Abstract
Background
Rotator cuff tendon tears represent a major component of reported orthopaedic injuries. In addition, more than one quarter of U.S. adults either currently have high cholesterol levels or have reduced their previously high cholesterol levels through the use of pharmaceuticals. Our clinical data have already linked hypercholesterolemia to full-thickness rotator cuff tears, and experimental data from our laboratory have shown effects on native tendon properties in multiple species. The objective of this study was to evaluate healing of supraspinatus tendons in our rat rotator cuff injury model. We hypothesized that tendon healing would be inferior in rats receiving a high-cholesterol diet for 6 months compared with those receiving standard chow.
Methods
All animals were subjected to a unilateral supraspinatus detachment and repair surgery, with contralateral limbs serving as within-animal comparative data. Animals continued their respective diet courses, and their supraspinatus tendons were biomechanically or histologically evaluated at 2, 4, and 8 weeks postoperatively.
Results
Biomechanical testing revealed a significant reduction in normalized stiffness in hypercholesterolemic rats compared with controls at 4 weeks after injury, whereas histologic analyses showed no significant differences in collagen organization, cellularity, or cell shape between groups.
Conclusion
On the basis of our findings, hypercholesterolemia may have a detrimental biomechanical effect on tendon healing in our rat rotator cuff injury and repair model.
Level of evidence
Basic Science Study, Animal Model.
Keywords: Hypercholesterolemia, tendon, biomechanics, injury, shoulder, rat, rotator cuff
In 2005 alone, more than 100 million American adults reported myriad musculoskeletal disorders.10 Rotator cuff tendon tears represent a major component of these disorders, with up to half of the population older than 50 years being afflicted.22 Moreover, more than 27% of the U.S. population aged 20 years or older have diagnosed hypercholesterolemia or have reduced their previously high cholesterol level with statins or other lipid-lowering medications.9 The detrimental effects of high total cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL) concentrations on cardiovascular health are well documented.13 From an orthopaedics perspective, hypercholesterolemia has also proved to be a risk factor for tendon rupture because of its link to Achilles tendon thickening,11 tendinopathy,12 and rupture16 and the formation of Achilles tendon xanthomas.6,12,17,20,21
Often, patients presenting to orthopaedic surgeons have little or no knowledge about their serum lipid profiles. One study has reported that orthopaedic patients with often unknown familial hypercholesterolemia typically present with Achilles tendon pain more than 20 years before being referred to a lipid clinic.6 Further, another study of patients presenting for Achilles tendon ruptures reported that 83% of those patients were hypercholesterolemic, and more than three quarters of those were unaware of this fact.16
Our previously reported clinical data have linked hypercholesterolemia to full-thickness rotator cuff tears,1 in that patients with full-thickness tears also tended to have increased concentrations of TC, TG, and LDL as well as decreased concentrations of high-density lipoprotein (HDL), commonly known as good cholesterol. Another clinical study,15 however, showed the opposite result of no clear correlation between patients’ plasma cholesterol level and rotator cuff tears.
Experimental data from our laboratory have shown effects on native tendon properties in the mouse,2 rat,3,4 pig,5 and nonhuman primate.4 Our mouse patellar tendon and pig biceps tendon studies in this area showed reduced stiffness and elastic modulus due to high cholesterol levels; however, in the rat, we found increased values for these measurements in the supraspinatus tendon after the completion of high-cholesterol diets.3,4
Despite this body of relevant knowledge, the effect of hypercholesterolemia on healing properties in the rat rotator cuff model remains unknown. Therefore, the objective of the current study was to evaluate healing of supraspinatus tendons with this model.18 We hypothesized that tendon healing as assessed through mechanical testing and histologic analysis would be inferior in hypercholesterolemic rats compared with normal controls.
Materials and methods
Of the 64 male Sprague-Dawley rats (400–450 g) procured for the study, 32 rats received a high-cholesterol diet consisting of 4% cholesterol and 1% sodium cholate (HC group). This experimental diet (Research Diets, Inc, New Brunswick, NJ, USA) was formulated as a modification of the standard rat chow and has been shown by us and others to produce elevated TC values in Sprague-Dawley rats after 3 months.3,14 The other 32 rats received the standard chow and served as controls (CTL group). During a period of 6 months, all rats were allowed food and water ad libitum and were weighed weekly. After the initial 6-month treatment period, all animals were subjected to a standard, unilateral supraspinatus detachment and repair surgery as described previously.19 Briefly, the supraspinatus tendon was surgically exposed and sharply detached at the humeral insertion with a scalpel. After detachment, the tendon was reattached to the greater tubercle of the humerus with a modified Mason-Allen technique. Overlying musculature was sutured back, and skin incisions were closed with staples. The contralateral limb remained uninjured and served as within-animal comparative data. Postoperatively, animals continued their respective diet courses and were allowed normal cage activity for 2 (n = 4 per group), 4 (n = 14), and 8 weeks (n = 14), at which points they were euthanized (Fig. 1). Immediately after sacrifice, blood was collected and plasma lipid panels were measured and assessed for TC, HDL, and TG. In addition, the ratio of TC to HDL (TC/HDL) was calculated as a clinically relevant parameter.
Figure 1.
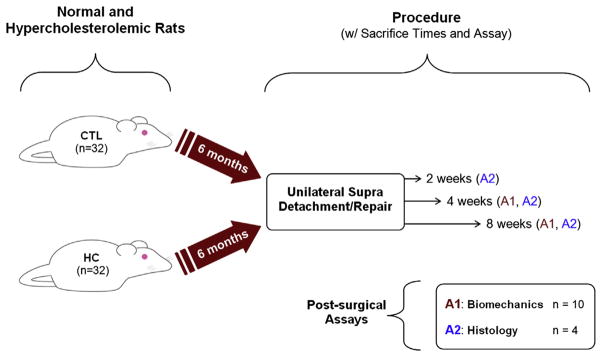
Study design. CTL, control group; HC, high-cholesterol diet.
For biomechanical evaluation (n = 10 per group), the supraspinatus tendon was finely dissected from the muscle under a stereomicroscope while preserving the humeral insertion. Verhoeff stain was placed on the tendon at the insertion as well as at 2 locations within the tendon midsubstance for optical measurement of local tissue strain with custom texture-correlation analysis software. Tendon cross-sectional area was measured near each stain line location with a custom device equipped with translational stages, 2 orthogonal linear variable differential transformers, and a charge-coupled device laser.8 The humeral diaphysis and head were potted in poly (methyl methacrylate) while keeping the insertion site undisturbed. The cylindrical potted end of the specimen was placed in a base fixture; the tendon end was glued between 2 layers of sandpaper and clamped. Specimens were submerged in a 37°C PBS bath and tensile tested with use of an Instron 5543 mechanical test frame (Instron Corp, Norwood, MA, USA). Tendons were initially preloaded to 0.1 N, followed by 10 cycles of preconditioning between 0.1 and 0.5 N at a strain rate of 0.4%/s. After a 300-second hold to achieve equilibrium, a 600-second stress-relaxation experiment began with a ramp to 5% strain at 5%/s, followed by a return to gauge length and 60-second hold. Finally, specimens were quasi-statically tested to failure at a rate of 0.3%/s. Key measurements included maximum (i.e., failure) load and stress, stiffness, elastic modulus, and percentage relaxation (percentage difference between peak and equilibrium stresses over the stress-relaxation).
For histologic assays (n = 4 per group), supraspinatus tendons were dissected, keeping the proximal humerus intact to preserve the tendon-to-bone insertion. The specimens were immediately fixed, decalcified, and processed with standard techniques. After paraffin embedding, 7-μm coronal sections were collected, dried, and stained with hematoxylin and eosin. Photomicrographs were blindly graded by 3 observers for assessment of cellularity and cell shape based on a scale of 0 (normal), 1 (mild changes), 2 (moderate changes), and 3 (marked changes), as done previously.7 The same images were also analyzed for collagen organization with quantitative polarized light microscopy. With use of this technique, angular deviation (a measure of disorganization) was calculated for each specimen.
Data were evaluated for differences in tendon healing between cholesterol groups. To assess healing in quantitative properties (i.e., biomechanical and organizational), each injured-limb parameter was normalized to that of the uninjured contralateral limb such that a value of 1.0 would represent full recovery and values of more or less than 1.0 would indicate altered healing. Comparisons were made between biomechanical and organizational data for CTL and HC groups by use of one-tailed t tests. For histologic grading, a single composite grade was derived from each set of 3 individual grades, as done previously.7 To evaluate healing, a change scores technique was employed, which calculated the difference between the injured and uninjured contralateral limb grades for each animal. The resulting values were compared between CTL and HC groups by a nonparametric Mann-Whitney test, except in cases in which there was no variation among grades within a group. In these cases, a Wilcoxon signed rank test was used in which the median of one group was compared with the value of the nonvarying group. Significance for all statistical analyses was set at P ≤ .05.
Results
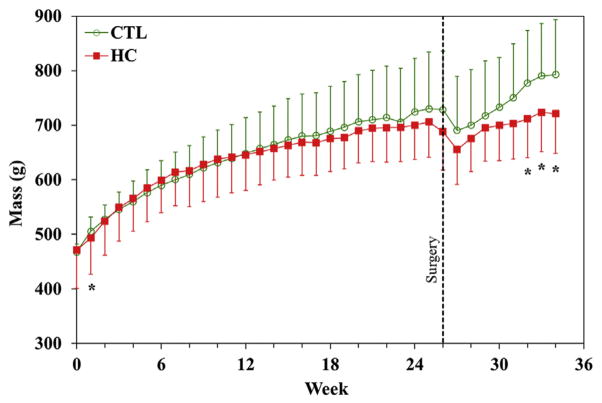
During the 6-month diet period, 2 animals (1 HC and 1 CTL) had to be euthanized for health reasons unrelated to the study design, reducing the sample size for 2-week histology to 3 per group. Lipid analysis (Table I) demonstrated that the HC diet produced significantly increased TC, HDL, and TC/HDL at 4 and 8 weeks and increased TC and HDL at 2 weeks, but there were no significant differences in TG. Animals in the HC group were significantly lighter than CTL rats at the first week of diet initiation as well as in the final 3 weeks for the animals in the 8-week postsurgical group (Fig. 2).
Table I.
Lipid panel results showing increased TC, HDL, and TC/HDL for HC groups at all time points
| TC (mg/dL) | HDL (mg/dL) | TG (mg/dL) | TC/HDL | |
|---|---|---|---|---|
| 2 weeks | ||||
| CTL | 106 ± 3 | 56.8 ± 6.3 | 153 ± 58 | 1.9 ± 0.2 |
| HC | 688 ± 329 | 121.1 ± 16.0 | 253 ± 180 | 5.9 ± 3.4 |
| P value | .02 | .001 | .2 | .06 |
| 4 weeks | ||||
| CTL | 102 ± 22 | 52.3 ± 13.4 | 195 ± 97 | 2.0 ± 0.2 |
| HC | 379 ± 115 | 97.9 ± 26.0 | 155 ± 55 | 4.1 ± 1.1 |
| P value | <.001 | <.001 | .09 | <.001 |
| 8 weeks | ||||
| CTL | 128 ± 36 | 54.2 ± 16.4 | 208 ± 76 | 2.3 ± 0.2 |
| HC | 577 ± 390 | 90.8 ± 30.0 | 221 ± 200 | 6.5 ± 2.6 |
| P value | <.001 | <.001 | .4 | <.001 |
CTL, control; HC, high-cholesterol diet; HDL, high-density lipoprotein; TC, total cholesterol; TG, triglyceride.
Data shown as means ± standard deviations.
Bold values in represent statistically significant P values.
Figure 2.
Masses of rats during the course of the study. Significant differences (*) were noted at the first week on the HC diet and in the final 3 weeks for the 8-week animals. CTL, control group; HC, high-cholesterol diet.
Geometrical and biomechanical results
Normalized (injured/native) cross-sectional area was closer to normal (1.0) in the HC group for the 8-week animals. Mechanical testing revealed a significant reduction in normalized stiffness compared with CTL rats at 4 weeks after injury but no difference at 8 weeks (Table II, Fig. 3). Because failure occurred at the insertion site for the injured limb and primarily at the grip for the uninjured limb, true normalized failure properties were not able to be reported.
Table II.
Biomechanical and organizational properties from rat supraspinatus tendons normalized to contralateral uninjured limb data
| Area | Percentage relaxation | Stiffness | Modulus | Angular deviation | |
|---|---|---|---|---|---|
| 4 weeks | |||||
| CTL | 5.11 ± 1.76 | 1.36 ± 0.29 | 0.28 ± 0.13 | 0.038 ± 0.008 | 0.71 ± 0.40 |
| HC | 4.87 ± 1.52 | 1.44 ± 0.24 | 0.20 ± 0.06 | 0.040 ± 0.027 | 6.08 ± 7.02 |
| P value | .4 | .3 | .05 | .4 | .1 |
| 8 weeks | |||||
| CTL | 4.93 ± 0.88 | 1.65 ± 0.34 | 0.31 ± 0.09 | 0.040 ± 0.020 | 1.70 ± 1.14 |
| HC | 3.64 ± 0.95 | 1.70 ± 0.37 | 0.33 ± 0.15 | 0.051 ± 0.037 | 2.65 ± 0.78 |
| P value | .004 | .4 | .4 | .2 | .1 |
Stiffness returned significantly closer to normal (1.0) for CTL rats at 4 weeks after injury; however, area was closer to normal in HC rats at 8 weeks after injury. Data as means ± standard deviations. P values are for high-cholesterol diet (HC) rats compared with control (CTL) rats.
Bold values represent statistically significant P values.
Figure 3.
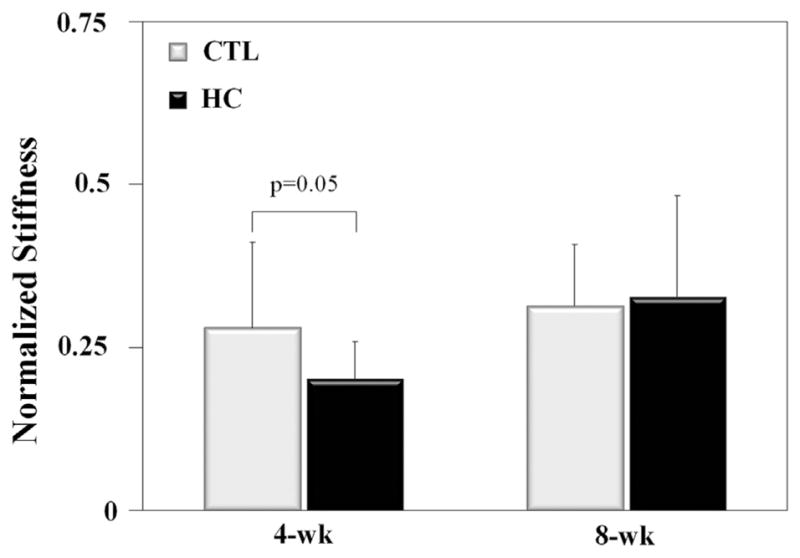
Normalized stiffness was lower (i.e., farther from normal) at 4 weeks after injury in the high-cholesterol diet (HC) group compared with the control (CTL) group but not at 8 weeks.
Histologic results
Polarized light microscopy showed no difference in normalized angular deviation between CTL and HC rats at 2 weeks after injury and repair. Collagen organization trended toward being closer to normal in CTL rats at 4 and 8 weeks (Table II); however, this finding was not significant (P = .1). Results for cellularity and cell shape also showed no significant differences between CTL and HC rats (Table III).
Table III.
Cellularity and cell shape results from grading of histologic samples
| Uninjured
|
Injured
|
Change scores
|
||||
|---|---|---|---|---|---|---|
| Cellularity | Cell shape | Cellularity | Cell shape | Cellularity | Cell shape | |
| 2 weeks | ||||||
| CTL | 1.00 ± 0.50 | 2.00 ± 1.00 | 3.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 1.00 | 0.00 ± 2.00 |
| HC | 1.00 ± 0.00 | 1.00 ± 1.00 | 3.00 ± 0.00 | 2.00 ± 0.50 | 2.00 ± 0.00 | 1.00 ± 3.00 |
| P value | 1 | .7 | ||||
| 4 weeks | ||||||
| CTL | 1.00 ± 0.50 | 3.00 ± 0.50 | 3.00 ± 0.00 | 1.00 ± 0.25 | 2.00 ± 1.00 | −2.00 ± 1.00 |
| HC | 0.00 ± 0.50 | 0.00 ± 1.50 | 3.00 ± 0.00 | 2.00 ± 0.50 | 3.00 ± 1.00 | 2.00 ± 5.00 |
| P value | .2 | .4 | ||||
| 8 weeks | ||||||
| CTL | 2.00 ± 0.50 | 2.00 ± 0.50 | 2.50 ± 1.00 | 1.00 ± 0.25 | 1.00 ± 2.00 | −1.00 ± 0.00 |
| HC | 0.50 ± 1.00 | 1.50 ± 3.00 | 2.00 ± 0.50 | 2.00 ± 0.00 | 1.50 ± 1.00 | 0.50 ± 3.00 |
| P value | .6 | .3 | ||||
Data as medians ± interquartile range. P values are for high-cholesterol diet (HC) rats compared with control (CTL) rats.
Discussion
Mechanical properties, collagen organization, and cell number and shape were assessed in healing supraspinatus tendons from normal (CTL) and high-cholesterol (HC) rats. As hypothesized, healing tendons from the HC group had reduced normalized stiffness at 4 weeks after injury; however, this finding was not present in the 8-week group. Unexpectedly, the 8-week HC animals weighed significantly less than the CTL animals in the 3 weeks before sacrifice. The difference in overall size of the animals at this time point may have contributed to the lack of 8-week stiffness differences. This diet course combined with this time point represents the farthest point to which our experiments have been carried out in this model. It is difficult to speculate as to whether the separation in masses would continue at a later time point or if the differences seen here were related to other more specific factors (e.g., animal husbandry).
Our current findings are generally in agreement with our previous clinical findings,1 which correlated hypercholesterolemia with rotator cuff injury. There are other clinical data,15 however, suggesting no relationship. This inconsistency in findings could potentially be due to differences in initial screening methodology (e.g., imaging, arthroscopy) used between the two clinical studies. The reduction in healing stiffness at 4 weeks is consistent with one of our previous studies in the mouse patellar tendon,2 which showed reduced elastic modulus due to lifelong exposure to high cholesterol.
Interestingly, tendon cross-sectional area was found to be significantly closer to normal in HC animals at 8 weeks but not at 4 weeks. This suggests a reduced inflammatory fibrotic response at 8 weeks or possibly reduced cell proliferation at 4 weeks, although these were not detected histologically. More time points would be needed to determine if the cross-sectional area measurements would continue to converge toward normal at a time point longer than 8 weeks.
In this study, injured tendon data were normalized to the contralateral limb to provide within-animal comparisons to preclude the need for analytical randomization techniques (e.g., bootstrapping) and thereby minimize data variation. Nevertheless, contralateral limb properties could have been affected by altered joint loading due to the injured limb and thus may not represent normal properties. However, purely native properties were not necessary to test our study hypothesis, which sought to assess the healing response of the tendon as opposed to establishing baseline magnitudes.
The HC diet used in this study during the course of 6 months resulted in a marked increase (roughly 300%) in TC concentration compared with normal. Levels of this magnitude would likely represent profound cardiovascular pathologic effects in a patient. HDL (also known as good cholesterol) was also significantly increased, resulting in a TC/HDL increase on the order of roughly 150%. Also, the time scale during which the HC rats were on the altered diet (6 months) could be considered much shorter in duration than that of a typical hypercholesterolemic patient, resulting in the relative exposures potentially being similar. Ultimately, although a direct clinical parallel could be beneficial in drawing conclusions and translating results to patient care, our focus in the current basic science study was to test our fundamental hypotheses related to healing ability in the presence of a high cholesterol level and to provide data and background information for future studies with more direct clinical parallels.
Although we did not collect blood for serum lipid profiles at the time of surgery, we are confident that the striking differences seen at the time of sacrifice were already in place 2 to 8 weeks earlier at the time of surgery. Previous work in our laboratory using this same model has shown that rats euthanized after only 3 months on the same diet (as opposed to 6 months in the current study) showed similar marked alterations in lipid profiles.3
Given that unsuspecting hypercholesterolemic patients often present to orthopaedists well before being screened for or diagnosed with hypercholesterolemia, our hope is that information gained from this study, future studies, and our overall body of work in this area could be beneficial to patients with previously undiagnosed hypercholesterolemia by providing their physicians an added opportunity for detection of this serious health condition before the appearance of any associated adverse cardiovascular symptoms. Conversely, knowledge that elevated serum cholesterol concentration could increase the risk or incidence of tendon injury or reduce the healing ability of existing injuries could be extremely valuable to patients with existing tendon injuries or noninjured high-risk patients who perform repetitive tasks, eccentric loading activities, or motions outside the normal range through occupational or other activities. Further clinical and basic science studies are warranted to reveal the mechanisms that control the current finding of interactions between tendon healing and hypercholesterolemia.
Conclusions
In summary, we have demonstrated decreased healing stiffness in hypercholesterolemic rats at 4 weeks after supraspinatus injury and repair. On the basis of this finding, hypercholesterolemia may have a detrimental effect on tendon healing in our rat rotator cuff injury and repair model. Work is currently being conducted to address biochemistry, vascularity, and other factors in these animals to elucidate the mechanisms involved in the change in stiffness reported here in addition to investigating the effects of hypercholesterolemia in a chronic rotator cuff injury model. Additional postinjury time points (e.g., 16 weeks) are also of interest in future studies to investigate longer term healing effects. Future work may also focus on the effect of statins and their potential for minimizing the detrimental changes seen here.
Acknowledgments
The authors gratefully acknowledge the contributions of Kyle Aune, Brianne Connizzo, Debra Cromley, Jason Hsu, Abbas Jawad, Daniel Rader, Allison Rozsits, and Stephen Thomas.
This work was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (Protocol: 802634).
This research was funded by the Orthopaedic Research and Education Foundation (08-022 to J.A. Abboud) and the NIH/NIAMS-supported Penn Center for Musculoskeletal Disorders (P30AR050950 to L.J. Soslowsky).
Footnotes
Disclaimer
The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
References
- 1.Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468:1493–7. doi: 10.1007/s11999-009-1151-9. http://dx.doi.org/10.1007/s11999-009-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res. 2011;29:380–3. doi: 10.1002/jor.21255. http://dx.doi.org/10.1002/jor.21255. [DOI] [PubMed] [Google Scholar]
- 3.Beason DP, Hsu JE, Edelstein L, Lee CS, Tucker JJ, Abboud JA, et al. Effect of diet-induced hypercholesterolemia on rotator cuff tendon mechanics in a rat model. Trans Orthop Res Soc. 2011;36:223. [Google Scholar]
- 4.Beason DP, Hsu JE, Marshall SM, McDaniel AL, Temel RE, Abboud JA, et al. Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. J Shoulder Elbow Surg. 2013;22:681–6. doi: 10.1016/j.jse.2012.07.008. http://dx.doi.org/10.1016/j.jse.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beason DP, Kuntz AF, Hamamdzic D, Wilensky RL, Mohler ERI, Abboud JA, et al. High cholesterol adversely affects biceps tendon mechanical properties in a porcine model. Trans Orthop Res Soc. 2009;34:184. [Google Scholar]
- 6.Beeharry D, Coupe B, Benbow EW, Morgan J, Kwok S, Charlton-Menys V, et al. Familial hypercholesterolaemia commonly presents with Achilles tenosynovitis. Ann Rheum Dis. 2006;65:312–5. doi: 10.1136/ard.2005.040766. http://dx.doi.org/10.1136/ard.2005.040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dourte LM, Perry SM, Getz CL, Soslowsky LJ. Tendon properties remain altered in a chronic rat rotator cuff model. Clin Orthop Relat Res. 2010;468:1485–92. doi: 10.1007/s11999-009-1206-y. http://dx.doi.org/10.1007/s11999-009-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favata M. Scarless healing in the fetus: implications and strategies for postnatal tendon repair [PhD dissertation] Philadelphia: University of Pennsylvania; 2006. [Google Scholar]
- 9.Health, United States, 2010: with special feature on death and dying. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 10.Jacobs JJ, Andersson GBJ, Bell J-E, Weinstein SL, Dormans JP, Gnatz SM, et al. Burden of musculoskeletal disease overview. In: Andersson G, editor. The burden of musculoskeletal diseases in the United States. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2008. pp. 1–20. [Google Scholar]
- 11.Kiortsis DN, Argyropoulou MI, Tsouli SG, Xydis V, Elisaf MS. Correlation of Achilles tendon thickness evaluated by ultrasonography with carotid intima-media thickness in patients with familial hypercholesterolemia. Atherosclerosis. 2006;186:228–9. doi: 10.1016/j.atherosclerosis.2006.02.002. http://dx.doi.org/10.1016/j.atherosclerosis.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Klemp P, Halland AM, Majoos FL, Steyn K. Musculoskeletal manifestations in hyperlipidaemia: a controlled study. Ann Rheum Dis. 1993;52:44–8. doi: 10.1136/ard.52.1.44. http://dx.doi.org/10.1136/ard.52.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langer T, Strober W, Levy RI. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972;51:1528–36. doi: 10.1172/JCI106949. http://dx.doi.org/10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Ryu JK, Piao S, Choi MJ, Kim HA, Zhang LW, et al. Efficient gene expression system using the RTP801 promoter in the corpus cavernosum of high-cholesterol diet-induced erectile dysfunction rats for gene therapy. J Sex Med. 2008;5:1355–64. doi: 10.1111/j.1743-6109.2008.00771.x. http://dx.doi.org/10.1111/j.1743-6109.2008.00771.x. [DOI] [PubMed] [Google Scholar]
- 15.Longo UG, Franceschi F, Spiezia F, Forriol F, Maffulli N, Denaro V. Triglycerides and total serum cholesterol in rotator cuff tears: do they matter? Br J Sports Med. 2010;44:948–51. doi: 10.1136/bjsm.2008.056440. http://dx.doi.org/10.1136/bjsm.2008.056440. [DOI] [PubMed] [Google Scholar]
- 16.Mathiak G, Wening JV, Mathiak M, Neville LF, Jungbluth KH. Serum cholesterol is elevated in patients with Achilles tendon ruptures. Arch Orthop Trauma Surg. 1999;119:280–4. doi: 10.1007/s004020050410. http://dx.doi.org/10.1007/s004020050410. [DOI] [PubMed] [Google Scholar]
- 17.Ozgurtas T, Yildiz C, Serdar M, Atesalp S, Kutluay T. Is high concentration of serum lipids a risk factor for Achilles tendon rupture? Clin Chim Acta. 2003;331:25–8. doi: 10.1016/s0009-8981(03)00075-5. http://dx.doi.org/10.1016/S0009-8981(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 18.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–92. doi: 10.1016/s1058-2746(96)80070-x. http://dx.doi.org/10.1016/S1058-2746(96)80070-X. [DOI] [PubMed] [Google Scholar]
- 19.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454–63. doi: 10.1016/S0736-0266(01)00144-9. http://dx.doi.org/10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 20.Tsouli SG, Kiortsis DN, Argyropoulou MI, Mikhailidis DP, Elisaf MS. Pathogenesis, detection and treatment of Achilles tendon xanthomas. Eur J Clin Invest. 2005;35:236–44. doi: 10.1111/j.1365-2362.2005.01484.x. http://dx.doi.org/10.1111/j.1365-2362.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- 21.von Bahr S, Movin T, Papadogiannakis N, Pikuleva I, Ronnow P, Diczfalusy U, et al. Mechanism of accumulation of cholesterol and cholestanol in tendons and the role of sterol 27-hydroxylase (CYP27A1) Arterioscler Thromb Vasc Biol. 2002;22:1129–35. doi: 10.1161/01.atv.0000022600.61391.a5. http://dx.doi.org/10.1161/01.atv.0000022600.61391.a5. [DOI] [PubMed] [Google Scholar]
- 22.Worland RL, Lee D, Orozco CG, SozaRex F, Keenan J. Correlation of age, acromial morphology, and rotator cuff tear pathology diagnosed by ultrasound in asymptomatic patients. J South Orthop Assoc. 2003;12:23–6. [PubMed] [Google Scholar]