Abstract
Branched chain fatty acids (BCFA) are primarily saturated fatty acids (FA) with a methyl branch, usually near the terminal methyl group. BCFA are abundant in bacteria, skin, and vernix caseosa but have seldom been studied with respect to human nutrition. They are constituents of the term newborn infant gut lumen, being swallowed as vernix particulate components of amniotic fluid in the last trimester of normal pregnancy. We recently showed that BCFA protect against Necrotizing Enterocolitis (NEC) in the rat pup model. Dietary BCFA at levels similar to those found in human vernix reduced NEC incidence by more than 50%, increased the abundance of BCFA-containing bacteria, and increased the expression of ileal anti-inflammatory IL-10. The few published reports of BCFA in human milk enable an estimate that breast fed infants consume 19 mg BCFA per 100ml milk. Dietary BCFA consumption from milkfat and other ruminant products, the main sources of dietary BCFA, is more than 400 mg BCFA per day in adult Americans. This estimate exceeds by several fold the average dietary intake of bioactive FA, such as docosahexaenoic acid. BCFA are bioactive, abundant but neglected components of the human food supply.
Introduction
Branched chain fatty acids (BCFA) are a class of primarily saturated fatty acids (FA) with a methyl branch on the carbon chain. The predominant branching is near the methyl end of the carbon chain. A FA with a methyl branch on the penultimate carbon, forming a terminal isopropyl group is referred to as an iso-BCFA. A FA with the methyl branch located on the antepenultimate carbon forming a terminal isobutyl group is referred to as an anteiso-BCFA. In this review, we will focus on these predominant iso- and anteiso-BCFA types.
BCFA are rare in internal mammalian tissues, but they are present in high amounts in skin and in vernix caseosa, the waxy substance that cover the skin of the late term fetus, where their concentrations is greater than 25%w/w [1]. BCFA are also an essential membrane component of many bacterial species. For instance, BCFA constitute 95% of the FA in several Bacilli and Lactobacilli [2]. Biophysically, BCFA function in the membranes similarly to cis unsaturated double bonds; both interfere with the ability of saturated FA to pack tightly to form rigid, high melting point extended structures. BCFA are thought to be synthesized from the catabolic products of the branched chain amino acids (BCAA) valine, leucine and isoleucine. In rats, injection of 14C labeled valine, leucine or isoleucine resulted in increase in skin iso- and anteiso-BCFA [3]. These amino acids undergo transamination and oxidative decarobxylation to iso-butyryl CoA, iso-valeryl CoA, and alpha-methyl-butyryl CoA, respectively, which are then elongated to even-numbered carbon iso-BCFA, odd-numbered carbon iso-BCFA, and odd-numbered carbon anteiso-BCFA, respectively.
To our knowledge, BCFA have not been studied for their role(s) in human nutrition. BCFA are not routinely reported in FA studies of different tissues, apparently because BCFA are trace or minor components of most internal tissues. In addition, most BCFA are obscured by straight chain saturated and monounsaturated peaks on many Gas Chromatography (GC) columns, the principle analytical method for FA.
Here, we first discuss the presence of BCFA in the newborn gastrointestinal (GI) tract and present evidence for their metabolism by the enterocyte. Then we will describe their possible role in prematurity-related intestinal inflammation, and finally we will evaluate their intake throughout the life cycle.
BCFA are normal constituents of the human neonatal gut and are metabolized by enterocytes
The fetus relies on placental transfer to fulfill its nutritional requirements for optimal growth and development. In addition to placental transfer, amniotic fluid (AF) contents are also known to contribute to the growth and maturation of the fetus [4].
Vernix caseosa is one of the lipid sources in AF and is produced by the fetal skin during the last trimester of pregnancy [5]. Late in gestation, vernix becomes suspended in AF and is swallowed by the late term fetus in increasing amounts as term birth approaches [6]. AF contains an estimated average 154 mg/L lipids overall [7], of which vernix BCFA are about 17 mg/L though there is wide variability in these figures [1]. The fetus swallows 200-500 ml/d of AF near term [8], enabling an estimate of 6 mg AF-suspended vernix BCFA per day, or about 180 mg BCFA in the last month of pregnancy [1].
We reported recently an analysis of BCFA in vernix (fetal gut input) and in meconium (newborn first output) of 18 healthy term infants [1]. Vernix BCFA concentrations totaled 29%w/w, and their chain length ranged from C11 to C26. Meconium, on the other hand, had 9 BCFA of total concentrations of 17%w/w, with chain lengths ranging from C16 to C26. We estimated that 180 mg BCFA were swallowed in the last month of pregnancy, but only 16mg of BCFA appeared in meconium. Taken together, these data indicate that BCFA are present throughout the length of the fetal and newborn GI tract and that short-chain BCFA entering the GI tract as AF-suspended vernix do not appear in meconium. The selective distribution of BCFA chain length between vermix and meconium and the difference between the estimated amount of BCFA ingested from AF-suspended vernix compared to the BCFA amount secreted in meconium imply that these FA are not inert components in the GI tract but rather are being metabolized.
To investigate human enterocyte metabolism of BCFA, we studied Caco-2 human colon carcinoma cells that differentiate into enterocyte cells [9], which, have characteristics common to fetal intestinal cells [10] and are used as a model for FA uptake [11]. Graded levels (0.1-0.5 mM) of a mixture of four pure BCFA with chain lengths of C14-C20 (iso-14:0, anteiso-17:0, iso-18:0 and iso-20:0) were delivered with bile salts in a micellar solution to simulate the postprandial intestine. Table 1 demonstrates that BCFA are taken up by Caco-2 in a dose responsive manner until they reached a plateau at around 0.50 mM. The evidence for both saturable and diffusive mechanisms for FA uptake by Caco-2 were described previously [11], and the saturable mechanism is consistent with the uptake pattern observed here. In addition, BCFA were incorporated into cell lipid classes in a selective manner (Table 2): anteiso-17:0 was unfavored in diacylglycerol (DAG) fraction compared to phospholipids (PL) and triacylglycerol (TAG). iso-18:0 was favored in PL and iso-20:0 was favored in DAG.
Table 1.
Caco-2 cell relative uptake of BCFA (%; mean ± SEM) dose-response
| Amount of total BCFA in the diet | ||||
|---|---|---|---|---|
| BCFA type | 0.1mM | 0.25mM | 0.4mM | 0.5mM |
| iso-14:0 | 12.6 ± 0.6a | 15.9± 0.2b | 22.1 ± 0.6c | 20.9. ± 0.4 c |
| anteiso-17:0 | 15.9 ± 0.2 a | 18.7 ± 0.5b | 25.6 ± 0.3c | 26.1 ± 0.3c |
| iso-18:0 | 19.2 ± 0.6 a | 20.9 ± 0.5a | 26.9 ± 0.3b | 26.4 ± 0.7b |
| iso-20:0 | 9.4 ± 0.3 a | 10.7 ± 0.3ab | 17.3 ± 0.2bc | 22.4 ± 2.8c |
The relative uptake of dietary BCFA was calculated as the percent of a BCFA in the cells (%w/w) divided by the percent of that BCFA in the dietary mixture (%w/w), × 100. Cells were incubated for 4 hours with graded levels (0.1-0.5 mM) of a mixture of 4 BCFA (iso-14:0, anteiso-17:0, iso-18:0, and iso-20:0). BCFA were delivered to the cells in fat-free media with 10 mM Taurocholate salt and 0.2 mM mono-olein, as described by Ho et al. [11]. Control cells were treated with 10 mM Taurocholate salt and 0.2 mM mono-olein, with and without graded levels of oleic acid corresponding to doses of BCFA. BCFA were negligible in Control cells (not shown). Comparisons were made among the relative uptake of individual BCFA across treatment groups and confirm dose-dependent uptake (different superscripts (a,b,c,d) indicate a statistically significant difference, p<0.05). Cells took up BCFA from the medium in a dose responsive manner until they reached a plateau at around 0.50 mM of BCFA in the dietary mixture.
Table 2.
BCFA uptake (%BCFA; mean ± SEM) into Caco-2 cells’ lipid classes
| FA | Diet | Phospholipids (PL) | Diacylglycerols (DAG) | Triacylglycerols (TAG) |
|---|---|---|---|---|
| iso-14:0 | 16.8 | 14.2 ± 1.6 a | 14.2 ± 2.7 a | 20.3 ± 1.5 a |
| anteiso-17:0 | 23.7 | 27.5 ± 0.4 a | 21.6 ± 0.6 b | 28.5 ± 1.0 a |
| iso-18:0 | 27.2 | 37.2 ± 0.4 a | 23.4 ± 0.7 b | 30.0 ± 1.2 c |
| iso-20:0 | 32.2 | 19.8 ± 1.7 a | 38.5 ± 0.8 b | 20.5 ± 1.0 a |
Cells were incubated as described in Table 1. Comparisons were made among individual BCFA across lipid classes (different superscripts (a,b,c,d) indicate a statistically significant difference between lipid classes, p<0.05). For example: anteiso-17:0 was unfavored in diacylglycerol (DAG) fraction compared to phospholipids (PL) and triacylglycerol (TAG). iso-18:0 was favored in PL and iso-20:0 was favored in DAG.
An early (1957) study demonstrated that weanling rats fed 100 mg / week anteiso-17:0 in an otherwise fat free diet excreted 8-10% of anteiso-17:0 in the feces, stored a similar amount in adipose tissue, and converted a small amount to anteiso-15:0 [12]. Our recent study showed that dietary BCFA fed to premature rat pups as a mixture of six pure BCFA (iso-14:0, anteiso-15:0, iso-16:0, anteiso-17:0, iso-18:0 and iso-20:0) and in concentrations of 20%w/w of rat formula fat, a similar concentration to human vernix, were taken up by ileum and were further transferred via the serum to the liver [13]. Dietary BCFA were incorporated in a structure-selective manner into the phospholipid (PL) fraction of the ileum [13]. The relative uptake of BCFA with fewer than 16 carbons, such as iso-14:0, was significantly lower in ileum PL compared to BCFA with 16 carbons and above (Table 3). The relative uptake of iso-18:0 in all tissues was exceptionally high, and more than twice as high as other BCFA with 16 carbons and above. The relative uptake pattern of BCFA in the liver was similar to their relative uptake pattern in the ileum PL. Taken together, these results indicate that BCFA are active components absorbed by intestine both in vitro and in vivo. The structure-selective uptake of BCFA suggests that there are different and specific roles for different BCFA. Selection against short BCFA for incorporation into the PL fraction was consistent with our previous observation showing a systematic shift of BCFA distribution between vernix and meconium [1].
Table 3.
The relative uptake of BCFA (%: mean ± SEM) in pups’ ileum phospholipids and liver
| BCFA | iso-14:0 | anteiso-15:0 | iso-16:0 | anteiso-17:0 | iso-18:0 | iso-20:0 |
|---|---|---|---|---|---|---|
| Ileum PL | 3.8 ± 1.7a | 15.0 ± 1.2ab | 24.3 ± 5.2b | 23.6 ± 3.1b | 57.0 ± 6.3c | 24.6 ± 4.5b |
| Liver | 10.6 ± 2.7a | 20.9 ± 1.8bc | 29.8 ± 1.6cd | 36.2 ± 3.0d | 74.4 ± 3.2e | 20.6 ± 0.3b |
The relative uptake of dietary BCFA was calculated as the percent of a BCFA in the tissue (%w/w) divided by the percent of that BCFA in the rat milk substitute (%w/w), × 100. Comparisons were made among individual BCFA within each tissue type (different superscripts (a,b,c,d) indicate a statistically significant difference, p<0.05). The concentrations of tissue BCFA in the Control animals (no dietary BCFA added) was negligible [13], thus all BCFA in the BCFA-fed pups’ tissues originated from the diet. The relative uptake of iso-14:0 in the ileum phospholipids (PL) was lower compared to longer BCFA (BCFA with 16 carbons and higher). The relative uptake of iso-18:0 was exceptionally high in all tissues. The relative uptake of BCFA in the liver was similar to the relative uptake in the ileum PL.
BCFA and Necrotizing Enterocolitis
A developmental role for BCFA was demonstrated recently by Kniazeva and colleagues working with Caenorhabditis elegans, a well studied nematode [14]. Both iso-15:0 and iso-17:0 were required for worm growth, and neither straight chain saturated or mono-unsaturated, or short or longer BCFA (iso-13:0, iso-18:0, iso-19:0) restored normal growth. The level of iso-15:0 and iso-17:0 in eggs was critical for growth and development, as animals depleted of these BCFA arrested at the first larva stage, and addition of iso-17:0 rescued larvae to full growth. These data demonstrate that at least one free-living animal has evolved a physiological requirement for BCFA of specific chain lengths and are essential for growth.
The presence of BCFA in the GI tract of the normal term newborn suggests that BCFA may have a bioactive role in the gut, possibly as a signal for gut maturation and preparing the intestine for extrauterine life. Gut disorders arising from the lack of vernix-BCFA ingestion might then be expected. Necrotizing enterocolitis (NEC) is an inflammatory disease in the intestine of premature infants. NEC is a major cause of morbidity in premature infants, with an estimated rate of death of 20-30% . The major risk factors include prematurity, enteral feeding—especially formula feeding—abnormal bacterial colonization, and intestinal hypoxia-ischemia [15]. AF-suspended vernix is swallowed by the term fetus in increasing amount as parturition approaches. The incidence of the prematurity-related gut inflammation disease NEC drops as gestational age approaches term [16], consistent with the increase in gut BCFA from ingested vernix. Thus, development of NEC could be related to the absence of vernix-BCFA ingestion.
BCFA are also a major component of a wide range of bacteria, about 15% of phyla [2]. BCFA constitute 95% of the FA in several Bacilli and Lactobacilli, including species such as Bacillus subtilis that have been shown to have a protective effect in colitis-induced animals [17]. We hypothesized that the presence of BCFA in the neonatal gut would alter the mix of dominant species, favoring those organisms that use BCFA in their membranes, thus maintaining species evolved to colonize the gut symbiotically. Moreover, we speculate also that the known greater diversity term infant microbiota compared to premature infant microbiota [18] may be enhanced by dietary BCFA, and inhibiting the increase in one or few dominant species and thus decreasing the risk for NEC.
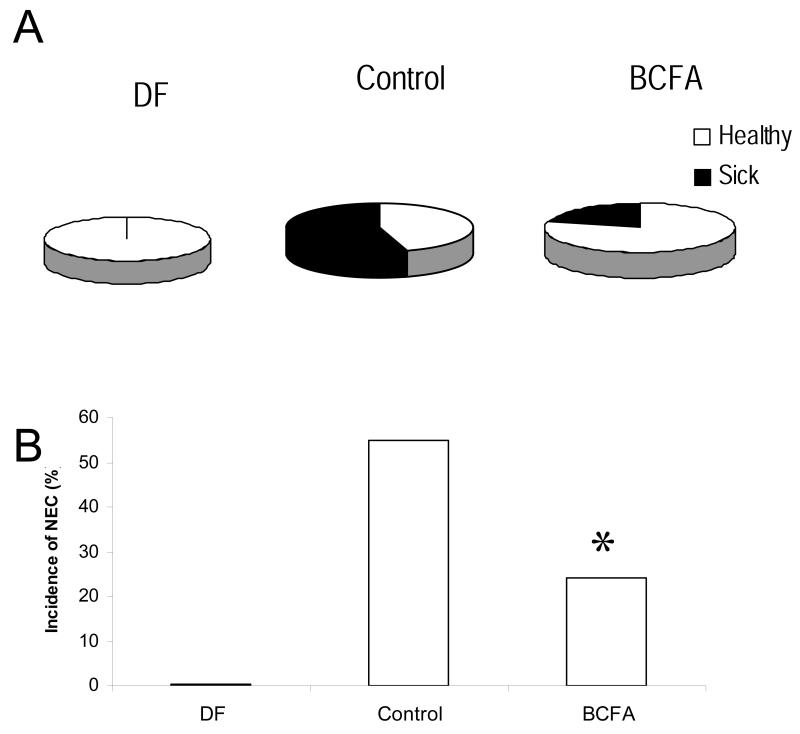
We tested this hypothesis recently, showing that feeding BCFA to rat pups reduces the incidence of NEC in a neonatal rat pup model [13]. When rat pups were exposed to ischemia-hypoxia conditions to induce NEC, four days with a rat milk formula with six BCFA substituted for 20%w/w of the fat reduced the incidence of NEC by more than 50% compared to Controls fed conventional rat milk substitute (Fig 1A and 1B).
Figure 1.
A. The proportions of animals that were healthy (white) and ill with NEC (black) in each treatment group: DF (Dam Fed; no sick animals), Control (Formula Fed, no BCFA; 17 of 31 animals were sick), BCFA (Formula Fed, 20%,w/w BCFA; 5 of 24 animals were sick). B. NEC incidence in rat pups in all experimental groups. NEC was significantly lower in the BCFA group compared to the Control group *p<0.05 Control vs. BCFA.
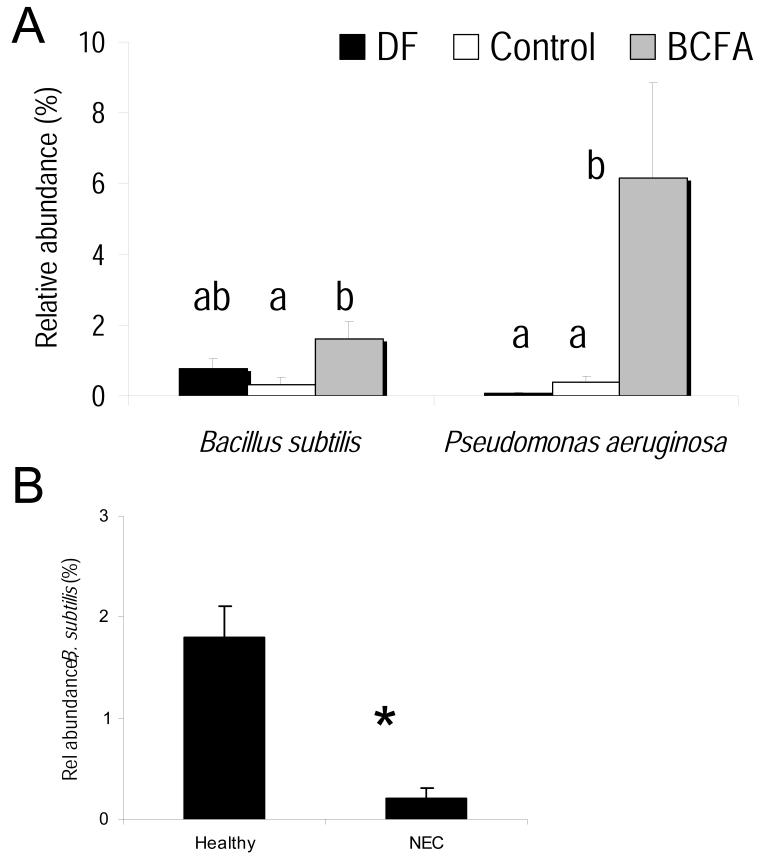
In addition, pup ceca was profiled by 454 pyrosequencing. The microbiota was altered by BCFA feeding, supporting a several fold greater relative abundance of the BCFA-containing bacteria Bacillus subtilis and Pseudomonas aeruginosa (Fig 2A). In addition, the relative abundance of B. subtilis was higher in healthy animals compared to sick animals regardless of treatment (Fig 2B), suggesting it may play an elementary role in reducing the incidence of NEC. B. subtillis contains over 90% of BCFA in its membranes [2]. Elevated B. subtilis have been associated with improved macroscopic lesion scores, reduced levels of pro-inflammatory cytokines, and increased anti-inflammatory cytokine levels in colitis-induced animals compared to untreated colitis-induced animals [17].
Figure 2.
A. The relative abundance (%; mean ± SEM) of Bacillus subtilis and Pseudomonas aeruginosa in the treatment groups: DF (Dam Fed), Control (Formula Fed, no BCFA), BCFA (Formula Fed, 20%,w/w BCFA). B. Bacillus subtilis in healthy animals and in animals with NEC. Data not marked by the same symbol are significantly different *(p<0.05).
The relative abundance of P. aeruginosa, normally considered a serious pathogen, increased in the BCFA-fed group compared to the Control group, despite lower incidence of NEC. Recent data show that BCFA, specifically iso-14:0, anteiso-15:0 iso-16:0, and anteiso-17:0, included in our study BCFA diet, repress motility and biofilm formation in P. aeruginosa without significantly inhibiting its growth in liquid cultures [19]. Both motility and biofilm formation are components of the virulence of P. aeruginosa. Thus, it is possible that BCFA in our study supported P. aeruginosa growth while repressing its motility suggesting yet another mechanism for a protective effect of BCFA. A plausible mechanism is that BCFA alter the gut microbiota of rat pups fed BCFA compared to Control by increasing BCFA-containing species, enhancing the diversity of the gut microbiota with some species exerting beneficial effects, reducing the pathogencity of pathogenic bacteria, or both.
Reductions in the BCFA-precursors iso-butyric, iso-valeric and alpha-methyl-butyric acids were reported recently in both ulcerative colitis (UC) and Irritable Bowel Syndrome (IBS) patients compared to healthy controls. While there were no differences in the levels of BCAA, the difference in BCFA-precursor levels was suggested to have resulted from a reduction in the abundance of BCFA-precursor forming bacteria [20]. This suggestion is consistent with our hypothesis that BCFA-dependent bacteria reduce intestinal disease states and warrants further investigation.
Our data also showed that dietary BCFA increased the expression of the anti-inflammatory cytokine, IL-10 compared with the Control group [13]. From our data we were not able to establish whether BCFA increased IL-10 thereby reducing inflammation, or reduced factors causing inflammation thereby reducing the need for inflammatory signaling. Reduced NEC incidence in rat-milk-fed versus formula-fed pups was associated with a twofold increase of ileal IL-10 mRNA [21]. Others showed that subcutaneous administration of recombinant human IL-10 to the intestine of NEC-induced neonate rats reduced the severity of microscopic ileal lesions compared with untreated rats [22]. Thus, the elevated expression we observed in ileal IL-10 may mediate NEC reduction in the BCFA group, possibly by opposing proinflammatory effects.
BCFA intake in infants and adults
The ingestion of BCFA is not limited to the perinatal period. The breastfed infant consumes BCFA since they are found in colostrum and mature milk at concentrations up to 1.5% w/w [23]. Taking 0.6% as a conservative estimate of mean BCFA concentration in American human milk [24] with 3.2%fat, we estimate that consumption of BCFA is about 19 mg BCFA per 100 mL human milk. Taking 600 mL as the mean intake of human milk by a one month-old infant, then a typical male infant weighing 4.5 kg <http://www.cdc.gov/growthcharts/data/who/GrChrt_Boys_24LW_100611.pdf> (Table 15-7) consumes about 114 mg total BCFA per day, or about 25 mg BCFA per kg body weight. A three-month-old infant consumes, on average, 800 mL human milk per day, ingesting about 152 mg BCFA/day or about 23 mg BCFA/kg body weight for a male infant weighing the median of 6.5kg. <http://www.cdc.gov/growthcharts/data/who/GrChrt_Boys_24LW_100611.pdf>. For comparison, the mean human milk concentrations of the bioactive long chain polyun-saturated fatty acid (LCPUFA) docosahexaenoic acid (DHA) is 0.32%w/w [25] and comparable total intakes for 1 and 3 month old infants would be 13 and 12 mg DHA per kg body weight per day, respectively. Thus, BCFA are consumed at levels that exceed DHA, a bioactive FA of great interest in infant nutrition. BCFA are not, however, a component of powdered milk formulas that use vegetable oils as their fat source, and in this sense these formula lipids differ from human milk.
BCFA are prominent in ruminant meat and milk, constituting 2%w/w of fat in the US retail cow’s milk supply [26]. Our preliminary analyses of foods from an Ithaca, New York, USA market show BCFA to be about 0.9-1.8% in various cheese and ground beef types (Ran-Ressler, et al., unpublished data). Based on these initial figures, and taking 1.3% and 0.9% as conservative estimates of the mean BCFA concentration in cheeses and in ground beef, respectively, we can estimate BCFA consumption in children. For children ages 2-11 years, the mean intake of beef is 43.5 g/d beef [27]. Children who consume a total of 28 g (1 oz) of cheese with an average of 27% fat, and 43.5 g/d of cooked beef with an average of 18% fat, consume about 168 mg BCFA per day. Addition of 1 cup of whole milk (155 mg BCFA) increases their daily BCFA intake to 323 mg. For comparison, the mean intake of DHA and arachidonic acid (ARA) in Canadian children, ages 4-7 years, was recently estimated to be 37 mg and 57mg per day [28]; both combined are only one third of the daily BCFA consumption from milk, cheese and beef.
Based on the same preliminary food data, the BCFA intake of an adult who consumes 28g (1oz) of cheese, 100 g of cooked beef and 1 cup of whole milk will total approximately 415 mg/day (Table 4) (Ran-Ressler, et al., unpublished data). BCFA intake is greater than the 100 mg average daily consumption of the DHA and eicosapentaenoic acid (EPA) reported in a survey of 8604 Americans between 1999 and 2000 and by women of child-bearing age, based on NHANES III data [29,30]. Thus, BCFA are being consumed in substantial amounts by most non-vegans, during all life stages.
Table 4.
Estimated BCFA content by representative serving size
| BCFA intake in common use serving sizes |
||||
|---|---|---|---|---|
| Food | Fat content (%wt) |
BCFA (%w/w) | Amount consumed, g |
BCFA content, mg |
| Cow’s milk | 3.2 | 2.0 | 240.0 | 155.0 |
| Cooked beef |
18.0 | 0.91 | 100.0 | 162.0 |
| Cheese | 27.02 | 1.32 | 28.0 | 98.0 |
| Total BCFA | 415.0 | |||
Preliminary determination of BCFA concentrations in beef, purchased from a local retail store.
Preliminary determination of the mean fat and BCFA concentrations in different cheeses, purchased from a local retail store.
In conclusion, BCFA are normal constituents in the gut from very early age, and they are present in the gut throughout the human life cycle. Studies in human cell and animal models show that BCFA are not inert components of the GI tract but are metabolized by enterocytes. BCFA have a beneficial role against inflammation in the premature intestine, alter the microbiota, and increase expression of anti-inflammatory cytokines in an animal model. These initial observations suggest that research is warranted to define with much more clarity BCFA bioactivity in infant and adult nutrition.
Acknowledgments
Original results were supported by NIH grant R21 HD064604 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The opinions expressed here are those of the authors alone. We thank Dong Sim for skilled technical support of fatty acid analyses.
Footnotes
Conflict of interest. The authors declare no conflict of interest.
References
- 1.Ran-Ressler RR, Devapatla S, Lawrence P, et al. Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr Res. 2008;64:605–609. doi: 10.1203/PDR.0b013e318184d2e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaneda T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigor MR, Dunckley GG, Purves HD. The synthesis of the branched-chain fatty acids of rat skin surface lipid. Biochim Biophys Acta. 1970;218:389–399. doi: 10.1016/0005-2760(70)90001-9. [DOI] [PubMed] [Google Scholar]
- 4.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: Not just fetal urine anymore. J Perinatol. 2005;25:341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 5.Rissmann R, Groenink HW, Weerheim AM, et al. New insights into ultrastructure, lipid composition and organization of vernix caseosa. J Invest Dermatol. 2006;126:1823–1833. doi: 10.1038/sj.jid.5700305. [DOI] [PubMed] [Google Scholar]
- 6.Narendran V, Wickett RR, Pickens WL, et al. Interaction between pulmonary surfactant and vernix: A potential mechanism for induction of amniotic fluid turbidity. Pediatr Res. 2000;48:120–124. doi: 10.1203/00006450-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Biezenski JJ, Pomerance W, Goodman J. Studies on the origin of amniotic fluid lipids. I. Normal composition. Am J Obstet Gynecol. 1968;102:853–861. doi: 10.1016/0002-9378(68)90514-0. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard JA. Fetal swallowing and amniotic fluid volume. Obstet Gynecol. 1966;28:606–610. [PubMed] [Google Scholar]
- 9.Pinto M, Robine-Leon S, Maric-Dominique A, et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 10.Blais A, Bissonnette P, Berteloot A. Common characteristics for na+-dependent sugar-transport in caco-2 cells and human-fetal colon. Journal of Membrane Biology. 1987;99:113–125. doi: 10.1007/BF01871231. [DOI] [PubMed] [Google Scholar]
- 11.Ho SY, Storch J. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal caco-2 cells. Am J Physiol Cell Physiol. 2001;281:C1106–1117. doi: 10.1152/ajpcell.2001.281.4.C1106. [DOI] [PubMed] [Google Scholar]
- 12.Livingston M, Bell ME, Shorland FB, et al. The metabolism in the rat of naturally occurring (+)-14-methylhexadecanoic acid. Biochem J. 1957;65:438–440. doi: 10.1042/bj0650438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ran-Ressler RR, Khailova L, Arganbright KM, et al. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS One. 2011;6:e29032. doi: 10.1371/journal.pone.0029032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kniazeva M, Crawford QT, Seiber M, et al. Monomethyl branched-chain fatty acids play an essential role in caenorhabditis elegans development. PLoS Biol. 2004;2:E257. doi: 10.1371/journal.pbio.0020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeby PJ, Jeffery H. Risk factors for necrotising enterocolitis: The influence of gestational age. Arch Dis Child. 1992;67:432–435. doi: 10.1136/adc.67.4_spec_no.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvam R, Maheswari P, Kavitha P, et al. Effect of bacillus subtilis pb6, a natural probiotic on colon mucosal inflammation and plasma cytokines levels in inflammatory bowel disease. Indian J Biochem Biophys. 2009;46:79–85. [PubMed] [Google Scholar]
- 18.Magne F, Suau A, Pochart P, et al. Fecal microbial community in preterm infants. J Pediatr Gastroenterol Nutr. 2005;41:386–392. doi: 10.1097/01.mpg.0000179855.38543.85. [DOI] [PubMed] [Google Scholar]
- 19.Inoue T, Shingaki R, Fukui K. Inhibition of swarming motility of pseudomonas aeruginosa by branched-chain fatty acids. FEMS Microbiol Lett. 2008;281:81–86. doi: 10.1111/j.1574-6968.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- 20.Le Gall G, Noor SO, Ridgway K, et al. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. Journal of Proteome Research. 2011;10:4208–4218. doi: 10.1021/pr2003598. [DOI] [PubMed] [Google Scholar]
- 21.Dvorak B, Halpern MD, Holubec H, et al. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal il-10 in a neonatal rat model. Pediatr Res. 2003;53:426–433. doi: 10.1203/01.PDR.0000050657.56817.E0. [DOI] [PubMed] [Google Scholar]
- 22.Ozturk H, Dokucu AI, Ogun C, et al. Protective effects of recombinant human interleukin-10 on intestines of hypoxia-induced necrotizing enterocolitis in immature rats. J Pediatr Surg. 2002;37:1330–1333. doi: 10.1053/jpsu.2002.35002. [DOI] [PubMed] [Google Scholar]
- 23.Egge H, Murawski U, Ryhage R, et al. Minor constituents of human milk. Iv. Analysis of the branched chain fatty acids. Chem Phys Lipids. 1972;8:42–55. doi: 10.1016/0009-3084(72)90042-4. [DOI] [PubMed] [Google Scholar]
- 24.Aitchison JM, Dunkley WL, Canolty NL, et al. Influence of diet on trans fatty acids in human milk. Am J Clin Nutr. 1977;30:2006–2015. doi: 10.1093/ajcn/30.12.2006. [DOI] [PubMed] [Google Scholar]
- 25.Brenna JT, Varamini B, Jensen RG, et al. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 26.Ran-Ressler RR, Sim D, O’Donnell-Megaro AM, et al. Branched chain fatty acid content of united states retail cow’s milk and implications for dietary intake. Lipids. 2011;46:569–576. doi: 10.1007/s11745-011-3530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel CR, Cross AJ, Koebnick C, et al. Trends in meat consumption in the USA. Public Health Nutr. 2011;14:575–583. doi: 10.1017/S1368980010002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lien VW, Clandinin MT. Dietary assessment of arachidonic acid and docosahexaenoic acid intake in 4-7 year-old children. J Am Coll Nutr. 2009;28:7–15. doi: 10.1080/07315724.2009.10719755. [DOI] [PubMed] [Google Scholar]
- 29.Ervin RB, Wright JD, Wang CY, et al. Dietary intake of fats and fatty acids for the united states population: 1999-2000. Adv Data. 2004:1–6. [PubMed] [Google Scholar]
- 30.Brenna JT, Lapillonne A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab. 2009;55:97–122. doi: 10.1159/000228998. [DOI] [PubMed] [Google Scholar]