Abstract
Background
Re-dissection of discarded lung resection specimens after routine pathology examination reveals missed lymph node metastasis. We sought to determine if size can be used to grossly select lymph nodes for microscopic examination.
Methods
Prospective cohort study of lymph nodes retrieved from discarded lung resection specimens. The association between size and histologic characteristics of retrieved material was compared by the Wilcoxon-Mann-Whitney test.
Results
We retrieved 1094 grossly ‘lymph node-like’ tissue from 112 remnant lung resection specimens, of which 345 (32%) proved not to be lymph nodes, and 71 of 749 (9%) lymph nodes had metastasis. Metastasis was present in discarded nodes in 26 of 112 (23%) patients. The non-lymph node tissue was significantly smaller than lymph nodes (p<0.0001); lymph nodes with metastases were significantly larger than those without metastases (p <0.0001). However, there was significant size overlap between the three types of grossly ‘lymph node-like’ tissue. Thirty two percent of nodes with metastasis were <1cm; 15% of patients had at least 1 lymph node <1cm with metastasis.
Conclusions
The size difference between lymph nodes with and without metastasis is clinically unhelpful because of broad overlap. Size is insufficiently discriminatory and cannot be relied on to select materials for histologic examination. A third of grossly retrieved material was non-lymph node tissue. This probably occurs during routine pathologic examination and likely contributes to the low N1 lymph node count.
Keywords: staging, nodal metastasis, quality of care
INTRODUCTION
Long-term survival after resection for non-small cell lung cancer (NSCLC) most closely correlates with the presence, extent, and number of lymph node metastasis.1–4 However, current pathologic nodal staging is insufficiently discriminatory of long-term lung cancer mortality risk. Forty four percent of patients with resected pathologic N0 (pN0) NSCLC die within 5 years.1 There is a direct correlation between the number of lymph nodes examined and survival in patients with pN0, despite similar stage.5–8 In those with known lymph node metastasis, the number and ratio of lymph nodes with metastasis is also of prognostic value.2–4,9–11 The thoroughness of pathologic nodal examination is therefore of paramount importance in the resection population, irrespective of nodal stage. This includes the thoroughness of examination of hilar/intrapulmonary (N1) lymph nodes. The Association of Directors of Anatomic and Surgical Pathology recommends that all lymph nodes within a lung resection specimen should be examined.12
Opportunity exists to improve the quality of nodal examination of lung resection specimens. In the US, 18% of patients with ‘node-negative’ NSCLC resections actually have no lymph nodes examined. These patients have a 5-year survival curve similar to patients with pN1.13 Only a median of 3–5 lymph nodes are examined in patients with resected pN0 NSCLC, significantly lower than the optimal number associated with the highest long-term survival, estimated to be between 11 and 21 nodes.5–8
The opportunity for quality improvement includes the intraoperative collection of hilar and mediastinal lymph nodes, to which pathologists have no direct access.14,15 However a thorough retrieval and examination of intrapulmonary lymph nodes can partially off-set the problem of incomplete intraoperative nodal mapping, because of strong correlation between intrapulmonary lymph node metastasis and the likelihood of mediastinal lymph node metastasis.2–4 Moreover, thorough examination of the resection specimen poses no additional risk to the patient.
Current pathology practice focuses the search for intrapulmonary lymph nodes mostly to the hilum of the resection specimen.16,17 Lymph nodes are grossly identified by visual (anthracotic appearance), textural and size characteristics. Because of the general belief that big lymph nodes are more likely to contain metastasis, large nodes are more likely to be retrieved for histologic examination. This principle is similar to radiologic nodal staging which primarily uses size criteria. However, CT prediction of lymph node metastasis is insensitive, non-specific, and unreliable.18
We previously demonstrated a high proportion of unexamined intrapulmonary lymph nodes in discarded lung resection specimens, some of which harbored metastasis.19 In the current report we evaluated the correlation between the size and histologic characteristics of the material retrieved from discarded lung resection specimens, which, but for our re-dissection protocol, would have been permanently lost to examination.
MATERIALS AND METHODS
With the permission of the Institutional Review Boards of the hospital systems from which the lung resection specimens were obtained, we tested the hypothesis that low N1 lymph node counts suggest incomplete retrieval of intrapulmonary lymph nodes during the routine pathology examination by re-dissecting discarded remnant lobar, or greater, lung cancer resection specimens. We have previously described our protocol.19 Briefly, we subjected remnant lung resection specimens to a thin (3–5mm) section re-dissection protocol designed to extract all material that grossly appeared to be lymph nodes, irrespective of their size.
We measured the largest diameter of all specimens and placed each in a separate cassette for processing for histology. A single hematoxylin and eosin (H&E) stained slide was made from each specimen, for examination by a pathologist who determined if the specimen was a lymph node, and if each lymph node had metastasis. We identified lymph nodes histologically by the combination of their rounded contour, the presence of a capsule, sub-capsular sinus and lymphoid follicles. Nests of cancer cells without all of these criteria were identified as satellite metastatic nodules and were not counted as lymph node metastasis. The presence of metastasis was identified solely by H&E staining.19
Specimen sizes were compared by histologic characteristics using the Wilcoxon Mann Whitney test. Associations between categorical variables were compared using the Chi-Square test. We set a p-value <0.05 as the level for rejection of the null hypothesis.
RESULTS
Tumor characteristics
We retrieved 1094 grossly lymph node-like materials from 112 lung resection specimens. Majority of cases (56%) were adenocarcinomas, 80% were T1 or T2 tumors and 70% were said to be pN0 after routine pathology examination (Table 1).
Table 1.
Clinical characteristics and findings on histologic examination.
| Characteristic | N (%) | |
|---|---|---|
| Histology | ||
| Adenocarcinoma | 63 (56) | |
| Squamous | 32 (29) | |
| Large Cell | 2 (2) | |
| Other | 15 (13) | |
| T-category | ||
| 1 | 49 (44) | |
| 2 | 40 (36) | |
| 3 | 18 (16) | |
| 4 | 4 (4) | |
| X | 1 (1) | |
| N-category (after routine examination) | ||
| 0 | 78 (70) | |
| 1 | 18 (16) | |
| 2 | 16 (14) | |
| Number of lymph nodes after routine examination | Mean (SD) | Median (range) |
| From N1 stations | 4.96 (3.51) | 4 (0, 18) |
| From N2 stations | 5.23 (4.14) | 4 (0, 19) |
| Total | 10.18 (5.87) | 10 (1, 30) |
| Number of additional material found on re-dissection | ||
| All (irrespective of histology) | 9.79 (7.35) | 8 (1, 51) |
| Lymph nodes | 6.73 (6.53) | 5 (0, 46) |
| Lymph nodes with metastasis | 0.62 (1.61) | 0 (0, 12) |
Histologic characteristics of retrieved grossly lymph node-like material
Of the 1094 materials retrieved, 345 (32%) were histologically determined not to be lymph nodes (including 11 satellite metastatic nodules), 749 (68%) were lymph nodes. Seventy-one of the 749 lymph nodes (9%) had metastasis (Table 2). Lymph nodes were found in 101 of 112 (90%) lung resection specimens, and lymph node metastasis was found in 26 specimens (23%). Seven of the 26 resections (27%) with lymph node metastasis were found in patients identified as pN0 after the routine pathology examination.
Table 2.
Size (in cm) distribution and histologic characteristics of retrieved lymph nodelike materials.
| Specimen histology | Retrieved material, n (%) | |||
|---|---|---|---|---|
| <0.5 | 0.5 – 0.9 | 1.0 – 1.4 | >1.5 | |
| All specimens | 390 (36)* | 488 (45)* | 157 (14)* | 59 (5)* |
| Non-lymph node | 199 (51)† | 112 (23)† | 24 (15)† | 10 (17)† |
| Lymph node | 191 (49)† | 376 (77)† | 133 (85)† | 49 (83)† |
| without metastasis | 188 (48)† | 356 (73)† | 111 (71)† | 23 (39)† |
| with metastasis | 3 (2)† | 20 (4)† | 22 (14)† | 26 (44)† |
percentage of all retrieved materials,
percentage of all retrieved materials of that size.
Size and histologic characteristics
The most common size of retrieved material was in the 0.5 – 0.9 cm range (45% of all specimens), but 19% of all materials were ≥1cm (Table 2). Twenty-two percent of all retrieved materials ≥1cm were lymph nodes with missed metastasis, and 26% of all retrieved lymph nodes ≥1cm had missed metastasis. Approximately half of all retrieved materials <0.5cm turned out not to be lymph nodes (Table 2).
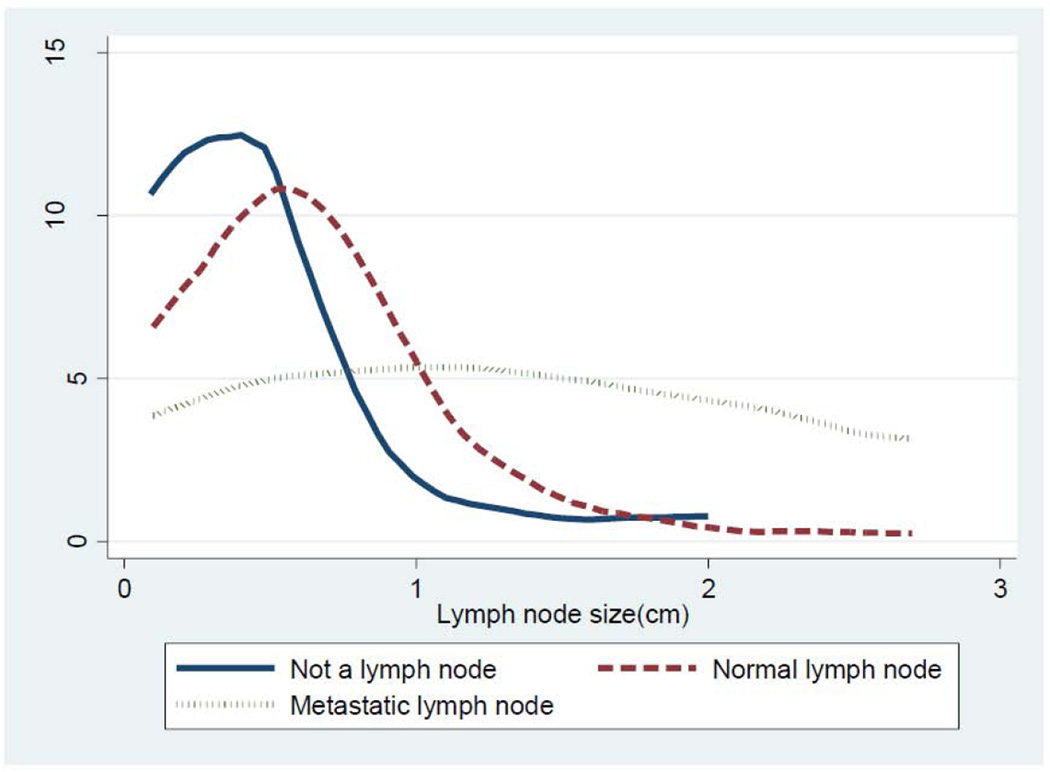
The median size of retrieved non-lymph node tissue was 4 mm, significantly less than the 7 mm median size of lymph nodes (p<0.0001). However, there was considerable overlap in the size range between the materials that were lymph nodes and non-lymph node tissue (Table 3, Figure 1). Similarly, although lymph nodes with metastasis were significantly larger than those without metastases (p <0.0001), there was significant overlap in the range of sizes (Table 3, Figure 1).
Table 3.
Size (in cm) and histologic characteristics of retrieved grossly lymph node-like material. All size units in cm.
| Histology | N (%) | Mean (SD) | Range | Median (IQR) |
|---|---|---|---|---|
| Non-lymph node | 345 (32) | 0.48 (0.36) | 0.09 – 2.0 | 0.4 (0.2 – 0.6) |
| Lymph node | ||||
| All | 749 (68) | 0.75 (0.45) | 0.1 – 2.7 | 0.7 (0.4 – 0.9) |
| No metastasis | 678 (91)* | 0.69 (0.39) | 0.1 – 2.7 | 0.6 (0.4 – 0.8) |
| With metastasis | 71 (9)* | 1.32 (0.62) | 0.1 – 2.7 | 1.2 (0.8 – 1.7) |
Percentage of all lymph node material.
IQR= interquartile range; SD= standard deviation.
Figure 1.
Size distribution of 3 different histologic types of ‘grossly lymph node-like’ tissue retrieved from discarded lung resection specimens.
Patient-level impact
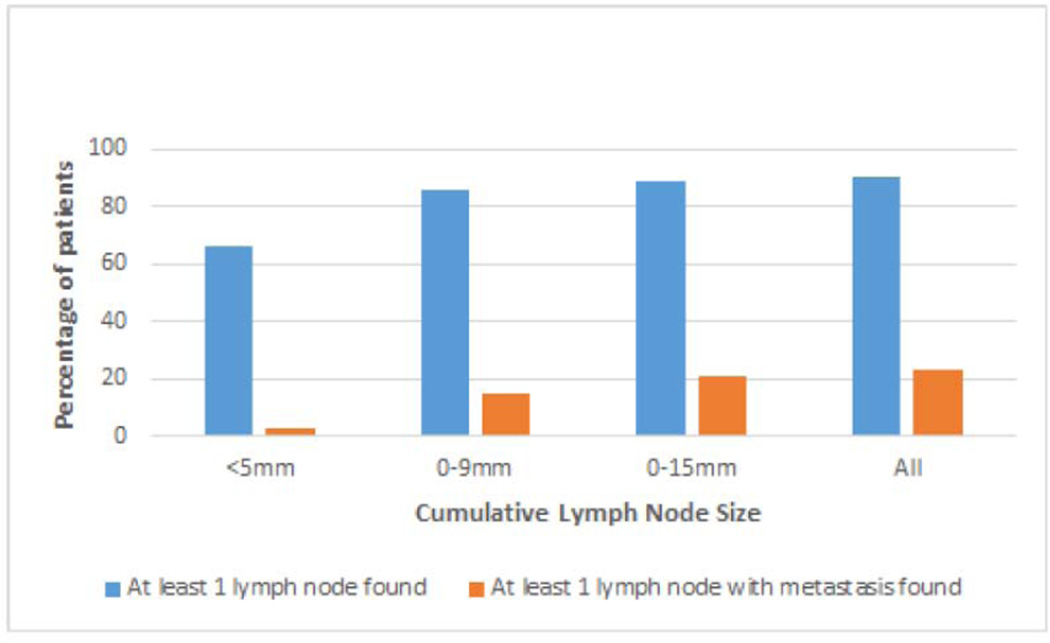
The most common size range of retrieved lymph nodes was 5 to 9 mm, found in 79% of patients (Table 4). In the whole cohort of 112 patients, 3% had at least 1 lymph node less than 5 mm which harbored metastasis and 13% had at least 1 lymph node with metastasis each in the 5 to 9 mm, 10 to 15 mm and greater than 15 mm range (Table 4). Grossly lymph node-like material was retrieved in all 112 remnant lung specimens, at least one of which was histologically confirmed to be a lymph node in 90% of specimens. At least 1 lymph node with metastasis was identified in 23% of all patients (Table 5, Figure 2).
Table 4.
Frequency of finding lymph node material of various sizes (in cm).
| Histologic variable | Number of patients affected (%) | |||
|---|---|---|---|---|
| <0.5 | 0.5 – 0.9 | 1.0 – 1.5 | > 1.5 | |
| At least 1 grossly lymph node-like material | 100 (89%) | 97 (87%) | 62 (55%) | 24 (21%) |
| At least 1 lymph node | 74 (66%) | 88 (79%) | 57 (51%) | 22 (20%) |
| At least 1 lymph node with metastasis | 3 (3%) | 15 (13%) | 14 (13%) | 15 (13%) |
Table 5.
Cumulative frequency distribution of patients with lymph node material of various sizes (in cm).
| Histologic variable | Cumulative number of patients affected, n (%) | |||
|---|---|---|---|---|
| <0.5 | 0 – 0.9 | 0 – 1.5 | All sizes | |
| At least 1 lymph node material | 100 (89%) | 111 (99%) | 112 (100%) | 112 (100%) |
| At least 1 lymph node | 74 (66%) | 96 (86%) | 100 (89%) | 101 (90%) |
| At least 1 lymph node with metastasis | 3 (3%) | 17 (15%) | 24 (21%) | 26 (23%) |
Figure 2.
Cumulative frequency of patients with discarded lymph nodes of various sizes.
DISCUSSION
The importance of accurate pathologic nodal staging of resected NSCLC is reflected in the Association of Directors of Anatomic Pathology recommendation for examination of all lymph nodes in the resection specimen.12 Despite this, the N1 lymph node yield from lung resection specimens is low, even when surgeons specifically dissect stations 10 (hilar), 11 (interlobar), and 12 (lobar) lymph nodes.20,21 There is no universally accepted minimum number of lymph nodes mandated for examination in lung cancer. For example, the International Association for the Study of Lung Cancer Staging Handbook recommends a minimum of 6 total lymph nodes, 3 from N1 stations and 3 from N2 stations.22 However, analyses of large US databases suggest that examination of more than 10 lymph nodes, possibly as many as 18–21, is associated with maximal survival of patients with pN0.5–8 Even in groups of patients with known nodal metastasis, the number (and ratio) of lymph nodes with metastasis is also of prognostic value.2–4,9–11
We found a large number of unexamined lymph nodes in our cohort, 9% of which had metastasis. Ninety percent of the cohort had one or more unexamined lymph nodes, and 23% had at least one unexamined lymph node with metastasis. We also found that one-third of all grossly identified lymph node specimens turned out not to be lymph nodes on microscopic examination. Although the non-lymph node specimens were significantly smaller than the lymph node specimens, the breadth of overlap means that size is relatively unreliable in distinguishing between lymph node and non-lymph node materials. We found the same difficulty in using size to distinguish between lymph nodes with and without metastasis.
Patients with intrapulmonary lymph node metastasis have a significantly worse survival than those with pathologic node-negative non-small cell lung cancer, although their survival is better than that of patients with hilar zone metastasis.23 This suggests the need for histologic examination of all materials that might be lymph nodes, irrespective of their size and location. However, this counsel of perfection raises a frustrating problem because almost half of the retrieved material <0.5cm were not lymph nodes, and only 2% were lymph nodes with metastasis. The quest for optimal thoroughness of examination is therefore associated with diminishing returns as smaller materials are retrieved.
Our findings are similar to those of Prenzel et al who, in their examination of 256 lung resection specimens, found a direct correlation between lymph node size and lymph node metastasis but also found that 44% of lymph nodes with metastasis were less than 10 mm in diameter and that 18% of patients with pathologic N2 disease had no lymph nodes greater than 10 mm in diameter.24 In our series, 23 of 71 (32%) lymph nodes with metastasis were less than 10 mm in diameter (Table 2). Similar findings have also been reported in colon cancer.25 The difference between these reports and ours is that we have examined lymph nodes retrieved after re-dissecting the discarded remains of previously examined lung resection specimens. The information from these lymph nodes would have been permanently lost to post-operative prognostication and clinical decision-making. Our findings further reinforce the unreliability of size in identifying the status of lymph nodes, and suggest a need to modify the current approach to gross retrieval of intrapulmonary lymph nodes (stations 11 to 14).
More speculatively, we hypothesize from our finding of a high percentage of non-lymph node material that a significant proportion of the material obtained during routine gross dissection of lung resection specimens may also turn out not to be lymph nodes. This may partially explain the low overall lymph node counts in routine pathologic examination of lung resection specimens. By extension, we also speculate that this may explain the baffling finding of Little et al that 52% of mediastinoscopy procedures performed in a cross-sectional sample of US lung resections in the National Cancer Data Base did not provide any lymph node material for examination.14 It is plausible that a significant proportion of the submitted specimens were found on microscopy to be non-lymph node tissue.
We recommend modification of the current protocol for gross dissection of lung cancer resection specimens to improve the examination of intrapulmonary lymph nodes. The improved protocol must avoid bias against the collection of small specimens.26 Although thorough examination may seem labor-intensive and time consuming, implementation is feasible even in clinically busy pathology departments, because the time required diminishes with experience.19 We are now testing a modified gross dissection protocol that can be used on fresh lung resection specimens, and which yields significantly more intrapulmonary lymph nodes than the traditional method of gross dissection.
Acknowledgments
Supported by NIH R01CA172253 (Osarogiagbon)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 15th World Conference on Lung Cancer, Sydney, Australia, October 2013.
Contributor Information
Raymond U. Osarogiagbon, Thoracic Oncology Research (ThOR) Group, Multidisciplinary Thoracic Oncology Program, Baptist Cancer Center, Memphis, TN.
Robert A. Ramirez, Louisiana State University, New Orleans, LA.
Christopher G. Wang, University of Alabama Birmingham, Birmingham, AL.
Laura E. Miller, Tulane University, New Orleans, LA.
Laura McHugh, Thoracic Oncology Research Group, Multidisciplinary Thoracic Oncology Program, Baptist Cancer Center, Memphis, TN.
Courtney A. Adair, Duckworth Pathology Group, Memphis, TN
Matthew P. Smeltzer, Department of Epidemiology and Biostatistics, School of Public Health, University of Memphis, Memphis, TN.
Xinhua Yu, Department of Epidemiology and Biostatistics, School of Public Health, University of Memphis, Memphis, TN
Allen Berry, Department of Pathology, St. Francis Hospital, Memphis, TN.
References
- 1.Rusch VW, Crowley J, Giroux DJ, et al. The IASLC lung cancer staging project: Proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603–612. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 2.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–125. [PubMed] [Google Scholar]
- 3.Lee JG, Lee CY, Park IK, Kim DJ, Park SY, Kim KD, Chung KY. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85:211–215. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Wei S, Asamura H, Kawachi R, Sakurai H, Watanabe S. Which is the better prognostic factor for resected non-small cell lung cancer: The number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol. 2011;6:310–318. doi: 10.1097/JTO.0b013e3181ff9b45. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig MS, Goodman M, Miller DL, Johnstone PAS. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 6.Ou SI, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol. 2008;3:880–886. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 7.Varlotto JM, Recht A, Nikolov M, Flickinger JC, DeCamp M. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer. 2009;115:851–858. doi: 10.1002/cncr.23985. [DOI] [PubMed] [Google Scholar]
- 8.Osarogiagbon RU, Ogbata OU, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg. 2013 Nov 20; doi: 10.1016/j.athoracsur.2013.09.058. [Epub ahead of print] PMID: 24266949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonnalagadda S, Smith C, Mhango G, et al. The Number of Lymph Node Metastases as a Prognostic Factor in Patients With N1 Non-small Cell Lung Cancer. CHEST. 2011;140:433–440. doi: 10.1378/chest.10-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonnalagadda S, Arcinega J, Smith C, Wisnivesky JP. Validation of the lymph node ratio as a prognostic factor in patients with N1 non-small cell lung cancer. Cancer. 2011;117:4724–4731. doi: 10.1002/cncr.26093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nwogu CE, Groman A, Hahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg. 2012;93:1614–1620. doi: 10.1016/j.athoracsur.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Association of Directors of Anatomic and Surgical Pathology. Recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Am J Clin Pathol. 2001;115:799–801. doi: 10.1309/685F-X6RF-6TBD-PY0P. [DOI] [PubMed] [Google Scholar]
- 13.Osarogiagbon RU, Yu X. Non-examination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96:1178–1189. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Little AG, Rusch VW, Bonner JA, Gaspar LE, Green MR, Webb R, Stewart AK. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80:2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 15.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early stage non-small cell lung cancer in the surveillance, epidemiology and end results database. J Thorac Oncol. 2012;7:1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 16.Hrubar, Westra, Pelps, Isacson, editors. Surgical Pathology Dissection. 1995. Lungs. [Google Scholar]
- 17.Lester SC, editor. Manual of Surgical Pathology. Second Ed Lung and pleura. [Google Scholar]
- 18.Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol. 2012;30:2823–2828. doi: 10.1200/JCO.2011.39.2589. [DOI] [PubMed] [Google Scholar]
- 20.Allen JW, Farooq A, O’Brien TF, Osarogiagbon RU. Quality of surgical resection for nonsmall cell lung cancer in a US metropolitan area. Cancer. 2011;117:134–142. doi: 10.1002/cncr.25334. [DOI] [PubMed] [Google Scholar]
- 21.Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy. Chest. 2010;138:1–6. doi: 10.1378/chest.10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstraw P. International Association for the Study of Lung Cancer Staging Handbook in Thoracic Oncology. Orange Park, FL: Editorial Rx Press; 2009. [Google Scholar]
- 23.Maeshima AM, Tsuta K, Asamura H, Tsuda H. Prognostic implication of metastasis limited to segmental (level 13) and/or subsegmental (level 14) lymph nodes in patients with surgically resected nonsmall cell lung carcinoma and pathologic N1 lymph node status. Cancer. 2012;118:4512–4518. doi: 10.1002/cncr.27424. [DOI] [PubMed] [Google Scholar]
- 24.Prenzel KL, Monig SP, Sinning JM, Baldus SE, Brochhagen H, Schneider PM, Holscher AH. Lymph node size and metastatic infiltration in non-small cell lung cancer. Chest. 2003;123:463–467. doi: 10.1378/chest.123.2.463. [DOI] [PubMed] [Google Scholar]
- 25.Monig SP, Baldus SE, Zierbes TK, Schroder W, Lindemann DG, Dienes HP, Holscher AH. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol. 1999;6:579–581. doi: 10.1007/s10434-999-0579-1. [DOI] [PubMed] [Google Scholar]
- 26.Arita T, Kuramitsu T, Kawamura M, et al. Bronchogenic carcinoma: incidence of metastases to normal sized lymph nodes. Thorax. 1995;50:1267–1269. doi: 10.1136/thx.50.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]