Abstract
Brentuximab vedotin (Adcetris, Seattle Genetics) is an antibody-drug conjugate (ADC) that joins an anti-CD 30 monoclonal antibody with the anti-tubulin agent monomethyl auristatin E, via a dipeptide linker. It has demonstrated significant activity in CD 30-positive lymphomas and is currently approved by the FDA for treatment of Hodgkin lymphoma that has relapsed following autologous stem-cell transplantation, or after two lines of chemotherapy in non-transplant candidates. Brentuximab vedotin has also been approved for the treatment of relapsed anaplastic large-cell lymphoma after front-line chemotherapy. We will briefly review the biology of Hodgkin lymphoma with a focus on the pathogenic role of CD 30 as well as the development of CD 30-targeted therapy. We will also discuss both the current role of brentuximab vedotin in the management of relapsed and refractory Hodgkin lymphoma, as well as likely future developments for this agent.
1 Classical Hodgkin lymphoma biology
Originally described in 1832 by Sir Thomas Hodgkin, the disease that bears his name was not classified as a lymphoproliferative disorder until recently [1]. Hodgkin lymphoma is divided into classical HL (cHL) and nodule lymphocyte-predominant HL (NLPHL), with the former being overwhelmingly more common [2]. cHL itself is further classified into four subtypes based on histology: nodular sclerosis (the most common subtype), mixed cellularity, lymphocyte-depleted, and lymphocyte-rich. One of the peculiar aspects of HL is that the neoplastic clone, also known as the Reed-Sternberg cell (HRS) in cHL and the lymphocyte predominant cell (LP) in NLPHL, is normally present only in small quantities in an affected lymph node, with the large majority of cells present in an inflammatory infiltrate comprised of various other immune cells. As significant differences exist between the HRS and LP cells, the rest of the discussion will be limited to the biology of cHL.
A detailed understanding of the underlying biology of cHL was hampered for years both by the paucity of the HRS cell as well as the uncertainty regarding its lineage. After years of controversy, the HRS cell was eventually shown to be an aberrant germinal or post-germinal B-cell, based on gene expression studies as well as the fact that it demonstrates immunoglobulin rearrangement and somatic hypermutation [3, 4]. One of the historic difficulties of identifying the precise lineage of the Reed-Sternberg cell lay in the fact that its immunophenotype differed considerably from that of normal B-cells. For instance, HRS cells often express markers that are not typically present on B-cells such as CD 15 and CD 30 but do not typically feature normal pan-B markers such as CD 19, CD 20, and CD 22 [5].
This highly aberrant situation raises the obvious question of how cells derived from B-cells end up being so different from their precursors. The answer appears to be due in large part to deregulated expression of various transcription factors. While the main B-cell lineage factor, PAX5, is still expressed in the HRS cell [6], many other transcription factors are significantly perturbed. For instance, the transcription factor NOTCH1, which normally directs immature lymphocytes towards the T-cell lineage while suppressing B-cell development, is aberrantly expressed in HRS and appears to play a significant role in the pathogenesis of cHL [7]. On the other hand, transcription factors that are involved in the expression of B-cell genes such as OCT2, BOB1, and PU.1 appear to be absent in the HRS cell [8, 9]. Other B-lineage transcription factors such as EBF1 and E2A may be present in low levels (in the case of EBF1) or are expressed but actively inhibited (in the case of E2A) [10].
Another important characteristic of cHL is the fact that the malignant HRS cell is present only in small quantities, while surrounded by an exuberant inflammatory background. In fact, the majority of the cells in cHL are normal reactive macrophages and T cells recruited by chemokines such as CCL17 that are secreted by HRS cells. The infiltrating T cells belong to the CD4+ helper T (Th) and regulatory T (Treg) phenotypes; the presence of Tregs may be one of the reasons that the HRS cell is able to escape immune surveillance [11]. There is significant crosstalk between the HRS cells and the other surrounding cells, and this signaling is mediated primarily by various chemokines and cytokines, such as CCL5, IL-5, and CCL20, produced by both the HRS cell as well as other cells in the microenvironment [12]. Although the increased understanding of the microenvironment has not thus far translated into therapeutic advancement, a number of associated biomarkers (various cytokines, NFkB, JAK/STAT 3, and various tyrosine kinases) have been found to be prognostic in cHL, and strategies targeting the microenvironment are under active development [13].
2 Overview of Hodgkin lymphoma treatment
The treatment of Hodgkin lymphoma (HL) has been one of the great successes of modern medicine. Previously an inevitably fatal disease, advances in radiation and chemotherapy have rendered it one of the most curable malignancies, a fact made particularly important by the average youth of patients with HL. Although the prognosis for patients with HL varies significantly depending on the stage of disease as well as other associated risk factors (low serum albumin, low hemoglobin, male gender, age > 45, stage IV disease, leukocytosis, and lymphopenia), the large majority of patients with HL achieve durable disease control by combination chemotherapy. Early-stage HL is associated with very high cure rates, and the five-year survival is well in excess of 90% across virtually all of the more recent studies [14]. However, in patients with advanced HL, the five-year freedom from progression rate can be as low as 62%, depending on their risk factors (see above), highlighting the need for improved therapies in this population [15].
Front-line therapy in HL generally consists of combination chemotherapy with a four-drug regimen of doxorubicin (adriamycin), bleomycin, vinblastine, and dacarbazine (ABVD), with or without radiation. ABVD was first described in 1975 [16] and has since evolved into the standard of care; a more intensive regimen which includes bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (escalated BEACOPP) decreases relapse rates but does not result in increased overall survival compared to ABVD,and has more toxicities [17]. As compared to ABVD, alternative regimens such as MOPP (meclorethamine, vincristine, procarbazine, and prednisone) and Stanford (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone) have been shown to be worse (in the case of MOPP) [18] or of similar efficacy (in the case of Stanford V) [19] in patients with advanced disease.
Despite the unequivocal successes that have been realized in the treatment of HL, a significant minority of patients, most of whom have poor-prognostic or advanced disease, will relapse after front-line therapy. Combination chemotherapy and radiation may be curative in a very selected subset of patients with localized, late relapse, but the standard approach for the large majority of patients with relapsed HL is high-dose chemotherapy followed by autologous stem cell transplantation (ASCT). This strategy can lead to a durable remission rate of 50% [20]. Unfortunately, the prognosis for patients who relapse following ASCT has historically been very poor with a median survival of 25 months [21]; while allogeneic stem cell transplantation (allo-SCT) can be performed in a group of these patients, relapse rates remain high even with this approach with a progression-free survival (PFS) at 4 years of 24% in one study [22]. Therefore new therapies are urgently needed for the population of refractory and relapsed HL.
3 The Role of CD 30 in Hodgkin Lymphoma
While CD15 negativity is seen in a small but substantial percentage of HL, CD 30 positivity is nearly universal [23]. Aside from its near-ubiquitous presence in HL and ALCL, CD 30 can also be expressed in a number of other lymphoma subtypes as well as germ cell tumors [24]. The physiologic role of CD 30 remains incompletely characterized, but the immediate downstream steps are well-described. CD 30 is a member of the tumor necrosis factor (TNF) receptor family that appears to be a marker for activated lymphocytes, and has only limited expression in normal cells [25]. It is activated upon binding by the CD 30 ligand (CD 30L, also known as CD 153), which is a surface glycoprotein belonging to the TNF family and expressed on multiple immune cells. Signal transduction mediated by CD 30 can result in activation of multiple downstream pathways (including NF-κB), which are dependent on various factors including the type of cell as well as the differentiation status, [26] The cytoplasmic domain of CD 30 itself has no intrinsic catalytic activity, and this process is contingent on recruitment of proteins belonging to the TNF receptor-associated factor (TRAF) family to the cytoplasmic portion of the receptors [27]. Of interest, CD 30 has been implicated both in cell proliferation as well as the seemingly opposite process of apoptosis with prominent involvement of the pro-survival protein NF-κB [28].
The role of CD 30-mediated cell signaling in HL HRS cells is unclear. Although HRS cells overexpress CD 30 and have activation of NF-κB pathways, stimulation of CD 30 in HL cell lines does not result in any change in either NF-κB activity or cell proliferation [29]. This suggests that NF-κB in HL is constitutively activated and independent of ligand receptor binding. In contrast to the lack of response to CD 30 stimulation in HL, activation of the CD 30 receptor in ALCL does result in downstream changes in gene expression as well as cell proliferation [30], and NF-κB is not constitutively active in ALCL [29]. The high level of CD 30 expression in HL cells in conjunction with its relatively limited presence in normal tissues has made it an attractive molecular target, and its importance in the pathophysiology of HL also suggests a potential therapeutic role for the abrogation of CD 30 signaling, an idea which has begun to be realized with brentuximab vedotin.
4 Targeting CD 30 in Hodgkin Lymphoma
Although various attempts at targeted therapies have been made in HL, the overwhelmingly greatest success has been achieved with the CD-30 targeted antibody-drug conjugate brentuximab vedotin, which is the subject of this review. In this section we will briefly outline the history of CD 30-directed therapy in HL.
Given the restricted expression of CD 30 in benign tissues as well as its high rate of expression in a number of lymphoid malignancies, it was quickly identified as a potential therapeutic target for a monoclonal antibody. At least two naked anti-CD 30 monoclonal antibodies have been tested in the phase I setting. SGN-30, a mouse-human chimeric monoclonal antibody against CD 30, had demonstrated significant preclinical activity which resulted in a phase I clinical trial of this agent in patients with refractory and relapsed CD 30+ hematologic malignancies. 24 patients, 21 of whom had HL, were enrolled, and SGN-30 was found to have only modest activity although one patient with cutaneous ALCL had a complete response. No responses were seen in the HL population, and only 4 out of 21 patients had disease stabilization with the longest duration of stable disease being 16 months [31]. A phase II study of this agent was conducted in patients with refractory HL and ALCL and again showed no responses in the HL cohort, although 11 out of 38 patients had disease stabilization for a period of up to 242 days [32]. MDX-060, a fully human monoclonal antibody against CD 30, was also assayed in a phase I/II trial and demonstrated similarly modest results in HL [33].
Building upon the naked monoclonal antibody, other CD 30-based strategies have also been attempted in HL including radioimmunotherapy and immunotoxins. Iodine-131-labeled murine anti-CD 30 was tested in 22 patients with refractory or relapsed HL and demonstrated a 27% response rate with a single CR, although all responses were fairly short-lived with a median duration of only 4 months. Moreover, the treatment proved to be difficult to tolerate as 7 patients experienced grade 4 hematologic toxicity, tempering enthusiasm for this approach [34]. Multiple attempts at constructing a CD 30-based immunotoxin have been made. While clinically meaningful activity was seen first in the Ber-H2-SO6 immunotoxin, consisting of a murine anti-CD 30 antibody conjugated to Saporin-S6 [35], as well as in Ki-4.dgA, consisting of a murine anti-CD 30 antibody conjugated to the ricin A-chain [36], response rates were low and duration of response modest. Finally, an anti-CD16/CD 30 bi-specific antibody designed to activate natural killer cells was also designed and tested in refractory cHL. Modest responses in early phase studies were seen, but further study was hampered by the development of human anti-mouse antibodies (HAMA) and difficulty in producing adequate quantities of the agent [37].
5 Brentuximab vedotin
Brentuximab vedotin is an ADC consisting of the chimeric monoclonal anti-CD 30 antibody SGN-30 conjugated via a valine-citrulline peptide linker to the anti-tubulin agent monomethyl auristatin E (MMAE) [38]. A number of features are notable regarding this compound.
Utilization of a chimeric rather than an entirely murine antibody decreased the development of HAMA, which was a significant problem with the previous agent Ki-4.dgA [36]. The choice of linker was also an important one; to minimize toxicity while preserving efficacy, the linker must remain stable while in circulation to prevent release of the free drug yet must be readily cleaved once the entire molecule is internalized into the target cell. Linkers that require protease activity are generally more stable than those that are chemically cleaved upon exposure to the acid milieu of the lysosome. As expected, the dipeptide linker in brentuximab vedotin is highly stable until internalization into the target cell [39].
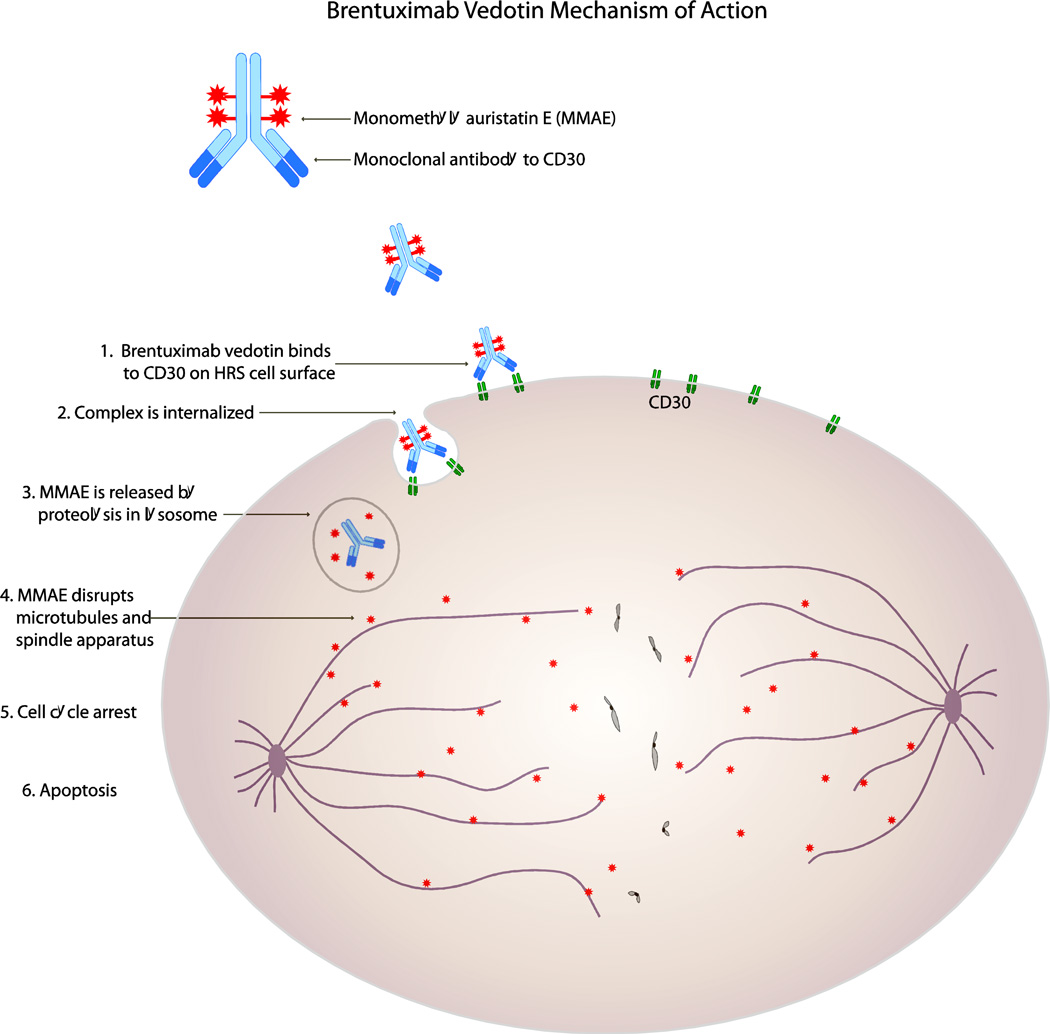
Also of importance is the actual cytotoxic portion of the ADC which, in the case of brentuximab vedotin, is MMAE, a synthetic derivative of dolastatin 10. Dolastatin 10 is an powerful anti-mitotic agent originally isolated from the sea hare Dolabella auricularia and had been previously shown to be a potent inhibitor of microtubule polymerization [40]. In clinical trials, however, it demonstrated only very modest single-agent activity while causing significant hematologic toxicity [41–43]. MMAE was specifically engineered via total synthesis to have high hydrophilicity and stability under physiologic conditions [44], and in vitro studies with the similar compound auristatin E (AE) showed it to be an extremely potent cytotoxic agent with activity in many different malignancies [45]. After demonstrating both in vitro and in vivo activity against CD 30-positive malignancies [46], the drug compound cAC10-vcMMAE (now brentuximab vedotin) entered human clinical trials. The mechanism of action for brentuximab vedotin is depicted in Figure 1.
Figure 1.
Brentuximab vedotin mechanism of action
6 Clinical data
The first phase I trial of brentuximab vedotin was published in 2010 and featured 45 patients with relapsed or refractory CD 30-positive lymphomas; 42 of these patients had HL, 2 patients had ALCL, and 1 had angioimmunoblastic T-cell lymphoma. The most common adverse events were fatigue, pyrexia, nausea, diarrhea, neutropenia, and peripheral neuropathy, and 1.8 mg per kilogram was found to be the maximum tolerated dose level [47]. Although obviously not the primary endpoint, the objective response rate (ORR) was 50% at this dose, and the impressive activity prompted multiple phase II trials of brentuximab vedotin in both HL as well as ALCL.
A multicenter open-label phase II study examining brentuximab vedotin in patients with HL that was relapsed or refractory after ASCT [48], demonstrated an ORR of 75% with 34% patients achieving a complete remission (CR). Crucially, the complete remissions proved to be durable, with a median CR duration of 20.5 months, although the median PFS was only 5.6 months. Although complete remissions seen with brentuximab vedotin were durable, the available survival data from the study indicate that patients who achieve only a partial remission (PR) will likely relapse. There are no clinical or biological factors that can predict for complete remission or durable responses as yet. This trial also showed that brentuximab vedotin is well tolerated, with few grade 3–4 adverse events. The most notable adverse events were the emergence of peripheral sensory neuropathy (53/102 patients). The majority of the events were grade 1–2 and 80% of the patients experienced improvement or resolution when the drug was discontinued. On the basis of this trial, brentuximab was granted accelerated approval for treatment of HL after failure of ASCT or after failure of two multi-agent chemotherapy regimens in patients who are not candidates for ASCT.
Retreatment with brentuximab vedotin has also been tested and found to be a viable strategy. A phase II trial was conducted in patients with CD 30 positive lymphomas who had previously achieved CR or PR on brentuximab vedotin [49]. These patients were taken off brentuximab vedotin for either toxicity, completion of 16 doses of therapy, or to proceed to autologous or allogeneic stem cell transplantation. They then developed relapsed CD 30 positive lymphomas, and were eligible to be retreated with brentuximab vedotin under this trial. Objective responses were seen in 17 out of 24 patients (70% ORR) and 39% of the patients were able to achieve CR again.
As allo-SCT is the only potentially curative treatment for HL which has relapsed after ASCT, there has also been significant interest in whether not brentuximab vedotin can serve as an adequate bridge to an allo-SCT. This issue was explored in a multicenter retrospective study (City of Hope Medical Center and Fred Hutchinson Cancer Center) of 18 patients who were treated with brentuximab vedotin after prior ASCT failure and subsequently were eligible for reduced-intensity allo-SCT [50]. Most patients received a graft from an unrelated donor, and every patient in the study engrafted without delays. The incidence of acute GVHD was 33.3%, and the incidence of chronic GVHD was 56.3%. Impressively, the overall survival after allo-SCT was 100% at one year and PFS of 92.3% at one year. This work was supported by another study using brentuximab vedotin as a bridge to allo-SCT in the UK [51]. Despite the brief follow-up period and small study size, these results are quite striking and suggest that brentuximab vedotin can be effectively utilized for disease cytoreduction prior to allo-SCT.
Finally, brentuximab vedotin has also been shown to be very active in patients relapsing after allo-SCT, a population that suffers from a particularly poor prognosis. In a multicenter retrospective analysis, the overall response rate was 50%, with a CR rate of 38%, and the PFS was 7.8 months. The results mirrored those of the trial examining brentuximab vedotin after failure of ASCT, as the CRs were again markedly more durable than partial responses; a PR was associated with a PFS of 34 weeks whereas over 80% of patients with a CR remained without evidence of disease at one year [52].
7 Future applications of brentuximab vedotin in HL
The available data clearly indicate that brentuximab vedotin has very impressive activity in relapsed and refractory HL. Complete remissions obtained can be durable especially given the extensive treatment history of the patients who have received this agent. Nevertheless, it also appears that in spite of its strong activity as a single agent in the relapsed and refractory setting, it is not curative for all patients. Progression eventually occurs in the majority of patients while on this therapy, and prolonged administration also tends to result in cumulative neuropathy [53]. As mentioned previously, there are no known clinical or biological factors that predict for CR or prolonged duration of response. There are also no validated resistance mechanisms. Investigators at City of Hope Medical Center showed that loss of CD 30 is probably not associated with resistance to brentuximab vedotin [54]. They performed core needle biopsies on patients who developed progressive disease while receiving brentuximab vedotin treatment, and the tumors showed persistent CD 30 expression by immunohistochemistry, despite their drug resistance. More translational research is needed to determine the resistance mechanisms to brentuximab vedotin. Another important research question is whether or not there is a role for brentuximab vedotin in patients with newly diagnosed HL or in patients who are experiencing a first relapse of their disease.
An open-label phase I study was conducted examining the combination of brentuximab vedotin with chemotherapy in the upfront treatment of advanced HL. Patients with advanced HL were either assigned to receive escalating doses of brentuximab vedotin (0.6, 0.9 or 1.2 mg/kg) along with standard doses of ABVD, or 1.2 mg/kg brentuximab vedotin along with AVD (withholding the bleomycin in this group). Both combinations were shown to be very active as all 22 patients receiving ABVD and brentuximab vedotin and 24 of 26 patients receiving AVD and brentuximab vedotin became PET-negative after 2 cycles. While these rates compare favorably with historical controls, the combination of ABVD and brentuximab vedotin was found to have prohibitive pulmonary toxicity with 2 patient deaths [55]. These results have prompted an ongoing open-label, randomized, multi-center phase III trial of AVD and brentuximab vedotin versus ABVD as front-line therapy for advanced HL (www.clinicaltrials.gov as NCT01712490). There is also an ongoing phase II trial for brentuximab vedotin as a treatment for the first relapse of HL prior to autologous transplantation (www.clinicaltrials.gov as NCT01393717). As patients with HL in first relapse with a reasonable performance status usually receive salvage chemotherapy followed by ASCT, it is hoped that the high response rates to brentuximab vedotin in later-line settings may result in its being an effective and relatively non-toxic bridge to ASCT.
One of the challenges in drug development for HL is that it is a disease in which the large majority of patients are cured, and consequently the therapeutic bar has been set high. Nonetheless, for the substantial number of patients with advanced stage disease who relapse, the available salvage therapies are clearly inadequate. Therefore, as the first rationally designed targeted agent approved in HL, brentuximab vedotin is a much-needed addition to the therapeutic arsenal in HL. In light of its striking activity in relapsed and refractory disease, multiple clinical trials are being conducted which may very well confirm a role in earlier-line settings, and these results are eagerly awaited.
Acknowledgements
Dr. Robert Chen is a consultant and speaker for Seattle Genetics and has received research funding from Seattle Genetics.
References
- 1.Kuppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9(1):15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 2.Diehl V, Sextro M, Franklin J, Hansmann ML, Harris N, Jaffe E, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin's disease and lymphocyte-rich classical Hodgkin's disease: report from the European Task Force on Lymphoma Project on Lymphocyte- Predominant Hodgkin's Disease. J Clin Oncol. 1999;17(3):776–783. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 3.Cossman J, Annunziata CM, Barash S, Staudt L, Dillon P, He WW, et al. Reed-Sternberg cell genome expression supports a B-cell lineage. Blood. 1999;94(2):411–416. [PubMed] [Google Scholar]
- 4.Marafioti T, Hummel M, Foss HD, Laumen H, Korbjuhn P, Anagnostopoulos I, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95(4):1443–1450. [PubMed] [Google Scholar]
- 5.Schmid C, Pan L, Diss T, Isaacson PG. Expression of B-cell antigens by Hodgkin's and Reed-Sternberg cells. Am J Pathol. 1991;139(4):701–707. [PMC free article] [PubMed] [Google Scholar]
- 6.Foss HD, Reusch R, Demel G, Lenz G, Anagnostopoulos I, Hummel M, et al. Frequent expression of the B-cell-specific activator protein in Reed-Sternberg cells of classical Hodgkin's disease provides further evidence for its B-cell origin. Blood. 1999;94(9):3108–3113. [PubMed] [Google Scholar]
- 7.Schwarzer R, Dorken B, Jundt F. Notch is an essential upstream regulator of NF-kappaB and is relevant for survival of Hodgkin and Reed-Sternberg cells. Leukemia. 2012;26(4):806–813. doi: 10.1038/leu.2011.265. [DOI] [PubMed] [Google Scholar]
- 8.Re D, Muschen M, Ahmadi T, Wickenhauser C, Staratschek-Jox A, Holtick U, et al. Oct-2 and Bob-1 deficiency in Hodgkin and Reed Sternberg cells. Cancer Res. 2001;61(5):2080–2084. [PubMed] [Google Scholar]
- 9.Torlakovic E, Tierens A, Dang HD, Delabie J. The transcription factor PU.1, necessary for B-cell development is expressed in lymphocyte predominance, but not classical Hodgkin's disease. Am J Pathol. 2001;159(5):1807–1814. doi: 10.1016/S0002-9440(10)63027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathas S, Janz M, Hummel F, Hummel M, Wollert-Wulf B, Lusatis S, et al. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat Immunol. 2006;7(2):207–215. doi: 10.1038/ni1285. [DOI] [PubMed] [Google Scholar]
- 11.Küppers R. New insights in the biology of Hodgkin lymphoma. ASH Education Program Book. 2012;2012(1):328–334. doi: 10.1182/asheducation-2012.1.328. [DOI] [PubMed] [Google Scholar]
- 12.Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. The classical Hodgkin's lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol. 2010;221(3):248–263. doi: 10.1002/path.2711. [DOI] [PubMed] [Google Scholar]
- 13.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin's lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol. 2011;29(14):1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 14.Armitage JO. Early-Stage Hodgkin's Lymphoma. New Engl J Med. 2010;363(7):653–662. doi: 10.1056/NEJMra1003733. [DOI] [PubMed] [Google Scholar]
- 15.Moccia AA, Donaldson J, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30(27):3383–3388. doi: 10.1200/JCO.2011.41.0910. [DOI] [PubMed] [Google Scholar]
- 16.Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C. Combination chemotherapy of Hodgkin's disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36(1):252–259. doi: 10.1002/1097-0142(197507)36:1<252::aid-cncr2820360128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, et al. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 18.Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478–1484. doi: 10.1056/NEJM199211193272102. [DOI] [PubMed] [Google Scholar]
- 19.Gordon LI, Hong F, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advancedstage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31(6):684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011;117(16):4208–4217. doi: 10.1182/blood-2010-09-288373. [DOI] [PubMed] [Google Scholar]
- 21.Moskowitz AJ, Perales MA, Kewalramani T, Yahalom J, Castro-Malaspina H, Zhang Z, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146(2):158–163. doi: 10.1111/j.1365-2141.2009.07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sureda A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin's lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97(2):310–317. doi: 10.3324/haematol.2011.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Wasielewski R, Mengel M, Fischer R, Hansmann ML, Hubner K, Franklin J, et al. Classical Hodgkin's disease. Clinical impact of the immunophenotype. Am J Pathol. 1997;151(4):1123–1130. [PMC free article] [PubMed] [Google Scholar]
- 24.Durkop H, Foss HD, Eitelbach F, Anagnostopoulos I, Latza U, Pileri S, et al. Expression of the CD 30 antigen in non-lymphoid tissues and cells. J Pathol. 2000;190(5):613–618. doi: 10.1002/(SICI)1096-9896(200004)190:5<613::AID-PATH559>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, et al. CD 30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85(1):1–14. [PubMed] [Google Scholar]
- 26.Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, et al. Tumor necrosis factor receptorassociated factor (TRAF) 5 and TRAF2 are involved in CD 30-mediated NFkappaB activation. J Biol Chem. 1997;272(4):2042–2045. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- 27.Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17(25):3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- 28.Duckett CS, Thompson CB. CD 30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 1997;11(21):2810–2821. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho DS, REa AJ, Abraham LJ. Functional aspects of the CD 30 gene in Hodgkin's lymphoma and anaplastic large cell lymphoma. Oncology Reviws. 2009;3:89–101. [Google Scholar]
- 30.Hirsch B, Hummel M, Bentink S, Fouladi F, Spang R, Zollinger R, et al. CD 30-induced signaling is absent in Hodgkin's cells but present in anaplastic large cell lymphoma cells. Am J Pathol. 2008;172(2):510–520. doi: 10.2353/ajpath.2008.070858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartlett NL, Younes A, Carabasi MH, Forero A, Rosenblatt JD, Leonard JP, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD 30+ hematologic malignancies. Blood. 2008;111(4):1848–1854. doi: 10.1182/blood-2008-01-127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, et al. A Phase II study of SGN-30 (anti-CD 30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146(2):171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 33.Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, et al. Phase I/II study of an anti-CD 30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25(19):2764–2769. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- 34.Schnell R, Dietlein M, Staak JO, Borchmann P, Schomaecker K, Fischer T, et al. Treatment of refractory Hodgkin's lymphoma patients with an iodine-131-labeled murine anti-CD 30 monoclonal antibody. J Clin Oncol. 2005;23(21):4669–4678. doi: 10.1200/JCO.2005.09.098. [DOI] [PubMed] [Google Scholar]
- 35.Falini B, Bolognesi A, Flenghi L, Tazzari PL, Broe MK, Stein H, et al. Response of refractory Hodgkin's disease to monoclonal anti-CD 30 immunotoxin. Lancet. 1992;339(8803):1195–1196. doi: 10.1016/0140-6736(92)91135-u. [DOI] [PubMed] [Google Scholar]
- 36.Schnell R, Staak O, Borchmann P, Schwartz C, Matthey B, Hansen H, et al. A Phase I study with an anti-CD 30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD 30+ Hodgkin's and non- Hodgkin's lymphoma. Clin Cancer Res. 2002;8(6):1779–1786. [PubMed] [Google Scholar]
- 37.Hartmann F, Renner C, Jung W, da Costa L, Tembrink S, Held G, et al. Anti-CD16/CD 30 bispecific antibody treatment for Hodgkin's disease: role of infusion schedule and costimulation with cytokines. Clin Cancer Res. 2001;7(7):1873–1881. [PubMed] [Google Scholar]
- 38.Brentuximab vedotin. Drugs R D. 2011;11(1):85–95. doi: 10.2165/11591070-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD 30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res. 2005;11(2 Pt 1):843–852. [PubMed] [Google Scholar]
- 40.Bai R, Pettit GR, Hamel E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem Pharmacol. 1990;39(12):1941–1949. doi: 10.1016/0006-2952(90)90613-p. [DOI] [PubMed] [Google Scholar]
- 41.Perez EA, Hillman DW, Fishkin PA, Krook JE, Tan WW, Kuriakose PA, et al. Phase II trial of dolastatin-10 in patients with advanced breast cancer. Invest New Drugs. 2005;23(3):257–261. doi: 10.1007/s10637-005-6735-y. [DOI] [PubMed] [Google Scholar]
- 42.von Mehren M, Balcerzak SP, Kraft AS, Edmonson JH, Okuno SH, Davey M, et al. Phase II Trial of Dolastatin-10, a Novel Anti-Tubulin Agent, in Metastatic Soft Tissue Sarcomas. Sarcoma. 2004;8(4):107–111. doi: 10.1155/2004/924913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saad ED, Kraut EH, Hoff PM, Moore DF, Jr, Jones D, Pazdur R, et al. Phase II study of dolastatin-10 as first-line treatment for advanced colorectal cancer. Am J Clin Oncol. 2002;25(5):451–453. doi: 10.1097/00000421-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30(7):631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 45.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21(7):778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 46.Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD 30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 47.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD 30-positive lymphomas. N Engl J Med. 2010;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 48.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients With Relapsed or Refractory Hodgkin's Lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartlett N, Brice P, Chen RW, Fanale MA, Gopal AK, Matous J, et al. Retreatment with brentuximab vedotin in CD 30-positive hematologic malignancies: A phase II study. ASCO Meeting Abstracts. 2012;30(15 suppl):8027. [Google Scholar]
- 50.Chen R, Palmer JM, Thomas SH, Tsai NC, Farol L, Nademanee A, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012;119(26):6379–6381. doi: 10.1182/blood-2012-03-418673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibb A, Jones C, Bloor A, Kulkarni S, Illidge T, Linton K, et al. Brentuximab vedotin in refractory CD 30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013;98(4):611–614. doi: 10.3324/haematol.2012.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gopal AK, Ramchandren R, O'Connor OA, Berryman RB, Advani RH, Chen R, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120(3):560–568. doi: 10.1182/blood-2011-12-397893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Donk NW, Dhimolea E. Brentuximab vedotin. MAbs. 2012;4(4):458–465. doi: 10.4161/mabs.20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nathwani N, Krishnan AY, Huang Q, Kim Y, Karanes C, Smith EP, et al. Persistence of CD expression in Hodgkin lymphoma following brentuximab vedotin (SGN-35) treatment failure. Leuk Lymphoma. 2012;53(10):2051–2053. doi: 10.3109/10428194.2012.666543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ansell SM, Connors JM, Park SI, O'Meara MM, Younes A. Frontline Therapy with Brentuximab Vedotin Combined with ABVD or AVD in Patients with Newly Diagnosed Advanced Stage Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2012;120(21):798. [Google Scholar]