Abstract
Jimpy is a murine mutation in myelin proteolipid protein, leading to premature death of oligodendrocytes and severe central nervous system hypomyelination. Jimpy is a bona fide model of human Pelizaeus-Merzbacher disease. This paper describes a severe reduction in expression of κ-opioid receptors (KOP) in oligodendrocytes of jimpy mice. A cell specific reduction of >90% is apparent by 5 days of age. Expression is not reduced in neurons, and μ-opioid receptor expression is normal. Mechanism(s) leading to deficient KOP expression in jimpy mice remain unclear. We speculate that loss of KOP may be related to increased [Ca2+]i and premature death of jimpy oligodendrocytes.
Keywords: Proteolipid protein, Pelizaeus-Merzbacher disease, dysmyelination, cell death, corpus callosum, striatum
Introduction
Jimpy is an X-linked mutation in the proteolipid protein (PLP) gene that causes a dysmyelinating phenotype in the central nervous system (CNS) [40, 50]. A single nucleotide change inactivates a splice acceptor site, resulting in excision of exon 5 from PLP mRNA [21, 32, 34]. The mutation also causes a frameshift, making the predicted COOH terminus of jimpy PLP completely abnormal. Jimpy is a bona fide model of dominant-negative forms of human Pelizaeus-Merzbacher disease, a rare, inherited, leukodystrophy associated with mutations in, or duplications of, the PLP gene [23, 48]. Jimpy mice produce little CNS myelin, likely due to premature death of oligodendrocytes (OLs) throughout the CNS coincident with active myelination [30]. Arborization, myelin membrane formation, and survival are adversely affected in vivo and in culture. Jimpy mice exhibit tremors by 8–10 days, followed by death at 20–28 days. OLs from jimpy mice show additional abnormalities that appear unrelated to loss of a myelin protein. These include altered pH, Em, [Ca2+]i, metabolic function, cAMP signaling, and proliferation/cell cycle [11, 12, 25, 26, 29, 42]. Many defects occur in situ or in culture long before the cells die, suggesting a direct or indirect contribution to OL death and dysmyelination. We previously showed that jimpy OLs grown in vitro also fail to express κ-opioid receptors (KOP), although most normal OLs express KOP throughout development [28]. The present study examined expression of KOP by jimpy OLs in vivo. Endogenous opioids normally modulate aspects of glial development [15, 16, 20, 28, 36, 38, 44]. Therefore, KOP loss might contribute to abnormalities in OL phenotype and function both in jimpy mice and in human diseases involving PLP mutations.
Methods
Jimpy mice were bred from carrier pairs (B6CBACa Aw-J/A-Plp1jp EdaTa/J) (Jackson Laboratory, Bar Harbor, ME). 16–18 day mutant mice were identified by characteristic tremors. Younger mutants were identified by DdeI restriction analysis after PCR amplification [26]. Mice were anaesthetized by halothane exposure, using procedures to minimize pain outlined in the NIH Guide for Care and Use of Laboratory Animals, then perfused transcardially with 4% Zamboni’s fixative. Cerebral hemispheres were postfixed (18h, 4ºC), infiltrated overnight sequentially in 10% and 30% sucrose, embedded in Tissue Tek OCT compound (Sacura Finetek, Torrance, CA) and stored at −80ºC.
Sections (7 μm) were immunostained sequentially for opioid receptors and antibodies specific for either OLs (APC) or neurons (NeuN). Tissue was permeabilized, incubated overnight at 4ºC in polyclonal antibody to either KOP-1 (Santa Cruz Biotechnology, Santa Cruz, CA) or μ-opioid receptor (MOP) (Chemicon, Temecula, CA), followed by monoclonal anti-APC/CC-1 (Oncogene, San Diego, CA) or anti-NeuN (Chemicon). Primary antibodies were visualized using appropriate fluorescent reagents. Sections were stained with Hoechst 33342 to identify nuclei (Molecular Probes), then mounted in ProLong antifade reagent (Molecular Probes).
OLs were quantified in the corpus callosum because of their high abundance in that region. Neurons were quantified in striatum since corpus callosum lacks neuron cell bodies, and a high percentage of striatal neurons express opioid receptors. 100 random OLs or neurons, identified by APC or NeuN, were selected per section at 2 ages. Hoechst staining was assessed, and cells were examined for KOP or MOP staining only if soma were associated with an intact Hoechst-labeled nucleus. Two sections per mouse were examined at 63X magnification for each staining regimen (200 total cells), then averaged as a single N for statistical purposes, with N=6–9 mice per group. Results were analyzed by ANOVA with Duncan’s post-hoc test (Statistica; StatSoft, Tulsa, OK).
KOP levels were also examined by Western blot of corpus callosum using standard procedures [26]. Tissue was homogenized in RIPA buffer with protease inhibitors (Roche, Indianapolis, IN) and protein concentrations determined by BCA Protein Assay (Pierce Chemical Co., Rockford, IL). Samples (5 μg) from 4–6 wild-type and jimpy mice were run on a single 10% Tris-HCl Criterion Precast Gel (Bio-Rad, Hercules, CA), transferred to PVDF membranes (Hybond-P; Amersham Biosciences, Piscataway, NJ), and stained using polyclonal anti-KOP (Santa Cruz) and monoclonal β-actin (Chemicon) antibodies. Blots were visualized with SuperSignal West Femto Substrate (Pierce), scanned and analyzed (Kodak Image Station 440CF). Integrated band volumes from the same gel, corrected for actin loading, were compared using a student’s t-test.
Results and Discussion
Tissue from 5–8 day jimpy animals contained a seemingly normal complement of OLs, consistent with reports that immature stages of the lineage are phenotypically normal, and that dysmyelination and OL death occur when OLs begin to produce myelin [13, 30]. Even though cell density and morphology appeared normal, both cell counts (Fig. 1) and immunostaining (Fig. 2) showed a substantial reduction in the percentage of APC+ OLs expressing KOP in 5–8 day corpus callosum. While 67.4% of OLs in wild-type tissue expressed detectable KOP, this was reduced to 4.5% in jimpy. The percent of OLs expressing KOP normally increased with age (Fig. 1). Instead, the discrepancy in KOP staining in jimpy at 16–18 days remained highly significant (85.1% in wild-type vs. 8.8% in jimpy; Fig. 1A), thus ruling out the explanation of a temporal delay in receptor expression. Later ages were not examined since jimpy mice die prematurely. Although corpus callosum was the only region where MOP and KOP were both quantified, OLs throughout the brain were KOP deficient. For example, APC+/KOP+ cells were reduced over 90% at both ages in striatum.
Figure 1.
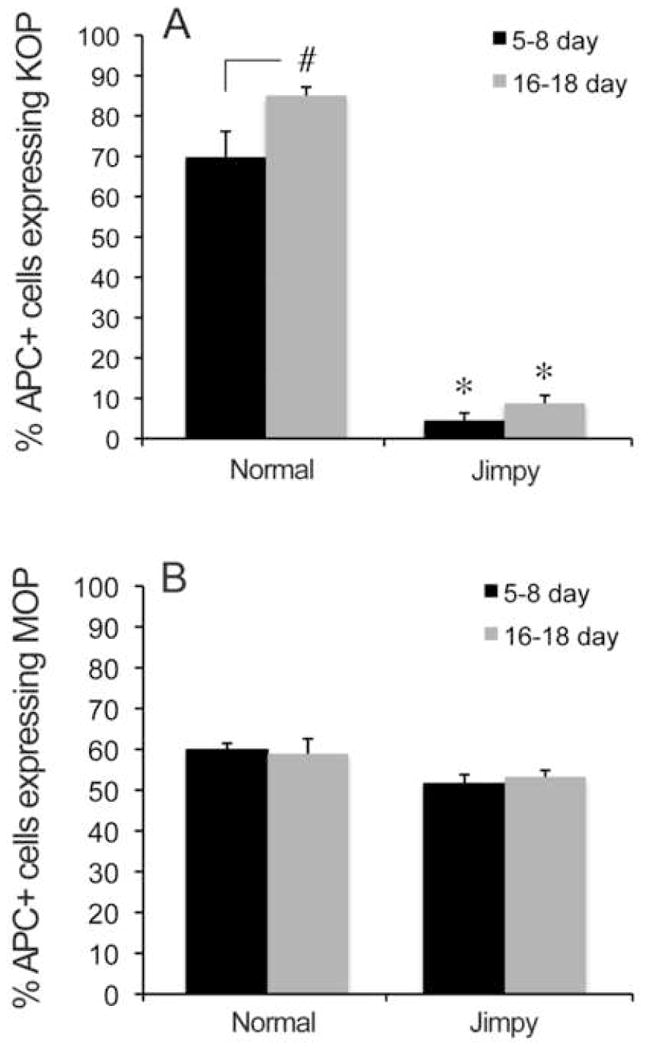
Expression of KOP and MOP in normal and jimpy (Jp) OLs. A. Most normal, APC+ OLs at 5–8 and 16–18 days express KOP. In contrast, <10% of jimpy OLs immunostain for KOP at either age (*p<0.0001 vs. normal, same age). KOP expression increases with age in normal but not jimpy OLs (# p<0.01). B. Unlike the situation for KOP, MOP expression was apparently not influenced by either genotype or age. Error bars represent S.E.M.
Figure 2.
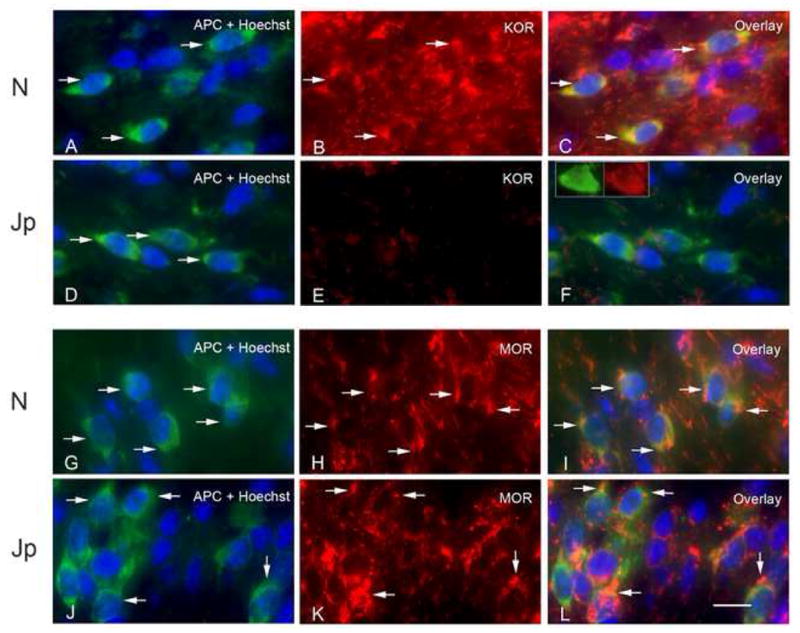
OLs from 16 day normal and jimpy (Jp) corpus callosum double-labeled for APC and either KOP (A–F) or MOP (G–L) and counterstained with Hoechst 33342. Many normal, APC+ OLs express KOP robustly (arrows indicate double-labeled cells in panels A–C). There is little KOP staining in jimpy corpus callosum (D–F). Although APC+ OLs are present (arrows in D), most are not KOP+ (E). The inset in F shows an APC+, KOP+ jimpy OL to illustrate morphology. In contrast, there is robust expression of MOP in APC+ OLs in both normal (G–I) and Jp (J–L) corpus callosum. Arrows in G–I indicate double-labeled cells in wild-type. Arrows in J–L indicate double-labeled cells in jimpy.
To test for a general defect in opioid receptors in jimpy OLs, we examined expression of MOP, which are expressed on large numbers of normal OLs both in vivo and in culture [28, 44]. Figure 1B shows no difference in MOP expression in wild-type versus jimpy OLs at either 5–8 days (59.8% vs. 51.1%) or 16–18 days (58.9% vs. 53.2%). MOP expression on normal OLs was somewhat reduced or delayed from in vitro findings [28] perhaps reflecting different milieus, or regional variations in timing of receptor expression. We also tested whether reduced KOP expression was specific to OLs by examining KOP expression in striatal neurons. Fig. 3 shows a significant increase in the percent of KOP+ neurons with age in both normal and jimpy striatum. There was no difference due to genotype at either age. Overall, the results indicate a severe reduction of KOP expression in jimpy CNS that is specific to OLs and not accompanied by decreased MOP.
Figure 3.
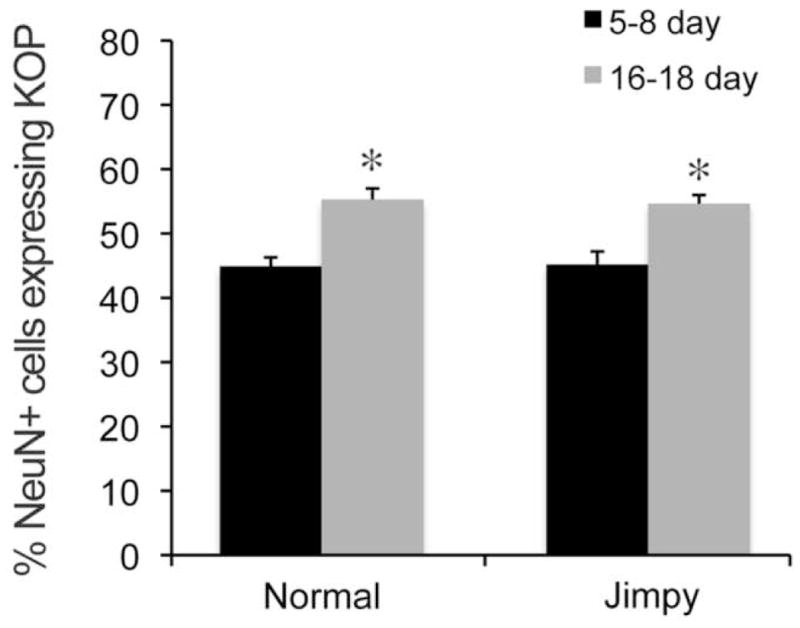
KOP expression in striatal neurons. KOP expression increases with age in both genotypes. There is no difference in the percentage of KOP-immunoreactive striatal neurons in jimpy as compared to wild-type mice at either 5–8 or 16–18 days. * p<0.005 vs. same genotype at 5–8 days of age. Error bars represent S.E.M.
Immunoblots from 5–8 day corpus callosum always showed a significant decrease in KOP (Fig. 4), mirroring the immunostaining. Results from 16–18 day samples were variable. A total of 3 sets of blots from 16–18 day old mice were performed, comparing “N”s of 4–6 jimpy and wild-type samples each. Only one experiment was significant. Since immunoblots reflect the aggregate expression on all KOP-expressing cells, which include astroglia and microglia as well as neurons and OLs, immunostaining likely provides a more accurate picture of KOP changes in OLs alone. Additionally, the jimpy CNS exhibits astrogliosis throughout the lifespan [43] and KOP expression in hypertrophied astroglia may offset KOP loss in the OL population
Figure 4.
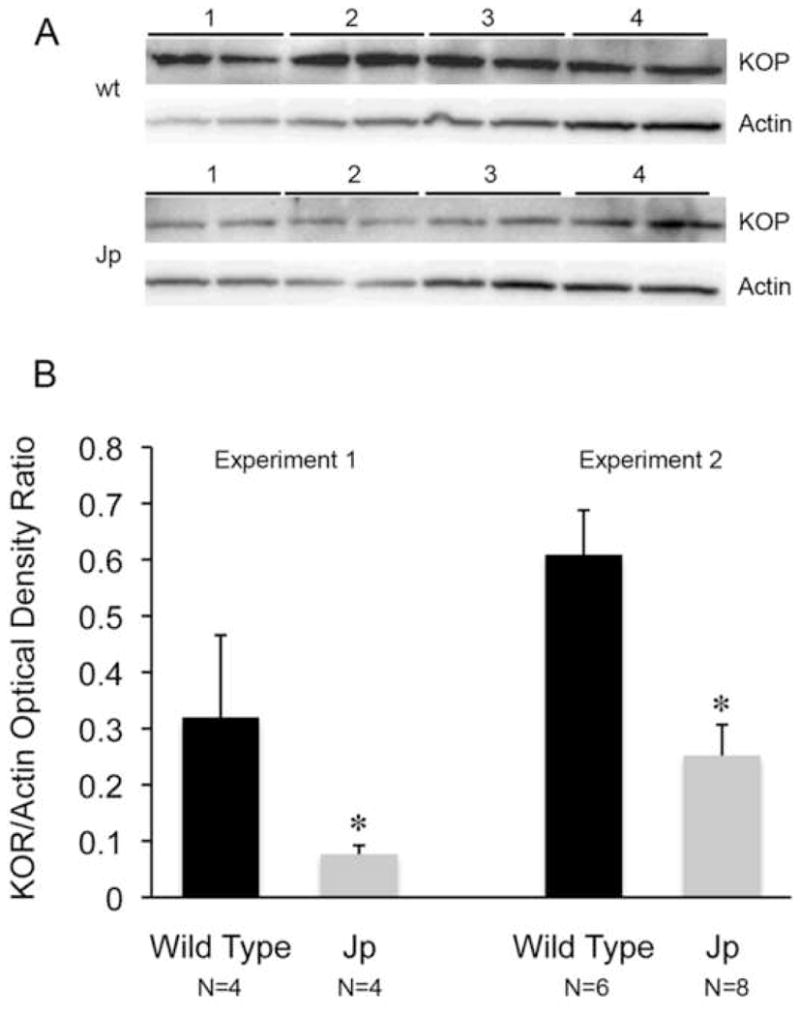
Immunoblot analysis of KOP in corpus callosum at 5–8 days. Panel A shows a representative immunoblot with duplicates of 4 wild-type (wt) and jimpy (Jp) samples stained for KOP and actin. KOP staining revealed a single band at 55 Kd. Panel B shows results of 2 separate experiments using different mice. Although absolute values differ between experiments, the jimpy KOR/actin ratio is significantly decreased from normal in both (* p<0.02 in experiment 1; * p<0.01 in experiment 2). Data in Experiment 1 are from panel A immunoblot. Error bars represent S.E.M.
Mechanism(s) underlying reduced KOP expression in jimpy mice are unclear, although maintained MOP expression argues against a general decline of opioid signaling in sick/dying jimpy OLs. The finding that KOP expression is significantly reduced by day 5 also counters this argument, since OLs in jimpy corpus callosum appear phenotypically normal and are not dying at that age [30]. As KOP levels are unaffected in neurons, the mutation appears specific for OLs. Ultimately, whether diminished KOP levels result from failure of production or failure to be inserted into OL membranes, they must stem from either loss of normal PLP or production of mutant jimpy PLP. One intriguing possibility comes from work showing that both PLP and its alternative splice product DM-20, bind cholesterol and are components of lipid rafts [41]. Missense PLP mutations similar to jimpy (jimpy-msd and rumpshaker) lead to impaired cholesterol binding and lipid raft association [31]. Lipid rafts can influence receptor function and turnover, including trafficking, stability, and internalization [1], which might in turn affect expression of KOP and other G-protein coupled receptors associated with lipid rafts [35, 51].
What are possible ramifications of reduced KOP levels in jimpy OLs? Overall, little is known about specific actions of KOP agonists on OL function or survival. In vivo studies generally suggest that selective KOP signaling is protective against CNS damage due to ischemia or trauma [3–5, 17, 18]. Much of this effect is probably indirect, mediated by reduced production of inflammatory agents or vascular changes [18, 37]. However, direct protective effects of KOP signaling may occur. Several studies with KOP agonists in vivo have shown specific sparing of white matter, suggesting direct or indirect effects on OLs [4, 18]. If direct protective effects occur through KOP signaling, loss of KOP might make jimpy OLs more vulnerable to injury. Our culture studies showed protective KOP signaling against glutamate toxicity in OLs [27], while KOP signaling has been variably effective against glutamate toxicity in neuron cell lines and cortical cultures [6, 7]. Studies discussed above use specific KOP agonists and antagonists. They do not cite studies with dynorphin peptides, whose interpretation is complex due to conflicting glutamatergic and KOP activities, as well as non-opioid effects [10, 14, 19, 46, 47, 49]. Taken together, discrepancies between in vivo and in vitro results suggest KOP signaling effects are cell and context specific, although the sparse data on hand suggests that KOP signaling may protect OLs. Supporting our concept that opioid status can affect myelination, the KOP antagonist and partial MOP agonist buprenorphine was recently shown to have multiple and complex actions on myelin and OLs during CNS development [39].
We previously showed increased baseline [Ca2+]i in jimpy OLs [26], and it is tempting to speculate a role for KOP since KOP-mediated signaling is implicated in regulating [Ca2+]i. Specific KOP agonists inhibit Ca2+ influx or increase extrusion in neurons [2, 7, 8, 33], and this might play a role in CNS protection observed with KOP signaling. KOP loss on jimpy OLs might thus elevate [Ca2+]i. Astroglia, in contrast, respond to KOP activation with increased [Ca2+]i, both through influx (L-type channels) and mobilization of intracellular stores [9, 16]. Another intriguing possibility is that loss of KOP-mediated protection evokes autocrine effects that elevate [Ca2+]i in jimpy OLs. OLs synthesize several opioids, including the KOP agonist dynorphin, which is detected even in immature OLs [27]. Some dynorphin peptides have excitotoxic, non-opioid activities via direct cell membrane effects or through interaction with glutamate receptors (reviewed in [19]) [22, 46, 47], including N-methyl-D-aspartate receptors, which are expressed by OLs [45]. If KOPs were reduced, the predominant dynorphin effects on OLs might be excitotoxicity and calcium influx, perhaps contributing to elevated [Ca2+]i and premature death. Alternatively, KOP can activate ERK in neural precursors, including those giving rise to OLs [24]. If KOP couples to protective ERK pathways in OLs, diminished KOP signaling might contribute to excitotoxic injury and/or a loss of ERK-mediated protection, promoting premature OL death.
In conclusion, jimpy mice exhibit a profound reduction in KOP expression in the CNS that is specific for OLs. Numbers of OLs expressing KOP are dramatically reduced by 5 days, and remain so throughout life. Jimpy OLs die prematurely, before forming myelin. Although reduced KOP levels probably do not directly cause OL death, we speculate that loss of KOP may increase vulnerability of jimpy OLs to other toxic events, perhaps by modulating [Ca2+]i or through loss of protective KOP-mediated signaling effects.
Acknowledgments
We acknowledge generous NIH support: R01 DA15097 and P01 DA19398
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 2.Attali B, Saya D, Nah SY, Vogel Z. Kappa opiate agonists inhibit Ca2+ influx in rat spinal cord-dorsal root ganglion cocultures. Involvement of a GTP-binding protein. J Biol Chem. 1989;264:347–353. [PubMed] [Google Scholar]
- 3.Baskin DS, Widmayer MA, Browning JL, Heizer ML, Schmidt WK. Evaluation of delayed treatment of focal cerebral ischemia with three selective kappa-opioid agonists in cats. Stroke. 1994;25:2047–2053. doi: 10.1161/01.str.25.10.2047. discussion 2054. [DOI] [PubMed] [Google Scholar]
- 4.Behrmann DL, Bresnahan JC, Beattie MS. A comparison of YM-14673, U-50488H and Nalmefene after spinal cord injury in the rat. Exp Neurol. 1993;119:258–267. doi: 10.1006/exnr.1993.1028. [DOI] [PubMed] [Google Scholar]
- 5.Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta 1-opioid agonists in a rat model of global ischemia. Physiol Behav. 2008;93:502–511. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Dawson G, Dawson SA, Goswami R. Chronic exposure to k-opioids enhances the susceptibility of immortalized neurons (F-11k7) to apoptosis-inducing drugs by a mechanism that may involve ceramide. J Neurochem. 1997;68:2363–2370. doi: 10.1046/j.1471-4159.1997.68062363.x. [DOI] [PubMed] [Google Scholar]
- 7.DeCoster MA, Conover JR, Hunter JC, Tortella FC. The neuroprotective kappa-opioid CI-977 alters glutamate-induced calcium signaling in vitro. Neuroreport. 1994;5:2305–2310. doi: 10.1097/00001756-199411000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Endoh T, Suzuki T. The regulating manner of opioid receptors on distinct types of calcium channels in hamster submandibular ganglion cells. Arch Oral Biol. 1998;43:221–233. doi: 10.1016/s0003-9969(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson PS, Nilsson M, Wagberg M, Hansson E, Ronnback L. Kappa-opioid receptors on astrocytes stimulate L-type Ca2+ channels. Neuroscience. 1993;54:401–407. doi: 10.1016/0306-4522(93)90261-d. [DOI] [PubMed] [Google Scholar]
- 10.Faden AI, Jacobs TP. Dynorphin-related peptides cause motor dysfunction in the rat through a non-opiate action. Br J Pharmacol. 1984;81:271–276. doi: 10.1111/j.1476-5381.1984.tb10074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feutz AC, Pham-Dinh D, Allinquant B, Miehe M, Ghandour M. Immortalized jimpy oligodendrocyte cell line: defects in cell cycle and cAMP pathway. Glia. 2001;2001:241–252. doi: 10.1002/glia.1058. [DOI] [PubMed] [Google Scholar]
- 12.Ghandour MS, Feutz AC, Jalabi W, Taleb O, Bessert D, Cypher M, Carlock L, Skoff RP. Trafficking of PLP/DM20 and cAMP signaling in immortalized jimpy oligodendrocytes. Glia. 2002;40:300–311. doi: 10.1002/glia.10122. [DOI] [PubMed] [Google Scholar]
- 13.Ghandour MS, Skoff RP. Expression of galactocerebroside in developing normal and jimpy oligodendrocytes in situ. J Neurocytol. 1988;17:485–498. doi: 10.1007/BF01189804. [DOI] [PubMed] [Google Scholar]
- 14.Goody RJ, Martin KM, Goebel SM, Hauser KF. Dynorphin A toxicity in striatal neurons via an α-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor mechanism. Neuroscience. 2003;116:807–816. doi: 10.1016/s0306-4522(02)00563-8. [DOI] [PubMed] [Google Scholar]
- 15.Gorodinsky A, Barg J, Belcheva MM, Levy R, McHale RJ, Vogel Z, Coscia CJ. Dynorphins modulate DNA synthesis in fetal brain cell aggregates. J Neurochem. 1995;65:1481–1486. doi: 10.1046/j.1471-4159.1995.65041481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurwell JA, Duncan MJ, Maderspach K, Steine-Martin A, Elde RP, Hauser KF. κ-opioid receptor expression defines a phenotypically distinct subpopulation of astroglia: relationship to Ca2+ mobilization, development, and the antiproliferative effect of opioids. Brain Res. 1996;737:175–187. doi: 10.1016/0006-8993(96)00728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall ED, Pazara KE. Quantitative analysis of effects of kappa-opioid agonists on postischemic hippocampal CA1 neuronal necrosis in gerbils. Stroke. 1988;19:1008–1012. doi: 10.1161/01.str.19.8.1008. [DOI] [PubMed] [Google Scholar]
- 18.Hall ED, Wolf DL, Althaus JS, Von Voigtlander PF. Beneficial effects of the kappa opioid receptor agonist U-50488H in experimental acute brain and spinal cord injury. Brain Res. 1987;435:174–180. doi: 10.1016/0006-8993(87)91599-x. [DOI] [PubMed] [Google Scholar]
- 19.Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, Woods AS, Yakovleva T, Shippenberg TS. Pathobiology of dynorphins in trauma and disease. Frontiers Biosci. 2005;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser KF, Steine-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+ dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudson LD, Berndt JA, Puckett C, Kozak CA, Lazzarini RA. Aberrant splicing of proteolipid protein mRNA in the dysmyelinating jimpy mutant mouse. Proc Natl Acad Sci U S A. 1987;84:1454–1458. doi: 10.1073/pnas.84.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hugonin L, Vukojevic V, Bakalkin G, Graslund A. Calcium influx into phospholipid vesicles caused by dynorphin neuropeptides. Biochim Biophys Acta. 2008;1778:1267–1273. doi: 10.1016/j.bbamem.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K. PLP1-related inherited dysmyelinating disorders: Pelizaeus-Merzbacher disease and spastic paraplegia type 2. Neurogenetics. 2005;6:1–16. doi: 10.1007/s10048-004-0207-y. [DOI] [PubMed] [Google Scholar]
- 24.Kim E, Clark AL, Kiss A, Hahn JW, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem. 2006;281:33749–33760. doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knapp PE. Proteolipid protein: Is it more than just a structural component of myelin? Developmental Neuroscience. 1996;18:297–308. doi: 10.1159/000111420. [DOI] [PubMed] [Google Scholar]
- 26.Knapp PE, Ismaili S, Hauser KF, Ghandour MS. Abnormal Ca++ regulation in oligodendrocytes from the dysmyelinating jimpy mouse. Brain Res. 1999;847:332–337. doi: 10.1016/s0006-8993(99)02012-0. [DOI] [PubMed] [Google Scholar]
- 27.Knapp PE, Itkis OS, Zhang L, Spruce BA, Bakalkin G, Hauser KF. Opiate signaling in oligodendrocytes: Possible autocrine effects on cell survival and development. Glia. 2001;35:156–165. doi: 10.1002/glia.1080. [DOI] [PubMed] [Google Scholar]
- 28.Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: μ and κ opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 29.Knapp PE, Skoff RP. A defect in the cell cycle of neuroglia in the myelin deficient jimpy mouse. Develop Brain Res. 1987;35:301–306. doi: 10.1016/0165-3806(87)90055-1. [DOI] [PubMed] [Google Scholar]
- 30.Knapp PE, Skoff RP, Redstone DW. Oligodendroglial cell death in jimpy mice: An explanation for the myelin deficit. J Neurosci. 1986;6:2813–2822. doi: 10.1523/JNEUROSCI.06-10-02813.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer-Albers EM, Gehrig-Burger K, Thiele C, Trotter J, Nave KA. Perturbed interactions of mutant proteolipid protein/DM20 with cholesterol and lipid rafts in oligodendroglia: implications for dysmyelination in spastic paraplegia. J Neurosci. 2006;26:11743–11752. doi: 10.1523/JNEUROSCI.3581-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macklin W, Gardinier M, King K, Kampf K. An AG->CG transition at a splice site in the myelin proteolipid protein gene in jimpy mice results in the removal of an exon. FEBS Letters. 1987;2:417–421. doi: 10.1016/0014-5793(87)80331-9. [DOI] [PubMed] [Google Scholar]
- 33.Moises HC, Rusin KI, Macdonald RL. Mu- and kappa-opioid receptors selectively reduce the same transient components of high-threshold calcium current in rat dorsal root ganglion sensory neurons. J Neurosci. 1994;14:5903–5916. doi: 10.1523/JNEUROSCI.14-10-05903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nave KA, Lai C, Bloom FE, Milner RJ. Jimpy mutant mouse: A 74 base deletion in the mRNA for myelin proteolipid protein and evidence for a primary defect in RNA splicing. Proc Natl Acad Sci U S A. 1986;83:9264–9268. doi: 10.1073/pnas.83.23.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handbook Exp Pharmacol. 2008:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 36.Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003;17:1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- 37.Qu ZX, Xu J, Hogan EL, Hsu CY. Effect of U-50488h, a selective opioid kappa receptor agonist, on vascular injury after spinal cord trauma. Brain Res. 1993;626:45–49. doi: 10.1016/0006-8993(93)90561-z. [DOI] [PubMed] [Google Scholar]
- 38.Reznikov K, Hauser KF, Nazarevskaja G, Trunova Y, Derjabin V, Bakalkin G. Opioids modulate cell division in the germinal zone of the late embryonic neocortex. Eur J Neurosci. 1999;11:2711–2719. doi: 10.1046/j.1460-9568.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56:1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidman RL, Dickie MM, Appel SH. Mutant mice (Quaking and Jimpy) with deficient myelination in the central nervous system. Science. 1964;144:309–311. doi: 10.1126/science.144.3616.309. [DOI] [PubMed] [Google Scholar]
- 41.Simons M, Kramer EM, Thiele C, Stoffel W, Trotter J. Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J Cell Biol. 2000;151:143–154. doi: 10.1083/jcb.151.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skoff RP. Increased proliferation of oligodendrocytes in the hypomyelinated mouse mutant-jimpy. Brain Res. 1982;248:19–31. doi: 10.1016/0006-8993(82)91143-x. [DOI] [PubMed] [Google Scholar]
- 43.Skoff RP. Myelin deficit in the jimpy mouse may be due to cellular abnormalities in astroglia. Nature. 1976;264:560–562. doi: 10.1038/264560a0. [DOI] [PubMed] [Google Scholar]
- 44.Stiene-Martin A, Knapp PE, Martin K, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- 45.Stys PK, Lipton SA. White matter NMDA receptors: an unexpected new therapeutic target? Trends Pharmacol Sci. 2007;28:561–566. doi: 10.1016/j.tips.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Tan-No K, Cebers G, Yakovleva T, Goh BH, Gileva I, Reznikov K, Aguilar-Santelises M, Hauser KF, Terenius L, Bakalkin G. Cytotoxic effects of dynorphins through nonopioid intracellular mechanisms. Exp Cell Res. 2001;269:54–63. doi: 10.1006/excr.2001.5309. [DOI] [PubMed] [Google Scholar]
- 47.Tang Q, Gandhoke R, Burritt A, Hruby VJ, Porreca F, Lai J. High-affinity interaction of (des-Tyrosyl)dynorphin A(2–17) with NMDA receptors. J Pharmacol Exp Therapeutics. 1999;291:760–765. [PubMed] [Google Scholar]
- 48.Trofatter JA, Dlouhy SR, DeMyer W, Conneally PM, Hodes ME. Pelizaeus-Merzbacher disease: tight linkage to proteolipid protein gene exon variant. Proc Natl Acad Sci U S A. 1989;86:9427–9430. doi: 10.1073/pnas.86.23.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker JM, Moises HC, Coy DH, Baldrighi G, Akil H. Nonopiate effects of dynorphin and des-Tyr-dynorphin. Science. 1982;218:1136–1138. doi: 10.1126/science.6128791. [DOI] [PubMed] [Google Scholar]
- 50.Willard HF, Riordan JR. Assignment of the gene for myelin proteolipid protein to the X chromosone: Implications for X-linked myelin disorders. Science. 1985;230:940–942. doi: 10.1126/science.3840606. [DOI] [PubMed] [Google Scholar]
- 51.Xu W, Yoon SI, Huang P, Wang Y, Chen C, Chong PL, Liu-Chen LY. Localization of the kappa opioid receptor in lipid rafts. J Pharmacol Exp Therapeutics. 2006;317:1295–1306. doi: 10.1124/jpet.105.099507. [DOI] [PubMed] [Google Scholar]


