Abstract
Purpose
Sorafenib and everolimus are both active against neuroendocrine tumors (NET). Because of potential synergy between VEGF pathway and mTOR inhibitors, we performed a phase I study to evaluate the safety and feasibility of combining sorafenib and everolimus in patients with advanced NET.
Methods
Patients were treated with everolimus 10 mg daily in combination with sorafenib (dose level 1: 200 mg twice daily; dose level 2: 200 mg per morning, 400 mg per evening) using standard phase I dose escalation design. Dose-limiting toxicity (DLT) was defined within the first cycle (28 days) of therapy. Treatment was continued until tumor progression, unacceptable toxicity, or withdrawal of consent. Twelve additional patients were treated at the maximum tolerated dose (MTD) level to further characterize safety and a preliminary assessment of activity.
Results
One patient in Cohort 1 experienced DLT (grade 3 skin rash); the cohort was expanded to 6 patients with no further DLTs. All 3 patients in Cohort 2 experienced DLT, consisting of thrombocytopenia, hand–foot skin reaction, and rash/allergic reaction. Sorafenib 200 mg twice daily in combination with everolimus 10 mg daily was established as the MTD. Independently reviewed best objective responses revealed that 62 % of patients had some degree of tumor shrinkage. By RECIST, we observed partial response in 1 patient, stable disease in 13 patients, and progressive disease in 3 patients.
Conclusion
Sorafenib 200 mg twice daily with everolimus 10 mg daily represents the MTD of this combination in patients with advanced NET. While the combination is active, toxicity concerns may preclude more widespread use.
Keywords: Neuroendocrine, Phase I, Sorafenib, Everolimus
Introduction
The recent introduction of targeted therapy has expanded the systemic treatment options for patients with advanced neuroendocrine tumors (NET). The mammalian target of rapamycin (mTOR) is a serine–threonine kinase that participates in the regulation of cell growth, proliferation, and apoptosis through modulation of the cell cycle [1]. mTOR mediates downstream signaling from a number of pathways, including VEGF, that are implicated in neuroendocrine tumor growth. A recent randomized study demonstrated improvement in progression-free survival (PFS) of patients with advanced pancreatic NET treated with the mTOR inhibitor everolimus [2]. In patients with carcinoid tumors, treatment with everolimus and octreotide was associated with a longer progression-free survival duration compared to octreotide alone when measured according to local investigator assessment [3].
A key role for angiogenesis and VEGF pathway signaling in NET has been suggested by clinical observations that neuroendocrine tumors are vascular tumors. Additionally, preclinical studies have demonstrated that neuroendocrine tumors have increased expression of VEGF, VEGF receptor-2 (VEGFR-2), and other growth factor receptors including platelet-derived growth factor receptors (PDGFRs) α and β, and stem-cell factor receptor (c-kit) [4–8]. A recent randomized, placebo-controlled trial demonstrated improved progression-free survival of patients with advanced pancreatic NET treated with the tyrosine kinase inhibitor sunitinib [9]. Sorafenib, which has activity against VEGFR-2, PDGFR-B, and b-Raf, has also demonstrated evidence of activity in neuroendocrine tumors. In a phase II study that included 43 patients with pancreatic NETs and 50 patients with carcinoid tumor, responses were observed in 7 % of the carcinoid patients and 11 % of the patients with pancreatic NET [10].
Because of possible synergistic effects of combining of an mTOR inhibitor with an inhibitor of the VEGF pathway, we performed a phase I study to evaluate the safety and feasibility of combining everolimus and sorafenib in patients with advanced NET. Cohorts of patients were treated with everolimus 10 mg daily and escalating doses of sorafenib beginning at a dose of 200 mg twice daily. Patients were followed for evidence of toxicity and preliminary evidence of efficacy. Treatment was continued until tumor progression, unacceptable toxicity, or withdrawal of consent.
Patients and methods
Patient population
All patients were required to be 18 years of age or older and have histologically documented, locally unresectable or metastatic carcinoid or pancreatic neuroendocrine tumors of low-or intermediate-grade histology. Patients with poorly differentiated or high-grade neuroendocrine carcinomas were not eligible. Treatment with prior chemotherapy was allowed, as was prior chemoembolization, cryotherapy, or radiofrequency ablation if measurable disease was not affected. Prior bevacizumab was permitted if last dose was greater than 6 weeks from initiation of protocol therapy. Patients were excluded if they had received prior treatment with everolimus or sorafenib. Mandated laboratory requirements included: aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5 times the upper limit of normal (ULN) (≤5 times ULN if liver metastasis was present), total bilirubin ≤1.5 times ULN, serum creatinine ≤1.5 times ULN, absolute neutrophil count (ANC) ≥1,500/mm3, and platelet count ≥100,000/mm3. All patients were required to have Eastern Cooperative Oncology Group (ECOG) performance status ≤1. Patients with a history of congestive heart failure, myocardial infarction, or unstable angina pectoris within 6 months preceding enrollment were excluded. Patients on chronic steroids or immunosuppressive therapy or with active or suspected chronic infections, including a history of chronic hepatitis B, were excluded. All patients provided written informed consent for participation in the study, which was approved by the institutional review boards of participating institutions. The study was registered with clinicaltrials.gov (NCT00942682).
Treatment and dose escalation
Patients were treated with oral everolimus at a dose of 10 mg once daily in combination with oral sorafenib twice daily. Patients enrolled at dose level 1 received sorafenib 200 mg twice daily, and patients enrolled at dose level 2 received sorafenib 200 mg in the morning and 400 mg in the evening.
A minimum of 3 and a maximum of 6 patients were enrolled in sequential cohorts according to a standard phase I dose escalation design. Dose escalation to the next cohort proceeded in the absence of more than one of six patients experiencing a dose-limiting toxicity (DLT). DLT was defined as grade 3 or higher non-hematologic toxicity (excluding untreated hyperglycemia; hyperlipidemia; or nausea, vomiting, and diarrhea uncontrolled by aggressive supportive measures), or grade 3 or higher hematologic toxicity lasting ≥7 days. Isolated grade 3 laboratory abnormalities were to be deemed clinically significant by the treating investigator to be considered DLT. DLT was defined within the first cycle (28 days) of treatment. Once the MTD was established, treatment of 12 additional patients at the MTD was planned to further characterize safety and toxicity.
Dose adjustments in sorafenib and everolimus were made for hematologic toxicity. Both agents were held if patients developed an absolute neutrophil count <1,000/mm3 or a platelet count <50,000/mm3. On recovery, treatment was resumed with dose reduction of everolimus to 5 mg daily (if receiving 10 mg daily) or to 5 mg every other day (if receiving 5 mg daily). For patients receiving sorafenib at a dose of 200 mg in the morning and 400 mg in the evening, sorafenib was reduced to 200 mg twice daily. Treatment with both drugs was also held for intolerable grade 2 or higher stomatitis, grade 2 or higher pneumonitis, or other non-hematologic toxicity that was grade 3 or higher (aside from hyperglycemia and hyperlipidemia, which were medically managed). Treatment was resumed with dose reduction pending resolution of non-hematologic toxicities to grade 1 or less. If non-hematologic toxicity did not recover within 3 weeks or if the patient experienced an unacceptable toxicity, study treatment was discontinued.
Treatment was continued until tumor progression, unacceptable toxicity, or withdrawal of consent. The study sponsors provided everolimus (Novartis) and sorafenib (Bayer).
Safety and response assessments
Patients were evaluated with a physical examination, serum chemistries, hematologic parameters, and for toxicity on days 1 and 15 of the first 28-day treatment cycle and on day 1 of subsequent treatment cycles. Toxicity was graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0. Serum chromogranin and fasting lipid panel were obtained at baseline and after every two treatment cycles. Tumor response was evaluated at the end of every other 28-day treatment period using multi-phasic computed tomography (CT), or magnetic resonance imaging (MRI).
Results
Patient characteristics
Characteristics of 21 patients enrolled in the study are listed in Table 1. The median age of the enrolled patients was 53 years, and 11 were males. Eighteen patients had carcinoid tumor, of which most were of small bowel origin, and three patients had pancreatic neuroendocrine tumor.
Table 1.
Patient characteristics
| Characteristics | N (%) |
|---|---|
| Total patients enrolled | 21 |
| Median age (range) | 53 |
| Gender | |
| Male | 11 (52) |
| Female | 10 (48) |
| ECOG performance status | |
| 0 | 9 (43) |
| 1 | 12 (57) |
| Tumor subtype | |
| Carcinoid | 18 (86) |
| Small bowel | 11 (52) |
| Bronchus | 3 (14) |
| Other or unknown primarya | 4 (19) |
| Pancreatic neuroendocrine tumor | 3 (14) |
| Tumor histology | |
| Well differentiated | 19 (90) |
| Moderately differentiated | 2 (10) |
Other primary sites of disease included: stomach (1), duodenum (1), thymus (1), unknown primary (1)
Dose escalation and dose-limiting toxicities
The dose escalation schema and associated DLTs are described in Table 2. One patient treated at dose level 1 experienced protocol-defined DLT (grade 3 skin rash); the cohort was expanded to 6 patients with no further observed DLTs. In the absence of additional DLT at dose level 1, enrollment to dose level 2 was initiated. All 3 patients treated at dose level 2 experienced DLT (grade 3 thrombocytopenia requiring holding treatment for >14 days, grade 3 hand–foot skin reaction, and grade 3 skin rash/allergic reaction). Sorafenib 200 mg twice daily in combination with everolimus 10 mg daily was established as the MTD. An additional 12 patients were treated at the MTD level to further characterize safety and toxicity.
Table 2.
Dosing schema and DLT summary
| Dose level | Sorafenib | Everolimus | N (21) | DLT |
|---|---|---|---|---|
| 1 | 200 mg twice daily | 10 mg daily | 6 + 12 (expansion cohort) | Grade 3 rash (n = 1) |
| 2 | 200 mg in the morning, 400 mg in the evening | 10 mg daily | 3 | Grade 3 thrombocytopenia lasting >14 days (n = 1) |
| Grade 3 hand–foot skin reaction (n = 1) | ||||
| Grade 3 skin rash/allergic reaction (n = 1) |
Exposure to treatment and clinical toxicities
All twenty-one enrolled patients received at least one dose of study treatment and were evaluable for toxicity. Patients received a median of four 28-day treatment cycles (range 0–26). Three patients discontinued treatment due to toxicity, including grade 3 pneumonitis (n = 1), grade 3 skin rash (n = 1), and grade 3 elevation in ALT/AST (n = 1) that resolved after discontinuation of study therapy. One patient treated at dose level 1 experienced fatal gastric perforation that occurred 37 days after following initiation of therapy and outside the DLT observation period. Disease progression was the most common reason for treatment discontinuation; of the 12 patients who discontinued therapy due to progression, six had documented radiologic progression by RECIST and six discontinued treatment due to clinical progression. Five patients discontinued treatment after withdrawing consent.
Suspected treatment-related adverse events across all treatment cycles are summarized in Table 3. Most of the observed toxicities were mild in nature, most commonly fatigue, nausea, rash, diarrhea, or electrolyte abnormalities. Treatment-related grade 3–4 non-hematologic adverse events observed in more than one patient at dose level 1 included diarrhea (n = 3), hypophosphatemia (n = 3), hypocalcemia (n = 2), and rash (n = 2). Grade 3–4 elevation in ALT/AST, hand–foot skin reaction, hyperglycemia, hypertension, hypertriglyceridemia, hypokalemia, hyponatremia, and pneumonitis occurred in one patient each. Grade 3–4 hematologic toxicities experienced at dose level 1 included thrombocytopenia (n = 2), neutropenia (n = 1), and leucopenia (n = 1). Treatment-related grade 3–4 non-hematologic adverse events observed at dose level 2 included rash/allergic reaction, anorexia, dehydration, hand–foot skin reaction, hypophosphatemia, and nausea. Grade 3–4 hematologic toxicities experienced by patients treated at dose level 2 included thrombocytopenia and lymphopenia.
Table 3.
Number of patients experiencing selected adverse events by dose level
| Dose level 1 (n = 18) |
Dose level 2 (n = 3) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AE grade |
AE grade |
||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | |
| Non-hematologic AE | |||||||||
| Elevated alkaline phosphatase | 3 | 2 | |||||||
| Allergic reaction | 1 | ||||||||
| Elevated ALT | 4 | 1 | |||||||
| Anorexia | 1 | 1 | |||||||
| Elevated AST | 7 | 1 | 1 | ||||||
| Dehydration | 1 | ||||||||
| Diarrhea | 2 | 7 | 3 | 1 | 1 | ||||
| Dry skin | 3 | ||||||||
| Dyspnea | 3 | 1 | |||||||
| Edema | 5 | 2 | 1 | ||||||
| Fatigue | 11 | 3 | 2 | ||||||
| Hand–foot skin reaction | 1 | 1 | 1 | ||||||
| Headache | 3 | 1 | |||||||
| Hypercholesterolemia | 4 | ||||||||
| Hyperglycemia | 9 | 4 | 1 | 1 | 1 | ||||
| Hypertension | 1 | 4 | 1 | 1 | |||||
| Hypertriglyceridemia | 5 | 1 | 1 | ||||||
| Hypoalbuminemia | 1 | 1 | |||||||
| Hypocalcemia | 9 | 1 | 2 | 3 | |||||
| Hypokalemia | 3 | 1 | |||||||
| Hypomagnesemia | 6 | 1 | |||||||
| Hyponatremia | 3 | 1 | |||||||
| Hypophosphatemia | 3 | 3 | 3 | 1 | 1 | ||||
| Infection | 2 | ||||||||
| Stomatitis | 7 | 1 | |||||||
| Nausea | 9 | 1 | 1 | 1 | |||||
| Neuropathy | 3 | ||||||||
| Mouth pain | 3 | 1 | |||||||
| Bowel perforation | 1 | ||||||||
| Pneumonitis | 1 | ||||||||
| Pruritus | 5 | ||||||||
| Rash | 8 | 5 | 2 | 1 | 1 | ||||
| Voice changes | 2 | ||||||||
| Vomiting | 6 | 1 | 2 | ||||||
| Weight loss | 3 | ||||||||
| Hematologic AE | |||||||||
| Anemia | 13 | 2 | 2 | 1 | |||||
| Leukopenia | 6 | 4 | 1 | 2 | 1 | ||||
| Lymphopenia | 2 | 1 | |||||||
| Neutropenia | 4 | 5 | 1 | 2 | |||||
| Thrombocytopenia | 11 | 3 | 2 | 1 | 1 | ||||
Treatment efficacy
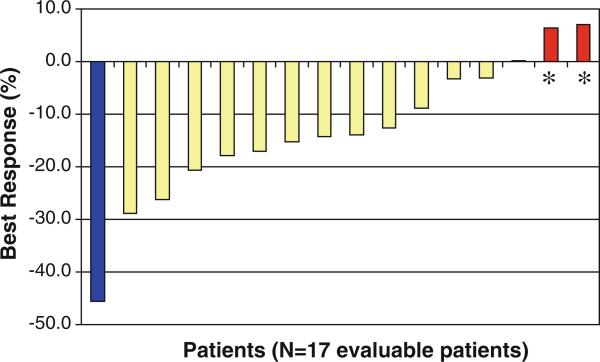
Patients were followed for radiographic response with cross-sectional imaging studies after every other cycle of treatment and for biochemical response with assessment of chromogranin A (CGA) levels after every cycle of treatment. Among 17 patients evaluable for radiographic response, one (6 %) experienced a partial response by RECIST as the best response to therapy, 13 (76 %) had stable disease, and 3 (18 %) had progressive disease. Including all enrolled patients, 13 (62 %) experienced some degree of tumor shrinkage during the course of treatment (Fig. 1). The proportion of patients on study who were progression-free at 6 months was 79 %. Nineteen patients had elevated CGA levels at baseline. Of these patients, 5 (26 %) had a CGA level decrease of 50 % or greater from baseline.
Fig. 1.
Best overall percentage change from baseline target lesion measurement by RECIST criteria. Asterisks indicates three patients had progressive disease as a result of the development of new lesions, rather than growth of the target lesions by 20 %. One patient not depicted developed new lesions; changes in other target lesions not assessed
Discussion
In this phase I study, we found that the combination of everolimus and sorafenib in patients with advanced NET was associated with toxicity that limited escalation to the anticipated full doses of both agents together. In our study, all patients receiving everolimus at a dose of 10 mg daily with more than 200 mg twice daily of sorafenib experienced DLT.
Electrolyte abnormalities, including hypophosphatemia and hypocalcemia, were common across all grades. Hypophosphatemia was also noted as one of the more common grade 3–4 adverse events in patients with pancreatic NET treated with everolimus in the RADIANT 3 study [2]. Furthermore, hypophosphatemia has also been observed in patients with advanced renal cell carcinoma receiving sorafenib (16 % grade 3–4) [11]. Possible mechanisms underlying the hypophosphatemia associated with sorafenib include development of pancreatic exocrine dysfunction and vitamin D deficiency [12]. The frequency of hypophosphatemia that we observed may be related to additive effects of sorafenib and everolimus. In addition, patients with neuroendocrine tumors possibly may be more susceptible to electrolyte abnormalities due to their underlying disease and concurrent therapy with somatostatin analogs that also can be associated with pancreatic insufficiency.
In our study, the combination of sorafenib and everolimus was associated with a RECIST-defined partial response in one patient and some degree of tumor regression in 13/21 (62 %) of patients. Due to possible synergistic effects, combining everolimus with an inhibitor of the VEGF pathway offers a promising treatment approach for patients with NET. However, our results suggest that combining everolimus with a tyrosine kinase inhibitor of the VEGF pathway is associated with treatment-related toxicity that requires dose modification of either or both agents. Attenuated dosing also has been required in other phase I studies of everolimus in combination with tyrosine kinase inhibitors. In a phase I trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma, dose-limiting toxicity, including thrombocytopenia, mucositis, and vomiting, was observed in the early cohorts of patients treated with everolimus 5 mg daily with sunitinib 37.5 mg daily [13]. Treatment was tolerated only at attenuated doses with a weekly, rather than daily, schedule of everolimus. Additionally, in a phase I study of everolimus plus sorafenib in patients with metastatic renal cell carcinoma, escalation of both agents to full monotherapy doses was not attempted due to concerns for toxicity; everolimus 5 mg daily with sorafenib 400 mg twice daily was established as the MTD in this study [14]. It is possible that synchronous blockade of the VEGF and mTOR pathways leads to synergistic toxicity. Alternatively, it is possible that drug interaction exits, leading to accumulation of sorafenib, everolimus, or active metabolites. We did not formally assess pharmacokinetic profiles in our study. However, in the phase I study of everolimus and sunitinib, the pharmacokinetic profiles for everolimus were similar to previous reports for it as a single agent, arguing against altered metabolism of everolimus due to sunitinib [13]. Similarly, there appeared to be no interaction between everolimus and sorafenib in patients with renal cell carcinoma [14].
The combination of everolimus with a VEGF inhibitor such as bevacizumab may represent an alternative and potentially more tolerable approach to dual inhibition of the mTOR and VEGF pathways. Interim analysis of a multicenter phase II trial of the mTOR inhibitor temsirolimus with bevacizumab suggests that this combination is relatively well tolerated with anticipated toxicity and also evidence of response in excess of what would be anticipated from single-agent therapy. Among the first 25 evaluable patients, a confirmed PR was documented for 13 patients (52 %) [15]. Additionally, the combination of everolimus and bevacizumab has been found to be well tolerated and associated with antitumor activity (overall response rate 26 %) in a phase II study of patients with low- or intermediate-grade neuroendocrine tumors [16]. The results of CALGB 80701 (NCT01229943), a randomized phase II study of everolimus versus everolimus plus bevacizumab in patients with advanced pancreatic NET, will provide valuable information regarding the relative benefit of dual inhibition of the VEGF and mTOR pathways. Additional studies in the future examining the efficacy of combining everolimus and inhibitors of the VEGF pathway are also warranted.
Acknowledgments
The authors gratefully acknowledge support from the Saul and Gitta Kurlat fund for neuroendocrine tumor research. This work was supported by Novartis, Bayer, and Onyx Pharmaceuticals.
Footnotes
Conflict of interest J. Chan: research funding from Novartis, Schering-Plough/Merck, Bayer, and Onyx Pharmaceuticals.
Contributor Information
Jennifer A. Chan, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA.
Robert J. Mayer, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA.
Nadine Jackson, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA; Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA.
Paige Malinowski, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA.
Eileen Regan, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA.
Matthew H. Kulke, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA.
References
- 1.Vignot S, Faivre S, Aguirre D, et al. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 4.Bowen KA, Silva SR, Johnson JN, et al. An analysis of trends and growth factor receptor expression of GI carcinoid tumors. J Gastrointest Surg. 2009;13:1773–1780. doi: 10.1007/s11605-009-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva SR, Bowen KA, Rychahou PG, et al. VEGFR-2 expression in carcinoid cancer cells and its role in tumor growth and metastasis. Int J Cancer. 2011;128:1045–1056. doi: 10.1002/ijc.25441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fjallskog ML, Hessman O, Eriksson B, et al. Upregulated expression of PDGF receptor beta in endocrine pancreatic tumors and metastases compared to normal endocrine pancreas. Acta Oncol. 2007;46:741–746. doi: 10.1080/02841860601048388. [DOI] [PubMed] [Google Scholar]
- 7.Fjallskog ML, Lejonklou MH, Oberg KE, et al. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res. 2003;9:1469–1473. [PubMed] [Google Scholar]
- 8.Hansel DE, Rahman A, Hermans J, et al. Liver metastases arising from well-differentiated pancreatic endocrine neoplasms demonstrate increased VEGF-C expression. Mod Pathol. 2003;16:652–659. doi: 10.1097/01.MP.0000077416.68489.50. [DOI] [PubMed] [Google Scholar]
- 9.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 10.Hobday TJ, Rubin J, Holen K, et al. MC044 h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): a Phase II Consortium (P2C) study. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007;25 Abstract 4504. [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 12.Mir O, Coriat R, Boudou-Rouquette P, et al. Sorafenib-induced diarrhea and hypophosphatemia: mechanisms and therapeutic implications. Ann Oncol. 2012;23:280–281. doi: 10.1093/annonc/mdr525. [DOI] [PubMed] [Google Scholar]
- 13.Molina AM, Feldman DR, Voss MH, et al. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012;118:1868–1876. doi: 10.1002/cncr.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harzstark AL, Small EJ, Weinberg VK, et al. A phase 1 study of everolimus and sorafenib for metastatic clear cell renal cell carcinoma. Cancer. 2011;117:4194–4200. doi: 10.1002/cncr.25931. [DOI] [PubMed] [Google Scholar]
- 15.Hobday T, Qin R, Reidy D, et al. Multicenter phase II trial of temsirolimus (TEM) and bevacizumab (BEV) in pancreatic neuroendocrine tumor (PNET). J Clin Oncol, 2012 ASCO Annual Meeting Proceedings. 2012;30(suppl 4) abstr 260. [Google Scholar]
- 16.Yao J, Phan A, Fogleman D, et al. Randomized run-in study of bevacizumab (B) and everolimus (E) in low- to intermediate-grade neuroendocrine tumors (LGNETs) using perfusion CT as functional biomarker. J Clin Oncol, 2010 ASCO Annual Meeting Proceedings. 2010;2815s(suppl) abstr 4002. [Google Scholar]