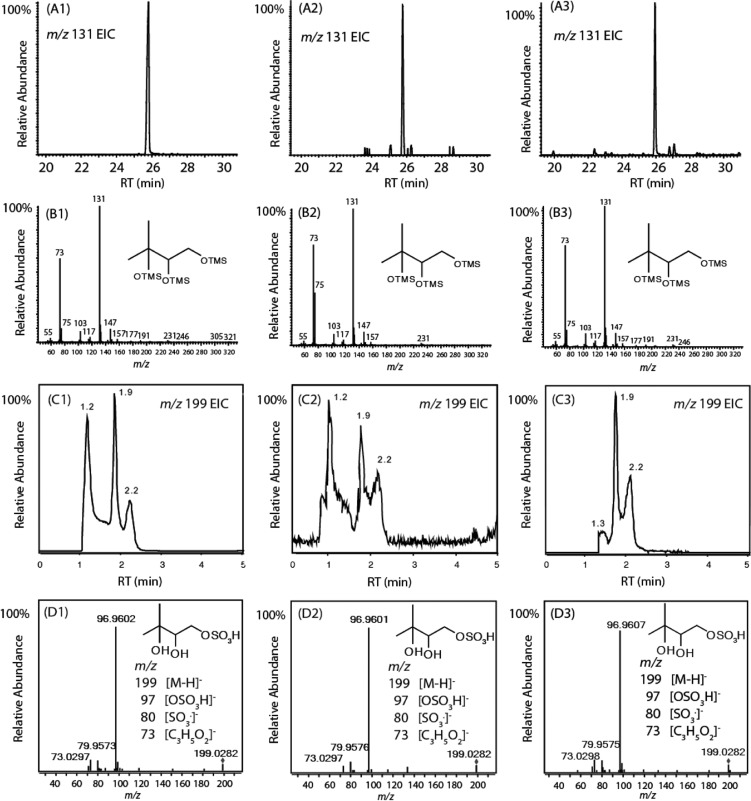
As suggested by previous studies,4,5 DHIP and the MBO-derived organosulfates are the predominant MBO SOA tracers in both chamber and field studies. Figure 2 presents the representative analytical results from the filter extractions for these two tracers. Shown are the reactive uptake of MBO epoxide (A1–D1), chamber photooxidation of MBO (A2–D2), and field studies (A3–D3). Jaoui et al.4 have shown that the base peak ion in the GC/EI-MS analysis of the trimethylsilylated DHIP appears at m/z 131, along with a less abundant product ion at m/z 231. Panels A1–A3 show that the respective extracted ion chromatograms (EICs) of the ion at m/z 131 are identical and along with the full-scan mass spectra in panels B1–B3 containing expected signature ions at m/z 131 and 231 (proposed fragmentation scheme shown in Figure S2 of the Supporting Information) strongly support the presence of DHIP in all studied aerosol samples.
Figure 2.
Comparison of chemical characterization of aerosol by GC-EI/MS collected from the reactive uptake of the MBO epoxide (A1–D1), MBO photooxidation chamber experiments (A2–D2), and ambient samples from the BEACHON-RoMBAS 2011 campaign (A3–D3). (A) Derivatization GC-EI/MS EIC of the ion at m/z 131 (signature ion of DHIP). (B) Full-scan mass spectrum of the peak in the EIC of the ion at m/z 131. (C) UPLC/(−)ESI-HR-Q-TOFMS EIC of the ion at m/z 199 ([M – H]− ion associated with the MBO-derived organosulfates) from aerosol samples. The accurate masses of all three peaks in panels C1–C3 indicate the C5H11O6S– composition (m/z 199.0276 ± 3 mDa). (D) MS2 spectra of the peak at ∼1.9 min from EIC of the ion at m/z 199 (C1–C3). The structure shown in panels D1–D3 is one of three possible regioisomeric esters.
Panels C1–C3 show the UPLC/(−)ESI-HR-Q-TOFMS EICs of the ion at m/z 199 ([M – H]− of the MBO organosulfates) and panels D1–D3 the respective tandem MS (MS2) data, exemplified by the peak at ∼1.9 min. The MS2 spectra of the peaks at ∼1.2 and ∼2.2 min have identical fragmentation patterns. Because of the low absolute abundance of the organosulfates in the MBO photooxidation chamber experiments, the signal-to-noise ratio in panel C2 is lower than that in panels C1 and C3. Traces C1–C3 are identical with respect to retention times, and all peaks have measured accurate masses consistent with the C5H11O6S– composition (199.0276 ± 3 mDa). Isomeric MBO epoxides are possible in the chamber and field studies (Figure S3 of the Supporting Information, proposed oxidation pathway), and consistent with this possibility, three peaks are resolved in organosulfate EICs18 C2 and C3. Because the reactive uptake of pure (3,3-dimethyloxiran-2-yl)methanol should initially yield two sulfate isomers, the presence of three isomeric esters in C1 likely suggests either an initial Payne rearrangement19,20 of the epoxide on reactive uptake or intramolecular transesterification21,22 promoted by the acidic seed aerosol may be involved in reactive uptake. Differences in the pattern of peak intensities of the EIC traces in Figure 2 are expected because isomer ratios from aerosol generated under different conditions are not anticipated to be identical. However, the presence of identical organosulfate isomers common to all EICs supports the presence of MBO epoxide in both chamber and field aerosol. It should also be noted that the MBO organosulfates were also observed at the BEARPEX (Biosphere Effects on Aerosols and Photochemistry Experiment) campaigns at modest concentrations (5 ng m–3 on average and up to 30 ng m–3).5
3.3. Relevance to Prior Laboratory and Field Studies
In the study presented here, reactive uptake chamber experiments using a synthetic MBO epoxide in the presence of acidic seed aerosol demonstrated SOA formation. Chemical characterization of the SOA from the reactive uptake of the MBO epoxide by acidic seed aerosol, smog chamber photooxidation of MBO, and field measurements revealed components common to all three samples, suggesting that epoxides are key intermediates, which can generally explain the observed acid enhancements of SOA derived from other biogenic hydrocarbons such as isoprene,6−9 α-pinene,8,10 and β-caryophyllene.8 This is further supported by the fact that ring-opening reactions of epoxides are kinetically favorable under typical tropospheric conditions.18,23,24 A second MBO epoxide isomer is possible from MBO photooxidation and should be explored in future studies. Because the focus of this study was to examine the involvement of an epoxide in MBO SOA formation rather than to determine the mechanism of MBO epoxide formation, we can only tentatively propose a mechanism for MBO epoxide formation at this time as shown in Figure S3 of the Supporting Information. Future work is also needed to detect the formation of MBO-derived epoxides in real time from laboratory and field studies. Currently, these epoxides cannot be observed by online gas chromatographic methods, and thus, alternative mass spectrometry approaches will be required.
Acknowledgments
This publication was made possible in part by Environmental Protection Agency (EPA) Grant 83540401. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the EPA. Further, the EPA does not endorse the purchase of any commercial products or services mentioned in the publication. UPLC/ESI-HR-Q-TOFMS analyses were conducted in the UNC-CH Biomarker Mass Facility located within the Department of Environmental Sciences and Engineering, which is a part of the UNC-CH Center for Environmental Health and Susceptibility and is supported by National Institute of Environmental Health Sciences (Grant 5P20-ES10126). Y.-H.L. and J.D.S. were supported in part by the Electric Power Research Institute. The BEACHON-RoMBAS measurements were supported by National Science Foundation (NSF) sponsorship of the National Center for Atmospheric Research. Y.-H.L. was also supported by a Dissertation Completion Fellowship from the UNC-CH Graduate School. J.L.J. was supported by NSF Grant AGS-1243354, National Oceanic and Atmospheric Administration Grant NA13OAR4310063, and U.S. Department of Energy (BER/ASR Program) Grant DE-SC0006035. We thank Thomas Karl for useful discussion.
Supporting Information Available
Additional information regarding the 1H NMR and 13C NMR spectra of the synthetic MBO epoxide (Figure S1), the fragmentation scheme proposed for major product ions in the GC/EI-MS data (Figure S2), and the tentatively proposed mechanism leading to MBO epoxide formation (Figure S3). This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
# H. Zhang: Department of Environmental Science, Policy and Management, University of California, Berkeley, CA 94720-3114.
Author Present Address
∇ A. Guenther: Atmospheric Sciences and Global Change, Pacific Northwest National Laboratory, Richland, WA 99354.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Hallquist M.; Wenger J. C.; Baltensperger U.; Rudich Y.; Simpson D.; Claeys M.; Dommen J.; Donahue N. M.; George C.; Goldstein A. H.; Hamilton J. F.; Herrmann H.; Hoffmann T.; Iinuma Y.; Jang M.; Jenkin M. E.; Jimenez J. L.; Kiendler-Scharr A.; Maenhaut W.; McFiggans G.; Mentel T. F.; Monod A.; Prevot A. S. H.; Seinfeld J. H.; Surratt J. D.; Szmigielski R.; Wildt J. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9145155–5236. [Google Scholar]
- Hoyle C. R.; Boy M.; Donahue N. M.; Fry J. L.; Glasius M.; Guenther A.; Hallar A. G.; Hartz K. H.; Petters M. D.; Petaja T.; Rosenoern T.; Sullivan A. P. A review of the anthropogenic influence on biogenic secondary organic aerosol. Atmos. Chem. Phys. 2011, 111321–343. [Google Scholar]
- Chan A. W. H.; Galloway M. M.; Kwan A. J.; Chhabra P. S.; Keutsch F. N.; Wennberg P. O.; Flagan R. C.; Seinfeld J. H. Photooxidation of 2-Methyl-3-Buten-2-ol (MBO) as a Potential Source of Secondary Organic Aerosol. Environ. Sci. Technol. 2009, 43134647–4652. [DOI] [PubMed] [Google Scholar]
- Jaoui M.; Kleindienst T. E.; Offenberg J. H.; Lewandowski M.; Lonneman W. A. SOA formation from the atmospheric oxidation of 2-methyl-3-buten-2-ol and its implications for PM2.5. Atmos. Chem. Phys 2012, 1242173–2188. [Google Scholar]
- Zhang H. F.; Worton D. R.; Lewandowski M.; Ortega J.; Rubitschun C. L.; Park J. H.; Kristensen K.; Campuzano-Jost P.; Day D. A.; Jimenez J. L.; Jaoui M.; Offenberg J. H.; Kleindienst T. E.; Gilman J.; Kuster W. C.; de Gouw J.; Park C.; Schade G. W.; Frossard A. A.; Russell L.; Kaser L.; Jud W.; Hansel A.; Cappellin L.; Karl T.; Glasius M.; Guenther A.; Goldstein A. H.; Seinfeld J. H.; Gold A.; Kamens R. M.; Surratt J. D. Organosulfates as Tracers for Secondary Organic Aerosol (SOA) Formation from 2-Methyl-3-Buten-2-ol (MBO) in the Atmosphere. Environ. Sci. Technol. 2012, 46179437–9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. H.; Zhang H. F.; Pye H. O. T.; Zhang Z. F.; Marth W. J.; Park S.; Arashiro M.; Cui T. Q.; Budisulistiorini H.; Sexton K. G.; Vizuete W.; Xie Y.; Luecken D. J.; Piletic I. R.; Edney E. O.; Bartolotti L. J.; Gold A.; Surratt J. D. Epoxide as a precursor to secondary organic aerosol formation from isoprene photooxidation in the presence of nitrogen oxides. Proc. Natl. Acad. Sci. U.S.A. 2013, 110176718–6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. H.; Zhang Z. F.; Docherty K. S.; Zhang H. F.; Budisulistiorini S. H.; Rubitschun C. L.; Shaw S. L.; Knipping E. M.; Edgerton E. S.; Kleindienst T. E.; Gold A.; Surratt J. D. Isoprene Epoxydiols as Precursors to Secondary Organic Aerosol Formation: Acid-Catalyzed Reactive Uptake Studies with Authentic Compounds. Environ. Sci. Technol. 2012, 461250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenberg J. H.; Lewandowski M.; Edney E. O.; Kleindienst T. E.; Jaoui M. Influence of Aerosol Acidity on the Formation of Secondary Organic Aerosol from Biogenic Precursor Hydrocarbons. Environ. Sci. Technol. 2009, 43207742–7747. [DOI] [PubMed] [Google Scholar]
- Surratt J. D.; Chan A. W. H.; Eddingsaas N. C.; Chan M. N.; Loza C. L.; Kwan A. J.; Hersey S. P.; Flagan R. C.; Wennberg P. O.; Seinfeld J. H. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl. Acad. Sci. U.S.A. 2010, 107156640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iinuma Y.; Böge O.; Gnauk T.; Herrmann H. Aerosol-chamber study of the α-pinene/O3 reaction: Influence of particle acidity on aerosol yields and products. Atmos. Environ. 2004, 385761–773. [Google Scholar]
- Paulot F.; Crounse J. D.; Kjaergaard H. G.; Kurten A.; St. Clair J. M.; Seinfeld J. H.; Wennberg P. O. Unexpected Epoxide Formation in the Gas-Phase Photooxidation of Isoprene. Science 2009, 3255941730–733. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Lin Y. H.; Zhang H.; Surratt J. D.; Ball L. M.; Gold A. Technical Note: Synthesis of isoprene atmospheric oxidation products: Isomeric epoxydiols and the rearrangement products cis- and trans-3-methyl-3,4-dihydroxytetrahydrofuran. Atmos. Chem. Phys. 2012, 12188529–8535. [Google Scholar]
- Fringuelli F.; Germani R.; Pizzo F.; Santinelli F.; Savelli G. Regioselective epoxidation of allylic alcohols with monoperoxyphthalic acid in water. J. Org. Chem. 1992, 5741198–1202. [Google Scholar]
- Nenes A.; Pandis S.; Pilinis C. ISORROPIA: A New Thermodynamic Equilibrium Model for Multiphase Multicomponent Inorganic Aerosols. Aquat. Geochem. 1998, 41123–152. [Google Scholar]
- Keene W. C.; Pszenny A. A. P.; Maben J. R.; Stevenson E.; Wall A. Closure evaluation of size-resolved aerosol pH in the New England coastal atmosphere during summer. J. Geophys. Res.: Atmos. 2004, 109D23D23307. [Google Scholar]
- Lin Y. H.; Knipping E. M.; Edgerton E. S.; Shaw S. L.; Surratt J. D. Investigating the influences of SO2 and NH3 levels on isoprene-derived secondary organic aerosol formation using conditional sampling approaches. Atmos. Chem. Phys. 2013, 1323095–3134. [Google Scholar]
- Ortega J.; Turnipseed A.; Guenther A. B.; Karl T. G.; Day D. A.; Gochis D.; Huffman J. A.; Prenni A. J.; Levin E. J. T.; Kreidenweis S. M.; DeMott P. J.; Tobo Y.; Patton E. G.; Hodzic A.; Cui Y.; Harley P. C.; Hornbrook R. H.; Apel E. C.; Monson R. K.; Eller A. S. D.; Greenberg J. P.; Barth M.; Campuzano-Jost P.; Palm B. B.; Jimenez J. L.; Aiken A. C.; Dubey M. K.; Geron C.; Offenberg J.; Ryan M. G.; Fornwalt P. J.; Pryor S. C.; Keutsch F. N.; DiGangi J. P.; Chan A. W. H.; Goldstein A. H.; Wolfe G. M.; Kim S.; Kaser L.; Schnitzhofer R.; Hansel A.; Cantrell C. A.; Mauldin Iii R. L.; Smith J. N. Overview of the Manitou Experimental Forest Observatory: Site description and selected science results from 2008–2013. Atmos. Chem. Phys. Discuss. 2014, 1421647–1709. [Google Scholar]
- Minerath E. C.; Elrod M. J. Assessing the Potential for Diol and Hydroxy Sulfate Ester Formation from the Reaction of Epoxides in Tropospheric Aerosols. Environ. Sci. Technol. 2009, 4351386–1392. [DOI] [PubMed] [Google Scholar]
- Totobenazara J.; Haroun H.; Remond J.; Adil K.; Denes F.; Lebreton J.; Gaulon-Nourry C.; Gosselin P. Tandem Payne/Meinwald versus Meinwald rearrangements on the α-hydroxy- or α-silyloxy-spiro epoxide skeleton. Org. Biomol. Chem. 2012, 103502–505. [DOI] [PubMed] [Google Scholar]
- Page P. C. B.; Rayner C. M.; Sutherland I. O. Isomer selectivity in stereocontrolled Payne rearrangement-epoxide cleavage of 2,3-epoxy alcohols in aprotic solvents: Application to an enantioselective total synthesis of (+)-exo-brevicomin. J. Chem. Soc., Perkin Trans. 1 1990, 51375–1382. [Google Scholar]
- Gibby S. G.; Younker J. M.; Hengge A. C. Investigation of the sulfuryl transfer step from substrate to enzyme by arylsulfatases. J. Phys. Org. Chem. 2004, 176–7541–547. [Google Scholar]
- Cameron D. R.; Thatcher G. R. J. Mechanisms of Reaction of Sulfate Esters: A Molecular Orbital Study of Associative Sulfuryl Group Transfer, Intramolecular Migration, and Pseudorotation. J. Org. Chem. 1996, 61175986–5997. [Google Scholar]
- Minerath E. C.; Schultz M. P.; Elrod M. J. Kinetics of the Reactions of Isoprene-Derived Epoxides in Model Tropospheric Aerosol Solutions. Environ. Sci. Technol. 2009, 43218133–8139. [DOI] [PubMed] [Google Scholar]
- Eddingsaas N. C.; VanderVelde D. G.; Wennberg P. O. Kinetics and Products of the Acid-Catalyzed Ring-Opening of Atmospherically Relevant Butyl Epoxy Alcohols. J. Phys. Chem. A 2010, 114318106–8113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.