Abstract
The female reproductive tract produces hormones for reproductive function and cardiovascular, bone and sexual health; the tract supplies a finite number of gametes, and it supports fetal development. Diseases that affect each of the female reproductive tract organs, along with treatments that have direct, deleterious effects on the reproductive tract (for example, chemotherapeutics), are understudied due to the lack of model systems that phenocopy in vivo function. This review describes a path toward developing female reproductive tract mimics. The models use isolated primary support cells cultured onto a biological scaffold and within a microfluidic system to create a niche and support the desired differentiation of epithelia, germ and somatic cells from patient-derived induced pluripotent stem cells. Improving our fund of knowledge about reproductive tract biology and creating reproductive organs for patients who have lost gonadal, uterine or vaginal/ cervical function is a major step forward in women's health and an important advancement in personalized medicine.
Introduction
The female reproductive tract produces hormones, supplies gametes and supports embryos through fetal development. Understudied and poorly understood diseases, including those contracted through sexual transmission, benign tumors and cancers, develop in or affect each of the female reproductive tract organs [1-4]. Advances in bioengineered tissue mimetics, including three-dimensional ovarian follicle culture, represent an important new avenue of investigation in the study of normal reproductive function and the regeneration of diseased tissues [5]. Great headway has been made in induced pluripotent stem cell (iPSC) derivation from human somatic cells for many organs, and new methods have been employed to derive these cells without integration of viral vector or transgene sequences [6-8]. Utilizing iPSCs to create the reproductive tract organ mimics would allow for new drug testing, and could provide personalized regenerative treatment options that restore fertility and/or endocrine function.
Recreating the female reproductive tract
The female reproductive tract organs are dynamic and require synchronization of movement and differentiation to guide ovulated oocytes, prepare for implantation and nurture a fetus to develop as an independent organism. It is necessary not only to see these organs as unique entities, but also as one cohesive system (Figure 1). Recently developed high-throughput drug screens utilize three-dimensional systems-level models that incorporate microfluidics to create a microenvironment that chemically and physically imitates the desired system (reviewed in [9]). Likewise, it is important to develop reproductive organs in a connected microfluidic system in order to provide the sequence of hormones that control biological function in a dynamic manner. Additionally, nonreproductive tract effects of the endocrine hormones produced by the ovaries are important to program into other organ systems in order to ensure normal function. Thus, while we have focused on the role of female sex hormones on the adjacent reproductive tissues, it is important to keep in mind the impact of the overall influence of estrogens and progesterones on all tissues of the body [10].
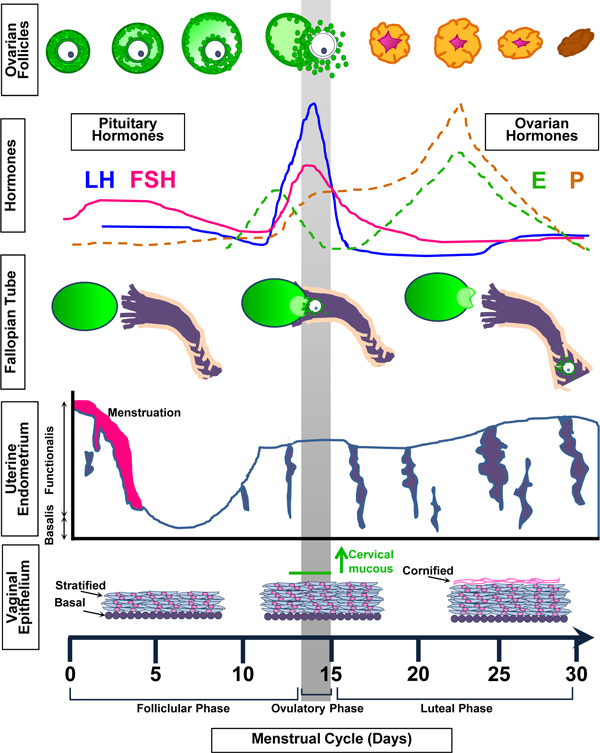
Figure 1.
Changes in the reproductive tract throughout the menstrual cycle. Follicle stimulating hormone (FSH) from the pituitary promotes follicle growth in the ovary. These follicles produce estrogen (E). In response to E, the functional is layer of the uterine epithelium and the stratified layer of the vaginal epithelium thicken as the infindibulum of the fallopian tube comes in contact with the ovary. The luteinizing hormone (LH) surge from the pituitary causes the dominant follicle to ovulate. The remaining follicular cells develop into a corpus luteum that produces E and progesterone (P). In turn, P promotes more proliferation within the uterus and vagina, and cornification in the vagina. The uterotubal junction of the fallopian tube widens to allow the passage of the ovulated oocyte or fertilized embryo. Reduced E and P levels induce atrophy of vaginal epithelium and menstruation of the uterine functionalis layer.
Each section below describes how the dynamic cell type within each female reproductive tract tissue could be replaced by patient-derived iPSCs that have been differentiated by the paracrine factors and cytokines of the supportive cell type or niche. A representative schematic is shown in Figure 2.
Figure 2.
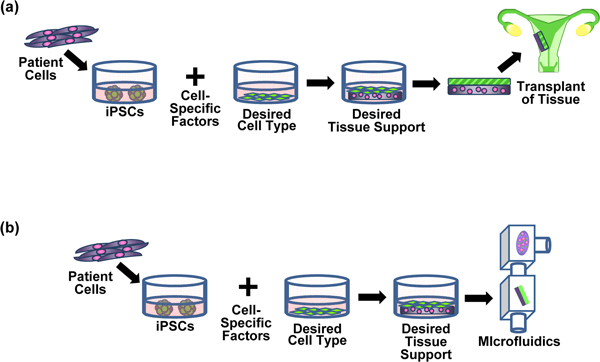
Example of induced pluripotent stem cell-derived tissues for use in tissue repair or drug discovery. (a) Restore uterine function: uterine tissue is transplanted to remedy frequent miscarriages due to uterine endometrium malformation. (b) Test drug efficacy: a similar uterine tissue mimic is used to screen potential drugs for treatment of frequent miscarriages. iPSCs, induced pluripotent stem cells.
Female reproductive tract organ mimics
The ovary: germ cells and somatic endocrine cells
The ovary is the central organ of the female reproductive tract because it produces a haploid gamete that can be fertilized to develop into a viable embryo. Oocytes do not develop in isolation but require close interactions with granulosa and theca cells to activate and mature. This somatic cell with germ cell unit is called the follicle.
Follicles are maintained in a hierarchy of developmental stages that regulate a woman's fertility during her reproductive life. The ability to recreate the germ cell and somatic cells of the follicle has progressed rapidly in recent years. Human iPSCs cultured with BMP-4, BMP-7 and BMP-8b for 1 to 2 weeks differentiated down the primordial germ cell lineage, as measured by VASA and deleted in azoospermia-like protein (DAZL) expression [11]. Moreover, mouse iPSCs that were reintegrated with ovarian somatic cells behaved as primordial germ cells and contributed to live offspring upon in vitro maturation and fertilization. The embryonic ovarian stromal cells surrounding the iPSC-derived cells induce expression of early and late stage primordial germ cell markers, such as Nanos, developmental pluripotency associated 3 (Dppa3, also known as Stella) and DazL, and contribute to the multi-layered follicle as the iPSC-derived cells mature into germinal vesicle stage oocytes [12]. The mechanical environment, which controls mechano transduction and physical forces, of the ovary is important to this process and can be engineered into the system using biomaterials [5,13,14]. The extracellular matrix contributes to the physically-distinct ovarian compartments, and is more dense and less vascularized in the rigid outer cortex, where primordial follicles reside, than the less dense medulla, where the recruited follicles grow, differentiate and prepare for ovulation [15-18].
Ultimately, the proper niche environment of support cells within a synthetic scaffold, that recreates both the cortex and medulla compartments, could be constructed to promote ordered iPSC-derived oocyte-containing follicle activation and sequential development of mature gametes. A functioning ovary mimic would then release the right hormones at the right time in the right amount to support endocrine function of reproductive and other target tissues.
The fallopian tubes: ciliated fimbria and muscular passages
The female reproductive tract organs - the fallopian tubes, uterus, cervix and vagina - develop from the Müllerian duct. The most anterior portion of the Müllerian duct develops into the fallopian tubes. These tubes are the site of fertilization and initial embryo development, and can be phenotypically and functionally divided into four segments, the infundibulum, ostium, ampulla and uterotubal junction. A three-dimensional microfluidic culture system is salient in maintaining the integrity of a fallopian tube mimic and ensuring response to estrogen signals from the ovary [19].
As in most organs, the oviduct mesenchyme determines the adjacent epithelial cell fate. Undifferentiated epithelial cells adjacent to the ampulla will differentiate into more ciliated cells, while those adjacent to the isthmus mesenchyme will form more secretory cells [20]. With this in mind, region-specific mesenchyme can be utilized to support and differentiate iPSCs into the appropriate epithelial cell type. Differentiation of the iPSCs into the desired epithelium can be monitored through expression of PAX8, forkhead box J1 (FOXJ1) and acetylated tubulin, and the proper response to paracrine signals from the ovary can be monitored through expression patterns and physiology as mentioned above. The constructed organ pieces can then be integrated to form the entire fallopian tube and assembled within the microfluidic system.
The uterus: cycling endometrium and contractile myometrium
The primary purpose of the uterus is to harbor and nurture the developing fetus throughout gestation. The dynamic and regenerating uterine endometrium potentially undergoes hundreds of cycles that involve differentiation, growth and shedding throughout a woman's reproductive lifespan. The uterus prepares for a potential blastocyst implantation by secreting glycogen and other histotrophic products [21]. Inappropriate remodeling of this tissue can lead to miscarriage or infertility. However, little is known about implantation of the embryo due to a lack of models that appropriately mimic the human menstrual cycle, implantation and pregnancy.
Human embryonic stem cells that were differentiated into embryoid bodies and cultured with neonatal mouse uterine mesenchyme differentiated into female reproductive tract-like cells that formed ductal glands, expressed PAX2 and homeobox A10 (HOXA10). Additionally, these cells secreted glycodelin A in response to cycling estrogen and progesterone [22]. A biological scaffold, such as a fibrin-alginate network, could be utilized to support mesenchymal cell expansion. While it would be ideal to create healthy and diseased uterine mimics from primary tissue biopsies, the types of tissue collected for research are mostly from older women undergoing hysterectomies or removal of leiomyomas. Myometrial cells may support iPSC differentiation in a similar manner to form a uterine mimic and provide a high-throughput screen for drug testing and/or tissue replacement with patient-specific phenotypes and genotypes.
The cervix and vagina: barrier and passage
Together the cervix and the vagina act as a barrier from potential exterior pathogens that may affect the more cranial reproductive tract organs. While the endocervix epithelium remains columnar like the uterine epithelium, the ectocervix is phenotypically similar to the vagina. In order to create a working vaginal mimic that can respond to hormones, it is important to establish an epithelium-stroma interaction that could be maintained within a biochemical scaffold. The Müllerian duct epithelium differentiates into stratified squamous epithelium along the ectocervix and vagina in response to paracrine signals from the mesenchyme. The basal layer of vaginal epithelium expresses the delta-N isoform of the tumor protein 63 (TP63), much like the basal layer of skin [23,24]. Because interaction with other undifferentiated cell types with the developing mesenchyme can induce the expression of delta-N-Trp63 in mice, the potential for the vaginal mesenchyme to induce a similar stratified squamous epithelium from iPSCs would be of interest [25]. The differentiated iPSC recombined with the vaginal mesenchyme could create the vaginal tissue mimic. Appropriate identification of these stratified squamous cell layers could be achieved by identifying expression of E-cadherin (CDH1) and K14.
Significance
The studies and concepts described here support the rationale for developing reproductive tract mimics. To create an ideal reproductive tract mimic, each tissue niche needs to be developed in order to support iPSC differentiation into the appropriate cell type. Given the hormonal response profile of these tissues, a microfluidic system is warranted. Establishing tissue banks of biopsies collected from both healthy and diseased patient tissues at various points in the menstrual cycle will provide a wide range of biological/fertility/infertility mimics.
The future of medical technology for the female reproductive tract will rely on the ability to accurately mimic these dynamic tissues in a system that can be adapted for genetic variations and diseased models, and can be replicated for high-throughput screens. While this concept may seem futuristic, recent advances in iPSC and microfluidic technologies indicate that organ mimic development is on the horizon to satisfy the urgent unmet needs of patients.
Abbreviations
iPSC: induced pluripotent stem cell.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
Funding for this review, and the costs of its publication, is supported by UH2 ES022920. A full version of this manuscript (length of review limited to 1,500 words) can be found at http://www.woodrufflab.org.
Declarations
Publication of this supplement has not been supported by sponsorship. Articles have undergone the journal's standard review process. The Editors declare that they have no competing interests.
This article has been published as part of Stem Cell Research & Therapy Volume 4 Supplement 1, 2013: Stem cells on bioengineered microphysiological platforms for disease modeling and drug testing. The full contents of the supplement are available online at http://www.stemcellres.com/supplements/4/S1.
References
- Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;4:253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Burdette JE. Evaluating the progenitor cells of ovarian cancer: analysis of current animal models. BMB Reports. 2011;4:435–445. doi: 10.5483/BMBRep.2011.44.7.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz KM, Rockey DD. Antibiotic resistance in Chlamydiae. Future Microbiol. 2010;4:1427–1442. doi: 10.2217/fmb.10.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrich C, Brummer O, Wasielewski Von R, Wegener G, Meijer C, Iftner T, Petry KU. Primary cervical cancer truly negative for high-risk human papillomavirus is a rare but distinct entity that can affect virgins and young adolescents. Eur J Gynaecol Oncol. 2009;4:45–48. [PubMed] [Google Scholar]
- Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;4:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy CA, Pauerstein CJ. Anatomy and physiology of the fallopian tube. Clin Obstet Gynecol. 1980;4:1177–1193. doi: 10.1097/00003081-198012000-00023. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;4:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler DJ, Ahmad FS, Ritz A, Hua H, Moroziewicz DN, Sproul AA, Dusenberry CR, Shang L, Paull D, Zimmer M, Weiss KA, Egli D, Noggle SA. Improved methods for reprogramming human dermal fibroblasts using fluorescence activated cell sorting. PLoS ONE. 2013;4:e59867. doi: 10.1371/journal.pone.0059867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;4:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;4:688–689. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, Byers B, Wilson KD, Wu JC, Simon C, Hovatta O, Reijo Pera RA. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;4:752–762. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Off spring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;4:971–975. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- Eppig J, Wigglesworth K. Development of mouse and rat oocytes in chimeric reaggregated ovaries after interspecific exchange of somatic and germ cell components. Biol Reprod. 2000;4:1014–1023. doi: 10.1095/biolreprod63.4.1014. [DOI] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;4:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;4:6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;4:613–620. doi: 10.1530/rep.0.1230613. [DOI] [PubMed] [Google Scholar]
- Yamada S, Fujiwara H, Honda T, Higuchi T, Nakayama T, Inoue T, Maeda M, Fujii S. Human granulosa cells express integrin alpha2 and collagen type IV: possible involvement of collagen type IV in granulosa cell luteinization. Mol Hum Reprod. 1999;4:607–617. doi: 10.1093/molehr/5.7.607. [DOI] [PubMed] [Google Scholar]
- Iwahashi M, Muragaki Y, Ooshima A, Nakano R. Type VI collagen expression during growth of human ovarian follicles. Fertil Steril. 2000;4:343–347. doi: 10.1016/S0015-0282(00)00618-X. [DOI] [PubMed] [Google Scholar]
- King SM, Quartuccio S, Hilliard TS, Inoue K, Burdette JE. Alginate hydrogels for three-dimensional organ culture of ovaries and oviducts. J Vis Exp. 2011;4:e2804. doi: 10.3791/2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi H, Umezu T, Tomooka Y. Reconstruction of oviduct and demonstration of epithelial fate determination in mice. Biol Reprod. 2010;4:528–533. doi: 10.1095/biolreprod.109.078329. [DOI] [PubMed] [Google Scholar]
- Slayden ODO, Brenner RMR. Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol. 2004;4:393–409. doi: 10.1679/aohc.67.393. [DOI] [PubMed] [Google Scholar]
- Ye L, Mayberry R, Lo CY, Britt KL, Stanley EG, Elefanty AG, Gargett CE. Generation of human female reproductive tract epithelium from human embryonic stem cells. PLoS ONE. 2011;4:e21136. doi: 10.1371/journal.pone.0021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mech Dev. 2005;4:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming M, Dotsch V, Andrews N, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;4:305–316. doi: 10.1016/S1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Dev Biol. 2001;4:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]