Abstract
The grapefruit juice-fexofenadine interaction involves inhibition of intestinal organic anion transporting polypeptide (OATP)-mediated uptake. Only naringin has been shown clinically to inhibit intestinal OATP; other constituents have not been evaluated. The effects of a modified grapefruit juice devoid of furanocoumarins (~99%) and polymethoxyflavones (~90%) on fexofenadine disposition were compared to effects of the original juice. Extracts of both juices inhibited estrone 3-sulfate and fexofenadine uptake by similar extents in OATP-transfected cells (~50% and ~25%, respectively). Healthy volunteers (n=18) were administered fexofenadine (120 mg) with water, grapefruit juice, or modified grapefruit juice (240 ml) by randomized, three-way crossover design. Compared to water, both juices decreased fexofenadine geometric mean AUC and Cmax by ~25% (p≤0.008 and p≤0.011, respectively), with no effect on terminal half-life (p=0.11). Similar effects by both juices on fexofenadine pharmacokinetics indicate furanocoumarins and polymethoxyflavones are not major mediators of the grapefruit juice-fexofenadine interaction.
Keywords: pharmacology, clinical pharmacology, clinical research, gastrointestinal, pharmacokinetics & drug metabolism
INTRODUCTION
The drug interaction liability of grapefruit juice (GFJ) and individual constituents has been studied extensively for more than 20 years.1 GFJ-drug interactions can manifest clinically as increased systemic drug exposure and subsequent adverse reactions due to mechanism-based inhibition of cytochrome P450 3A (CYP3A)-mediated metabolism in the intestine by GFJ.2,3 A class of constituents, furanocoumarins, was established as major mediators of this effect.3–5 A different GFJ-type interaction was reported in 2002, when the non-sedating, minimally metabolized antihistamine fexofenadine was used as a probe substrate for examining the effect of GFJ on the efflux transporter, P-glycoprotein (P-gp), in the intestine.6 Healthy volunteers administered fexofenadine with GFJ exhibited an unexpected mean 63% decrease in fexofenadine exposure. This seemingly paradoxical effect was observed consistently in four independent clinical studies reported over the subsequent five years.7–10 The underlying mechanism was postulated to involve inhibition of fexofenadine active uptake in the intestine by organic anion transporting polypeptides (OATPs). Three clinical studies demonstrated that the size and flare of histamine-induced skin wheals were increased after administration of fexofenadine with GFJ or orange juice but not water.11 Population pharmacokinetic analysis of the combined data from these studies and a bioequivalence study showed that the oral availability of fexofenadine was reduced by 36%. Due to the potential for reduced therapeutic efficacy, the labeling of all fexofenadine products recommends taking the drug with water.
Constituents in GFJ have been identified as OATP inhibitors in vitro,10,12 but the flavanone, naringin, is the only single constituent tested clinically.10 Relative to water, GFJ and an aqueous solution of naringin at the same concentration as that in GFJ (~1,200 μmol/l) decreased fexofenadine mean exposure by 42 and 22%, respectively. A suspension of a particulate fraction of GFJ containing three one-hundredths the concentration of naringin (34 μmol/l) had no effect on fexofenadine exposure. The investigators concluded that naringin was a major causative ingredient inhibiting enteric OATP. However, the ~50% difference in fexofenadine exposure between GFJ and naringin suggests other constituents contributed to the interaction.
The furanocoumarins, bergamottin and 6′,7′-dihydroxybergamottin (DHB), and the polymethoxyflavones, tangeretin and nobiletin, have been reported to inhibit human enteric OATP activity in vitro.12 Whether these observations translate to the clinic is not known. Based on the cumulative data, these two classes of GFJ constituents were evaluated as candidate OATP inhibitors by comparing the effect of a GFJ devoid of furanocoumarins and polymethoxyflavones (i.e., modified GFJ, mGFJ) with that of the original GFJ on fexofenadine disposition in both OATP-transfected cells and healthy volunteers.
METHODS
Materials and chemicals
[3H]Estrone 3-sulfate (E1S) ammonium salt (54.3 Ci/mmol) was purchased from Perkin Elmer (Waltham, MA). [3H]Fexofenadine (6 Ci/mmol), originally a gift from GlaxoSmithKline (Research Triangle Park, NC) and custom synthesized by Amersham Life Sciences (Piscataway, NJ), was provided by Dr. Dhiren Thakker (Eshelman School of Pharmacy, Chapel Hill, NC). E1S potassium salt, verapamil (VER), bromosulfophthalein (BSP), bergamottin, DHB, naringin, hesperidin, tangeretin, and nobiletin were purchased from Sigma-Aldrich (St. Louis, MO). Fexofenadine was obtained from Tocris Bioscience (Minneapolis, MN). COS-1 and HEK293T/17 cells were obtained from American Type Culture Collection (Manassas, VA). Cell culture plates were purchased from Corning Life Sciences (Tewksbury, MA). Opti-MEM and Lipofectamine2000 were purchased from Invitrogen (Carlsbad, CA). X-tremeGENE 9 was purchased from Roche Applied Science (Indianapolis, IN). Human OATP1A2 (variant 1; accession number NM_134431.1) and OATP2B1 expression plasmids were obtained from Origene Technologies (Rockville, MD). Plasmids for mock-transfected cells, pEYFP-C1 and pcDNA3.1/Hygro(+), were purchased from Clontech (Mountain View, CA) and Invitrogen, respectively. MDCKII parental cells and stably transfected MDCKII-OATP2B1 cells were provided by Dr. Markus Grube (Ernst-Moritz-Arndt University, Greifswald, Germany). All other chemicals and reagents were purchased from Fisher Scientific (Pittsburgh, PA).
Preparation of whole and modified grapefruit juice
A commercial GFJ concentrate was obtained from a Florida processing facility and prepared as described previously.5,13 The GFJ and mGFJ were re-pooled, re-pasteurized at 71.7°C for 6 s, poured into sterilized bottles, and stored at −20°C until use. Representative compounds from the furanocoumarin, polymethoxyflavone, and flavanone classes were re-measured by HPLC as described previously.5,13
Preparation of whole and modified grapefruit juice extracts
Concentrated extracts of GFJ and mGFJ were prepared by adding 20 ml ethyl acetate to a 50-ml conical polypropylene tube containing 25 ml juice. Contents were shaken vigorously for 30 s and centrifuged (2500 x g for 30 min at 25 °C). The resulting organic layer was transferred to a 250-ml round-bottom glass flask. The extraction procedure was repeated twice, with each resultant organic layer combined in the flask. Organic layers were evaporated in vacuo. The residue was transferred to a 2-ml vial using methanol as a rinse and evaporated to dryness under air. The dried material was resuspended with 500 μl methanol, yielding a 200-fold concentrated extract of the starting juice volume (100 ml).
Transient transfection of OATP1A2 and OATP2B1 into COS-1 and HEK293T/17 cells
Human OATP1A2 and OATP2B1 expression plasmids were verified by DNA sequencing prior to use. COS-1 cells were maintained, seeded, and transfected transiently as described previously.14 HEK293T/17 cells were cultured and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum at 37°C and 5% CO2. Cells were seeded on Day 0 onto 100-mm dishes and transfected on Day 1 using the X-tremeGENE9 transfection reagent per manufacturer instructions. Transfection efficiency was assessed on Day 2 with fluorescence microscopy by estimating the percentage of cells expressing EYFP (mock). On Day 3, transfected cells were trypsinized, seeded onto 24-well plates, and incubated for approximately 48 h before commencing the uptake assays. MDCKII parental cells and stably transfected MDCKII-OATP2B1 cells were cultured and maintained in a similar manner as HEK293T/17 cells. Cells were passaged once, seeded onto 24-well plates, and incubated for approximately 48 h prior to initiating uptake studies.
Uptake and inhibition assays
On Day 4 or 5, cells were washed and preincubated for 30 min at 37°C in uptake buffer (pH 6).14 Buffer was replaced, and cells were incubated with a dosing solution (200 μl) consisting of radiolabeled (plus unlabeled) E1S (total: 5 μmol/l) or fexofenadine (total: 5 μmol/l) in the presence of vehicle (1% methanol), VER (250 μmol/l) and/or BSP (250 μmol/l), or diluted juice extract (1:200 and 2:200 to approximate concentrations in the clinical juice). After 3 (E1S) or 30 (fexofenadine) min, cells were washed thrice with ice-cold phosphate-buffered saline and lysed with either 0.1 N sodium hydroxide (COS-1 cells) or 1% Triton X-100 in phosphate-buffered saline (HEK293T/17 cells). Liquid scintillation cocktail (5 ml) was added to 200-μl aliquots of the cell lysates, and radioactivity was counted. Protein concentrations were determined with a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Waltham, MA). Uptake was linear over the selected times for each substrate (data not shown).
Clinical study protocol
The study protocol was reviewed and approved by the University of North Carolina Biomedical Institutional Review Board and Clinical and Translational Research Center (CTRC) Committee.
Participants
All subjects provided written informed consent prior to participation. Healthy volunteers (9 women, 9 men) were enrolled. The median age (range) of the women and men was 30 (23–54) and 37 (23–60) y, respectively. Participants were self-identified as Caucasian (7 women, 5 men), African-American (2 women, 2 men), Asian (1 man), or Hispanic (1 man). Concomitant medications included oral/vaginal ring contraceptive therapy (2 Caucasian women), hydrochlorothiazide (1 Caucasian woman), low-dose aspirin (1 African-American man), multivitamin, calcium, folate, and ω-3 fatty acid supplements (3 women, 3 men). Prior to enrollment, each volunteer underwent a medical history, physical examination, and laboratory tests (i.e., liver function tests, basic metabolic panel, complete blood count). All women underwent a serum pregnancy test. Subjects were instructed to abstain from all fruit juices for ≥7 d prior to and during the study and to abstain from alcohol- and caffeine-containing beverages the evening before each admission. Each subject was assigned randomly to 1 of 6 treatment sequences: ABC, CAB, BCA, CBA, BAC, or ACB (A = water, B = GFJ, C = mGFJ).
Study design
Eligible volunteers were admitted to the CTRC the evening prior to each of three study phases, which were separated by ≥10 d. All women underwent a repeat serum pregnancy test on the evening of each admission. Following an overnight fast beginning at midnight and placement of an indwelling venous catheter in an antecubital vein, each subject ingested two 60 mg fexofenadine tablets (Prasco Laboratories, Mason, OH) with 240 ml water, GFJ, or mGFJ. Blood (7 ml) was collected into EDTA-containing tubes (Becton-Dickinson, Franklin Lakes, NJ) before and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12 h after fexofenadine administration. Subjects continued to fast until after the 4-h blood collection, after which meals and snacks, devoid of fruit juices and caffeinated beverages, were provided. Vital signs were recorded at baseline and monitored periodically. Subjects were discharged after the 12-h blood collection and returned for outpatient blood draws at 24, 36, 48, and 72 h post-fexofenadine administration. Plasma was separated from blood cells by centrifugation within 1 h of collection, transferred into cryovials, and stored at −80°C pending analysis for fexofenadine.
Analysis of plasma for fexofenadine
Plasma collections were processed by transferring 50 μl to a 96-well plate insert and precipitating proteins with 150 μl of methanol containing fexofenadine-d6 (1 nmol/l) as internal standard (Toronto Research Chemicals Inc., Toronto, ON, Canada). The mixtures were vortex-mixed for 5 min and centrifuged (3000 x g for 10 min at 4°C). Calibration solutions (0.0012–7.2 μmol/l) and quality controls (0.005, 0.05, 0.1, 0.5, 1 μmol/l) were prepared similarly using fexofenadine and multiple-donor pooled plasma (Biological Specialty Corporation, Colmar, PA). Plasma was analyzed for fexofenadine by HPLC-tandem mass spectrometry using an API 4000 triple quadrupole with TurboIonSpray interface (Applied Biosystems/MDS Sciex, Concord, ON, Canada) as described.15 Briefly, 5 μl were injected, and fexofenadine and fexofenadine-d6 were eluted from an Aquasil C18 column (2.1 × 50 mm, particle diameter = 5 μm; Thermo Fisher Scientific, Waltham, MA) using a mobile phase gradient (A: 0.1% formic acid in water; B: 0.1% formic acid in methanol) at a flow rate of 0.75 ml/min. The mass spectrometer was operated in positive-ion mode. Multiple reaction monitoring was used to detect fexofenadine (502 → 466 m/z) and fexofenadine-d6 (508 → 472 m/z). The lower limit of quantification was 0.0012 μmol/l; inter- and intra-day coefficients of variation were 12 and <15%, respectively, for the quality controls.
Data analysis
OATP1A2- and OATP2B1-mediated uptake, in the absence or presence of inhibitor, was normalized for protein content. Net uptake was determined by subtracting the uptake in mock-transfected cells from that in OATP1A2- and OATP2B1-expressing cells incubated under parallel conditions.
Fexofenadine pharmacokinetics were evaluated by non-compartmental methods using WinNonlin (v 5.2, Pharsight Corp., Mountain View, CA). The terminal elimination rate constant (λz) was estimated by log-linear regression of at least the last three data points in the terminal phase of the plasma concentration-time profile. The terminal half-life (t½) was calculated as 0.693/λz. The maximum concentration (Cmax), time to reach Cmax (tmax), and the last measured concentration (Clast) were determined visually from the concentration-time profile. AUC0-last was calculated using the trapezoidal rule with linear up/log down interpolation. AUC from zero to infinite time (AUC0-∞) was calculated as the sum of AUClast and Clast/λz. Apparent oral clearance (Cl/F) was calculated as Dose/AUC0-∞. Below limit of quantification concentrations were excluded from data analysis.
Statistical analysis
Statistical analysis employed SigmaPlot (v 11, Systat Software, Inc., San Jose, CA). In vitro data are presented as means ± SDs of triplicate incubations. Two-way analysis of variance followed by Tukey’s test was used to test for differences between vehicle and inhibitor treatments. Student’s unpaired t-test was used to test for differences between mock- and OATP-transfected cells. A p-value < 0.05 was considered statistically significant. Pharmacokinetic outcomes (t½, Cmax, AUC0-∞, Cl/F) are reported as geometric means with coefficients of variation (CV%). Medians and ranges are reported for tmax. The Wilcoxon signed-rank test was used to compare tmax. Pharmacokinetic outcomes, expressed as the ratio of GFJ to water or GFJ to mGFJ, are reported as geometric means with 90% confidence intervals. Comparisons using one-tailed paired Student’s t-tests (given the uni-directional nature of the interaction) with Bonferroni correction (i.e., 0.05/2) were used to detect differences between water and GFJ and between GFJ and mGFJ treatments. A p-value ≤ 0.025 was considered statistically significant. Based on the method proposed by Lauzon and Caffo,16 for the primary pharmacokinetic measure of interest, AUC, a minimum of 18 subjects who completed all treatment phases was deemed adequate for this study, assuming a within-subject CV of 23% for fexofenadine determined from a previous study.10 The randomization scheme was generated using SAS (v 9.2, SAS Institute Inc., Cary, NC).
RESULTS
Representative furanocoumarin, polymethoxyflavone, and flavanone concentrations in grapefruit juice and modified grapefruit juice
Due to the >5-y lapse since last use,5,13 GFJ and mGFJ were re-analyzed for representative compounds from each of three phytochemical classes shown to inhibit intestinal OATP activity in vitro (Table 1). Relative to GFJ, the representative furanocoumarins DHB and bergamottin in mGFJ were reduced by >99% and 95%, respectively. The representative polymethoxyflavones nobiletin and tangeretin were reduced by 95 and 73%, respectively. The representative flavanones naringin, narirutin, and hesperidin were reduced by ~30, 32, and 44%, respectively. Mean (±SD) aggregate representative furanocoumarins, polymethoxyflavones, and flavanones measured in GFJ (48 ± 1, 0.48 ± 0.03, and 1,064 ± 10 μmol/l, respectively) and in mGFJ (0.6 ± 0.1, 0.05 ± 0.02, and 727 ± 14 μmol/l, respectively) were consistent with those measured initially (GFJ: 59 ± 2, 0.71 ± 0.03, and 1,012 ± 24 μmol/l; mGFJ: 0.3 ± 0.01, 0.03 ± 0.01, and 787 ± 69 μmol/l).5,13 The net loss of ≤3% indicated negligible degradation over the >5-y storage period.
Table 1.
Concentrations of representative furanocoumarins, polymethoxyflavones, and flavanones in grapefruit juice (GFJ) and modified GFJ (mGFJ)
| Mean Concentration (μmol/l) (SD) | ||
|---|---|---|
| Constituent | GFJ | mGFJ |
| Furanocoumarins | ||
| DHB | 38.8 (0.3) | 0.13 (0.01) |
| Bergamottin | 9.07 (0.04) | 0.50 (0.08) |
| Polymethoxyflavones | ||
| Nobiletin | 0.37 (0.02) | 0.02 (0.01) |
| Tangeretin | 0.11 (0.01) | 0.03 (0.01) |
| Flavanones | ||
| Naringin | 770 (5) | 531 (8) |
| Narirutin | 271 (4) | 183 (5) |
| Hesperidin | 24.6 (1.2) | 14.7 (0.6) |
DHB, 6′,7′-dihydroxybergamottin
Effects of grapefruit juice and modified grapefruit juice extracts on estrone 3-sulfate and fexofenadine uptake in OATP-transfected cells
Ethyl acetate extracts of GFJ and mGFJ were evaluated as inhibitors of OATP1A2- and OATP2B1-mediated uptake in COS-1 and HEK293T/17 cells using the probe substrate estrone 3-sulfate (E1S) to assess functional activity of the juices prior to clinical study conduct. Both juice extracts inhibited E1S uptake in COS-1 cells by at least 50% relative to vehicle (Figure 1A and 1B). Single-strength GFJ and mGFJ extracts inhibited OATP1A2-mediated uptake by 70 and 80%, respectively; double-strength extracts inhibited uptake by 84 and 50%, respectively (Figure 1A). Single-strength GFJ and mGFJ extracts inhibited OATP2B1-mediated uptake by 78 and 61%, respectively; double-strength extracts inhibited uptake by 52 and 74%, respectively (Figure 1B). Both juice extracts inhibited E1S uptake in HEK293T/17 cells by at least 45% relative to vehicle (Figure 1C and 1D). Single-strength GFJ and mGFJ extracts inhibited OATP1A2-mediated uptake by 90 and 62%, respectively; double-strength extracts inhibited uptake by 80 and 74%, respectively (Figure 1C). Single-strength GFJ and mGFJ extracts inhibited OATP2B1-mediated uptake by 74 and 59%, respectively; double-strength extracts inhibited uptake by 60 and 45%, respectively (Figure 1D). BSP, a non-specific OATP inhibitor, inhibited uptake by >80% in all cell systems.
Figure 1.
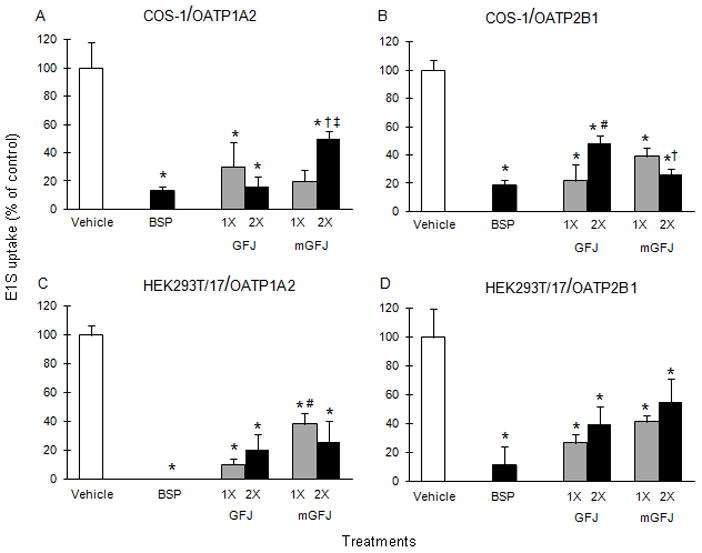
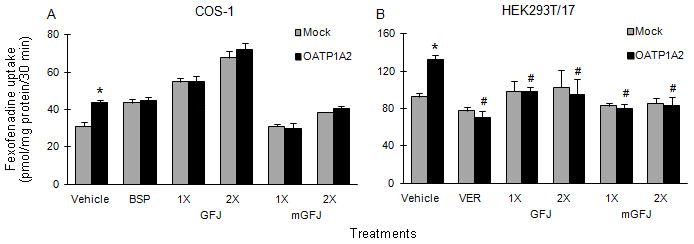
COS-1 cells transiently overexpressing OATP1A2 were employed initially to gain mechanistic insight into inhibition of fexofenadine uptake by GFJ and mGFJ. Due to high uptake activity in mock (EYFP)-transfected cells with the juice extracts and BSP (Figure 2A), OATP1A2 was expressed transiently in HEK293T/17 cells, in which uptake in mock-transfected cells was more consistent (Figure 2B). Relative to vehicle-treated OATP1A2-transfected cells, both single- and double-strength GFJ extract inhibited fexofenadine uptake by ~27%. Both single- and double-strength mGFJ extract inhibited uptake by ~40%. Because BSP did not inhibit fexofenadine uptake in HEK293T/17 cells, VER was used as an alternate OATP1A2 inhibitor, inhibiting by ~50%.
Figure 2.
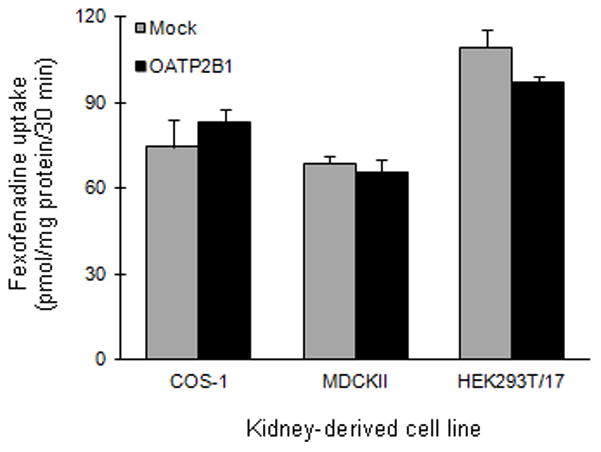
OATP2B1-mediated uptake of fexofenadine was evaluated in transiently transfected COS-1 and stably transfected MDCKII cells (Figure 3). Due to the possibility of cell line-dependent expression/uptake, OATP2B1 was expressed in HEK293T/17 cells. Fexofenadine uptake was not observed with any cell line.
Figure 3.
Effects of grapefruit juice and modified grapefruit juice on fexofenadine pharmacokinetics in healthy volunteers
The effects of GFJ and mGFJ on fexofenadine pharmacokinetics were compared in 18 healthy participants. None of the participants withdrew from the study. Each treatment was well tolerated by all participants. No side effects were reported.
The geometric mean concentration-time profiles of fexofenadine in the presence of GFJ and mGFJ were nearly superimposable (Figure 4). The percentage of fexofenadine AUC extrapolated to infinite time (AUC0-∞) was <10% in all subjects and in all phases. Relative to water, GFJ and mGFJ decreased geometric mean AUC0-∞ by ~25% (Table 2). The geometric mean AUC0-∞s were similar between GFJ and mGFJ (Table 2). Both juices increased geometric mean Cl/F by ~33% (Table 2). Relative to water, both juices decreased geometric mean Cmax by ~23% (Table 2). The geometric mean terminal t½ and median tmax did not differ between treatments (Table 2).
Figure 4.
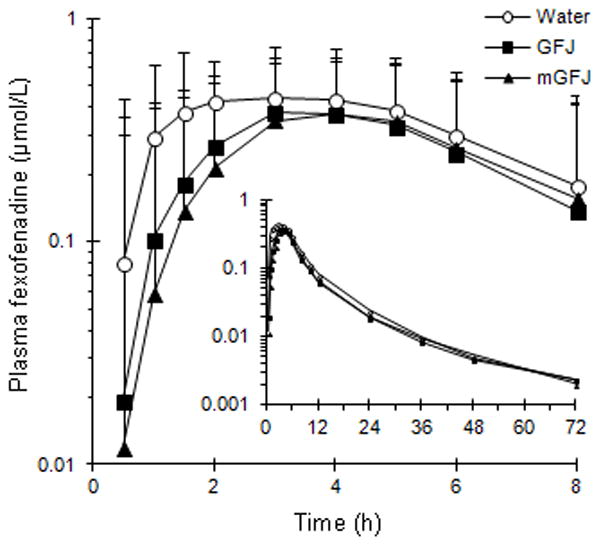
Table 2.
Pharmacokinetics of fexofenadine (120 mg) in 18 healthy volunteers after administration with 240 ml of water, grapefruit juice (GFJ), or modified GFJ (mGFJ)
| Outcome | Geometric Mean (CV%) | Geometric Mean Ratio (90% CI) | |||
|---|---|---|---|---|---|
| Water | GFJ | mGFJ | GFJ/Water | GFJ/mGFJ | |
| AUC0-∞ (μmol/l ·h) | 4.22 (40.0) | 3.22a (33.5) | 3.15 (28.6) | 0.76 (0.3–1.4) | 0.98 (0.4–1.5) |
| Cl/F (l/h) | 52.8 (40.0) | 69.3b (33.5) | 70.9 (29.6) | 1.31 (0.70–1.92) | 1.02 (0.45–1.60) |
| Cmax (μmol/l) | 0.57 (52.2) | 0.45c (43.7) | 0.44 (32.4) | 0.78 (0.17–1.39) | 0.97 (0.35–1.60) |
| t½ (h) | 11.9 (36.5) | 10.3d (37.6) | 10.3 (29.7) | 0.86 (0.28–1.45) | 1.01 (0.43–1.58) |
| tmax (h) [median (range)] | 3 (1–6) | 3.5e (1.5–5) | 4 (2–6) | ||
AUC0-∞, area the curve from time zero to infinity; Cl/F, apparent oral clearance; Cmax, maximum concentration; t½, terminal half-life; tmax, time to Cmax
Statistical comparisons for all outcomes except tmax were made between water and GFJ and between GFJ and mGFJ using a one-tailed paired Student’s t-test with Bonferroni correction (p < 0.025).
p = 0.008
p = 0.023
p = 0.011
p = 0.11
p = 0.135 (Wilcoxon signed-rank test)
DISCUSSION
Since discovery of the fruit juice-OATP interaction, candidate inhibitors of OATP isoforms such as OATP1A2 and OATP2B1 have been proposed and evaluated for effects on intestinal and hepatic drug uptake.17–20 The GFJ and mGFJ tested in the current work were compared previously in two clinical studies involving the CYP3A substrate felodipine and the dual CYP3A/P-gp substrate cyclosporine. The felodipine study established furanocoumarins, in aggregate, as major inhibitors of enteric CYP3A.5 The cyclosporine study further substantiated furanocoumarins as major inhibitors of enteric CYP3A, and likely P-gp; in addition, polymethoxyflavones were ruled out as inhibitors of enteric P-gp.13 Based on these observations, this unique GFJ-mGFJ combination permitted both in vitro and clinical evaluation of the collective impact of furanocoumarins and polymethoxyflavones on the absorption of a growing class of drugs whose enteric uptake depends on OATPs. Because no “clean” OATP substrates suitable for human use have been identified, coupled with prior knowledge of the GFJ-fexofenadine interaction,6–10 fexofenadine was selected as a third prototypic probe substrate to test with this GFJ and mGFJ.
Before clinical testing, both the original GFJ and mGFJ were evaluated for OATP inhibitory activity using OATP1A2- and OATP2B1-overexpressing cell systems and the probe substrate E1S. Compared to vehicle, both juice extracts inhibited both OATP isoforms by >50% in COS-1 and HEK293T/17 cells. Inhibition of E1S uptake by both extracts also was observed in stably transfected MDCKII-OATP2B1 cells (data not shown) and was consistent with previous observations with stably transfected HEK293 cells and dilutions of whole GFJ.12 The similar extents of OATP inhibition by the two extracts in HEK293T/17 cells predicted that systemic fexofenadine exposure in the clinical study would be comparable between juice treatments.
As anticipated, relative to water, GFJ decreased the geometric mean systemic exposure (AUC, Cmax) to fexofenadine. This observation, coupled with the lack of an effect on geometric mean terminal half-life, was consistent with inhibition of uptake in the intestine by GFJ. The decrease in AUC (~25%) was lower than that reported in previous GFJ-fexofenadine studies (31 to 67%),6–10 which could be attributed to the highly variable concentrations of bioactive ingredients among commercial brands and batches of GFJ.21,22 The modest decrease in AUC reflects considerable interindividual variability in magnitude of effect, which ranged from −68 to +57% among the 18 subjects. This variability could be attributed in part to polymorphisms in the genes that encode for OATP (SLCO), as well as P-gp (MDR1); however, genotyping for relevant polymorphisms was beyond the scope of this study.
As predicted by the enteric OATP-transfected cells using E1S as the probe substrate, mGFJ decreased geometric mean systemic fexofenadine exposure to a similar extent as GFJ. The nearly identical effects by both juices on fexofenadine pharmacokinetics indicated furanocoumarins and polymethoxyflavones are not major mediators of the GFJ-fexofenadine interaction. Elimination of furanocoumarins as major in vivo inhibitors of enteric OATPs is consistent with the single clinical study involving 12 healthy volunteers administered fexofenadine (120 mg) and an aqueous suspension (300 ml) of a particulate fraction of GFJ, which contained mostly furanocoumarins (measurements not provided) and a relatively trivial concentration (34 μmol/l) of the flavanone naringin.10 Fexofenadine AUC in the presence of the particulate fraction was similar to that in the presence of water. Naringin also was tested in the same clinical study10 at the same concentration as that in whole GFJ (~1,200 μmol/l); the single constituent explained only half of the reduction in fexofenadine exposure caused by GFJ, indicating other constituents, possibly other flavanones (e.g., narirutin, hesperidin), contribute to the interaction.
As aforementioned, fexofenadine is not a clean OATP substrate. Numerous investigators have shown that fexofenadine also is a P-gp substrate.23–27 The diluted extracts of the clinically administered juices were tested as inhibitors of fexofenadine uptake in various OATP1A2- or OATP2B-transfected kidney-derived cell lines. Initial optimization studies with COS-1 cells demonstrated that fexofenadine was a weak substrate for OATP1A2. Low fexofenadine uptake by OATP1A2, coupled with high background activity, rendered difficulty in ascertaining the effects of the juice extracts. However, unlike COS-1 cells, the juice extracts did not significantly alter fexofenadine uptake in mock-transfected HEK293T/17 cells. Due to higher fexofenadine uptake activity in OATP1A2-transfected HEK293T/17 cells, comparable inhibition by both juice extracts was demonstrated. In addition to P-gp and OATP, apical and basolateral efflux mediated by multidrug resistant protein (MRP) 2 and MRP3, respectively, have been shown to contribute to fexofenadine transport in vitro.28 Studies with transiently transfected COS-1 and HEK293T/17 cells, as well as stably transfected MDCKII cells, demonstrated that fexofenadine is not a substrate for OATP2B1. Conflicting in vitro data have been reported regarding OATP2B1-mediated transport of fexofenadine. Fexofenadine uptake in transiently transfected HeLa and stably transfected HEK293 cells either was not evident or was weak.9,29,30 However, an independently generated MDCKII-OATP2B1 cell line showed an ~3-fold difference in fexofenadine uptake compared to mock-transfected cells.28 Fexofenadine has been shown to be a substrate of OATP2B1 in a Xenopus laevis oocyte system.31 The discrepancy between in vitro experiments with fexofenadine may be attributed to multiple binding sites on OATP2B1 with different affinities, but to date, substrate-dependent binding has been demonstrated only with transfected oocytes.32
In summary, current in vitro and clinical observations collectively indicate that furanocoumarins and polymethoxyflavones are not major mediators of the GFJ-fexofenadine interaction. Flavanones are likely candidate inhibitors of enteric OATPs in vivo. In vitro and clinical studies accounting for natural product ingredient composition are essential for appropriate evaluation, translation, and optimal pharmacotherapeutic management of grapefruit juice- and other natural product-drug interactions. A bioactivity-guided fractionation approach similar to that used to identify intestinal CYP3A inhibitors in cranberry33 and hepatic OATP inhibitors in Rollinia emarginata34 can be used to identify specific causative ingredients. Identification of such OATP inhibitors would permit evaluation and prediction of the interaction liability of GFJ and other foods containing the same compounds with other existing and potential OATP substrates, including some fluoroquinolones, beta-blockers, and statins.35 Finally, confirmation of causative mediators may impact food manufacturing practices, leading to advances designed to minimize interference with drugs, including removal of compounds via chemical processing5 or plant gene engineering.36
Acknowledgments
This work was supported by National Institutes of Health grants R01 GM077482, R01 DK047987, and UL1RR025747 (now UL1TR000083). WinNonlin software was provided to the Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, by Certara as a member of the Pharsight Academic Center of Excellence Program. The authors thank Dr. Arlene S. Bridges (UNC ADME Mass Spectrometry Center) for assistance with the development of the HPLC-tandem mass spectrometry assay and analysis of plasma; the staff and study subjects of the University of North Carolina Hospital CTRC; Dr. Markus Grube (Ernst-Moritz-Arndt University, Greifswald, Germany) for providing MDCKII cells; Dr. Dhiren R. Thakker (UNC Eshelman School of Pharmacy) for providing [3H]fexofenadine and scientific advice with respect to the in vitro studies; and Dr. J. Heyward Hull (UNC Eshelman School of Pharmacy) for guidance with respect to clinical study design. MFP dedicates this article to Dr. David P. Paine.
Footnotes
The authors have no disclosures and declare no personal or financial conflicts of interest.
References
- 1.Hanley MJ, Cancalon P, Widmer WW, Greenblatt DJ. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7(3):267–286. doi: 10.1517/17425255.2011.553189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99(10):2545–2553. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmiedlin-Ren P, Edwards DJ, Fitzsimmons ME, et al. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos. 1997;25(11):1228–1233. [PubMed] [Google Scholar]
- 4.Guo LQ, Fukuda K, Ohta T, Yamazoe Y. Role of furanocoumarin derivatives on grapefruit juice mediated inhibition of human CYP3A activity. Drug Metab Dispos. 2000;28(7):766–771. [PubMed] [Google Scholar]
- 5.Paine MF, Widmer WW, Hart HL, et al. A furanocoumarin-free grapefruit juice establishes furanocoumarins as the mediators of the grapefruit juice-felodipine interaction. Am J Clin Nutr. 2006;83(5):1097–1105. doi: 10.1093/ajcn/83.5.1097. [DOI] [PubMed] [Google Scholar]
- 6.Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71(1):11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 7.Banfield C, Gupta S, Marino M, Lim J, Affrime M. Grapefruit juice reduces the oral bioavailability of fexofenadine but not desloratadine. Clin Pharmacokinet. 2002;41(4):311–318. doi: 10.2165/00003088-200241040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Dresser GK, Kim RB, Bailey DG. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: possible role of organic anion transporting polypeptides. Clin Pharmacol Ther. 2005;77(3):170–177. doi: 10.1016/j.clpt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362–370. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 10.Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81(4):495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 11.Allegra [package insert] Bridgewater, NJ: sanofi-aventis; 2008. Dec, [Google Scholar]
- 12.Satoh H, Yamashita F, Tsujimoto M, et al. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–523. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- 13.Paine MF, Widmer WW, Pusek SN, et al. Further characterization of a furanocoumarin-free grapefruit juice on drug disposition: studies with cyclosporine. Am J Clin Nutr. 2008;87(4):863–871. doi: 10.1093/ajcn/87.4.863. [DOI] [PubMed] [Google Scholar]
- 14.Lan T, Rao A, Haywood J, et al. Interaction of macrolide antibiotics with intestinally expressed human and rat organic anion-transporting polypeptides. Drug Metab Dispos. 2009;37(12):2375–2382. doi: 10.1124/dmd.109.028522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R, Kalvass JC, Yanni SB, Bridges AS, Pollack GM. Fexofenadine brain exposure and the influence of blood-brain barrier P-glycoprotein after fexofenadine and terfenadine administration. Drug Metab Dispos. 2009;37(3):529–535. doi: 10.1124/dmd.107.019893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauzon C, Caffo B. Easy multiplicity control in equivalence testing using two one-sided tests. Am Stat. 2009;63(2):147–154. doi: 10.1198/tast.2009.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenblatt DJ. Analysis of drug interactions involving fruit beverages and organic anion- transporting polypeptides. J Clin Pharmacol. 2009;49(12):1403–1407. doi: 10.1177/0091270009342251. [DOI] [PubMed] [Google Scholar]
- 18.Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70(5):645–655. doi: 10.1111/j.1365-2125.2010.03722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica. 2008;38(7–8):778–801. doi: 10.1080/00498250801986951. [DOI] [PubMed] [Google Scholar]
- 20.König J. Uptake transporters of the human OATP family: molecular characteristics, substrates, role in drug-drug interactions, and functional consequences of polymorphisms. Handb Exp Pharmacol. 2011;(201):1–28. doi: 10.1007/978-3-642-14541-4_1. [DOI] [PubMed] [Google Scholar]
- 21.Ho PC, Saville DJ, Coville PF, Wanwimolruk S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharm Acta Helv. 2000;74(4):379–385. doi: 10.1016/s0031-6865(99)00062-x. [DOI] [PubMed] [Google Scholar]
- 22.De Castro WV, Mertens-Talcott S, Rubner A, Butterweck V, Derendorf H. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006;54(1):249–255. doi: 10.1021/jf0516944. [DOI] [PubMed] [Google Scholar]
- 23.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27(8):866–871. [PubMed] [Google Scholar]
- 24.Perloff MD, von Moltke LL, Greenblatt DJ. Fexofenadine transport in Caco-2 cells: inhibition with verapamil and ritonavir. J Clin Pharmacol. 2002;42(11):1269–1274. doi: 10.1177/009127002762491370. [DOI] [PubMed] [Google Scholar]
- 25.Tannergren C, Petri N, Knutson L, Hedeland M, Bondesson U, Lennernäs H. Multiple transport mechanisms involved in the intestinal absorption and first-pass extraction of fexofenadine. Clin Pharmacol Ther. 2003;74(5):423–436. doi: 10.1016/S0009-9236(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 26.Petri N, Tannergren C, Rungstad D, Lennernäs H. Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm Res. 2004;21(8):1398–1404. doi: 10.1023/b:pham.0000036913.90332.b1. [DOI] [PubMed] [Google Scholar]
- 27.Tahara H, Kusuhara H, Fuse E, Sugiyama Y. P-glycoprotein plays a major role in the efflux of fexofenadine in the small intestine and blood-brain barrier, but only a limited role in its biliary excretion. Drug Metab Dispos. 2005;33(7):963–968. doi: 10.1124/dmd.105.004192. [DOI] [PubMed] [Google Scholar]
- 28.Ming X, Knight BM, Thakker DR. Vectorial transport of fexofenadine across Caco-2 cells: involvement of apical uptake and basolateral efflux transporters. Mol Pharm. 2011;8(5):1677–1686. doi: 10.1021/mp200026v. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu M, Fuse K, Okudaira K, et al. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33(10):1477–1481. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- 30.Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004;308(2):438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 31.Imanaga J, Kotegawa T, Imai H, et al. The effects of the SLCO2B1 c. 1457C>T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet Genomics. 2011;21(2):84–93. doi: 10.1097/fpc.0b013e32834300cc. [DOI] [PubMed] [Google Scholar]
- 32.Shirasaka Y, Mori T, Shichiri M, Nakanishi T, Tamai I. Functional pleiotropy of organic anion transporting polypeptide OATP2B1 due to multiple binding sites. Drug Metab Pharmacokinet. 2012;27(3):360–364. doi: 10.2133/dmpk.dmpk-11-sh-080. [DOI] [PubMed] [Google Scholar]
- 33.Kim E, Sy-Cordero A, Graf TN, Brantley SJ, Paine MF, Oberlies NH. Isolation and identification of intestinal CYP3A inhibitors from cranberry (Vaccinium macrocarpon) using human intestinal microsomes. Planta Med. 2011;77(3):265–270. doi: 10.1055/s-0030-1250259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth M, Araya JJ, Timmermann BN, Hagenbuch B. Isolation of modulators of the liver-specific organic anion-transporting polypeptides (OATPs) 1B1 and 1B3 from Rollinia emarginata Schlecht (Annonaceae) J Pharmacol Exp Ther. 2011;339(2):624–632. doi: 10.1124/jpet.111.184564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Lima Toccafondo Vieira M, Huang SM. Botanical-drug interactions: a scientific perspective. Planta Med. 2012;78(13):1400–1415. doi: 10.1055/s-0032-1315145. [DOI] [PubMed] [Google Scholar]
- 36.Greenblatt DJ, Zhao Y, Hanley MJ, et al. Mechanism-based inhibition of human cytochrome P450-3A activity by grapefruit hybrids having low furanocoumarin content. Xenobiotica. 2012;42(12):1163–1169. doi: 10.3109/00498254.2012.700428. [DOI] [PubMed] [Google Scholar]


