Abstract
Background
Opioid dependence is a major risk factor for HIV infection, however, the impact of buprenorphine/naloxone treatment on HIV risk behaviors among HIV-infected opioid-dependent patients is unknown.
Methods
We conducted a longitudinal analysis of 303 HIV-infected opioid-dependent patients initiating buprenorphine/naloxone treatment. Outcomes included self-reported past 90-day needle-sharing and non-condom use. We assessed trends over the 12 months using the Cochran-Armitage trend test. Using Generalized Estimating Equations, after multiple imputation, we determined factors independently associated with needle-sharing and non-condom use, including time-updated variables. We then conducted a mediation analysis to determine whether substance use explained the relationship between time since treatment initiation and needle-sharing.
Results
Needle-sharing decreased from baseline to the fourth quarter following initiation of buprenorphine/naloxone (9% vs. 3%, p<0.001), while non-condom use did not (23% vs. 21%, p=0.10). HIV risk behaviors did not vary based on the presence of a detectable HIV-1 RNA viral load. Patients who were homeless and used heroin, cocaine/amphetamines or marijuana were more likely to report needle-sharing. Heroin use fully mediated the relationship between time since treatment initiation and needle-sharing. Women, patients who identified as being gay/lesbian/bisexual, those married or living with a partner and who reported heroin or alcohol use were more likely to report non-condom use. Older patients were less likely to report non-condom use.
Conclusions
While buprenorphine/naloxone is associated with decreased needle-sharing among HIV-infected opioid-dependent patients, sexual risk behaviors persist regardless of viral load. Targeted interventions to address HIV risk behaviors among HIV-infected opioid-dependent populations receiving buprenorphine/naloxone are needed.
Keywords: buprenorphine, HIV, opioid-related disorders, risk behaviors
1. INTRODUCTION
Opioid dependence continues to fuel HIV transmission, with injection drug use accounting for over 4 million cases of HIV worldwide (Center for Strategic and International Studies (CSIS) Task Force on HIV/AIDS, 2008; Mathers et al., 2008). HIV transmission can occur either through the sharing of drug-injecting equipment or sexual risk behaviors (Des Jarlais et al., 2007; Strathdee et al., 2010). Established interventions for HIV prevention for these populations and their partners include needle and syringe exchange programs; condoms; and expanded combination antiretroviral therapy for HIV-infected individuals (Marshall et al., 2010). In addition, there is growing support for the effectiveness of opioid agonist treatment (OAT), including methadone and buprenorphine, at decreasing HIV risk behaviors among uninfected patients (Sullivan et al., 2008; Gowing et al., 2011; MacArthur et al., 2012).
Available in the United States since 2002, buprenorphine is a partial mu-receptor agonist effective at treating opioid dependence (Mattick et al., 2008) and is included in the World Health Organization’s Model List of Essential Medicines (Center for Strategic and Internationa Studies (CSIS) Task Force on HIV/AIDS, 2008). Commonly administered in combination with naloxone, buprenorphine may be prescribed by providers who have obtained a special registration from the Drug Enforcement Agency and have appropriate linkages to clinical services (Sullivan et al., 2008). Recent data demonstrate the impact of integrated buprenorphine/naloxone and HIV treatment on improving HIV, drug treatment outcomes (Altice et al., 2011; Fiellin et al., 2011) and quality of life (Korthuis et al., 2011) for some individuals. Meanwhile, cross-sectional data reveal that ongoing HIV risk behaviors among HIV-infected, opioid-dependent patients occur frequently and may be associated with infection with resistant virus (Chaudhry et al., 2011; Tetrault et al., 2013). However, the impact of buprenorphine/naloxone on HIV risk behaviors over time among HIV-infected opioid-dependent patients is unknown.
Therefore, the purpose of the current study was to begin to address this gap, focusing on needle-sharing and non-condom use, over a one year period among HIV-infected opioid-dependent patients initiating buprenorphine/naloxone treatment. Given the increased likelihood of HIV transmission in the setting of a detectable HIV viral load, we also examined whether needle-sharing and non-condom use differed based on the presence of a detectable HIV viral load.
2. METHODS
2.1 Study Overview
The Buprenorphine-HIV Evaluation and Support (BHIVES) Project1 was funded by the HIV/AIDS Bureau of the Health Resources and Services Administration from 2004 to 2009 as a Special Project of National Significance, and the design and patient characteristics are described in detail elsewhere (Chaudhry et al., 2011; Weiss et al., 2011). Through the collaboration of multiple partners, BHIVES led to the creation and evaluation of the integrated provision of buprenorphine and HIV treatment services in 10 HIV primary care settings across the United States. The current analysis relies on data from nine sites as one site was unable to meet the integrated care requirement (Weiss et al., 2011). Among those contributing data to these analyses, six were located in academic medical centers; one in a public hospital; one in a community health center; and one in a community health center located within an academic medical system. Sites developed their own protocols for the delivery of these services, which included integrated HIV and buprenorphine/naloxone treatment, counseling, and linkage to supportive services along with a comparison arm, which varied across sites. This study was approved by the Institutional Review Boards at The New York Academy of Medicine and each of the demonstration sites. Patients were reimbursed for their participation with an in-kind incentive for completing assessments.
2.2 Participants
Participants were identified by providers and through self-referral based on both clinic-based and community-based recruitment efforts. Participants were: 18 years and older; HIV-infected; met Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; American Psychiatric Association, 2000) criteria for opioid dependence; were initiating buprenorphine/naloxone treatment at baseline; and willing and able to provide written informed consent in English or Spanish. Patients were excluded if they were pregnant; had transaminases five times the normal level or greater; met criteria for benzodiazepine abuse or dependence in the prior six months; met criteria for alcohol dependence; received high doses of methadone; were suicidal or severely psychiatrically impaired; or were inappropriate based on the provider’s clinical judgment (Weiss et al., 2011). These criteria were chosen to be consistent with existing guidelines for providers who were new to offering office-based buprenorphine (Center for Substance Abuse Treatment, 2004) and the sites were able to apply the exclusion criteria with some flexibility based on their expertise (Weiss et al., 2011). Our analytic sample was restricted to patients who received at least one dose of buprenorphine/naloxone at study initiation.
2.3 Data Sources
Data were collected through interview and medical chart abstraction at baseline and then quarterly for the period of 12 months after buprenorphine/naloxone initiation. Data sources included patient surveys and patients’ medical records.
2.4 HIV Risk Behaviors
To determine the impact of buprenorphine/naloxone on HIV risk behaviors, we assessed self-reports of needle-sharing behaviors and non-condom use at each interval. Specifically, patients were asked: When was the last time you shared needles or works with anyone?, and among those who reported being sexually active during the past 90 days before the baseline and quarterly follow-up interviews, When was the last time you had vaginal or anal sex without using a condom? We categorized the response options accordingly: within the past 90 days as recent; greater than 90 days ago and never as not recent; and unknown or refuse to answer as missing.
2.5 Covariates
Survey data were used to determine demographic information, education, employment, housing status, incarceration history, HIV disease history including likely mode of acquisition, antiretroviral use and adherence. The presence of a detectable viral load was defined as >400 copies/mL (Chaudhry et al., 2011). Substance use behaviors were assessed using the Addiction Severity Index-Lite (ASI-Lite) at baseline (lifetime and past 30 days) and each interval (past 30 days; McLellan, 1985). Time-updated variables for employment, housing status, incarceration, antiretroviral use, HIV viral load and substance use were included in the models. Data were abstracted from the medical chart to determine years since HIV diagnosis, CD4 count, HIV-1 RNA viral load, use of antiretroviral therapy, hepatitis B and C serologies, and presence of an AIDS-defining illness. To account for changes over time, time since treatment initiation (quarter) was included as a covariate (SAS Institute Inc., 2009).
2.6 Statistical Analysis
We performed descriptive statistics to characterize the demographic and clinical characteristics of patients receiving buprenorphine/naloxone at baseline. The proportion of patients with needle-sharing and non-condom use over time was determined and assessed for a trend using Cochran-Armitage trend test (Cochran, 1954; Armitage, 1955). We assessed bivariate associations between demographic and clinical characteristics and HIV risk behaviors and defined statistical significance based on a threshold of p<0.05. To further identify factors associated with each HIV risk behavior, we constructed multivariate Generalized Estimating Equations (GEE) models to account for within-individual correlation as a result of repeated measures from the same participants over time. Variables included in the multivariate models were those which yielded p<0.25 in bivariate models (Mickey et al., 1989; Demchuk et al., 1999; Bursac et al., 2008) and those thought to be clinically relevant. Potential collinearity was assessed by calculating the correlation between independent variables and covariates, and no pair had a Spearman’s correlation >0.40. All analyses were conducted using SAS version 9.3 (Cary, NC). We used multiple imputation with Markov chain Monte Carlo method to handle missing data (Rubin, 1996; McPherson et al., 2013). Twenty imputed datasets were generated using PROC MI and the outcome variables (needle sharing and non-condom use) were dichotomized using adaptive rounding (Bernaards, 2007). Results of the GEE analysis on each of the datasets, including 303 participants from five time points (1,515 observations each), were combined using PROC MIANALYZE. Given our hypothesis that changes in needle-sharing are due to changes in substance use, we then conducted a mediation analysis to assess whether changes in substance use mediated changes in needle-sharing over time (Baron et al., 1986; Vyavaharkar et al., 2010). To complete this, we assessed whether 1) time since treatment initiation was associated with substance use; 2) time since treatment initiation was associated with needle-sharing; and 3) the relationship between time since treatment initiation and needle-sharing was attenuated after adjusting for substance use.
3. RESULTS
3.1 Participant Characteristics
Three hundred and three participants received at least one dose of buprenorphine/naloxone and were included in the analytic sample (Table 1). Detailed demographic and clinical characteristics have been previously published (Chaudhry et al., 2011). The majority of patients were men, black, heterosexual, single, and had completed at least a high school education. At baseline, 74% were unemployed, 25% were homeless and 13% had been incarcerated in the past 30 days. At treatment entry, patients had a mean CD4 count of 355 cells/μL, 58% had a detectable HIV-1 RNA viral load and 61% were prescribed antiretroviral therapy. Heroin (70%) was the most commonly used substance followed by cocaine/amphetamines (59%) and alcohol (49%). In addition, marijuana/cannabis (29%), methadone/other opiate analgesics (24%), and barbiturates/sedatives/hypnotics/tranquilizers (9%) were also used by a significant proportion of the participants.
Table 1.
Patient Demographic and Clinical Characteristics, n=303, 1515 observations
| Characteristic | Buprenorphine/Naloxone Treatment
|
||||
|---|---|---|---|---|---|
| Baseline | Q1 | Q2 | Q3 | Q4 | |
|
| |||||
| Age at baseline (Mean, SD) | 45.2 (8.1) | ||||
|
| |||||
| Men, % | 67.7 | ||||
|
| |||||
| Sexual Orientation, % | |||||
| Heterosexual | 81.0 | ||||
| Gay/lesbian/bisexual | 19.0 | ||||
|
| |||||
| Race/Ethnicity, % | |||||
| White | 22.7 | ||||
| Black | 51.5 | ||||
| Latino | 22.4 | ||||
| Other races | 3.3 | ||||
|
| |||||
| Marital Status, % | |||||
| Married/living with partner | 15.8 | ||||
| Single/separated/divorced/widowed | 84.2 | ||||
|
| |||||
| Education, % | |||||
| Less than high school | 42.4 | ||||
| High school graduate/GED | 34.4 | ||||
| Some college/college graduate | 23.2 | ||||
|
| |||||
| Most recent CD4 at baseline (Mean, SD) | 355 (258) | ||||
|
| |||||
| Most recent viral load at baseline (Mean, SD) | 41,736 (194,991) | ||||
|
| |||||
| Hepatitis C antibody, % | |||||
| Negative | 19.0 | ||||
| Positive | 67.9 | ||||
| No document of test | 5.2 | ||||
| Unknown | 7.8 | ||||
|
| |||||
| Risk factor†, % | |||||
| Employment* | 25.7 | 23.8 | 26.2 | 28.7 | 26.3 |
| Homeless** | 25.1 | 24.3 | 23.7 | 19.3 | 21.4 |
| Incarceration in past 30 days | 12.9 | 13.7 | 13.1 | 13.3 | 12.8 |
| Prescribed ART** | 60.5 | 66.5 | 66.3 | 67.8 | 66.6 |
| Detectable viral load** | 58.0 | 45.2 | 47.5 | 45.1 | 46.3 |
|
| |||||
| Substance use within past 30 days†, % | |||||
| Heroin** | 70.3 | 38.4 | 35.5 | 35.7 | 35.7 |
| Methadone, Other opiate analgesics** | 23.7 | 10.4 | 8.0 | 10.1 | 9.0 |
| Alcohol** | 49.0 | 40.1 | 43.8 | 42.7 | 41.1 |
| Barbiturates, Sedatives, Hypnotics, Tranquilizers** | 9.3 | 3.7 | 2.9 | 4.1 | 3.0 |
| Cocaine, Amphetamines** | 59.1 | 43.9 | 45.4 | 45.3 | 43.1 |
| Marijuana* | 29.1 | 19.6 | 23.9 | 24.3 | 23.6 |
Note:
p-values are based on results from the Cochran-Armitage Trend Test
asterisk (*) indicates p<0.001;
indicates p<0.0001
3.2 Trends in Risk Factors over Time
The majority of patients remained unemployed over the one year period, with a substantial number experiencing homelessness and incarceration (Table 1). An increasing number of patients were prescribed antiretroviral therapy and had an undetectable viral load over the one year period. Substance use decreased across all substances over time.
3.3 Trends in HIV Risk Behaviors over Time
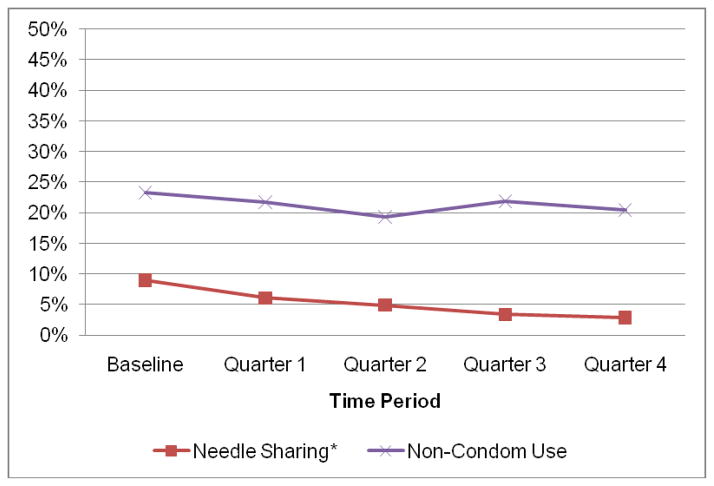
At baseline, 9% of patients reported needle-sharing behaviors, which decreased to 3% at quarter 4 (p<0.001; Figure 1). Among those who were sexually active, non-condom use decreased from 23% at baseline to 20% at quarter 4; however, this change was not significant (p=0.10).
Figure 1.
Prevalence of Risk Behaviors during the First Year after Buprenorphine/Naloxone Initiation, n=303, 1515 observations
Note: asterisk (*) indicates p<0.05.
3.4 Factors Associated with Needle-Sharing
In the bivariate analyses, homelessness and use of heroin, methadone/other opiates, barbiturates/sedatives/hypnotics/tranquilizers, cocaine/amphetamines, and marijuana were each associated with needle-sharing (Table 2). In contrast, time since treatment initiation was inversely associated with needle sharing. The presence of a detectable HIV-1 RNA viral load was not associated with needle-sharing.
Table 2.
Factors Associated with Needle-Sharing During the First Year after Buprenorphine/Naloxone Initiation, Multivariate Analysis (n=303 participants, 1515 observations)
| Characteristic | Unadjusted Odds Ratio, 95% CI | Adjusted Odds Ratio, 95% CI |
|---|---|---|
|
| ||
| Period of Observation (ref=baseline) | ||
| Quarter 1 | 0.66 (0.38, 1.14) * | 1.17 (0.62, 2.20) |
| Quarter 2 | 0.52 (0.26, 1.03) * | 0.91 (0.42,1.95) |
| Quarter 3 | 0.34 (0.14, 0.85) ** | 0.60 (0.24, 1.53) |
| Quarter 4 | 0.29 (0.11, 0.75) ** | 0.49 (0.18, 1.35) |
|
| ||
| Age (10 year increments) | 0.74 (0.50, 1.09) * | 0.95 (0.65, 1.39) |
|
| ||
| Women (ref=men) | 0.83 (0.43, 1.62) | |
|
| ||
| Sexual Orientation (ref=heterosexual) | ||
| Gay, Lesbian, Bisexual | 1.61 (0.79, 3.27) * | 0.93 (0.45, 1.94) |
|
| ||
| Race/Ethnicity (ref= white) | ||
| Black | 0.61 (0.27, 1.34) * | 0.64 (0.29, 1.44) |
| Latino | 0.93 (0.41, 2.13) | 1.07 (0.48, 2.39) |
| Other Race | 1.64 (0.41, 6.19) | 1.42 (0.39, 5.12) |
|
| ||
| Marital Status ref=single/separated/divorced/widowed) | ||
| Married/Live with Partner | 0.95 (0.43, 2.06) | |
|
| ||
| Education (ref=less than high school) | ||
| High School graduate/GED | 1.31 (0.66, 2.60) | |
| Some college/college graduate | 0.95 (0.40, 2.27) | |
|
| ||
| Employed (ref=unemployed) | 0.79 (0.41, 1.52) | |
|
| ||
| Homeless (ref=housed) | 2.26 (1.18, 4.32) ** | 2.05 (1.04, 4.06) ** |
|
| ||
| Incarcerated within Past 30 days (ref=no) | 1.69 (0.78, 3.65) * | 1.77 (0.81, 3.87) |
|
| ||
| Prescribed Antiretroviral Therapy (ref=no) | 0.62 (0.35, 1.10) * | 0.75 (0.41, 1.39) |
|
| ||
| Substance Use within Past 30 days (ref=no) | ||
| Heroin | 4.03 (2.14, 7.57) ** | 2.99 (1.54, 5.81) ** |
| Methadone, Other opiates | 2.17 (1.13, 4.16) ** | 1.42 (0.72, 2.80) |
| Alcohol | 1.40 (0.80, 2.44) * | 0.87 (0.48, 1.56) |
| Barbiturates, Sedatives, Hypnotics, Tranquilizers | 2.40 (1.08, 5.37) ** | 1.73 (0.73, 4.11) |
| Cocaine, Amphetamines | 3.37 (1.79, 6.36) ** | 2.02 (1.01, 4.03) ** |
| Marijuana | 3.34 (1.88, 5.95) ** | 2.49 (1.38, 4.48) ** |
| HIV Biomarkers (ref: undetectable viral load) | ||
| Detectable viral load | 1.01 (0.56, 1.83) | |
Notes: CI = 95% Confidence Interval;
asterisk (*) indicates p<0.25;
indicates p<0.05.
In the multivariate analysis, compared to those who reported having housing, patients experiencing homelessness were more likely to share needles (OR 2.05 [95% CI 1.04, 4.06]). In addition, heroin (OR 2.99 [95% CI 1.54, 5.81]), cocaine/amphetamine (OR 2.02 [95% CI 1.01, 4.03]), and marijuana use (OR 2.49 [95% CI 1.38, 4.48) were positively associated with needle sharing. Time since treatment initiation was not associated with needle-sharing practices.
Heroin use was a complete mediator in the relationship between time since treatment initiation and needle-sharing (data not shown). Alcohol use did not mediate the relationship at all. Each of the other substances were partial mediators as they attenuated the relationship between time since treatment initiation and needle-sharing.
3.5 Factors Associated with Non-Condom Use
In the bivariate analyses, women; those who identified as gay, lesbian or bisexual (vs. heterosexual); being married or living with a partner; and use of heroin, alcohol; and marijuana were associated with non-condom use (Table 3). Increasing age and being prescribed antiretroviral therapy was protective against non-condom use. Neither time since treatment initiation nor the presence of a detectable HIV-1 RNA viral load was associated with non-condom use.
Table 3.
Factors Associated with Non-Condom Use During the First Year after Buprenorphine/Naloxone Initiation, Multivariate Analysis (n=303 participants, 1515 observations)
| Characteristic | Unadjusted Odds Ratio, 95% CI | Adjusted Odds Ratio, 95% CI |
|---|---|---|
|
| ||
| Period of Observation (ref=baseline) | ||
| Quarter 1 | 0.92 (0.63, 1.34) | 1.16 (0.74, 1.81) |
| Quarter 2 | 0.79 (0.53, 1.18) * | 0.97 (0.61, 1.52) |
| Quarter 3 | 0.92 (0.61, 1.38) | 1.16 (0.73, 1.84) |
| Quarter 4 | 0.85 (0.57, 1.25) | 1.06 (0.68, 1.66) |
|
| ||
| Age (10 year increments) | 0.66 (0.54, 0.82) ** | 0.96 (0.94, 0.98) ** |
|
| ||
| Women (ref=men) | 1.78 (1.21, 2.62) ** | 1.71 (1.15, 2.55) ** |
|
| ||
| Sexual Orientation (ref=heterosexual) | ||
| Gay, Lesbian, Bisexual | 1.97 (1.23, 3.16) ** | 1.87 (1.15, 3.04) ** |
|
| ||
| Race/Ethnicity (ref= white) | ||
| Black | 1.24 (0.77, 1.97) | 1.63 (0.98, 2.72) |
| Latino | 1.48 (0.84, 2.59) * | 1.55 (0.86, 2.80) |
| Other Race | 0.61 (0.18, 2.10) | 0.47 (0.12, 1.86) |
|
| ||
| Marital Status (ref=single/separated/divorced/widowed) | ||
| Married/Live with Partner | 1.86 (1.11, 3.11) ** | 2.01 (1.17, 3.46) ** |
|
| ||
| Education (ref=less than high school) | ||
| High School graduate/GED | 0.73 (0.47, 1.12) * | 0.78 (0.50, 1.23) |
| Some college/college graduate | 0.65 (0.40, 1.05) * | 0.77 (0.47, 1.26) |
|
| ||
| Employed (ref=unemployed) | 1.19 (0.83, 1.70) | |
|
| ||
| Homeless (ref=housed) | 0.97 (0.65, 1.43) | |
|
| ||
| Incarcerated within Past 30 days (ref=no) | 1.24 (0.72, 2.12) | |
|
| ||
| Prescribed Antiretroviral Therapy (ref=no) | 0.67 (0.45, 0.98) ** | 0.81 (0.55, 1.21) |
|
| ||
| Substance Use within Past 30 days (ref=no) | ||
| Heroin | 1.89 (1.29, 2.77) ** | 1.77 (1.19, 2.64) **
|
| Methadone, Other opiates | 0.91 (0.57, 1.46) | |
| Alcohol | 1.83 (1.25, 2.67) ** | 1.67 (1.11, 2.51) **
|
| Barbiturates, Sedatives, Hypnotics, Tranquilizers | 1.21 (0.65, 2.25) | |
| Cocaine, Amphetamines | 1.33 (0.97, 1.84) * | 0.86 (0.60, 1.22) |
| Marijuana | 1.53 (1.03, 2.26) ** | 1.31 (0.86, 1.98) |
|
| ||
| HIV Biomarkers (ref: undetectable viral load) | ||
| Detectable viral load | 0.96 (0.68, 1.37) | |
Notes: CI = 95% Confidence Interval;
asterisk (*) indicates p<0.25;
indicates p<0.05.
In the multivariate analysis, women were more likely to report non-condom use than men (OR 1.71 [95% CI 1.15, 2.55]), as were patients who identified as being gay, lesbian or bisexual (vs. heterosexual) (OR 1.87 [95% CI 1.15, 3.04]); being married or living with a partner (vs. never married, separated, divorced or widowed) (OR 2.01 [95% CI 1.17, 3.46]). Heroin use (OR 1.77 [95% CI 1.19, 2.64]) and alcohol use (OR 1.67 [95% CI 1.11, 2.51]) were also positively associated with non-condom use. Older patients were less likely to report non-condom use (OR 0.96 [95% CI 0.94, 0.98]). Time since treatment initiation remained unassociated with non-condom use.
4. DISCUSSION
These data are the first to examine the potential longitudinal impact of office-based buprenorphine/naloxone treatment on HIV risk behaviors among HIV-infected opioid-dependent patients. This study offers several notable findings. First, buprenorphine/naloxone is associated with decreased heroin use leading to decreased needle-sharing over time; in contrast, non-condom use persisted and was reported by approximately one in five patients at each interval. Second, neither needle-sharing nor non-condom use practices varied based on the presence of a detectable HIV-1 RNA viral load. Third, we identified modifiable factors associated with these HIV risk behaviors, which differed for needle-sharing and non-condom use.
Our findings are consistent with prior work in HIV-uninfected populations (Sullivan et al., 2008). Specifically, two recent systematic reviews found that among opioid-dependent injection drug users, opioid agonist treatment was associated with decreased injection drug use, needle-sharing behavior and an average 54% reduction in the risk of new HIV infections (Gowing et al., 2011; MacArthur et al., 2012). On the other hand, opioid agonist treatment was not found to increase condom use though these studies only included patients receiving methadone (Gowing et al., 2011). Our results extend the existing evidence by providing data on HIV risk behaviors among HIV-infected patients receiving office-based buprenorphine/naloxone in the era of combination antiretroviral therapy.
Since HIV transmission risk increases in the presence of a detectable viral load, it is concerning that over half of our patients had a detectable viral load. This is likely explained by the fact that at baseline 40% of patients were not on antiretroviral therapy, consistent with previous observations that treatment is often deferred in patients with active substance use (Mathers et al., 2010; Westergaard et al., 2012). In addition, active substance use contributes to non-adherence (Braithwaite et al., 2005). The fact that patients with a detectable viral load had ongoing HIV risk behaviors including needle-sharing and non-condom use raises concern regarding HIV transmission risk. Our findings support results from the AIDS Linked to the IntraVenous Experience (ALIVE) study, which found that moderate or high baseline viral loads were associated with an increased risk of needle-sharing and any sex after antiretroviral treatment initiation, respectively (Fu et al., 2012). While HIV risk behaviors decreased overall in this cohort after antiretroviral treatment initiation, needle-sharing practices increased among those who continued to inject (Fu et al., 2012). As HIV risk behaviors often occur in the context of serodiscordant partnerships and can occur in the presence of antiretroviral resistance (Tetrault et al., 2013), developing strategies to promote and reinforce risk reduction continually as HIV-infected patients interface with the medical system is essential.
Homelessness and substance use with heroin, cocaine/amphetamines and marijuana emerged as important factors associated with needle-sharing. Patients who were homeless were almost twice as likely as those who were housed to report needle-sharing, consistent with prior work (Kidder et al., 2008). In addition to heroin use, stimulant and marijuana use were each associated with a two-fold increased odds of needle-sharing. Marijuana has been found to be independently associated with needle-sharing in prior studies (Bouhnik et al., 2004; Walley et al., 2008; Tyurina et al., 2013).
While older patients were less likely to report non-condom use (McHugh et al., 2012), females; those who identified as being gay, lesbian or bisexual; and married or living with a partner were more likely to report non-condom use. These findings may reflect factors contributing to differences by gender, such as the role of intimate partner violence, sexual risk reduction self-efficacy and partner-level factors (Somlai et al., 2003; Kapadia et al., 2007; Engstrom et al., 2011; Kapadia et al., 2011). Recent heroin and alcohol use were also associated with non-condom use in our study, which is consistent with existing literature (Metzger et al., 1993; Kalichman et al., 2002; Des Jarlais et al., 2007; Theall et al., 2007).
Our findings should be interpreted in the context of a few limitations. First, HIV risk behaviors were determined based on self-report. Second, we were unable to account for mental health variables, such as anxiety, which may be associated with our outcomes as we lacked longitudinal data on these variables (Derogatis et al., 1983). Third, due to limited longitudinal HIV-1 RNA viral load data, we only assessed this at baseline and cannot assess whether changes in viral load were associated with changes in HIV risk behaviors over time. Finally, the external validity of our study is unclear since systematic information was not collected by each site on the numbers or characteristics of patients who were evaluated for eligibility and/or deemed ineligible.
Despite these limitations, this study provides important data demonstrating ongoing HIV risk behaviors among an HIV-infected opioid-dependent population during their first year after buprenorphine/naloxone initiation. In particular, these data support the need for ongoing comprehensive approaches to HIV prevention (Crawford et al., 2010). Interventions grounded in the information-motivation-behavioral skills model of behavioral change which integrate education, motivational exercises and skills training (e.g. proper needle cleaning, correct use of latex products) regarding HIV transmission have been found to be efficacious in uninfected opioid-dependent populations (Calsyn et al., 2009, 2010; Copenhaver et al., 2013; Edelman et al., 2013). Future studies based on these principles should be tailored to the specific needs of HIV-infected, opioid-dependent patients.
Supplementary Material
Acknowledgments
Role of the Fu0nding Source: This work was generously supported by the Health Resources Services Agency and the Special Projects of National Significance (Grant Number H97HA03793) and the Robert Wood Johnson Foundation Clinical Scholars Program. Dr. L. Fiellin was a Robert Wood Johnson Physician Faculty Scholar during the conduct of this study. Dr. Edelman was funded as a Robert Wood Johnson Foundation- VA Clinical Scholar and DAHRS Scholar (1K12DA033312-01A1) during her involvement with this study. The funders had no further role in the study design, the collection, analysis and interpretation of data, in the writing, or the in the decision to submit the paper for publication.
The BHIVES Collaborative members are listed in Appendix 1. We would like to acknowledge Dr. Brent Moore and Dr. Tassos C. Kyriakides for their thoughtful input and assistance in revising this manuscript.
Footnotes
More information on the BHIVES Collaborative can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors: E.J. Edelman contributed to the proposal for the current analysis, the interpretation of data, and wrote the first draft of the manuscript. T. Chantarat conducted the analyses, participated in the interpretation of the data and the drafting of the manuscript. S. Caffrey contributed to the drafting of the first draft of the manuscript. A. Chaudhry contributed to data collection and drafting of the manuscript. P.G. O’Connor, L. Weiss, D.A. Fiellin and L.E. Fiellin contributed to the design and implementation of the BHIVES study, the interpretation and preparation of this manuscript. All authors contributed to have and have approved the manuscript.
Conflicts of Interest: Dr. D. Fiellin has received honoraria from Pinney Associates for serving on External Advisory Boards to review the diversion and abuse of buprenorphine. The authors have no additional conflicts of interest.
An earlier version of this work was presented as a poster presentation at the National Annual Society of General Internal Medicine Meeting. Orlando, FL, May 9th, 2012.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies or the US government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, Cunningham CO, Sullifan LE, Vergara-Rodriguea Pm, Fiellin DA, Cajina A, Botsko M, Nandi V, Gourevitch MN, Finkelstein R for the BHIVES Collaborative. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22–32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing; Arlington, VA: 2000. [Google Scholar]
- Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bernaards CA, Bellin TR, Schafer JL. Robustness of a multivariate normal approximation for imputation of incomplete binary data. Stat Med. 2007:261368–1382. doi: 10.1002/sim.2619. [DOI] [PubMed] [Google Scholar]
- Bouhnik AD, Carrieri MP, Rey D, Spire B, Gastaut JA, Gallais H, Obadia Y, MANIF Study Group, 2004. Drug injection cessation among HIV-infected injecting drug users. Addict Behav. 2000;29:1189–1197. doi: 10.1016/j.addbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Dayn N, Cook RL, Gordon a, Bridges MW, Seiler JF, Justice AC. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsyn DA, Crits-Christoph P, Hatch-Maillette MA, Doyle SR, Song YS, Coyer S, Pelta S. Reducing sex under the influence of drugs or alcohol for patients in substance abuse treatment. Addiction. 2010;105:100–108. doi: 10.1111/j.1360-0443.2009.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsyn DA, Hatch-Maillette M, Tross S, Doyle SR, Crits-Christoph P, Song YS, Harrer JM, Lalos G, Berns SB. Motivational and skills training HIV/sexually transmitted infection sexual risk reduction groups for men. J Subst Abuse Treat. 2009;37:138–150. doi: 10.1016/j.jsat.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Strategic and Internationa Studies (CSIS) Task Force on HIV/AIDS. Combating the Twin Epidemics of the HIV/AIDS and Drug Addiction: Opportunities for Progress and Gaps in Scale. CSIS; Washington, DC: 2008. [Google Scholar]
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Imprvement Protocol (TIP) Series 40. Susbstance Abuse and Mental Health Service Administration; Rockville, MD: 2004. [PubMed] [Google Scholar]
- Chaudhry AA, Botsko M. Participant characteristics and HIV risk behaviors among individuals entering integrated buprenorphine/naloxone and HIV care. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S14–21. doi: 10.1097/QAI.0b013e318209d3b9. [DOI] [PubMed] [Google Scholar]
- Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–451. [Google Scholar]
- Copenhaver MM, Lee IC, Baldwin P. A randomized controlled trial of the Community-friendly Health Recovery Program (CHRP) among high-risk drug users in treatment. AIDS Behav. 2013;17:2902–2913. doi: 10.1007/s10461-013-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford ND, Vlahov D. Progress in HIV reduction and prevention among injection and noninjection drug users. J Acquir Immune Defic Syndr. 2010;55(Suppl 2):S84–87. doi: 10.1097/QAI.0b013e3181fbca5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchuk AM, Morgenstern LB, Krieger DW, Chi TL, Hu W, Wein TH, Hardy RJ, Grotta JC, Buchan AM. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–39. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, Perlis T, Hagan H, Abdul-Quader A, Heckathorn DD, McKnight C, Bramson H, Nemeth C, Torian LV, Friedman SR. Convergence of HIV seroprevalence among injecting and non-injecting drug users in New York City. AIDS. 2007;21:231–235. doi: 10.1097/QAD.0b013e3280114a15. [DOI] [PubMed] [Google Scholar]
- Edelman EJ, Moore BA, Caffrey S, Sikkema KJ, Jones ES, Schottenfeld RS, Fiellin DA, Fiellin LE. HIV testing and sexual risk reduction counseling in office-based buprenorphine/naloxone treatment. J Addict Med. 2013;7:410–416. doi: 10.1097/ADM.0b013e3182a3b603. [DOI] [PubMed] [Google Scholar]
- Engstrom M, Shibusawa T, El-Bassel N, Gilbert L. Age and HIV sexual risk among women in methadone treatment. AIDS Behav. 2011;15:103–113. doi: 10.1007/s10461-009-9625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB, Chaudhry A, Cunningham CO, Gourevitch MN, Lum PJ, Sullivan LE, Schottenfeld RS, O’Connor PG, BHIVES Collaborative. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S33–38. doi: 10.1097/QAI.0b013e3182097537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu TC, Westergaard RP, Lau B, Celentano DD, Vlahov D, Mehta SH, Kirk GD. Changes in sexual and drug-related risk behavior following antiretroviral therapy initiation among HIV-infected injection drug users. AIDS. 2012;26:2383–2391. doi: 10.1097/QAD.0b013e32835ad438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Bomemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011:CD004145. doi: 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Weinhardt L, DiFonzo K, Austin J, Luke W. Sensation seeking and alcohol use as markers of sexual transmission risk behavior in HIV-positive men. Ann Behav Med. 2002;24:229–235. doi: 10.1207/S15324796ABM2403_08. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Latka MH, Hudson SM, Golub ET, Campbell JV, Bailey S, Frye V, Garfein RS DUIT STudy Team. Correlates of consistent condom use with main partners by partnership patterns among young adult male injection drug users from five US cities. Drug Alcohol Depend. 2007;91(Suppl 1):S56–63. doi: 10.1016/j.drugalcdep.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Latka MH, Wu Y, Strathdee SA, Mackesy-Amiti ME, Hudson SM, Thiede H, Garfein RS. Longitudinal determinants of consistent condom use by partner type among young injection drug users: the role of personal and partner characteristics. AIDS Behav. 2011;15:1309–1318. doi: 10.1007/s10461-009-9569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder DP, Wolitski RJ, Pals SL, Campsmith ML. Housing status and HIV risk behaviors among homeless and housed persons with HIV. J Acquir Immune Defic Syndr. 2008;49:451–455. doi: 10.1097/qai.0b013e31818a652c. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, Tozzi MJ, Nandi V, Fiellin DA, Weiss L, Egan JE, Botsko M, Acosta A, Gourevitch MN, Hersh D, Hsu J, Boverman J, Altice FL, BHIVES Collabortive. Improved quality of life for opioid-dependent patients receiving buprenorphine treatment in HIV clinics. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S39–45. doi: 10.1097/QAI.0b013e318209754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, Degenhardt L, Hickman M. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BD, Wood E. Toward a comprehensive approach to HIV prevention for people who use drugs. J Acquir Immune Defic Syndr. 2010;55(Suppl 1):S23–26. doi: 10.1097/QAI.0b013e3181f9c203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, Myers B, Ambekar A, Strathdee SA. Reference Group to the UN on HIV and Injecting Drug Use, 2010. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2009;375:1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP. Reference Group to the UN on HIV and Injecting Drug Use, 2008. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2007;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davioli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Weitzman M, Safren SA, Murray HW, Pollack MH, Otto MW. Sexual HIV risk behaviors in a treatment-refractory opioid-dependent sample. J Psychoactive Drugs. 2012;44:237–242. doi: 10.1080/02791072.2012.703507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New Data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McPherson S, Barbosa-Leiker C, McDonell M, Howell D, Roll J. Longitudinal missing data strategies for substance use clinical trials using generalized estimating equations: an example with a buprenorphine trial. Hum Psychopharmacol. 2013;28:506–515. doi: 10.1002/hup.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, O’Brien CP, Druley P, Navaline H, DePhilippis D, Stolley P, Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6:1049–1056. [PubMed] [Google Scholar]
- Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- SAS Institute Inc. Longitudinal Data Analysis with Discrete and Continuous Responses Course Note. SAS Institute Inc; Cary, NC: 2009. [Google Scholar]
- Somlai AM, Kelly JA, McAuliffe TL, Ksoblech K, Hackl KL. Predictors of HIV sexual risk behaviors in a community sample of injection drug-using men and women. AIDS Behav. 2003;7:383–393. doi: 10.1023/b:aibe.0000004730.62934.ed. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Stockman JK. Epidemiology of HIV among injecting and non-injecting drug users: current trends and implications for interventions. Curr HIV/AIDS Rep. 2010;7:99–106. doi: 10.1007/s11904-010-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA. Narrative review: buprenorphine for opioid-dependent patients in office practice. Ann Intern Med. 2008;148:662–670. doi: 10.7326/0003-4819-148-9-200805060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, Chawarski MC, Pantalon MV, Barry D, O’Connor PG, Schottenfield RS, Fiellin DA. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. J Subst Abuse Treat. 2008;35:87–92. doi: 10.1016/j.jsat.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetrault JM, Kozal MJ, Chiarella J, Sullivan LE, Dinh aT, Fiellin DA. Association between risk behaviors and antiretroviral resistance in HIV-infected patients receiving opioid agonist treatment. J Addict Med. 2013;7:102–107. doi: 10.1097/ADM.0b013e31827f9bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theall KP, Clark RA, Powell A, Smith H, Kissinger P. Alcohol consumption, ART usage and high-risk sex among women infected with HIV. AIDS Behav. 2007;11:205–215. doi: 10.1007/s10461-006-9159-6. [DOI] [PubMed] [Google Scholar]
- Tyurina A, Krupitsky E, Cheng DM, Coleman SM, Walley AY, Bridden C, Gnatienko N, Zvartau E, Raj A, Samet JH. Is cannabis use associated with HIV drug and sex risk behaviors among Russian HIV-infected risky drinkers? Drug Alcohol Depend. 2013;132:74–80. doi: 10.1016/j.drugalcdep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyavaharkar M, Moneyham L, Corwin S, Saunders R, Annang L, Tavakoli A. Relationships between stigma, social support, and depression in HIV-infected African American women living in the rural Southeastern United States. J Assoc Nurses AIDS Care. 2010;21:144–152. doi: 10.1016/j.jana.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, Krupitsky EM, Cheng DM, Raj A, Edwards EM, Bridden C, Egorova VY, Zvartau EE, Woody GE, Samet JH. Implications of cannabis use and heavy alcohol use on HIV drug risk behaviors in Russian heroin users. AIDS Behav. 2008;12:662–669. doi: 10.1007/s10461-007-9243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Egan JE, Botsko M, Netherland J, Fiellin DA, Finkelstein R. The BHIVES collaborative: organization and evaluation of a multisite demonstration of integrated buprenorphine/naloxone and HIV treatment. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S7–13. doi: 10.1097/QAI.0b013e3182097426. [DOI] [PubMed] [Google Scholar]
- Westergaard RP, Ambrose BK, Mehta SH, Kirk GD. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc. 2012;15:10. doi: 10.1186/1758-2652-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.