Abstract
Pathologists evaluate histology sectioned perpendicular to the tissue surface, or vertical cross-section. This orientation (XZ-plane) enables evaluation of mucosal differentiation in the basilar-to-luminal direction. Current endomicroscopes use a conventional (single axis) optical design.1 Imaging is limited to horizontal cross-sections (XY-plane) where the micro-anatomy is frequently similar across the field-of-view (FOV). In the dual axes configuration, light is delivered and collected off-axis, and images can be detected over a much larger range of intensities.2 Molecular images collected using fluorescence can improve specificity for disease detection and reveal functional properties about tissue.3 Proper interpretation of these images requires correlation with the micro-anatomy. We aim to demonstrate the simultaneous collection of two fluorescence images in vivo in vertical cross-sections using a dual axes confocal endomicroscope. An overlay of molecular and anatomical images from normal and dysplastic mouse colonic mucosa will be displayed in real time.
Description of Technology
In the dual axes confocal endomicroscope, the illumination and collection beams travel along different paths, and the region of overlap defines the focal volume (4 μm lateral, 5 μm axial resolution),4 Fig. 1A. A parabolic mirror weakly focuses the folded beams to create a long working distance. A tiny scan mirror (3×2 mm2) is used to perform large deflections (±6°) at resonance (3.01 kHz) and achieve a large FOV (800 μm). A bulk piezoelectric (PZT) actuator moves the scan mirror and hence focal point perpendicular to the tissue surface (400 μm depth) at 5 frames/second. Laser beams at λex = 671 and 785 nm are delivered into the illumination fiber to excite a Cy5.5-labeled (AKPGYLS) peptide,5 hereafter AKP*-Cy5.5 and IRDye 800, respectively. These fluorophores were chosen to minimize hemoglobin absorption and tissue scattering, reduce background from tissue autofluorescence, and provide maximum light penetration depth. Fluorescence is collected off-axis and travels along a symmetric optical path before being focused into a collection fiber. Fluorescence from the two fluorophores is separated by a dichroic mirror (740 nm) and detected by two separate photomultiplier tubes (PMT). The complete packaged instrument is shown, Fig. 1B.
Fig 1. Dual axes confocal endomicroscope.
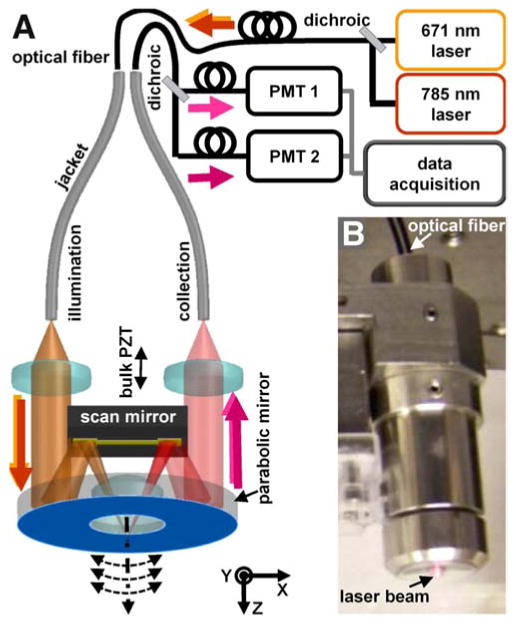
A) Schematic show laser excitation at 671 and 785 nm delivered into the illumination fiber. The beams are focused by a parabolic mirror and delivered off-axis into the tissue, and the fluorescence is collected along the same path. A scan mirror deflects the beams in the XY-plane, and a bulk PZT actuator moves the focus perpendicular to the tissue surface. B) Instrument.
Mice that have been genetically engineered with a somatic inactivation of Apc to spontaneously develop adenomas in the distal colon were anesthetized with isofluorane (2% at 0.5 L/min).6 The Cy5.5-labeled peptide (600 μM, 200 μL) was injected via the tail vein. After 150 min to clear non-specific binding, IRDye 800 (600 μM, 200 μL) was then administered. The distal tip of the dual axes confocal endomicroscope was placed in contact with a prolapsed region of colonic mucosa, and ∼2 mW of excitation at each wavelength was delivered into the tissues. Images from either fluorescence channel were recorded at the same PMT gain. Video streams that showed minimum motion artifact, lack of debris (stool, mucus) covering the mucosal surface, and recognizable crypt morphology were identified.
Video description
In the dual axes confocal design, the illumination and collection beams are separated and intersect at the focus below the tissue surface (video1). In this geometry, sub-cellular resolution can be achieved, and very little of the light scattered by tissue along the illumination path is collected. Thus light over a large range of intensities can be collected to produce vertical cross-sections. A long working distance is achieved using a parabolic mirror that weakly focuses the beams. The space created is used by a tiny scan mirror that deflects the beams horizontally, while a PZT actuator moves the focus vertically.
The anatomic images (λex = 785 nm, IRDye 800) from normal colonic mucosa (FOV 800×400 μm2) reveal vertically-oriented crypts with regular architecture and similar dimensions in height and width (video2). A layer of water on the mucosal surface helps couple the light into the tissue. The contrast agent appears to fill the lamina propria (lp) and surround individual colonocytes (c) along the crypt periphery, Fig. 2A. The fluorescence intensity decreases with tissue depth. The molecular images (λex = 671 nm, AKP*-Cy5.5) show weak signal in the epithelium (background), Fig. 2B. The overlay images are shown in pseudocolor, and are dominated by the normal crypt anatomy (green) with minimal contributions from the molecular image (orange), Fig. 2C. The corresponding histology over a similar FOV shows normal-appearing crypts lined by epithelium with colonocytes that have uniform, basally-oriented nuclei (arrows) and abundant goblet-cells with mucin-filled vacuoles, scale bar 100 mm, Fig. 2D.
Fig. 2. Vertical cross-sectional images.
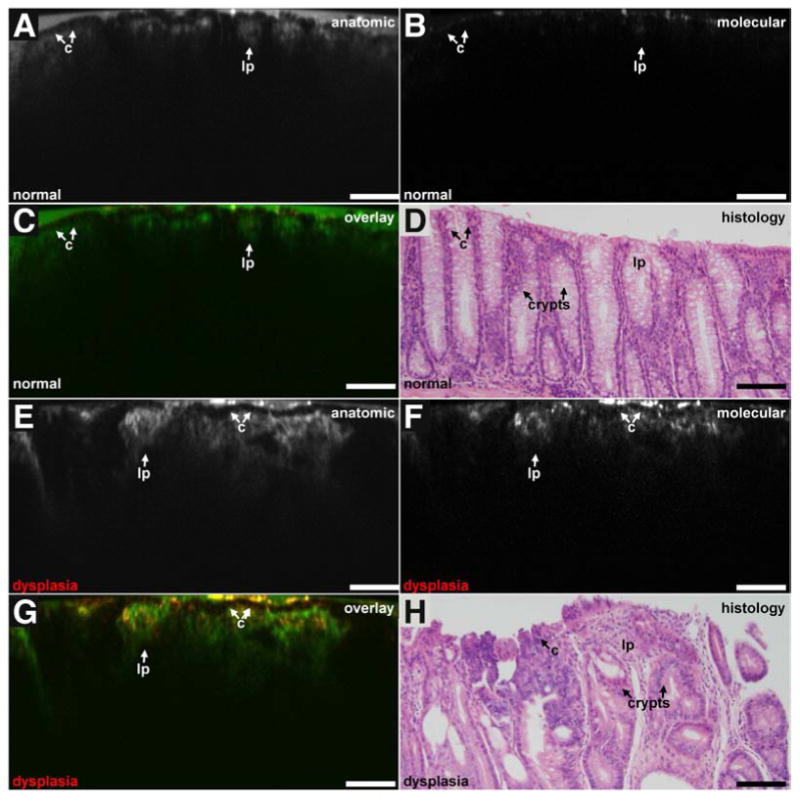
Anatomic, molecular, overlay, and histology images for A-D) normal and E-H) dysplastic mouse colonic mucosa are shown in vertical cross-sections, scale bar 100 μm.
The anatomic images from dysplastic colonic mucosa show irregular crypt architecture with variable dimensions and a complex branching pattern with an occasional cribriform arrangement, Fig. 2E. The contrast agent appears to extravasate from the lamina propria. The molecular images show much greater fluorescence intensity around the surface of individual colonocytes (target), Fig. 2F. The target-to-background ratio (average from 5 cells in each image) is 2.2. The overlay images show co-registration of peptide binding (orange) with colonocytes along the crypt periphery in the epithelium, Fig. 2G. The corresponding histology shows dysplastic crypt epithelium with enlarged, hyperchromatic and occasional vesicular nuclei that are no longer basally-oriented (arrows), scale bar 100 mm, Fig. 2H. Mucin production in the goblet cells is markedly decreased. An area of pronounced nuclear pseudo-stratification is apparent near the center.
Take home message
The dual axes confocal endomicroscope can collect vertical cross-sectional images over a large FOV (800×400 μm2). Anatomic and molecular fluorescence images can be acquired simultaneously and co-registered in real time to identify the signal location. Images that reveal the molecular properties of tissues can be detected in vivo with the same orientation as that typically used by pathologists.
Supplementary Material
Acknowledgments
Funding: TDW received funding from NIH R01 CA142750 and U54 CA136429.
Abbreviations
- FOV
field-of-view
- PMT
photomultiplier tube
- PZT
piezoelectric
Footnotes
Conflicts of Interest: Authors (ZQ, MJM, KK, KO, TDW) are inventors on patent applications filed on the instrument.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Piyawattanametha W, Wang TD. Vivo Microendoscopy. In: Tunnell J, editor. Vivo Clinical Imaging and Diagnosis. McGraw Hill Professional; New York, NY: 2011. pp. 45–76. [Google Scholar]
- 2.Liu JTC, Mandella MJ, Crawford JM, Friedland S, Soetikno R, Contag CH, Kino GS, Wang TD. A dual-axes confocal reflectance microscope for distinguishing colonic neoplasia. J Biomedical Optics. 2006;11:054019-1–10. doi: 10.1117/1.2363363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsiung P, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH, Wang TD. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nature Medicine. 2008;14:454–58. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu Z, Liu Z, Duan X, Khondee S, Joshi BP, Mandella MJ, Oldham K, Kurabayashi K, Wang TD. Targeted vertical cross-sectional imaging with handheld near-infrared dual axes confocal fluorescence endomicroscope. Biomedical Optics Express. 2013;4:322–330. doi: 10.1364/BOE.4.000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller SJ, Lee CM, Joshi BP, Gaustad A, Seibel EJ, Wang TD. Targeted detection of murine colonic dysplasia in vivo with flexible multi-spectral scanning fiber endoscopy. J Biomedical Optics. 2012;17:021103. doi: 10.1117/1.JBO.17.2.021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, Cho KR, Fearon ER. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67:9721–30. doi: 10.1158/0008-5472.CAN-07-2735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


