Abstract
Objectives
To model, in rats, the development of chronic trigeminal nociceptive hypersensitivity seen in patients with recurrent headache.
Background
Pathophysiology studies suggest that patients with recurrent migraine headache experience repeated bouts of dural nociceptor activation. In some patients, the severity and frequency of headache attacks increase over time. Patients with recurrent headache are hypersensitive to nitric oxide donors, such as glyceryl trinitrate (GTN).
Current trigeminal pain models do not reflect the repeated episodic nature of dural nociceptor activation in patients with recurrent headache. Repeated nociceptor activation creates long-lasting changes in the periphery and brain due to activity-dependent neuronal plasticity. An animal model of repeated activation of dural nociceptors will facilitate the study of the physiological changes caused by repeated, episodic pain and the factors important for the transition of episodic to chronic migraine.
Methods
We induced dural inflammation by infusing an inflammatory soup (IS) through a cannula on the dura in awake behaving rats. This was repeated 3 times per week for up to 4 weeks. Periorbital pressure sensory testing was used to monitor the change in trigeminal sensitivity. Rats were challenged with GTN to test the hypothesis that many dural stimulations are required to model the hypersensitivity of migraine patients. Quantitative trigeminal sensory testing and microdialysis in the trigeminal nucleus caudalis (TNC) were used to measure GTN hypersensitivity.
Results
Multiple infusions of IS (>8), over weeks, induced a long-lasting decrease in periorbital pressure thresholds that lasted >3 weeks after the last infusion. In contrast, IS infusion in IS-naive rats and those that received 3 IS infusions produced only short-lasting decreases in periorbital pressure thresholds.
Rats that received more than 8 IS infusions showed a marked increase in their neurochemical and behavioral responses to GTN. In these rats, GTN induced a decrease in periorbital von Frey thresholds that lasted >5 hours. In contrast, in rats that received only 3 IS infusions, GTN caused a threshold decrease for 1.5 hour.
In vivo microdialysis in the TNC showed that GTN increased extracellular glutamate levels in rats with more than 8 IS infusions to 7.7 times the basal levels. In IS-naive rats and those that received only 3 IS infusions, the extracellular glutamate levels rose to only 1.7 and 1.9 times the basal level, respectively.
Conclusions
Repeated IS stimulation of the dura produces a chronic state of trigeminal hypersensitivity and potentiates the response to GTN. This hyperresponsiveness outlasts the last IS infusion and is the basis of our rat model of recurrent headache. This model can be used to study the changes in the brain and periphery induced by repeated trigeminovascular nociceptor activation and has the potential to elucidate the mechanisms for the transition of episodic to chronic headache.
Keywords: pathophysiology, migraine, chronic daily headache, inflammation, microdialysis, sensory testing, pain, dura
This study describes a new model of recurrent headache in rats. Our model uses repeated inflammatory dural stimulation to mimic the repeated activation of dural afferents believed to occur in patients with recurrent migraine headache. After multiple inflammatory stimulations of the dura, there is an unique physiological state generated in the rats that is stable, and long outlasts the last stimulation. This state is characterized by sensory and physiological hyperresponsiveness of the trigeminal neurovascular system that significantly differs from the relatively short-lasting and smaller magnitude changes induced by a single acute stimulation of the dura.
The pain phase of migraine involves nociceptor activation due to either neurogenic inflammation or another, unknown, mechanism.1–4 According to this theory, patients experience repeated attacks of nociceptor activation on the dura over time, separated by pain-free periods.4–6 Current animal model of headache do not reflect the repeated trigeminovascular nociceptor activation in patients with recurrent headache. Repeated, episodic nociceptor activation creates long-lasting changes in the periphery and the brain and can increase the perception of pain in secondary or referred areas due to activity-dependent neuronal plasticity.7–12 An animal model of repeated activation of the dural nociceptors is needed to reveal new mechanisms of trigeminal pain chronification and the factors important for the transition of episodic to chronic headache.
Electrophysiology and microdialysis studies in the trigeminal nucleus caudalis (TNC) demonstrate that an acute chemical stimulation of the rat dura, using a mixture of inflammatory mediators, is a nociceptive stimulus that both activates and sensitizes dural C and Aδ nociceptors for hours.13,14 There are no published reports of the effects of repeated chemical dural stimulation on rat behavior and physiology.
The nitric oxide (NO) donor, glyceryl trinitrate (GTN), induces migraine-like headaches in patients with migraine.15 In healthy subjects, only a short-lasting headache is induced that resolves after the first hour.16,17 In migraine patients, the NO-induced headache lasts >5 hours and can even be associated with premonitory symptoms.18 Because very few healthy subjects experience migraine-like headache following GTN injection, it has been suggested that GTN can be used to diagnose migraine.18,19 Hypersensitivity to exogenous NO experienced by patients with recurrent headaches suggests that repeated episodes of headache pain sensitizes the brain to NO and vasodilation of the dural blood vessels.20,21 Although there is little experimental evidence, it is believed that increased neuronal excitability in the brain and periphery, due to prolonged or repeated nociceptive input, is important in the pathophysiology of chronic pain and recurrent headache.22,23
Studies of GTN’s effects in acute, one-time trigeminal pain models in rats show that it has little or no effect. One study showed that GTN did not induce c-FOS expression or cause the release of calcitonin gene-related peptide (CGRP) in an intact animal, but GTN facilitated the release of CGRP in response to capsaicin.24 Two studies showed that GTN potentiated sensory responses, but the effect of GTN alone was weak in one study and no different than control in another.25,26 A study using a hemisected skull showed that the NO donor NONOate caused an increase in CGRP release in a dose-dependent manner.27 These studies demonstrate that GTN facilitates sensory responses in rats, but the effect of GTN alone is minimal.28,29 We challenged rats that received many inflammatory stimulations of the dura with GTN and found that their response was much higher than rats that received only 3 stimulations. This demonstrates that many inflammatory stimulations of the dura are required to model the hyperresponsiveness of humans with recurrent headache to GTN.
The aim of this study was to demonstrate that repeated episodic activation of the trigeminovascular system leads to chronic trigeminal hypersensitivity that models the hypersensitivity of patients with recurrent headache. Our goal was to demonstrate, using sensory and physiological measures, that our model provides a system for the study of episodic and chronic headache disorders.
METHODS
Rats were housed individually in a temperature-controlled (21–22 °C) environment under a 12-hour light/dark cycle and given food and water ad libitum (total n = 28). All procedures on the animals were approved by the Thomas Jefferson University I.A.C.U.C. committee.
Implantation of the Cannula Used to Infuse Inflammatory Soup
Male Sprague Dawley rats (250–300 g, Harlan) were fitted with a cannula under isoflurane anesthesia (3% induction, 1.5% during surgery) mixed with compressed air. A ~3-mm wide craniotomy was performed above the junction of the superior sagittal and transverse sinuses, and a plastic cap with a stainless steel cannula (26 gauge, Plastics One Inc., Roanoke, VA, USA) was affixed to the bone around the opening in the skull using small screws and dental cement. The cannula’s end opened onto the dura over the transverse sinus along the midline. The cannula was sealed with an obturator that extends just beyond the end of the cannula over the dura. This prevented scar tissue from growing over the hole of the cannula, which would obstruct the flow of the inflammatory soup (IS) onto the dura. After surgery, rats recovered for at least 1 week before sensory testing. Trigeminal pressure thresholds were monitored during the recovery period to ensure that the thresholds returned back to their presurgery baseline.
Infusion of the Inflammatory Soup or Saline
Rats were placed in an 18-inch diameter lucite bowl that allows for free movement for the infusion of IS or saline to the dura. The IS contained 1-mM histamine, serotonin, bradykinin, and 0.1-mM prostaglandin E2 in phosphate-buffered saline (PBS) pH 7.4 (adapted from Strassman 1996).13 A polyethylene tube (PE50), connected to a microinfusion pump (WPI Inc., Sarasota, FL, USA), was attached to the top of the exposed cannula. The pump provided a steady infusion of 20 μL of IS or saline over 5 minutes while the rat was freely moving. The tube was left attached for at least 15 minutes after the infusion to allow for diffusion into the surrounding tissue of the dura. The obturators were returned to the cannula after the last sensory test of the day, ~5 hours after infusion. A visual inspection of the skin and dental cement seal around the cap was done to ensure that there was no irritation due to leakage of the IS soup outside of the dura onto skin of the animal.
Rats were infused with IS or saline 3 times per week to approximate the headache frequency of patients with chronic daily headache, ~13–14 stimulations in 30 days. The International Headache Society classifies chronic migraine or chronic tension-type headache as >15 days per month.30 There was an interstimulus interval of 48–72 hours in this study. Control rats received sham stimulation of 20-μL PBS, pH 7.4.
All cannula placements on top of the dura were verified post mortem. The cannula was flushed to ensure it was not clogged and a visual inspection was done on the dura to make sure it was properly placed over the junction of the superior sagittal and transverse sinuses.
Tactile Sensory Testing
Rats were tested during the daylight portion of their circadian cycle. Rats were placed in a plastic tube restraint (inner diameter 8 cm, length 25 cm; see Fig. 1). The restraint was atraumatic and the animals entered the device uncoaxed. The restraint prevented the rat from walking away from the sensory testing, but it was large enough for the animals to turn around in with some difficulty. Rats were acclimated to the testing apparatus through training periods before and after the cannula implantation surgery.
Fig. 1.
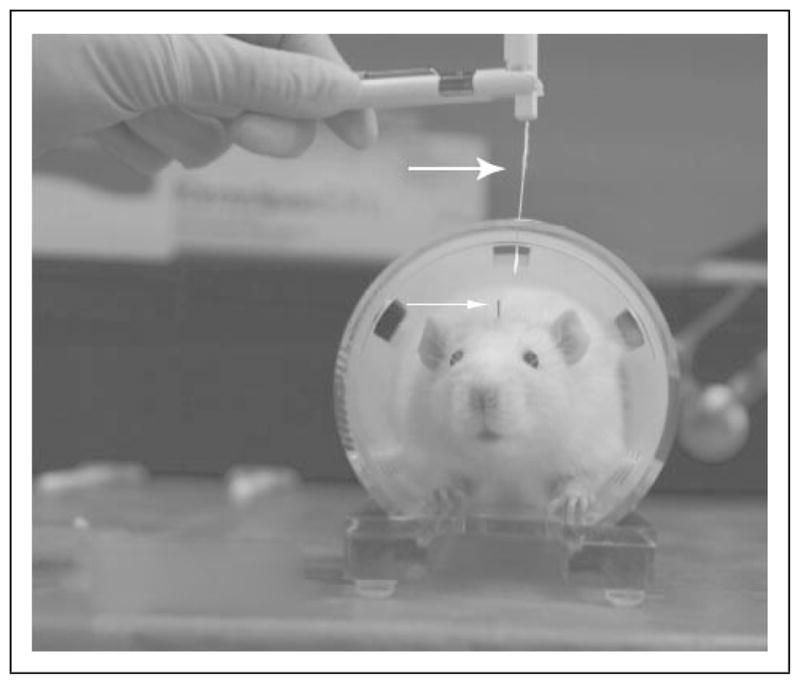
Picture of a rat fitted with a dura cannula for IS infusion. This picture shows a rat fitted with a cannula (see small arrow) for IS infusion in the plastic tube restraint used for sensory testing on the face. The picture shows the von Frey monofilament (see large arrow) used to test the von Frey pressure threshold on the face. Threshold was noted when rats quickly retracted their head away from the bending von Frey monofilament. The restraint is atraumatic and it is used only to reduce the movement of the animal during sensory testing.
Pressure thresholds were determined by applying the von Frey monofilaments (Stoelting Co., Wood Dale, IL, USA) to the periorbital region of the rat on the right and left side of the face over the rostral portion of the eye. The von Frey hairs are calibrated nylon monofilaments that generate a reproducible buckling stress and are identified by manufacturer-assigned force values (15, 10, 8, 6, 4, 2, 1.4, 1, 0.6, 0.4, 0.07 g). The higher the force value of the monofilament, the stiffer and more difficult it is to bend. The data for the right and left side are recorded separately for each timepoint. The von Frey stimuli were presented in a sequential ascending or descending order, as necessary, to determine the threshold of response, as described in Chapan et al. and Vos et al.31,32 A positive response led to the presentation of the next weaker stimulus, and lack of a positive response led to the presentation of the next stronger stimulus. Results were presented as the threshold in grams ± SD or as a percent change from baseline on the side that had the lowest value. A positive response for the von Frey test was considered to be present when the rat vigorously stroked its face with the ipsilateral forepaw, a quick recoil of the head away from the stimulus, or vocalization. Rats that did not respond to the 15-g stimulus were assigned 15 g as their threshold for analysis.
The 66% probability thresholds of positive response to von Frey stimuli were determined prior to infusion of IS or saline, and at 0.33, 1.5, and 2.5, and 5 hours thereafter. The 66% probability threshold is defined as a positive response to 2 of 3 trials of the von Frey monofilament.
Microdialysis in TNC
CMA11 microdialysis probes (CMA Microdialysis Inc., Solna, Sweden) were customized with an active dialyzing membrane length of 1 mm especially for microdialysis in small regions of the brain. The probe cuprophan membrane had a molecular weight cut off of 6000 Dalton and the outer diameter of the probe was 0.24 mm. The tip of the microdialysis probe was placed into the TNC: −2.6 mm to −2.9 mm from obex; 1.7 mm to 1.9 mm lateral to the midline. Probes were inserted 2–3 hours before the microdialysis study, vitreous silica tubing (1.2 μL/100mm) connected the probe to 2.5-mL glass syringes mounted on a CMA/100 Microinjection Pump (CMA Microdialysis AB, North Chelmsford, MA, USA). The dialysis system was perfused at 2.0 μL/min with sterilized, pyrogen-free artificial extracellular fluid (aECF; composition in mM/L: NaCl, 135; KCl, 3; MgCl2, 1.0; CaCl2, 1.2; ascorbate, 0.2 and 2 mM sodium mono- and dibasic phosphate to pH 7.4; Sigma, St. Louis, MO). The collection period was every 10 minutes. Total collection time was ~5 hours, which included at least 3 hours after the injection of GTN.
Location of the Microdialysis Probes
At the end of experiments, the microdialysis probes were removed and stored in distilled water between experiments. The animals were sacrificed with euthasol and perfused intracardially with 0.01-M phosphate-saline buffer followed by 4% paraformaldehyde. The brain was removed and cut into 20-μm sections using a cryostat (Leica CM 3000, Leica Inc., Wetzlar, Germany). Cresyl violet Nissl staining was performed and the position of the microdialysis probes or the recording electrode was noted. All animals presenting improperly positioned microdialysis probes were eliminated from our study.
HPLC Measurement of Amino Acids
The amino acids content of each sample (specifically glutamate, glutamine and GABA, and aspartate) was analyzed by using a binary gradient high-performance liquid chromatography (HPLC) device (Shimadzu, Columbia, MD) with fluorescence detection and precolumn derivatization O-phthal aldehyde (OPA; Pierce, Rockford, IL). In this article, we report the changes in extracellular glutamate. The measurements of the other amino acids act as a control for the changes in extracellular glutamate.33 A sample to reagent ratio of 1:2 (v/v) was used (10-μL dialysate sample + 20-μL OPA). After a 60-second reaction, 20-μL of each sample was auto-injected into the column (100 × 4.6 mm, 3 μm, 120 Å, Keystone, Bellefonte, PA, USA). The mobile phases used for separation were A: 0.03M sodium acetate, 1.0% tetrahydrofuran solution (pH 6.88), and B: 0.02M sodium acetate, 80.0% acetonitrile solution (pH 6.82). The flow rate was 0.6 mL/min. The column temperature was 35°C. The concentrations of neurotransmitters were determined using the Shimadzu Class VP5 software (Shimadza Scientific Instruments, Columbia, MD, USA).
Injection of Glyceryl Trinitrate
To test the effects of GTN on pressure thresholds on the face of the rat, GTN (0.1 mg/kg, ip) was injected following baseline sensory tests, and periorbital thresholds were monitored at 0.33, 1.5, 2.5, and 5 hours after the injection.
During the microdialysis experiments, GTN (0.1 mg/kg) was injected into the tail vein of the rat, at a rate of 0.02 mg/min for 5 minutes, after 1 hour of baseline measurements were obtained that confirmed stable extracellular amino acid levels. Samples were collected for at least 4 hour after the injection.
Statistical Analysis
All thresholds, duration values, and amino acid concentrations were collected in Microsoft Excel and tested in SPSS 14.0 (SPSS Inc., Chicago, IL, USA) using a general linear model ANOVA for significance.
RESULTS
Figure 1 illustrates the rat fitted with a cannula for IS infusion (small arrow head) in the plastic tube restraint used during pressure threshold testing on the face. The von Frey monofilament (large arrow head) was used to test the pressure threshold in the periorbital region of the rat above the rostral part of the eye. Threshold was noted when rats quickly retracted their head away from the bending von Frey monofilament or performed a long brush of its face with the ipsilateral paw.
Infusion of the IS onto the dura did not initiate long periods of spontaneous behaviors, such as rigorous scratching, grooming, or escape behaviors. In order to quantify the effects of IS infusion on the dura, we used evoked nociceptive-like behaviors, such as paw swipe, head withdrawal, or vocalization in response to von Frey monofilaments.
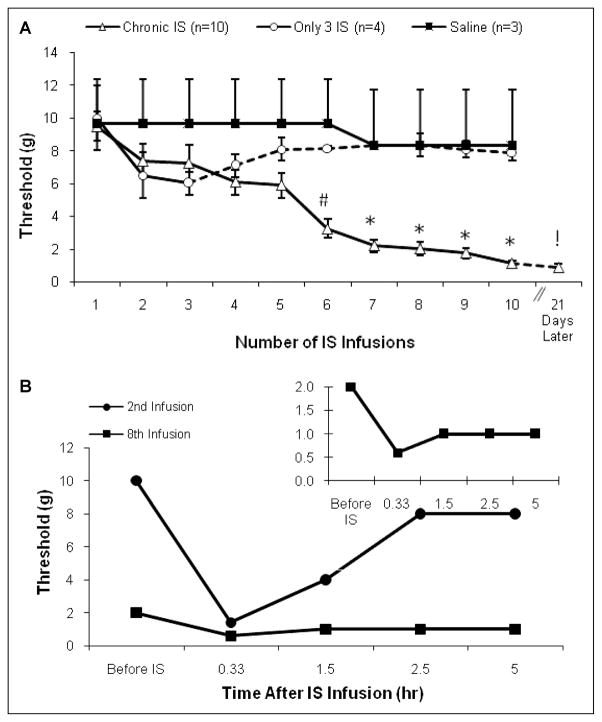
To demonstrate that chronic IS infusion decreases the baseline periorbital pressure thresholds, we plotted the von Frey threshold before the animals received IS or saline infusion over 3–4 weeks (Fig. 2A). IS infusions were done 3 times per week on Monday, Wednesday, and Friday, therefore there were 48–72 hours of no stimulation between each IS infusion.
Fig. 2.
(A) Basal threshold before IS infusion. The mean ± SD baseline von Frey periorbital pressure thresholds in 3 groups of rats. The testing was done before the animal received the inflammatory soup or saline infusion for that day. The dotted line shows the results from the continued monitoring of the rats without dural IS stimulation. The significant decrease in the thresholds noted in the more than 8 IS infusion group is due to the successive stimulation with IS. (# P <.05, *P <.01 ANOVA). (B) Von Frey threshold change in response to IS infusion: The typical response to IS infusion in one rat on 2 different infusion days. The graph shows the periorbital von Frey thresholds for a rat during the second and the eighth times the rat received IS stimulation, which corresponds to the 7th and 24th day after implantation of the cannula. Thresholds from the eighth infusion are plotted in the inset with a different scale.
The periorbital von Frey thresholds decreased significantly in the chronic IS group that received at least 8 IS infusions following repeated IS infusions. During the first week (infusion days 1–3) there was a small decrease in threshold, but this was not significantly different between the 2 IS groups and the saline group (ANOVA, P > 0.05). After the fifth infusion, the chronic IS infusion rats began transition from normal pressure thresholds to an hypersensitive state where the thresholds for eliciting paw swiping and head withdrawal behaviors were significantly lower (<2 g; Fig. 2A). In many of the rats, the thresholds decreased rapidly between 4 and 5 infusions to below 2 g and did not drop gradually over many infusions. The last point in Fig. 2A shows that the threshold did not recover even 21 days following the last IS infusion. Only in the chronic IS group were infusion days 1–3 significantly higher than days 6–10 (ANOVA, #P < .05, *P < .01).
In order to demonstrate that the chronic, low trigeminal thresholds in the rats were due to repeated stimulation of the dura and not due to a delayed action of the first few IS infusions, we included a group of rats that received only 3 IS infusions over 1 week. In this group, we continued tracking thresholds in parallel with the long-term infusion group with no further infusions (Fig. 2A, dotted line).
The infusion of IS on the dura decreased periorbital sensory thresholds for weeks following surgery. Figure 2B shows the response to IS infusion in one rat on 2 different infusion days measured with von Frey calibrated monofilaments. The threshold pressure that elicit nociceptive-like behaviors was measured before and at 20 minutes, 1.5, 2.5, and 5 hours after IS infusion. The second infusion showed a significant recovery after 1.5 hours. Although prior to the eighth IS infusion the baseline threshold was much lower than before the second infusion, the rats still responded quickly to IS on both days. Following the eighth infusion, the induced decrease in threshold persisted for >5 hours (Fig. 2B, inset).
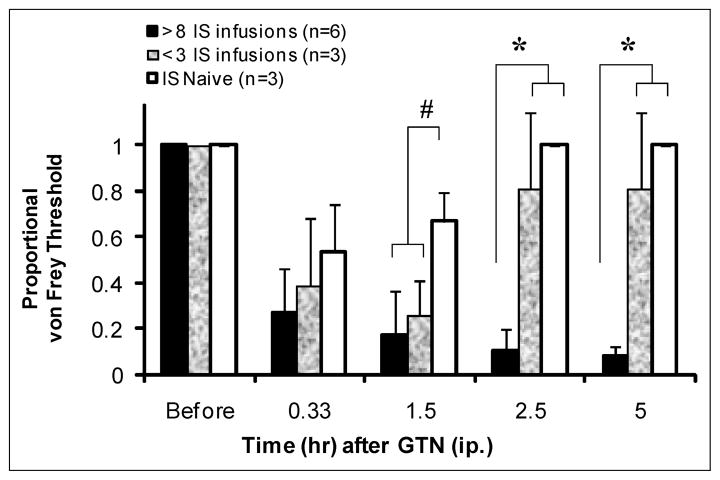
Repeated episodic activation of the dura with IS hypersensitized the trigeminal neurovascular system and potentiated the decrease in periorbital von Frey thresholds following GTN injection (Fig. 3). Rats were tested with GTN 12–31 days after their last IS infusion. GTN (0.1 mg/kg, ip) elicited a decrease in von Frey thresholds in IS-naive rats or rats that had received 3 IS infusions for a shorter duration compared to rats with a history of more than 8 IS infusions. Because the baseline thresholds were lower in the rats that received more than 8 chronic infusions of IS, we normalized the data by calculating the proportional change in threshold using the baseline thresholds as a reference. All of the rats reacted to the GTN injection with a decrease in periorbital thresholds. In the IS-naive rats and rats that received 3 IS infusions, the threshold decreased for ~1.5 hours. In rats that had a history of more than 8 IS infusions, the von Frey threshold decreased for >5 hours. There was a significant difference between the IS-naive rats and 3 IS rats compared to those with a history of more than 8 IS infusions at 2.5 hours and 5 hours (*P < .01, one-way ANOVA).
Fig. 3.
Glyceryl trinitrate (GTN) von Frey response. The effects of GTN (0.1 mg/kg, ip) on the periorbital von Frey thresholds (mean ± SD) in 3 groups of rats: naive rats, rats that had received 3 IS infusions, and rats with a history of >10 IS infusions. Rats were tested 12–31 days after their last IS infusion. All of the rats reacted to the GTN injection with a decrease in periorbital thresholds. In the naive rats and rats with 3 IS infusions, the threshold decreased for ~1.5 hours and then recovered. In the rats with more than 8 IS infusions, the duration of the von Frey threshold decrease was >5 hours. There was a significant difference between the IS-naive rats and rats with few IS infusions vs those with a history more than 8 IS infusions at 2.5- and 5-hour time points (1.5 hour #P = .055, 2.5 and 5 hour P < .01, one-way ANOVA).
In vivo microdialysis in the deep lamina of the TNC showed that the response to GTN (0.1 mg/kg, iv) is potentiated in rats following more than 8 IS infusions (Fig. 4). Rats were tested 12–31 days after the last IS infusion. Extracellular glutamate levels increased in all 3 groups of rats following GTN (groups = 1: naive rats that had not received IS, 2: rats with 3 IS infusions, and 3: rats with a history of more than 8 IS infusions). The extracellular glutamate levels in the IS-naive rats increased from 0.0812 ± 0.0019 μM to 0.1585 ± 0.0130 μM, which corresponds to a peak increase of 1.7 from the basal level. In rats that received 3 IS infusions the extracellular glutamate level increased from 0.0940 ± 0.0033 μM to 0.1638 ± 0.0073 μM, which is 1.9 times the basal level. There was no statistical difference in the peak levels of these 2 groups (P > .05). In rats with more than 8 IS infusions, there was a significantly greater increase in glutamate after 1.5 hours compared to naive rats or rats with 3 IS infusions. The extracellular glutamate increased from a mean of 0.0542 ± 0.0034 μM to 0.4178 ± 0.0892 μM, which is 7.7 times the basal level in rats that received more than 8 IS infusions. This is a very large increase and it matches the timing of the change in periorbital pressure thresholds (Fig. 3). This difference was maintained for at least 5 hours after the GTN injection (#P = .055, *P < .01, univariate ANOVA).
Fig. 4.
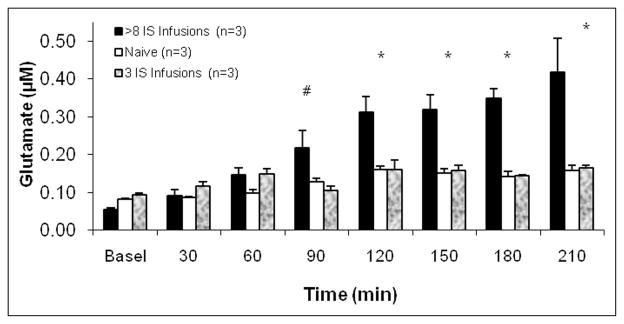
GTN effects on the extracellular glutamate in the TNC. The effects of GTN (0.1 mg/kg, iv) on the extracellular glutamate levels in (i) naive rats that had not received IS, (ii) rats with a history of more than 8 IS infusions, and (iii) rats with 3 IS infusions. Glutamate levels increased in all of the rats following GTN. In the rats with more than 8 IS infusions, there was a significantly greater increase in glutamate after 1.5 hours compared to rats with 3 IS infusions. This difference was maintained for the duration of the experiment (# P = .074, * P < .01, univariate ANOVA).
COMMENTS
Episodic activation of dural nociceptors in patients with recurrent headache can create long-lasting changes in the periphery and brain that increases their pain perception.34 A recent study of patients with transformed migraine (chronic migraine) found that recurrent headache patients with frequent headache exhibit brush allodynia on days when they do not have a headache.35
Using repeated dural IS infusions in rats, we modeled the repeated episodic activation of the trigeminovascular system seen in patients with recurrent migraine headache. Rats that received more than 8 IS infusions developed chronic periorbital tenderness with low pressure thresholds for eliciting pain-like behaviors below 2 g. In comparison, rats that were IS infusion-naive and rats that received 3 IS infusions had periorbital pressure thresholds between 8 g and 15 g. Because the recovery of the baseline thresholds of rats that receive 3 IS infusions begins soon after the last IS infusion, the decrease measured in rats with more than 8 IS infusions was not the result of a delayed effect of the earlier IS infusions (Fig. 2B). The transition from normal interstimulus periorbital thresholds to very low thresholds (<2 g) was dependent on repeated dural stimulation and began in most animals after the fifth IS infusion. This stable low threshold state of the rats was maintained even 21 days following the last IS infusion (Fig. 2A).
Recurrent Headache and Repeated Activation of Dural Nociceptors
Most animal studies of trigeminal neurovascular pain involve animals that have had no history of trigeminal pain and use a single nociceptive event to model the physiological response of the trigeminal system to recurrent headache.13,33,36–39 An important difference between these models of trigeminal neurovascular pain and the clinical presentation of headache is that patients with recurrent headache have histories of repeated episodic trigeminal nociceptive activation.
Patients with transformed migraine often have a history of episodic migraine that began in their teens or twenties.40,41 The process of transformation is characterized by headaches that become more frequent over months to years.42,43 It is not practical to test the effects of acute migraine medications on patients before they transform compared to afterward. In addition, a primary reason for treating an episodic migraineur with a preventive medication is to prevent the episodic headaches from worsening and transforming into a daily headache.44 These assertions and rationales for preventive treatment are essentially untested, because it is impractical (or nearly impossible) to perform a large clinical study that would track patients from early adolescence, when migraine often begins, to middle age.45,46 Another common assertion, which is untested, is that repeated migraines decrease the effectiveness of acute medication.47 The lack of a model system to test questions of the progressive nature of migraine was the motivation for the development of our chronic model.
Glyceryl Trinitrate Response
The physiological response to NO donors is different in human subjects with and without recurrent migraine headache. In subjects with recurrent migraine headache, NO donors induce long-lasting severe headaches. In contrast, NO donors induce only a short duration mild headache in subjects without migraine headache. GTN, an injectable NO donor, caused a stronger and longer-lasting neurochemical and behavioral responses in rats with many IS infusions.
NO donors facilitate sensory responses in rats.24,27 Previous studies of GTN’s effects on nociception showed minimal or undetectable responses.25,26 Our data are consistent with these studies for the IS-naive rats and those that received 3 IS infusions. In contrast, rats that received more than 8 IS infusions showed a very large increase in extracellular glutamate and very long-lasting decreases in periorbital pressure thresholds in response to GTN compared with IS-naive rats or rats with few IS infusions (Fig. 4). Because the glutamate response of the TNC to GTN was tested 12–31 days after the last IS infusion, these data demonstrate that hypersensitivity to GTN injection is maintained long after the last IS infusion. Rats that are hyperresponsive to GTN following repeated IS infusions, may serve as a model for recurrent headache patients’ hypersensitivity to GTN.18,48,49
Although there is a strong link between glutamate and migraine, animal models of trigeminal pain have only shown small changes in glutamate following nociceptive stimulation.50 In an acute model of dural inflammation, Oshinsky and Luo (2006) found that IS produces a 2.5-fold increase in extracellular glutamate in IS-naive rats.33 This is very close to the peak glutamate levels of 1.7 and 1.9 in the IS-naive and 3-IS-infusion groups in this study (Fig. 4). In contrast, rats that received more than 8 IS infusions had a 7.7 times increase in extracellular glutamate. This huge increase points to a significant role of a history of repeated dural nociceptor activation in the physiological response to further stimulation. Future microdialysis studies in the TNC and other areas of the brain in rats following repeated dural stimulation can reveal the neurochemical basis of hypersensitivity in the neurovascular system. This would lead to a better understanding of the pathophysiology of recurrent headache and could lead to new targets for treatment of chronic headache.
The different responses to GTN noted in rats with no or few previous IS stimulations compared with rats that received more than 8 IS stimulations parallels the different responses to NO donors (such as GTN) seen in migraine sufferers compared with healthy individuals.15,51,52 We believe that these data support using this model in future studies to characterize the pathophysiology of changes that occur in the brain following repeated trigeminovascular nociceptor activation.
Trigeminal Pain Behaviors
To date, there are no published reports of repeated inflammatory dural stimulation in rats. In a behavior study of rats following a single stimulation of the dural with an IS, Malick et al showed that the rats ate ~30% less food during the 4 hours after stimulation.36 After this 4-hour period, the rats recovered and actually ate more than the control animals. The results of our study confirm that in IS-naive rats, there is a quick recovery following dural stimulation.
In another migraine model, Kemper et al injected capsaicin into the cisterna magna in awake behaving rats. These rats were sacrificed within 2 hours due to the severe spontaneous behavioral expressions of pain. Chronic constriction injury of the infraorbital nerve also induces many spontaneous pain behaviors.32,53,54
In our model, IS infusions did not noticeably alter face grooming patterns or vigorous scratching in our model. For this reason, we used the evoked paw swipe, head withdrawal, or vocalization in response to von Frey monofilaments to monitor changes in trigeminal thresholds. These evoked behavioral withdrawal behaviors were so strong that they appeared as an expression of mechanical allodynia in the rats.
Because IS infusion on a small area of the dura is a weak stimulus, we were able to repeat the infusions over days and weeks without significant distress to the animal. The animals in this study maintained a groomed, healthy appearance in addition to steady increases in body weight throughout the study.
CONCLUSION
Repeated inflammation of the dura provides a novel system to study the effects of episodic painful stimuli on plasticity in the brain. Our study shows that this model reproduces the transition of episodic to chronic trigeminal pain seen in most patients with chronic daily headache. This model can be used to find new targets for treatment and test potential acute and preventive migraine medications.
Acknowledgments
This study was funded by a C.U.R.E. grant from the PA Department of Health and Pfizer Inc. The authors would like to thank Jia Luo and Menka Sangvhi for help with the experiments and Stephen Silberstein, James Schwaber, Greg Gonye, and Lynn Kaiser for reviewing the manuscript.
Abbreviations
- GTN
glyceryl trinitrate
- NO
nitric oxide
- IS
inflammatory soup
- TNC
trigeminal nucleus caudalis
- CGRP
calcitonin gene-related peptide
Footnotes
Conflict of Interest: None
References
- 1.Reuter U, Bolay H, Jansen-Olesen I, et al. Delayed inflammation in rat meninges: Implications for migraine pathophysiology. Brain. 2001;124:2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007;13:39–44. doi: 10.1016/j.molmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Burstein R, Jakubowski M. Unitary hypothesis for multiple triggers of the pain and strain of migraine. J Comp Neurol. 2005;493:9–14. doi: 10.1002/cne.20688. [DOI] [PubMed] [Google Scholar]
- 4.Burstein R, Jakubowski M, Levy D. Anti-migraine action of triptans is preceded by transient aggravation of headache caused by activation of meningeal nociceptors. Pain. 2005;115:21–28. doi: 10.1016/j.pain.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 6.Reuter U, Bolay H, Jansen-Olesen I, et al. Delayed inflammation in rat meninges: Implications for migraine pathophysiology. Brain. 2001;124:2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- 7.Giamberardino MA. Referred muscle pain/ hyperalgesia and central sensitisation. J Rehabil Med. 2003;41(Suppl):85–88. doi: 10.1080/16501960310010205. [DOI] [PubMed] [Google Scholar]
- 8.Bolay H, Moskowitz MA. Mechanisms of pain modulation in chronic syndromes. Neurology. 2002;59(5 Suppl 2):S2–S7. doi: 10.1212/wnl.59.5_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 9.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 10.Piovesan E, Young B, Werneck L, Kowacs P, Oshinsky M, Silberstein S. Recurrent extratrigeminal stabbing and burning sensation with allodynia in a migraine patient. Cephalalgia. 2003;23:231–234. doi: 10.1046/j.1468-2982.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- 11.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 12.Dubner R. Plasticity in central nociceptive pathways. In: Merskey H, Loeser JD, Dubey R, editors. The Paths of Pain 1975–2005. Seattle, WA: IASP Press; 2005. pp. 101–115. [Google Scholar]
- 13.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 14.Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- 15.Ashina M, Simonsen H, Bendtsen L, Jensen R, Olesen J. Glyceryl trinitrate may trigger endogenous nitric oxide production in patients with chronic tension-type headache. Cephalalgia. 2004;24:967–972. doi: 10.1111/j.1468-2982.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 16.Olesen J, Thomsen LL, Iversen H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol Sci. 1994;15:149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 17.Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol. 1994;1:73–80. doi: 10.1111/j.1468-1331.1994.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 18.Sances G, Tassorelli C, Pucci E, Ghiotto N, Sandrini G, Nappi G. Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia. 2004;24:110–119. doi: 10.1111/j.1468-2982.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 19.Dalsgaard-Nielsen T. Migraine diagnostics with special reference to pharmacological tests. Int Arch Allergy Immunol. 1955;7:312–322. doi: 10.1159/000228235. [DOI] [PubMed] [Google Scholar]
- 20.Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity: A possible molecular mechanism of migraine pain. NeuroReport. 1993;4:1027–1030. doi: 10.1097/00001756-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–918. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 22.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 23.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 24.Offenhauser N, Zinck T, Hoffmann J, et al. CGRP release and c-fos expression within trigeminal nucleus caudalis of the rat following glyceryltrinitrate infusion. Cephalalgia. 2005;25:225–236. doi: 10.1111/j.1468-2982.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 25.Costa A, Smeraldi A, Tassorelli C, Greco R, Nappi G. Effects of acute and chronic restraint stress on nitroglycerin-induced hyperalgesia in rats. Neurosci Lett. 2005;383:7–11. doi: 10.1016/j.neulet.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Jones MG, Lever I, Bingham S, Read S, McMahon SB, Parsons A. Nitric oxide potentiates response of trigeminal neurons to dural or facial stimulation in the rat. Cephalalgia. 2001;21:643–655. doi: 10.1046/j.1468-2982.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- 27.Strecker T, Dux M, Messlinger K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephalic promoting increases in meningeal blood flow. J Vasc Res. 2002;39:489–496. doi: 10.1159/000067206. [DOI] [PubMed] [Google Scholar]
- 28.Kitto KF, Haley JE, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett. 1992;148:1–5. doi: 10.1016/0304-3940(92)90790-e. [DOI] [PubMed] [Google Scholar]
- 29.Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci. 1998;18:7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramadan NM, Olesen J. Classification of headache disorders. Semin Neurol. 2006;26:157–162. doi: 10.1055/s-2006-939915. [DOI] [PubMed] [Google Scholar]
- 31.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 32.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oshinsky ML, Luo J. Neurochemistry of trigeminal activation in an animal model of migraine. Headache. 2006;46(Suppl 1):S39–S44. doi: 10.1111/j.1526-4610.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 34.Biondi DM. Is migraine a neuropathic pain syndrome? Curr Pain Headache Rep. 2006;10:167–178. doi: 10.1007/s11916-006-0042-y. [DOI] [PubMed] [Google Scholar]
- 35.Cooke L, Eliasziw M, Becker WJ. Cutaneous allodynia in transformed migraine patients. Headache. 2007;47:531–539. doi: 10.1111/j.1526-4610.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 36.Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001;98:9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergerot A, Holland PR, Akerman S, et al. Animal models of migraine: Looking at the component parts of a complex disorder. Eur J Neurosci. 2006;24:1517–1534. doi: 10.1111/j.1460-9568.2006.05036.x. [DOI] [PubMed] [Google Scholar]
- 38.Goadsby PJ, Classey JD. Evidence for serotonin (5-HT)1B, 5-HT1D and 5-HT 1F receptor inhibitory effects on trigeminal neurons with craniovascular input. Neuroscience. 2003;122:491–498. doi: 10.1016/s0306-4522(03)00570-0. [DOI] [PubMed] [Google Scholar]
- 39.Goadsby PJ, Duckworth JW. Low frequency stimulation of the locus coeruleus reduces regional cerebral blood flow in the spinalized cat. Brain Res. 1989;476:71–77. doi: 10.1016/0006-8993(89)91537-0. [DOI] [PubMed] [Google Scholar]
- 40.Bigal ME, Lipton RB. When migraine progresses: Transformed or chronic migraine. Exp Rev Neurother. 2006;6:297–306. doi: 10.1586/14737175.6.3.297. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65–72. doi: 10.1111/j.1526-4610.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 42.Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R. Frequency of headache is related to sensitization: A population study. Pain. 2006;123:19–27. doi: 10.1016/j.pain.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 43.Kropp P, Linstedt U, Gerber W. Duration of migraine disease correlates with amplitude and habituation of event-related potentials. Schmerz. 2005;19:489–496. doi: 10.1007/s00482-005-0386-y. [DOI] [PubMed] [Google Scholar]
- 44.Loder E, Biondi D. General principles of migraine management: The changing role of prevention. Headache. 2005;45(Suppl 1):S33–S47. doi: 10.1111/j.1526-4610.2005.4501002.x. [DOI] [PubMed] [Google Scholar]
- 45.Katsarava Z, Schneeweiss S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788–790. doi: 10.1212/01.wnl.0000113747.18760.d2. [DOI] [PubMed] [Google Scholar]
- 46.Limmroth V, Biondi D, Pfeil J, Schwalen S. Topiramate in patients with episodic migraine: Reducing the risk for chronic forms of headache. Headache. 2007;47:13–21. doi: 10.1111/j.1526-4610.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 47.Silberstein SD, Saper JR, Freitag FG. Migraine: Diagnosis and treatment. In: Silberstein S, Lipton RB, Dalessio, editors. Wolff’s Headache and Other Head Pain. New York: Oxford University Press; 2001. pp. 121–237. [Google Scholar]
- 48.Pardutz A, Szatmári E, Vecsei L, Schoenen J. Nitroglycerin-induced nNOS increase in rat trigeminal nucleus caudalis is inhibited by systemic administration of lysine acetylsalicylate but not of sumatriptan. Cephalalgia. 2004;24:439–445. doi: 10.1111/j.1468-2982.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- 49.Van Der Kuy P-M, Lohman JJHM. The role of nitric oxide in vascular headache. Pharm World Sci. 2003;25:146–151. doi: 10.1023/a:1024800512790. [DOI] [PubMed] [Google Scholar]
- 50.Ramadan NM. The link between glutamate and migraine. CNS Spectr. 2003;8:446–449. doi: 10.1017/s1092852900018757. [DOI] [PubMed] [Google Scholar]
- 51.Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19:660–667. doi: 10.1046/j.1468-2982.1999.019007660.x. [DOI] [PubMed] [Google Scholar]
- 52.Vincent V. GTN in migraine. Cephalalgia. 1999;19:626. [Google Scholar]
- 53.Kemper RHA, Meijler WJ, Ter Horst GJ. Trigeminovascular stimulation in conscious rats. NeuroReport. 1997;8:1123–1126. doi: 10.1097/00001756-199703240-00012. [DOI] [PubMed] [Google Scholar]
- 54.Ter Horst GJ, Meijler WJ, Korf J, Kemper RHA. Trigeminal nociception-induced cerebral fos expression in the conscious rat. Cephalalgia. 2001;21:963–975. doi: 10.1046/j.1468-2982.2001.00285.x. [DOI] [PubMed] [Google Scholar]