Abstract
The stability of citrate-capped silver nanoparticles (AgNPs) and the embryonic developmental toxicity were evaluated in the fish test water. Serious aggregation of AgNPs was observed in undiluted fish water (DM-100) in which high concentration of ionic salts exist. However, AgNPs were found to be stable for 7 days in DM-10, prepared by diluting the original fish water (DM-100) with deionized water to 10%. The normal physiology of zebrafish embryos were evaluated in DM-10 to see if DM-10 can be used as a control vehicle for the embryonic fish toxicity test. As results, DM-10 without AgNPs did not induce any significant adverse effects on embryonic development of zebrafish determined by mortality, hatching, malformations and heart rate. When embryonic toxicity of AgNPs was tested in both DM-10 and in DM-100, AgNPs showed higher toxicity in DM-10 than in DM-100. This means that the big-sized aggregates of AgNPs were low toxic compared to the nano-sized AgNPs. AgNPs induced delayed hatching, decreased heart rate, pericardial edema, and embryo death. Accumulation of AgNPs in the embryo bodies was also observed. Based on this study, citrate-capped AgNPs are not aggregated in DM-10 and it can be used as a control vehicle in the toxicity test of fish embryonic development.
Keywords: Silver Nanoparticles, Nanoparticle Stability, Embryonic Zebrafish Toxicity, Nanoparticle Exposure Media
INTRODUCTION
Silver nanoparticles (AgNPs) have been widely used in inks, microelectronics, drug-delivery agents, biosensors, and medical imaging due to their distinctive physico-chemical properties including high electrical and thermal conductivity, chemical stability, catalytic activity, and non-linear optical behavior. In particular, the broad spectrum of bactericidal activity of silver has made AgNPs extremely popular in a diverse range of consumer products including personal care items, plastics, soaps, pastes, textile and medical science (Ahamed et al., 2010; Harper et al., 2010). With the wide increase in AgNPs applications, the concerns on the release of AgNPs to the environment and their adverse health effects are also increasing. Consumer products containing AgNPs are likely to release AgNPs over their lifetime, resulting in human and environmental exposures, which is of concern since the cause of toxicity of AgNPs has not been well characterized (Fabrega et al., 2011).
Several studies have reported that AgNPs significantly induced cell necrosis/apoptosis in several cell types and toxicity in vivo. For example, AgNPs less than 3 nm induced cytotoxicity in macrophages (Yen et al., 2009). Decreased cell viability was also observed in liver and neuron cells treated with AgNPs. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of AgNPs in rats were investigated. Subchronic inhalation toxicity of AgNPs was also investigated. Histopathological examinations indicated a dose-dependent increase in lesions related to AgNPs exposure, including mixed inflammatory cell infiltration, chronic alveolar inflammation, and small granulomatous lesions (Kim et al., 2008; Sung et al., 2009; Park et al., 2010). Only a few studies have investigated the effects of AgNPs on fish. It was shown that AgNPs have adverse effects on the early life stage development that include spinal cord deformities, cardiac arrhythmia and mortality. Accumulated AgNPs in the gills and liver induced oxidative stress and blocked the uptake of oxygen (Yeo and Pak, 2008; Bilberg et al., 2010; Scown et al., 2010).
Recent publications utilizing fish models have focused on the toxicity potency of silver ions compared to AgNPs. They showed that AgNPs are less potent than Ag+ with respect to dysmorphology and loss of viability of aquatic organisms (Griffitt et al., 2008; Powers et al., 2011; Yeo and Yoon 2009). Bilberg and colleagues (2012) found a similar response in their study assessing acute exposure to adult zebrafish. Juvenile zebrafish and Japanese medaka have been shown to be more susceptible to AgNO3 than to an equal mass concentration of AgNPs, at least under the conditions that maximize free Ag+ concentrations. Factors that may contribute to the differences in toxicity include agglomeration dynamics, differential uptake, biodistribution and physiological or behavioral responses (Harper et al., 2008). In addition, small differences in exposure conditions (media, temperature, and pH) can significantly change the behavior of nanoparticles. Thus, characterizing the physicochemical properties and behavior of AgNPs in the exposure solution for toxicological studies becomes apparently critical.
Recently, the aggregation and dispersion of AgNPs in different dilutions of the media recommended by OECD for Daphnia magna toxicity testing was studied (Romer et al., 2011). They reported that all citrate-stabilized AgNPs aggregated quickly in the media with high ionic strength, which is not optimal for the toxicity test of nanomaterials.
In this study, the stability/aggregation of 10 and 100 nm citrate-stabilized AgNPs was evaluated with dynamic light scattering (DLS) for 7 days in different dilutions of zebrafish embryo media under static conditions. The toxicity of the 10 nm sized- and 100 nm sized-AgNPs was evaluated under the two different media using embryonic zebrafish starting at 8 hours post fertilization (hpf) and concluding at 120hpf.
METERIALS AND METHODS
Materials
AgNPs at a concentration of 1.0 mg/mL with the presence of citrate as a capping agent were purchased from NanoComposix, Inc. (San Diego, CA, USA). Two batches of spherical AgNPs were tested with average particle sizes reported by the manufacture of 10 nm ± 2 nm and 100 nm ± 8 nm. Fish water consisted of Instant Ocean salts (Aquatic Ecosystems, Apopka, FL, USA) dissolved in reverse osmosis water at 0.3 g/L. Pronase was purchased from Sigma-Aldrich Company (St Louis, MO, USA). Adult fish for spawning were fed with crushed TetraMin Tropical Flake or live Artemia from INVE (Salt Lake City, UT, USA).
Preparation of the AgNPs suspension
The exposure media was prepared using reverse-osmosis water with 0.3 g/L Instant Ocean Salts. Conductivity was adjusted to 475 ± 50 μS and pH was adjusted to 7.25 ± 0.25 using sodium carbonate. The AgNPs mass concentrations (mg/L) were used to prepare the AgNPs test suspensions. Dilute embryo media (DM-10) was prepared by diluting the original embryo media (DM-100) with RO water by 10 %. AgNPs stock solutions were stored at 4 °C in the dark when not in use. Prior to dilution, AgNPs stock solutions were allowed to come to room temperature. AgNPs stock solutions designated for embryo exposures were not sonicated or vortexed, but instead, homogenized by gentle inverting and rocking before diluting. AgNPs suspensions were prepared by diluting appropriate amounts of 1,000 mg/L AgNPs stock solutions with embryo media in polypropylene tubes to obtain the desired test concentrations.
Characterization of AgNPs
Aliquots of 3 ml from each test suspension were obtained to conduct particle size characterization, which included measures of hydrodynamic diameter and zeta potential. A ZetaPALS particle size analyzer (Brookhaven Instruments, Holtsville, NY, USA) was used to determine the hydrodynamic size distribution of AgNPs suspensions using dynamic light scattering at 1 min intervals and the surface charge of AgNPs suspensions was measured using a zeta potential probe at 3 min intervals. Mean values were obtained from three independent measurements for each parameter over the course of the toxicity experiments.
Zebrafish husbandry and embryo collection
Adult zebrafish Danio rerio were reared in the standard laboratory condition of 28 °C with a pH of 7± 0.2 on a 14-h light/10-h dark photoperiod. The fish were fed twice daily with either crushed or live fish food. For spawning, male and female zebrafish were placed into a spawning basket in polycarbonate tanks the afternoon before the embryos were needed. Zebrafish typically spawn when the lights come on after the 10-h dark period. The following morning, newly fertilized eggs were collected, rinsed several times in system water, and placed in fresh media in a 150-mm platic petri dish. Unfertilized or necrotic embryos were removed prior to placing the petri dish into the incubator to keep the embryos warm until they reached 8 hpf (Truong et al., 2011).
Exposure
AgNPs suspensions were loaded into 96-well plates at a volume of 150 μL per well with rows containing a single concentration. The order of the rows was randomized for each experimental replicate to minimize potential plate effects. Embryos were transferred to wells containing AgNPs suspensions or media only without AgNPs as a negative control when the average age of the embryos reached ~7 hpf (between 6 hpf and 8 hpf). Exposure solutions were not exchanged or refreshed during the test period. The embryos were incubated at 28 °C with a 16-h light/8-h dark cycle. Assessments were performed at 24, 48, 72 and 120 hpf.
Embryo toxicity
Embryo viability was assessed at 24 and 120 hpf. At 48 hpf, heart-rate was counted during 10 second intervals and recorded for each embryo. Hatching was recorded at 72 hpf and again at 120 hpf. Pericardial edema and circulation were evaluated and recorded at 120 hpf (Usenko et al., 2007, 2008).
Statistical analysis
Data are presented as average mean value ± standard deviation. A standard student t-test was used for statistical analysis.
RESULTS
Stability/Aggregation of AgNPs in exposure culture media
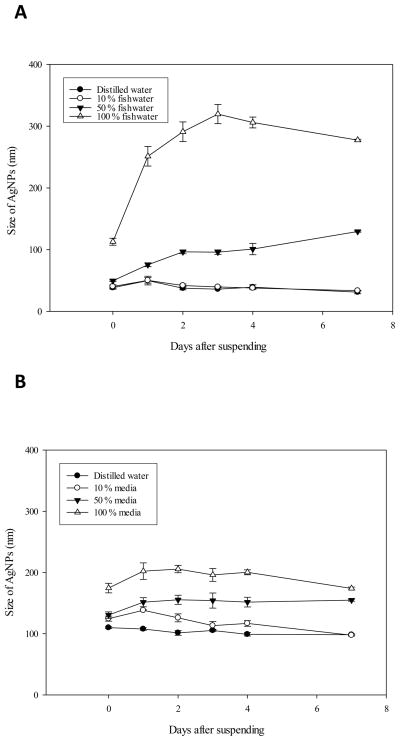
AgNPs were characterized using DLS and zeta potential. Figure 1 shows the size changes of AgNPs (4 mg/L)in differently diluted embryo culture media. In deionized water, AgNPs seemed to be stable and the aggregates of the nanoparticles were not formed. In Figure 1A, the average size of AgNPs in deionized water measured by DLS was about 30–40 nm, which is bigger than the original size of 10 nm certified by the supplier, and the size distribution was maintained during the monitoring period of 7 days. The stability of AgNPs in the DM-10 was comparable to those dispersed in deionized water. AgNPs aggregated significantly when suspended in the 50% diluted media (DM-50) and DM-100 (undiluted), in which the concentration of ionic salts was higher than in the DM-10. In DM-100, aggregates of AgNPs (original size; 10 nm) were increased over the seven day time course and reached maximal 300 nm-sized particles on day 3. When the monitoring was performed for the original 100 nm-sized AgNPs in different media dilutions, the pattern of particle stability seemed to be similar to those of original 10 nm-sized AgNPs (Fig. 1B). The AgNPs of 100 nm-sized nanoparticles also seemed to be stable both in deionized water and in the DM-10, but aggregated in DM-100 of higher salt solution (Fig. 1B). Interestingly, the aggregated formed from 100 nm-sized AgNPs in DM-100 was not as large as those formed from the 10 nm-sized AgNPs. The size of aggregates from 100 nm-sized AgNPs was maintained around a mean of 200 nm, while 10 nm-sized AgNPs aggregated up to a mean size of 320 nm (Fig. 1A and 1B).
Fig. 1. Size monitoring of AgNPs in embryo media over seven days.
AgNPs suspension was prepared in cuvettes for the DLS measurement and the cuvettes were maintained at room temperature during the monitoring period. Agitation or sonication was not performed after fresh preparation. A; 10 nm sized-AgNPs, B: 100 nm sized-AgNPs
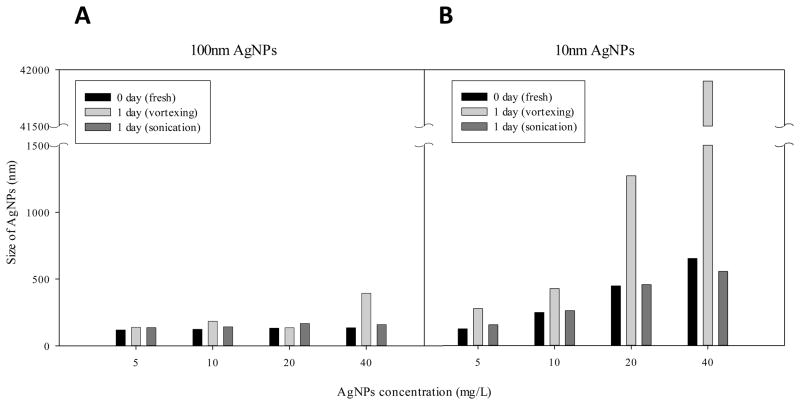
AgNPs seemed to aggregate and precipitate not only at increasing salt concentrations, but also when the concentration of AgNPs in the media was increased (Fig. 2). This suggests that some difference may exist between the nominal concentration which assumes an even distribution of particles and the actual concentration of AgNPs at a given location in the well if the AgNPs fall out of the suspension during the exposure period. In order to address this, AgNPs suspension was prepared in a DLS cuvette with a nominal concentration of 5 ~ 40 ppm and the average mean size was measured. Briefly, after measuring the fresh AgNPs suspension of the media in the cuvettes, the cuvettes were left to stand for 24 hours and then vigorously vortexed. After this, the size of AgNPs in the suspension was measured. If AgNPs were precipitated and the precipitates were re-suspended by vortexing, then the average mean size of the particles should be observed by an increase in the mean aggregate size. As shown in Figure 2, the mean aggregate size was significantly increased after vortexing the precipitates in the bottom of the cuvette. The size of the aggregates that precipitated in the bottom of the cuvette was also seen to increase with increasing concentration. However, when the re-suspended solution was sonicated which gives more powerful agitation than vortexing, the aggregates were dispersed and the sizes were reduced.
Fig. 2. Size monitoring of AgNPs in embryo culture media with vortexing or sonication.
AgNPs suspension was prepared in cuvettes and the size was measured using DLS. When the cuvettes were left standing for 1 day at room temperature, sedimentation was observed in some cuvettes. The cuvettes were vortexed for 15 second and the size of AgNPs in the suspension was measured. The same AgNPs suspension was sonicated for 30 seconds and measured again (VWR Model B1500A-MTH, 50W 42 KHz). The experiments were performed and representative result was shown (n=3). A; 100 nm sized-AgNPs, B: 10 nm sized-AgNPs
Effects of media dilution on embryo development
Development of embryos was observed for 5 days in both the DM-10 and DM-100. No statistical differences were found for mortality and touch response between diluted media (DM-10) and undiluted media (DM-100), and all surviving embryos hatched normally within 72 hpf. As shown in Table 1, there were no statistical differences in spontaneous movements or heart rate between the embryos cultured in either media.
Table 1.
Effects of AgNPs on the spontaneous tail movement and heart beat of zebrafish embryos
| 10% Diluted media | 100% non-diluted media | ||
|---|---|---|---|
| Spontaneous Movements for 2 min at 24 hpf | 6.2 ± 2.5 | 7.2 ± 2.6 | |
|
| |||
| Heartbeat for 1 min | at 48 hpf | 159.1± 15.7 | 155.1±16.1 |
| at 72 hpf | 174.5 ± 18.7 | 186.0 ±18.6 | |
No statistically significance was found between two media by student t-test
Effects of AgNPs on embryo development
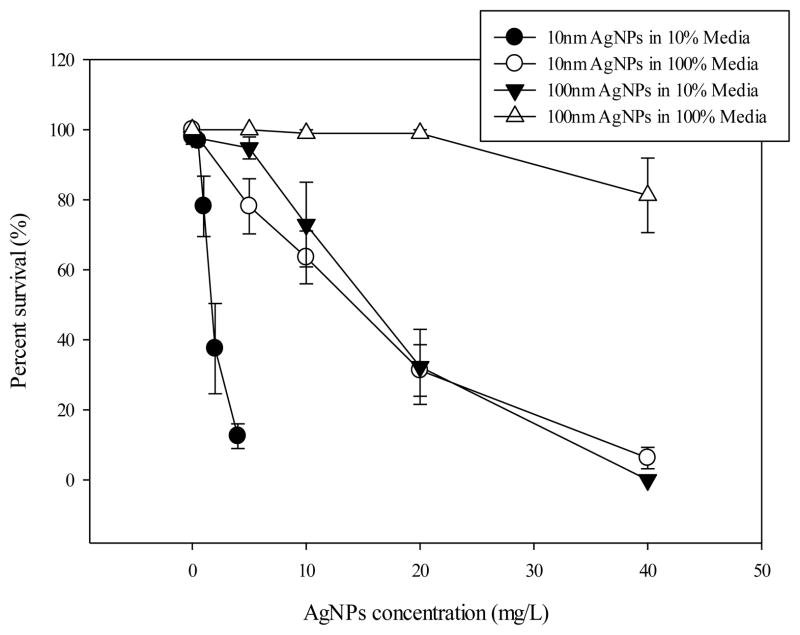
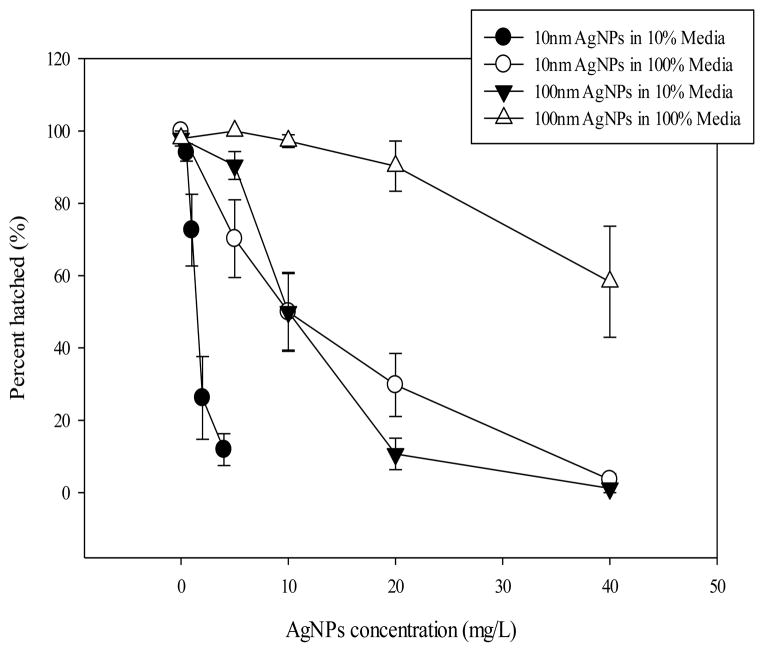
Embryos in the non-treated control groups of the DM-10 or DM-100 were healthy and hatched normally by 72 hpf with almost all embryos surviving until 120 hpf. When treated with AgNPs, embryo survival (Fig. 3), hatching of embryos by 72 hpf (Fig. 4), and the heart rate at 48 hpf was decreased (Figure 5), and the incidence of pericardial edema at 120 hpf was increased (Fig. 6) compared to control embryos. For these endpoints, higher toxicity was observed when AgNPs were suspended in the DM-10 compared to those in the DM-100 media for both 10 nm-sized AgNPs and 100 nm-sized AgNPs (Fig. 3). A similar effect was observed for the 100 nm-sized AgNPs in which the majority of embryos (81±11 %) survived in DM-100 when exposed at 40 ppm, but no embryos survived the treatment at 40 ppm in the DM-10 (Fig. 3). While all control embryos hatched normally by 72 hpf (day 3), those treated with AgNPs showed a concentration dependent decrease in the number of embryos hatched (either from delayed hatching or failure to hatch) (Fig. 4).
Fig. 3. Mortality of embryonic zebrafish exposed to AgNPs.
The proportion of embryos surviving at 120 hpf for each treatment type was shown. Data are shown as the mean ± SEM of four experimental replicates (n=4). 100 % media means undiluted original culture media and 10 % media means diluted the original culture media by one to ten.
Fig. 4. Embryonic hatching after AgNP exposures.
The proportion of embryos hatched by 72 hpf for each treatment type. Data are shown as mean ± SEM of four experimental replicates (n=4).
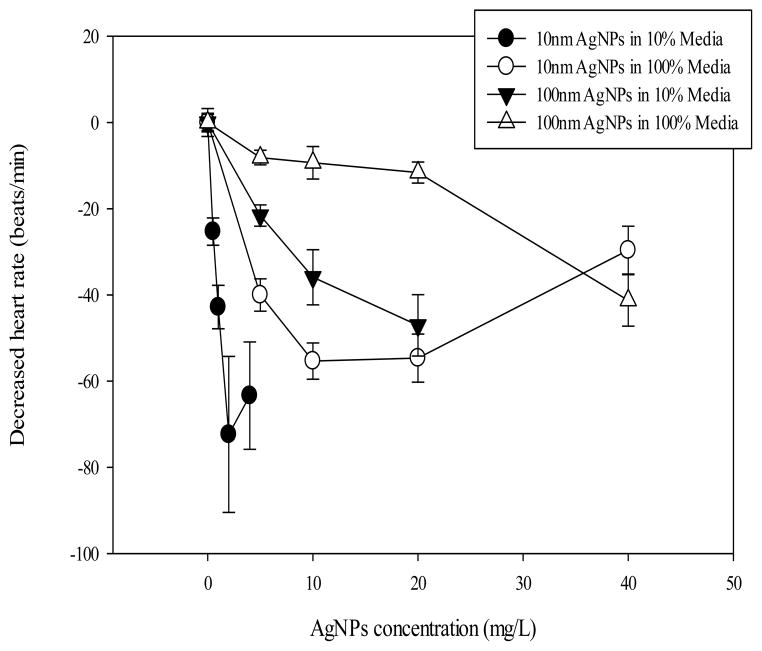
Fig. 5. Decreased heart rate of zebrafish embryos to AgNP exposure.
Graphs are the differences in mean heart rate from control embryos at 48 hpf for each treatment type. Individual means were calculated for each of four experimental replicates and the mean of the control animals were subtracted from each of the replicates and a new mean was calculated from those values. Plotted is mean ± SEM of four experimental replicates (n=4).
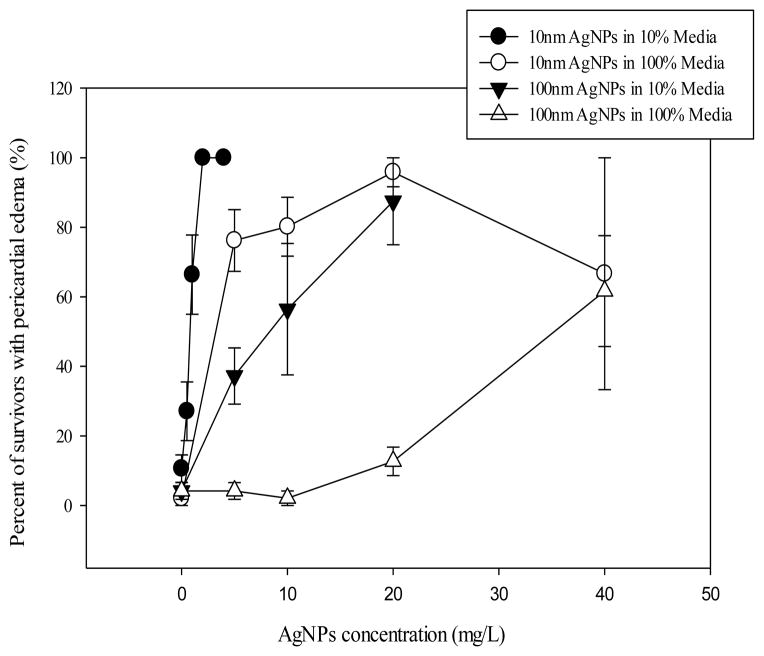
Fig. 6. Morphological impacts of embryonic exposure to AgNPs.
Graph shows the proportion of embryos surviving at 120 hpf that also had pericardial edema (PE) for each treatment type. Plotted is mean ± SEM of four experimental replicates (n=4).
It is generally known that the chorion may block the chemical transport from the outer environment into the inner core to protect the embryos. When the sensitivity of two types of embryos (with - and without the chorion) was compared, it was found that the embryos with the chorion were more sensitive to AgNPs compared to the dechorinated embryos. The mortality of embryos with the intact chorion was about 95 – 100 % at 4 ppm of the 10 nm sized-AgNPs in DM-10. However, the mortality of the dechorinated embryos was 0–25 % at the same exposure condition. It seemed that the chorion may not play a role as a blockage of AgNPs exposure to the larvae. AgNPs seemed to be accumulated in the embryos through the chorion and thus hatching was not accomplished in most damaged embryos (Fig. 7). The AgNPs-exposed dechorinated embryos showed more deformity compared to the embryos with the chorion. Deformities of larvae included yolk sac edema, curved or bent axes, swelling around the yolk sac and pericardial edema. Delayed development and lack blood circulation (or slowed circulation) with weak heart beats were also observed.
Fig. 7. Effects of 10 nm sized-AgNPs on the developmental stage of zebrafish embryos.
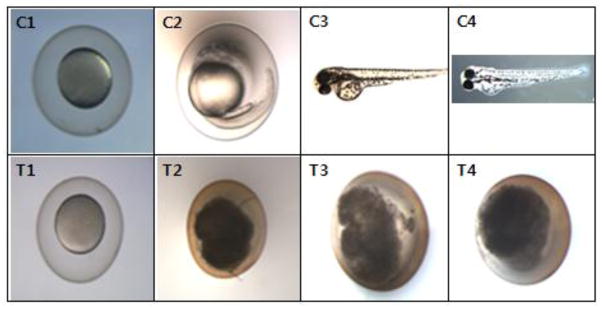
Embryos were cultured in 10 % diluted embryo media (DM-10) and a representative photo is shown (5X). C stands for control group and T for AgNPs-treated group. AgNPs in treated group seemed to be accumulated in the core of embryos with a black color. C1 and T1 at 8 hpf, C2 and T2 at 1 dpf, C3 and T3 at 2 dpf, C4 and T4 at 3 day post fertilization (dpf).
Effects of AgNPs on heart and vascular formation
Heart rate was recorded as a measurement of physiological response to AgNPs exposure. Heart rate decreased in a concentration dependent manner in the AgNPs-treated embryos at 48 hpf (Fig. 5). Other negative cardiovascular impacts were also observed including thrombosis in the heart, decreased heart stroke volume, and decreased circulating blood flow (data not shown). A concentration dependent increase was shown in the percentage of embryos displaying pericardial edema that survived until 120 hpf (Fig. 6).
DISCUSSION
With the wide use of AgNPs, it is more than possible that they will be released to the environment either by direct application or during the life cycle of products (Fabrega et al., 2011). The release of AgNPs in the environment could cause potential impacts on the ecosystem. When AgNPs are released into the environment, their mobility, bioavailability, and toxicity are mainly affected by particle stability (Powers et al., 2011). The stability of nanoparticles is a function of many factors including the type of capping agents, dispersants, the surrounding environmental conditions, such as pH, ionic strength, and the background electrolyte composition (Chen et al., 2006; El et al., 2010). Among these factors, different types of capping agents have been widely investigated to enhance the stability of AgNPs. Capping agents may prevent the aggregation of AgNPs through electrostatic repulsion, steric repulsion or both. In the case of AgNPs, citrate, polyvinylpyrrolidone (PVP), and sodium borohydride (NaBH4) are the most widely used capping agents. In toxicity tests, particle size and surface charge may have a significant impact on the toxic responses. For example, if the nanoparticles are aggregated, the toxic response may be affected.
Recently, aquatic toxicology studies of AgNPs have reported on the toxicities to algae, daphnids, worms and fish (Bielmyer et al., 2006; Lee et al., 2007; Rho et al., 2009). Although most toxicity studies of AgNPs on aquatic organisms involve potential hazards to the organisms, little information is available on the physicochemical stability of AgNPs in the test system. Furthermore, the precipitation of aggregates in test media has not been adequately considered. Nominal concentrations of nanoparticles may not be used in test media for the identification of toxicity levels such as LC50. To express the concentration of AgNPs, ICP-MS measurement was widely used for the supernatant of test media to analyze the Ag+ concentration. The nominal silver concentration of the particles in the embryo medium was measured by taking an aliquot near the top of the suspensions to mimic exposures (Bar-Ilan et al., 2009; Power et al., 2011). However, dilution of test media containing high salts has never been tried in fish embryo tests to avoid the aggregation of AgNPs coated with hydrophilic chemicals such as citrate.
In this study, embryo media was diluted to prevent the aggregation of citrated stabilized AgNPs. As shown in Fig. 1, AgNPs were very stable in diluted media with low salt concentrations. In the DM-10, AgNPs were as stable as those in deionized water for 7 days, which is a long enough time to evaluate embryo toxicity by this method. Because the salt concentration in DM-50 or DM-100 was higher than DM-10, the size of the aggregates in DM-50 or DM-100 was increased. The maximal size of 10 nm-sized AgNPs reached to 320 nm by day 3 after freshly preparing the AgNPs suspension and then the size of the aggregates did not increase thereafter. The size of suspending AgNPs in deionized water or in DM-10 was bigger than the labeled size in the stock solution supplied by the manufacture. We do not know the reason at this moment but we assume the decreased concentration of citrate, which is a stabilizer of AgNPs stock solution, could be one possible reason.
The 100 nm-sized AgNPs were more stable in the test media compared 10 nm-sized AgNPs. It seemed that the physicochemical properties of the 100 nm-sized AgNPs were totally different from the 10 nm-sized AgNPs and the size was not the only factor to affect the stability. The size of the aggregates seemed to be bigger in higher concentrations of AgNPs and this was more evident for the 10 nm AgNPs (Fig. 2B). The size of AgNPs at 5 ppm in DM-100 was 125 nm but it was 625 nm at 40 ppm in the same condition when they were measured immediately after preparation. When the fresh prepared suspension was left standing in the cuvette for 1 day and measured after vortexing, the size of AgNPs was significantly increased to microsize, indicating that the large aggregates had precipitated in the bottom of the cuvette. The size increase of AgNPs in the cuvette after vortexing was because the big precipitated aggregates were re-suspended and measured. In the re-suspension of the 40 ppm AgNPs (10 nm) after 1 day of standing, the size of the aggregates reached about 4 μm. However, the precipitated aggregates were fully re-suspended by sonication which is more powerful than vortexing. This phenomenon shows that nanoparticles may be precipitated in the test media and this precipitation may affect the actual concentration of the test material spatially and temporally. This also suggests that maximal test concentration of AgNPs in the test media should be limited based on the stability of the particles to maintain the same concentration during the exposure period. In the case of citrate-stabilized AgNPs, high ionic salts interacted with citrate and changed the electro-repulsion capacity which might have caused the aggregation of AgNPs. When the salt concentration of embryo culture media is decreased, the repulsion capacity of citrate may be decreased hence the aggregation of AgNPs was reduced. The impact of dilute media was therefore investigated to determine if the diluted embryo media affect the developmental process or the normal physiology of embryos. Mortality, hatching, blood formation, blood circulation, touch response, and deformity were not influenced in the DM-10. Furthermore, spontaneous movement and heartbeat were not changed compared to the embryos cultured in DM-100 (Table 1), showing that diluted media can be applied to embryo toxicity tests to stabilize nanoparticle exposures.
Notably, this work shows that the impact of AgNPs treatment was dependent on both the size of the AgNPs and the concentration of dissolved salts in the exposure media and that these variables had a significant effect on all the endpoints evaluated (Fig. 3, 4, 5 and 6). The smaller 10 nm-sized AgNPs were more toxic compared to the larger 100 nm-sized AgNPs in either undiluted DM-100 or diluted media DM-10. The difference in toxicity between the two sizes could potentially be explained under consideration of alternative dose metrics other than mass in which toxicity could be measured against the total surface area of the particles or the approximate number of particles or perhaps the rate of particle dissolution. Also, the toxicity of both the 10 nm and 100 nm particles were more pronounced when embryos were exposed in DM-10 compared to the embryos exposed to the same sized particle but in DM-100. This shows that the toxicity of the particles are influenced by their environment, affecting particle behavior in solution such as the degree of polydispersity, the rates of aggregation or agglomeration, or the potential dissolution properties of particles. Primary particle size may still represent a stronger contributing factor to AgNPs toxicity than hydrodynamic diameter measured by DLS because the aggregates of the 10 nm AgNPs in undiluted media were larger and still more toxic compared to the aggregates of the 100 nm AgNPs under the same conditions. AgNPs at higher concentrations were also found to be less stable than those at lower concentrations in undiluted media as evident in Fig. 2 which shows that larger numbers of aggregates were formed in response to the increasing concentration. This effect was also more pronounced for the smaller 10 nm-sized AgNPs than for the larger 100 nm-sized AgNPs (Fig. 2). This data suggest that the No Observed Effect Concentration (NOEC) value of AgNPs in aquatic toxicity tests should be carefully evaluated in high test concentrations and in media with high concentrations of salt or other dissolved solutes because the formation of nanoparticles aggregates may effectively change the actual concentration in suspension.
The chorion is considered to be an important barrier to some chemicals, protecting embryos from direct exposure to environmental toxicants (Kimmel et al., 1995; Lee et al., 2007). The chorion also plays a role in metal trafficking from the environment to embryo. When embryos with the chorion were treated with AgNPs, the chorion did not seem to block the passage of AgNPs transport, but instead, the particles may have acted to block the passage of oxygen through the chorion and caused fatal effects on the embryos. When the toxicity of AgNPs to embryos with the chorion was compared to that observed in dechorinated embryos, dechorinated embryos were more resistant to AgNPs and survived as described before. AgNPs may also have accumulated inside the chorion (Fig. 7), increasing the mortality of embryos.
Citrate-stabilized AgNPs were easily aggregated in undiluted media and this seemed to clearly affect the observed toxicity in comparison to the AgNPs in diluted media which were stable during the exposure period and likely better represent the toxic potential of these particles. Here we showed that AgNPs caused mortality, delayed hatching and failure to hatch, general dysmorphology, heart dysfunction and slow blood circulation. This work also suggests that the toxicity of AgNPs in particular and perhaps many other classes of nanomaterials seem to be strongly influenced by primary particle size, media characteristics, and particle behavior and that particle characterization throughout the exposure period is a necessary requisite to accurately estimate the potential hazard of nanomaterials in a context dependent manner.
Acknowledgments
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government to K.P. (NRF-2010-013-E00035). Support to SLH was provided in part by National Institute of Environmental Health Sciences (NIEHS) grants ES017552-01A2, ES016896-01 and P30 ES03850, and the Air Force Research Laboratory (AFRL) FA8650-05-1-5041.
References
- Ahamed M, Alsalhi MS, Siddiqui MK. Silver nanoparticle applications and human health. Clin Chim Acta. 2010;411:1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5:1897–1910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- Bielmyer GK, Grosell M, Brix KV. Toxicity of silver, zinc, copper, and nickel to the copepod Acartia tonsa exposed via a phytoplankton diet. Environ Sci Technol. 2006;40:2063–2068. doi: 10.1021/es051589a. [DOI] [PubMed] [Google Scholar]
- Bilberg K, Malte H, Wang T, Baatrup E. Silver nanoparticles and silver nitrate cause respiratory stress in Eurasian perch (Perca fluviatilis) Aquat Toxicol. 2010;96:159–165. doi: 10.1016/j.aquatox.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Bilberg K, Hovgaard MB, Besenbacher F, Baatrup E. In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio) J Toxicol. 2012:1–9. doi: 10.1155/2012/293784. Article ID. 293784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KL, Elimelech M. Aggregation and deposition kinetics of fullerene (C) nanoparticles. Langmuir. 2006;22:10994–11001. doi: 10.1021/la062072v. [DOI] [PubMed] [Google Scholar]
- El BAM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol. 2010;44:1260–1266. doi: 10.1021/es902240k. [DOI] [PubMed] [Google Scholar]
- Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR. Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int. 2011;37:517–531. doi: 10.1016/j.envint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem. 2008;27:1972–1978. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- Harper SL, Usenko CY, Hutchison J, Maddux BLS, Tanguay RL. In vivo biodistribution and toxicity depends on nanomaterial composition, size, surface functionalization and route of exposure. J Exp Nanosci. 2008;3:195–206. [Google Scholar]
- Harper SL, Hutchison J, Maddux BLS, Tanguay RL. Integrative strategies to understand nanomaterial-biological interactions. International Perspectives on Environmental Nanotechnology: Applications and Implications. 2010;2:51–56. [Google Scholar]
- Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon IH, Jeong J, Han BS, Yu IJ. Twenty-eight-day oral toxicity, genotoicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XHN. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Yi J, Kim Y, Choi K, Park K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol In Vitro. 2010;24:872–878. doi: 10.1016/j.tiv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Powers CM, Slotkin TA, Seidler FJ, Badireddy AR, Padilla S. Silver nanoparticles alter zebrafish development and larval behavior: Distinct roles for particle size, coating and composition. Neurotoxicol Teratol. 2011;33:708–714. doi: 10.1016/j.ntt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JY, Sim SJ, Yi J, Park K, Chung KH, Ryu DY, Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol. 2009;43:3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- Römer I, White TA, Baalousha M, Chipman K, Viant MR, Lead JR. Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests. J Chromatogr A. 2011;1218:4226–4233. doi: 10.1016/j.chroma.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Scown T, Santos E, Johnston B, Gaiser B, Baalousha M, Mitov S. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol Sci. 2010;115:521–534. doi: 10.1093/toxsci/kfq076. [DOI] [PubMed] [Google Scholar]
- Sung JH, Ji JH, Park JD, Yoon JU, Kim DS, Jeon KS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Chang HK, Lee JH, Cho MH, Kelman BJ, Yu IJ. Subchronic inhalation toxicity of silver nanoparticles. Toxicol Sci. 2009;108:452–461. doi: 10.1093/toxsci/kfn246. [DOI] [PubMed] [Google Scholar]
- Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Methods Mol Biol. 2011;691:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. Exposure to C60 elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol. 2008;229:44–55. doi: 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo MK, Pak SW. Exposing zebrafish to silver nanoparticles during caudal fin regeneration disrupts caudal fin growth and p53 signaling. Mol Cell Toxicol. 2008;4:311–317. [Google Scholar]
- Yeo MK, Yoon JW. Comparison of the effects of nano-silver antibacterial coatings and silver ions on zebrafish embryogenesis. Mol Cell Toxicol. 2009;5:23–31. [Google Scholar]
- Yen HJ, Hsu SH, Tsai CL. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 2009;5:1553–1556. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]