Abstract
Background
Health-related quality of life (QoL) has prognostic value in many cancers. A recent study found that the performance of prognostic systems for metastatic colorectal cancer (mCRC) were improvable. We evaluated the independent prognostic value of QoL for overall survival (OS) and its ability to improve two prognostic systems’performance (Köhne and GERCOR models) for patients with mCRC.
Methods
The EQ-5D questionnaire was self-completed before randomization in the OPTIMOX1, a phase III trial comparing two strategies of FOLFOX chemotherapy which included 620 previously untreated mCRC patients recruited from January 2000 to June 2002 from 56 institutions in five countries. The improvement in models’ performance (after addition of QoL) was studied with Harrell’s C-index and the net reclassification improvement.
Results
Of the 620 patients, 249 (40%) completed QoL datasets. The Köhne model could be improved by LDH, mobility and pain/discomfort; the C-index rose from 0.54 to 0.67. The associated NRI for 12-month death was 0.23 [0.05; 0.46]. Mobility and pain/discomfort could be added to the GERCOR model: the C-index varied from 0.63 to 0.68. The NRI for 12 months death was 0.35 [0.12; 0.44].
Conclusions
Mobility and pain dimensions of EQ5D are independent prognostic factors and could be useful for staging and treatment assignment of mCRC patients. Presented at the 2011 ASCO Annual Meeting (#3632).
Background
Colorectal cancer (CRC) is the third most diagnosed cancer in men and the second most diagnosed in women, with over 1.2 million new cases and 608 700 deaths worldwide in 2008 [1]. About up to half (20% to 50%) of CRC patients will develop metastases during the course of their disease [2] and approximately 35% are diagnosed with synchronous metastases [2,3]. Standard treatments for metastatic CRC (mCRC) are based on chemotherapy.
As is the case for many cancers, CRC staging is essential for optimal patient management. Accurate prognostication facilitates both therapeutic decisions and stratification in randomized clinical trials of cancer treatments. In CRC, the well-known TNM staging system is predominantly used [4]. In mCRC, two validated prognostic classification systems can be applied: Köhne prognostic index [5] for patients receiving front-line fluoropyrimidine mono-chemotherapy and GERCOR (Groupe Coopérateur Multidisciplinaire en Oncologie) prognostic index [6] for patients with oxaliplatin-based or irinotecan-based regimens. However, the models’ ability to discriminate between patients on the basis of their prognosis (as measured by the C-index [7]) is still relatively modest. Thus, improvement of these prognostic indicators is required [6].
In palliative care patients, the prognostic value of health-related quality of life (QoL) has been demonstrated for several types of cancer [8-10]. For mCRC patients, QoL is known to be an independent prognostic factor for overall survival (OS) [8,11]. Hence, QoL is a candidate for the improvement of existing prognostic indices. Given that QoL is a multidimensional concept, there is a need to identify the QoL dimensions associated with OS for each specific type of cancer. The results of a recent study showed that social functioning (as measured with the EORTC QLQ-C30 tool) is an independent prognostic factor for survival in mCRC patients [12]. The objective of the present study was to assess the independent prognostic value of QoL in mCRC and evaluate its ability to improve the Köhne and GERCOR prognostic indices.
Methods
Patients
Individual patient data from the OPTIMOX1 phase III trial were analysed. The 620 evaluable patients from OPTIMOX1 were recruited from January 2000 to June 2002 from 56 institutions in five countries. In this trial, the oxaliplatin stop-and-go strategy proved to be as good as a continuous oxaliplatin-based chemotherapy strategy in previously untreated mCRC patients. The trial's inclusion and exclusion criteria are detailed elsewhere [13].
Quality of life assessment
Quality of life was self-reported by the patient using the generic EQ-5D questionnaire (also known as EuroQol) [14], which has five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) rated as one of three levels ("no problems",”some problems" and ”extreme problems", coded as 1, 2 and 3, respectively). The EQ-5D also includes a 100-centimetre visual analogue scale (VAS) for the self-assessment of overall health (0 = worst possible score; 100 = best possible score).
The GERCOR and Köhne prognostic indices
The Köhne prognostic index [5] comprises four variables: performance status (PS), number of metastatic sites, alkaline phosphatase (ALP) level and white blood cell (WBC) count. The GERCOR prognostic index [6] is based on two variables: PS and serum lactate dehydrogenase (LDH) level. Patients are classified into three risk groups (low, intermediate and high) in both models.
Statistical analysis
Demographic and clinical characteristics were summarized as frequency and percentage. In order to check whether selection bias was present, the patients’clinical characteristics were compared (with chi-squared test or Fisher's exact test) as a function of the available QoL data at baseline.
Overall survival was defined as the time from randomization to death (regardless of the cause) or last follow-up (censored data). All randomized patients with complete QoL data were included in the statistical analysis.
Univariate and multivariate analysis were performed using Cox proportional hazards modelling, with calculation of the hazard ratio (HR) and the corresponding 95% two-sided confidence intervals (95%CI).
In order to evaluate the independent prognostic value of QoL, we built two multivariate models with backward selection. The first model included all demographic and clinical variables associated with OS (p<0.1) in univariate analysis. The second model included demographic, clinical and QoL variables with p<0.1 in univariate analysis.
Improvements in the prognostic index was evaluated by adding clinical variables (other than those used to build the prognostic index) and QoL variables (with p<0.1 in univariate analysis) to a model with backward selection (Köhne or GERCOR index being forced in the model). Patients with available QoL data for whom Köhne and GERCOR indices could be calculated were considered for prognostic systems’ improvement.
The models were compared by calculating the Schemper statistic [15] and Harrell’s C index [7]. The Schemper statistic is equivalent to R2 in linear regression and quantifies the proportion of the survival variability that is explained by the model. Briefly, the higher the Schemper statistic is, the more accurate the OS predictions would be. Harrell’s C index estimates discriminate capability, i.e. the ability to distinguish between high-risk and low-risk patients. The C-index varies from 0.5 (no discrimination) to 1 (perfect discrimination). Optimism-corrected C-index was calculated using 200 bootstrap replications.
Category-free net reclassification improvement [16] (NRI) was also calculated at various moments (12, 24 and 36 months), in order to evaluate the additional utility of QoL domains and other clinical factors. NRI quantifies”the correctness of upward and downward reclassification or movement of predicted probabilities as a result of adding a new marker”. The confidence interval for NRI was calculated using the percentiles of 1000 bootstrap replications.
We also performed a sensitivity analysis using the multiple-imputation technique [17,18] (with 10 replications) for missing QoL data. The choice of 10 replications was prompted by the large amount of missing QoL data in the trial (60%). In line with Van Buuren’s method [19], the demographic and clinical variables initially included in the final complete-data model, those associated with the lack of QoL data and those strongly associated with OS (albeit absent from the final model) were used as predictors for the imputation of missing QoL data using a logistic regression model (QoL coded as 2–3 vs. 1). Multiple imputation with 10 replications (of the original database) consisted in creating 10 plausible values for each missing data and thus generating 10 new complete databases. For each of the new databases, a standard analysis was performed and the results were combined into a single estimation of the parameter of interest, while taking account of the uncertainty of the imputation technique [20]. Variables selected more than 5 times out of 10 replications were included in the multivariate model after multiple imputations.
Since there was no within-imputation variance according to the Schemper statistic, the pooled estimate was presented as the median [range] [20].
Construction of the a modified prognostic index was based on linear transformation as follows: The regression coefficient for each variable selected in the final multivariate complete case Cox model was divided by the lowest coefficient and rounded to the nearest integer [21]. The sum of these integers is the maximum score (M) for the modified index; hence the new score varied from zero to M. According to the score, the modified prognostic index was then arbitrary divided into three risk groups: good prognostic, intermediate prognostic and poor prognostic.
Survival distributions were estimated using the Kaplan-Meier method [22] and compared with the log-rank test.
All statistical analyses were carried out using SAS® software (version 9.2, SAS Institute Inc., Cary, NC) and R.2.12.0 software (free) using the Design, SurvIDINRI (for NRIs) and Multivariate Imputation by Chained Equations packages ( http://www.multiple-imputation.com/). P-values were two-sided and variables with p<0.05 were considered significantly associated with OS in multivariate models.
Results
Patient characteristics
The patient baseline characteristics are summarized in Table 1, most of them were male (59%) and 43% were over the age of 65. Synchronous metastasis was predominant (68%) and most of the patients with metachronous metastasis received adjuvant chemotherapy (66%, 130/196).
Table 1.
Baseline demographic, clinical and laboratory variables for patients with and without available QoL data
| All patients | Available QoL | Missing QoL | All patients | |||||
|---|---|---|---|---|---|---|---|---|
|
Variable |
Class |
N |
% |
N |
% |
N |
% |
P |
|
Age |
≤65 |
353 |
57 |
138 |
55 |
215 |
58 |
|
| |
>65 |
267 |
43 |
111 |
45 |
156 |
42 |
0.2900 |
|
Gender |
Male |
367 |
59 |
151 |
61 |
216 |
58 |
|
| |
Female |
252 |
41 |
98 |
39 |
154 |
42 |
0.5739 |
|
PS |
0 |
333 |
54 |
122 |
49 |
211 |
57 |
|
| |
1 |
239 |
38 |
110 |
44 |
129 |
35 |
|
| |
2 |
48 |
8 |
17 |
7 |
31 |
8 |
0.0611 |
|
Number of sites |
1 |
354 |
58 |
147 |
59 |
207 |
57 |
|
| |
>1 |
260 |
42 |
102 |
41 |
158 |
43 |
0.5672 |
|
Liver involvement |
No |
149 |
24 |
52 |
21 |
97 |
27 |
|
| |
Yes |
460 |
76 |
197 |
79 |
263 |
73 |
0.0872 |
|
Metastases |
Synchronous |
415 |
68 |
168 |
68 |
247 |
68 |
|
| |
Metachronous |
196 |
32 |
80 |
32 |
116 |
32 |
0.9374 |
|
Adjuvant chemotherapy |
No |
488 |
79 |
200 |
81 |
288 |
78 |
|
| |
Yes |
130 |
21 |
48 |
19 |
82 |
22 |
0.4013 |
|
Tumour site |
Colon |
398 |
64 |
160 |
64 |
238 |
64 |
|
| |
Rectum |
211 |
34 |
86 |
35 |
125 |
35 |
|
| |
both |
11 |
2 |
3 |
1 |
8 |
1 |
0.6730 |
|
LDH |
≤1xULN |
380 |
61 |
134 |
56 |
246 |
66 |
|
| |
>1xULN |
240 |
39 |
115 |
44 |
125 |
34 |
0.0017 |
|
ALP |
≤1xULN |
350 |
56 |
129 |
52 |
221 |
60 |
|
| |
>1xULN |
270 |
44 |
120 |
48 |
150 |
40 |
0.0560 |
|
CEA |
≤1xULN |
177 |
28 |
61 |
25 |
116 |
31 |
|
| |
>1xULN |
443 |
72 |
188 |
75 |
255 |
69 |
0.0673 |
|
EuroQoL |
|
|
|
|
|
|
|
|
|
Mobility |
1 |
223 |
81 |
223 |
81 |
|
|
|
| |
2-3 |
54 |
19 |
54 |
19 |
|
|
|
|
Self-care |
1 |
255 |
93 |
255 |
93 |
|
|
|
| |
2-3 |
19 |
7 |
19 |
7 |
|
|
|
|
Usual activities |
1 |
193 |
71 |
193 |
71 |
|
|
|
| |
2-3 |
79 |
29 |
79 |
29 |
|
|
|
|
Pain/discomfort |
1 |
137 |
50 |
137 |
50 |
|
|
|
| |
2-3 |
138 |
50 |
138 |
50 |
|
|
|
|
Anxiety/depression |
1 |
145 |
53 |
145 |
53 |
|
|
|
| |
2-3 |
130 |
47 |
130 |
47 |
|
|
|
| VAS score | 70 [10–100] ** | |||||||
** Median (range).
ULN= Upper Limit of Normal.
VAS= visual analogue scale.
PS= performance status.
ALP= alkaline phosphatase.
LDH= serum lactate dehydrogenase.
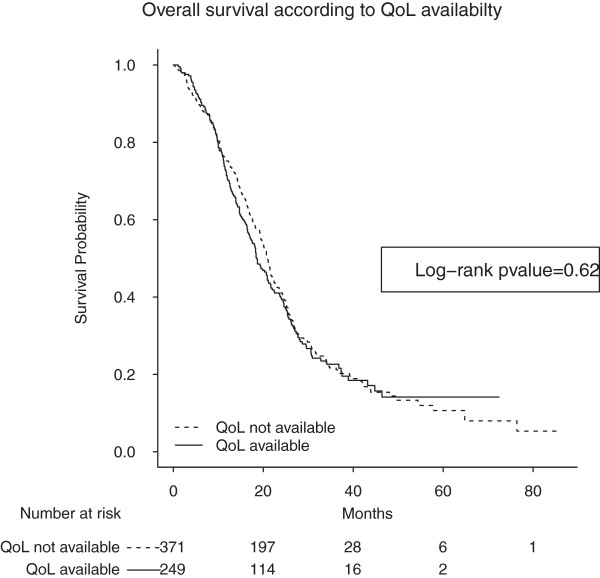
Data on QoL was available for 249 of the 620 patients in the original OPTIMOX1 cohort (40%). Normal serum LDH was significantly more frequent in patients with missing QoL data. Patients with missing QoL data also tended to have lower serum ALP levels, a better PS and less liver involvement compared to patients with available QoL. Of the 249 patients, 75% died after a median follow-up period of 35.8 months (95% CI = [33.8–38.4]). There was no apparent correlation between the availability of QoL datasets and OS (Log-rank pvalue = 0.62; Figure 1).
Figure 1.
Overall survival (in months) of patients lacking QoL data (dotted line; n = 371) and patients with available QoL data (solid line; n = 249). Log-rank p value = 0.62. The median survival times for patient with and without QoL datasets were 18.6 months (95% CI [17.0 - 21.6]) and 20.8 months (95% CI = [19.5–22.2]), respectively.
Most of the patients had good QoL: 81%, 93%, 71%, 50% and 53% had no problems in terms of mobility, self-care, usual activities, pain/discomfort and anxiety/depression, respectively. The median VAS score was 70 (range = [10–100]).
Univariate analysis
Given that”extreme problems” (coded as 3) were infrequent, QoL item scores were pooled into two classes (i.e. a score of 1 vs. a score of 2 or 3). We also combined PS into 2 classes (0 vs. 1–2), due to the low proportion of patients with a PS score of 2.
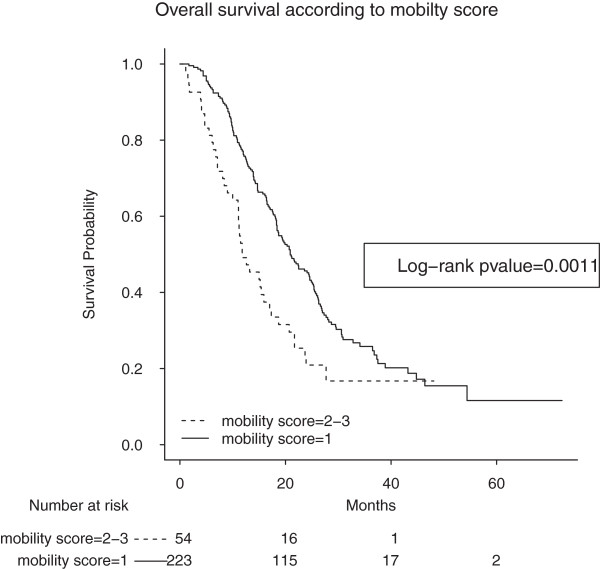
Univariate analyses of clinical and QoL variables are summarized in Table 2. High serum LDH, poor PS, high serum ALP, >1 metastatic sites, age>65, high serum CEA, mobility problems (as coded 2–3) (Figure 2), pain/discomfort problems (as coded 2–3) and anxiety/depression problems (as coded 2–3) were associated with a poorer prognosis.
Table 2.
Univariate and multivariate Cox analyses
| Univariate analysis |
Multivariate analysis |
Multivariate analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model not including QoL | Full model, including QoL | |||||||||
|
Variable |
Class |
HR |
95% CI |
P |
HR |
95% CI |
P |
HR |
95% CI |
P |
|
Age |
≤65 |
1 |
|
|
|
|
|
|
|
|
| |
>65 |
1.42 |
1.06 – 1.89 |
0.0178 |
|
|
|
|
|
|
|
Gender |
Male |
1 |
|
|
|
|
|
|
|
|
| |
Female |
1.06 |
0.79 – 1.42 |
0.6945 |
|
|
|
|
|
|
|
PS |
0 |
1 |
|
|
1 |
|
|
1 |
|
|
| |
1-2 |
1.84 |
1.38 – 2.46 |
<0.0001 |
1.98 |
1.44 – 2.73 |
<0.0001 |
1.87 |
1.35 – 2.59 |
0.0002 |
|
Number of sites |
1 |
1 |
|
|
1 |
|
|
1 |
|
|
| |
>1 |
1.47 |
1.10 – 1.97 |
0.0094 |
1.48 |
1.08 – 2.05 |
0.0160 |
1.48 |
1.07 – 2.04 |
0.0176 |
|
Liver involvement |
No |
1 |
|
|
|
|
|
|
|
|
| |
Yes |
1.14 |
0.795 – 1.65 |
0.4699 |
|
|
|
|
|
|
|
Metastases |
Synchronous |
1 |
|
|
|
|
|
|
|
|
| |
Metachronous |
0.89 |
0.61 – 1.29 |
0.5403 |
|
|
|
|
|
|
|
Adjuvant chemotherapy |
No |
1 |
|
|
|
|
|
|
|
|
| |
Yes |
0.95 |
0.76 – 1.19 |
0.68 |
|
|
|
|
|
|
|
LDH |
≤1xULN |
1 |
|
|
1 |
|
|
1 |
|
|
| |
>1xULN |
2.04 |
1.48 – 2.80 |
<0.0001 |
1.93 |
1.39 – 2.68 |
<0.0001 |
1.83 |
1.31 – 2.55 |
0.0004 |
|
APL |
≤1xULN |
1 |
|
|
|
|
|
|
|
|
| |
>1xULN |
1.60 |
1.20 – 2.14 |
0.0016 |
|
|
|
|
|
|
|
CEA |
≤1xULN |
1 |
|
|
|
|
|
|
|
|
| |
>1xULN |
1.48 |
1.01 – 2.18 |
0.0444 |
|
|
|
|
|
|
|
EuroQoL |
|
|
|
|
|
|
|
|
|
|
|
Mobility |
1 |
1 |
|
|
|
|
|
1 |
|
|
| |
2-3 |
1.90 |
1.33 – 2.71 |
0.0004 |
|
|
|
1.66 |
1.12 – 2.48 |
0.0117 |
|
Self-care |
1 |
1 |
|
|
|
|
|
|
|
|
| |
2-3 |
1.52 |
0.88 – 2.62 |
0.1322 |
|
|
|
|
|
|
|
Usual activities |
1 |
1 |
|
|
|
|
|
|
|
|
| |
2-3 |
1.20 |
0.88 – 1.64 |
0.2553 |
|
|
|
|
|
|
|
Pain/discomfort |
1 |
1 |
|
|
|
|
|
|
|
|
| |
2-3 |
1.39 |
1.04 – 1.86 |
0.0239 |
|
|
|
|
|
|
|
Anxiety/depression |
1 |
1 |
|
|
|
|
|
|
|
|
| |
2-3 |
1.45 |
1.09 – 1.93 |
0.0116 |
|
|
|
|
|
|
|
VAS score |
|
1.001 |
0.996 – 1.005 |
0.7975 |
|
|
|
|
|
|
|
Harrell’s C index |
|
|
|
|
0.65 [0.61 – 0.69] |
|
0.67 [0.63 – 0.71] |
|||
| 0.65* |
0.66* |
|||||||||
| Schemper statistic | 9.32% | 10.42% | ||||||||
ULN = Upper Limit of Normal.
* = Optimism-corrected C-index.
Figure 2.
Overall survival (in months) of patients with mobility problems (as coded 2–3) (dotted line; n = 54) and patients without mobility problems (as coded 1) (solid line; n = 223). Log-rank p value = 0.0011. The median survival times were 20.9 (95% CI = [18.6–24.9]) months and 11.8 (95% CI = [11.1–17.3]) months for patients without problems (coded as 1) and those with problems (as coded 2–3), respectively.
There were no significant associations between the risk of death and self-care (p = 0.1322), usual activities (p = 0.2553) and the VAS score (p = 0.1280) QoL scales on the other.
Multivariate analysis
The results for multivariate analyses are summarized on Table 2.
In the first model, high LDH, >1 metastatic sites and poor PS were associated with a shorter survival.
In the second model, high LDH, >1 metastatic sites, poor PS and mobility problems were associated with a shorter survival.
After multiple imputations, the pooled HR for mobility was 1.57 (95% CI = [1.16–2.12]) (p = 0.0043) in the model including LDH, the number of metastatic sites, PS, ALP, pain/discomfort and mobility (Additional file 1).
Improvement of prognostic indices
In order to evaluate improvements in performance of the Köhne and GERCOR prognostic indices, we first calculated their performance in our population (Table 3).
Table 3.
Improvement of Köhne prognostic index
| Köhne prognostic index | |||||
|---|---|---|---|---|---|
| Variable |
HR (95% CI) |
P value |
c-index |
Schemper (%) |
NRI (95% CI) |
| Köhne (2 vs. 1) |
1.18 [0.96 – 1.47] |
=0.1200 |
|
|
|
| Köhne (3 vs. 1) |
2.66 [1.84 – 3.85] |
<0.0001 |
0.54 [0.51 -0.57] *0.54 |
1.6 |
|
|
Improvement of the Köhne prognostic index with clinical and QoL factors: complete-case analysis | |||||
| Köhne (2 vs. 1) |
1.11 [0.80 – 1.55] |
=0.5114 |
|
|
NRI at 12 months = 0.23 ([0.07; 0.46]) |
| |
NRI at 24 months = 0.31 ([0.16; 0.44]) |
||||
| Köhne (3 vs. 1) |
2.17 [1.25 – 3.75] |
=0.0056 |
|
|
|
| |
NRI at 36 months = 0.27 ([0.02; 0.50]) |
||||
| LDH (>1ULN vs. ≤ 1ULN) |
2.09 [1.53 – 2.87] |
<0.0001 |
0.67 [0.63 -0.71] |
10.8 |
|
| Mobility (2–3 vs. 1) |
1.56 [1.05 – 2.32] |
=0.0266 |
*0.66 |
|
|
| Pain/discomfort (2–3 vs. 1) |
1.60 [1.17 – 2.18] |
=0.0031 |
|
|
|
|
Improvement of the Köhne prognostic index with clinical and QoL factors after multiple imputation | |||||
| Köhne (2 vs. 1) |
1.24 [0.97 – 1.58] |
=0.0780 |
|
|
|
| Köhne (3 vs. 1) |
2.15 [1.43 – 3.24] |
=0.0002 |
|
|
|
| LDH (>1ULN vs. ≤ 1ULN) |
1.99 [1.61 – 2.46] |
<0.0001 |
0.66 [0.59 -0.73] |
8.63 [7.74 – 10.8] |
|
| Mobility (2–3 vs. 1) |
1.39 [1.06 – 1.83] |
=0.0191 |
R = 65% |
|
|
| Pain/discomfort (2–3 vs. 1) | 1.67 [1.20 – 2.31] | =0.0031 | R = 113% | ||
LDH = lactate dehydrogenase.
ULN = Upper Limit of Normal.
* = bootstrap C-index.
R = relative increase in variance due to missing data.
QoL = Quality of Life.
HR = Hazard ratio.
NRI = net reclassification improvement.
For multiple imputations, a logistic model was used: response variable = QoL scale (2–3 vs. 1) and exploratory variables were number of metastatic sites, liver involvement, WHO Performance Status, CEA, APL and LDH.
Variables considered in the imputation method (last model) were selected more than 5 times among the 10 replications of multiple imputations (see statistical method).
Improvement of the Köhne prognostic index
After addition of QoL and clinical variables to the Köhne prognostic index in a complete-case analysis (N = 236), high LDH, mobility and pain/discomfort problems appeared to be related to a shorter survival (Table 4). The C-index and Schemper statistic were improved while the NRIs were significantly different from zero (Table 3). A modified Köhne prognostic index was built using the above variables (Table 5).
Table 4.
Improvement of the GERCOR prognostic index
| GERCOR prognostic index | |||||
|---|---|---|---|---|---|
| Variable |
HR (95% CI) |
P value |
c-index |
Schemper (%) |
NRI (95% CI) |
| GERCOR (2 vs. 1) |
1.82 [1.43 – 2.33] |
<0.0001 |
|
|
|
| GERCOR (3 vs. 1) |
3.10 [2.38 – 4.05] |
<0.0001 |
0.63 [0.61 -0.66] *0.63 |
6.44 |
|
|
Improvement of the GERCOR prognostic index clinical and QoL factors: complete-case analysis | |||||
| GERCOR (2 vs. 1) |
1.70 [1.14 – 2.54] |
=0.0090 |
|
|
NRI at 12 months = 0.35 [0.06; 0.44] |
| GERCOR (3 vs. 1) |
3.35 [2.20 – 5.10] |
<0.0001 |
0.67 [0.63 -0.71] *0.67 |
11.52 |
NRI at 24 months = 0.27 [0.04; 0.38] |
| |
NRI at 36 months = 0.28 [0.01; 0.45] |
||||
| Mobility (2–3 vs. 1) |
1.77 [1.19 – 2.62] |
=0.0047 |
|
|
|
| Anxiety/depression (2–3 vs. 1) |
1.41 [1.03 – 1.92] |
=0.0314 |
|
|
|
|
Improvement of the GERCOR prognostic index clinical and QoL factors: multiple imputation | |||||
| GERCOR (2 vs. 1) |
1.77 [1.36 – 2.30] |
<0.0001 |
|
|
|
| GERCOR (3 vs. 1) |
2.49 [1.84 – 3.38] |
<0.0001 |
|
|
|
| ALP (>1ULN vs. ≤ 1ULN) |
1.25 [1.00 – 1.57] |
=0.0480 |
0.67 [0.64 -0.71] |
9.56 [8.76 – 11.52] |
|
| Mobility (2–3 vs. 1) |
1.42 [1.08 – 1.86] |
=0.0120 |
R = 60% |
|
|
| Pain/discomfort (2–3 vs. 1) | 1.55 [1.10 – 2.20] | =0.0140 | R = 138% | ||
LD = lactate dehydrogenase.
ULN = Upper Limit of Normal.
* = bootstrap C-index.
R = relative increase in variance due to missing data.
QoL = Quality of Life.
HR = Hazard ratio.
NRI = net reclassification improvement.
For multiple imputations, a logistic model was used: response variable=QoL scale (2–3 vs. 1) and exploratory variables were number of metastatic sites, liver involvement, WHO Performance Status, CEA, APL and LDH.
Variables considered in the imputation method (last model) were selected more than 5 times among the 10 replications of multiple imputations (see statistical method).
Table 5.
Modified Köhne prognostic index
| 0 point | 1 point | 2 points | 3 points | 4 points | 5 points | 6 points | 7 points | |
|---|---|---|---|---|---|---|---|---|
| Köhne |
Köhne I |
Köhne II |
|
|
|
|
|
Köhne III |
| LDH |
≤ 1ULN |
|
|
|
|
|
|
>1ULN |
| Mobility score |
1 |
|
|
|
2-3 |
|
|
|
| Pain/discomfort score | 1 | 2-3 |
The modified Köhne index varied from 0 to 22 points.
Poor prognosis: 15 to 22 points.
Intermediate prognosis: 8 to 14 points.
Good prognosis: 0 to 6 points.
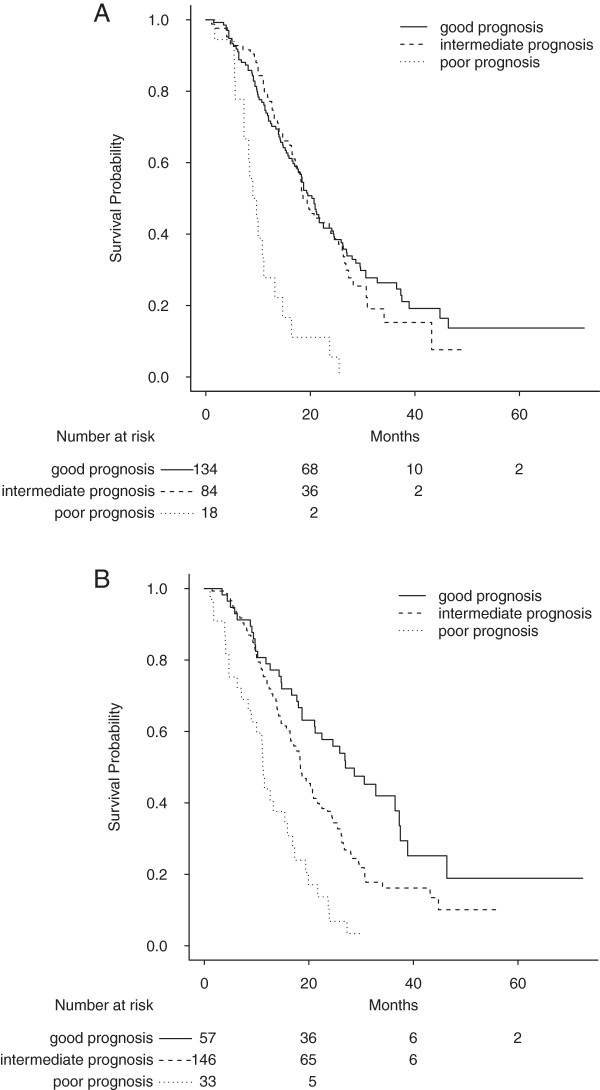
Survival distributions for the Köhne and improved Köhne prognostic systems are shown in Figure 3 A&3B.
Figure 3.
Survival strata according to the Köhne prognostic model before and after improvement. A: Overall survival (in months) for good, intermediate and poor prognosis according to the Köhne prognostic model. Median survival = 20.7 [17.7 – 24.4] for the group with good prognosis (n = 134); Median survival = 18.6 [17.1 – 25.4] for the group with intermediate prognosis (n = 84); Median survival = 9.0 [7.3 -14.7] for the group with poor prognosis (n = 18). Log-rank p = 0.0013. Optimism corrected C-index = 0.54. B: Overall survival (in months) for good, intermediate and poor prognosis according to the modified Köhne group. Median survival = 27.0 [21.1 – 37.5] for the group with good prognosis (n = 57); Median survival = 18.4 [16.5 – 21.6] for the group with intermediate prognosis (n = 146); Median survival = 11.3 [9.0 – 16.9] for the group with poor prognosis (n = 33). Log-rank p<0.0001. Optimism corrected C-index = 0.60.
The Results of multiple imputations are summarized in Table 3.
A complete-case analysis of the GERCOR prognostic classification revealed that mobility and Anxiety/depression could improve performance: the C-index, Schemper statistic, and NRI are summarized in Table 4.
Based on these two new QoL scales, a modified GERCOR prognostic system was built using the above variables (Table 6).
Table 6.
Modified GERCOR prognostic index
| 0 point | 1 point | 2 points | 3 points | 4 points | |
|---|---|---|---|---|---|
| GERCOR |
GERCOR I |
|
GERCOR II |
GERCOR III |
|
| Mobility score |
1 |
2-3 |
|
|
|
| Pain/discomfort score | 1 | 2-3 |
The modified GERCOR index varied from 0 to 5 points.
Poor prognosis: 4 to 5 points.
Intermediate prognosis: 2 or 3 points.
Good prognosis: 0 or 1 point.
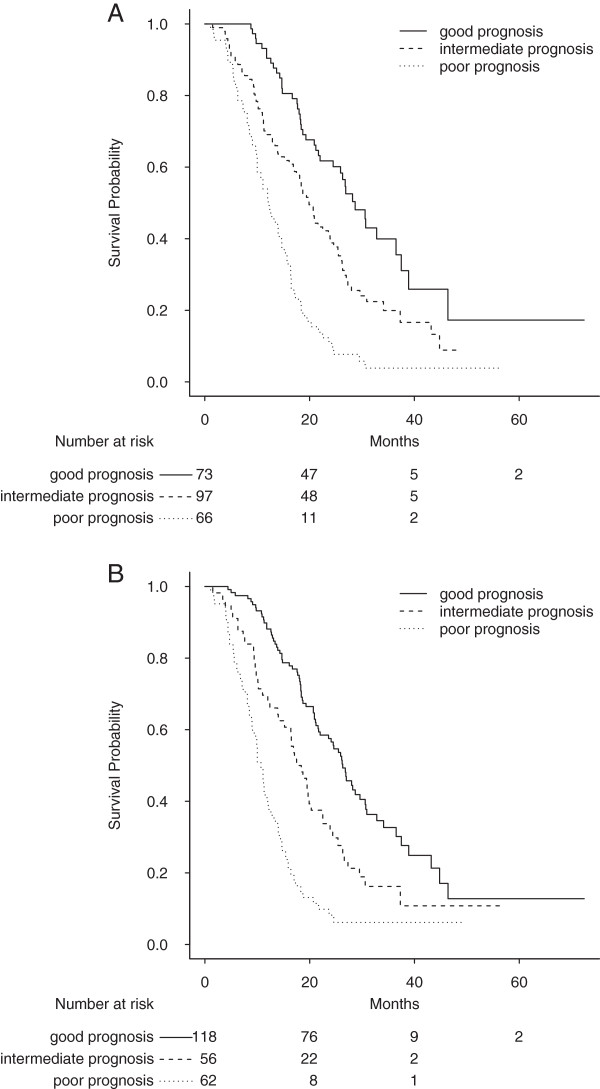
Survival distributions for the GERCOR and improved GERCOR prognostic systems are shown in Figure 4A and Figure 4B.
Figure 4.
Survival strata according to the GERCOR prognostic model before and after improvement. A: Overall survival (in months) for good, intermediate and poor prognosis according to the GERCOR prognostic system. Median survival = 28.7 [24.5 – 38.9] for the group with good prognosis (n = 73); Median survival = 19.9 [18.1 – 23.9] for the group with intermediate prognosis (n = 97); Median survival = 12.1 [10.0 – 15.4] for the group with poor prognosis (n = 66). Log-rank p<0.0001. Optimism corrected C-index = 0.65. B: Overall survival (in months) for good, intermediate and poor prognosis according to the modified GERCOR prognostic system. Median survival = 28.2 [24.5 – 37.5] for the group with good prognosis (n = 68); Median survival = 21.6 [18.7 – 26.2] for the group with intermediate prognosis (n = 90); Median survival = 11.5 [10.0 – 14.7] for the group with poor prognosis (n = 78). Log-rank p<0.0001. Optimism corrected C-index = 0.66.
The Results of multiple imputations are summarized in Table 4.
Discussion
In this study, EuroQol mobility dimension appeared to be the third most important prognostic factor (measured by the hazard ratio) for overall survival in unresectable mCRC, after serum LDH level and ECOG performance status. Self-reported QoL is known to be associated with OS in several types of cancer [8,9,11,12]. Our present results confirmed the independent prognostic value of QoL scales in patients with mCRC [8,11,12]. Our first multivariate model (including clinical and biochemical variables) revealed the prognostic value of LDH, PS and the number of metastatic sites, whereas our second model (with the addition of QoL) confirmed the value of LDH, PS and the number of metastatic sites and further identified the QoL”mobility" scale as an independent prognostic factor.
After multiple imputations, the mobility QoL scale remained significant despite its high associated relative increase in variance due to missing data imputation. Pain/discomfort was not significant but showed a prognostic value after the multiple- imputation analysis; this may be partially related to the high increase in variance due to missing QoL data.
We found that the Köhne prognostic system could be improved by including LDH, mobility and pain/discomfort in both complete-case and imputation analyses. Moreover, the GERCOR prognostic index was improved by mobility and anxiety/depression in a complete-case analysis and by ALP, mobility and pain/discomfort after multiple imputations. This difference in the selection of variables may be due to lack of power in the complete-case analysis albeit ALP was at the limit of statistical significance. Therefore the GERCOR prognostic index was essentially improved by QoL scales. The added value of QoL scales (completed by the patient) for improvements of the two prognostic systems revealed that the patient’s perception of his/her disease was an important information to record for prognosis assessment in addition to the clinician’s evaluation [23].
Despite a marked increase in variance due to missing data, the mobility and pain/discomfort QoL dimensions significantly improved the Köhne and GERCOR staging systems. This result comforted the independent prognostic value of these QoL scales in mCRC patients. The results for complete-case and multiple-imputation analysis were very similar. QoL significantly improved the prognostic indices with both methods (complete-case and multiple-imputation analyses). This may be related to the fact that the compete-case analysis was not biased. In fact, patients with and without QoL data at inclusion did not differ in terms of the median survival time [24] (i.e. missingness was not related to outcome).
It should be noted that such a large improvement in the C-index from 0.54 to 0.66 for the Köhne prognostic index has rarely been reported in prognostic studies. After the addition of both clinical and QoL factors, the NRIs were also statistically significant for both the Köhne and the GERCOR prognostic systems (95% CIs did not contained zero). The independent prognostic value of mobility and pain/discomfort QoL scales (using the EQ-5D) for mCRC is compatible with the result of Efficace [12] regarding the prognostic value of social functioning scale (using the EORTC QLQ-C30). In fact, mobility and pain problems could impair the social functioning QoL dimension.
One of the present study's strengths relates to its use of the easily understood and rapidly completed EQ-5D. The EQ-5D was chosen because it was expected to be less time consuming and could prevent missing data. However, EQ-5D is not a cancer-specific questionnaire like the EORTC QLQ-C30 and it constitutes a limitation of our study. The high proportion of missing data (60%) and its large variability between countries (ranged from 5% to 66%) constitute another limitation in the generalizability of our results. Such a large heterogeneity in missing data might be related to the trial logistic and/or each country’s culture. It is also important to note than our population came from a randomized controlled trial with restrictive inclusion and non inclusion criteria and might not be representative of mCRC patients in general [25]. Quality of Life may be an important parameter to record when assessing the situation of mCRC patients, since it improved the accuracy of OS prediction and greatly improved the two best-known prognostic classification systems for mCRC. We consider that QoL domains are important factors in the field of stratified therapy in the sense that knowing some aspect of the patient’s self-reported QoL level could be decisive in the choice of different treatment options in the area of tailored medicine. By way of an example, a clinician might wish to avoid a treatment with pain as side-effect if the patient reported preexisting pain symptoms. Pain and mobility could also serve as an inclusion and/or stratification factor in randomized, controlled trials in mCRC.
Conclusion
Our results confirmed the prognostic value of QoL in mCRC patients. Thus, QoL scores should be recorded as it could give supplementary information to the clinician regarding the prognosis of a patient as well as in the judgment of an acceptable treatment side effect.
Abbreviations
QoL: Quality of life; mCRC: Metastatic colorectal cancer; OS: Overall survival; EQ-5D: Generic measure of health status developed by the EuroQol Group; HR: Hazard ratio; CI: Confidence interval; CRC: Colorectal cancer; TNM: Tumor Node Metastasis; GERCOR: Groupe Coopérateur Multidisciplinaire en Oncologie; EORTC: European Organization for Research and Treatment of Cancer; VAS: Visual analogue scale; PS: Performance status; ALP: Alkaline phosphatase; LDH: Lactate dehydrogenase; ITT: Intention to treat; NRI: Net reclassification improvement; CEA: Carcinoembryonic antigen; HDL: High density lipoprotein.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MD, BC, FB, AG, CL, CT, TF, NP, SD, AH, MH and MG the seven authors are justifiably credited with authorship, according to the authorship criteria. In detail: MD BC TF FB – conception, design, analysis and interpretation of data, drafting of the manuscript, final approval given; AG CL CT SD NP AH MH MG BC – acquisition of data, interpretation of data, critical revision of manuscript, final approval given. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Dr. David Fraser for advice in English language.
Supplementary Material
Results of the multivariate analysis after QoL imputation.
Contributor Information
Momar Diouf, Email: diouf.momar@chu-amiens.fr.
Benoist Chibaudel, Email: benoist.chibaudel@sat.aphp.fr.
Thomas Filleron, Email: Filleron.Thomas@claudiusregaud.fr.
Christophe Tournigand, Email: christophe.tournigand@sat.aphp.fr.
Marine Hug de Larauze, Email: marine.hugdelarauze@gercor.com.fr.
Marie-Line Garcia-Larnicol, Email: marie-line.garcia@sat.ap-hop-paris.fr.
Sarah Dumont, Email: SARAHNDUMONT@YAHOO.FR.
Christophe Louvet, Email: CHRISTOPHE.LOUVET@IMM.FR.
Nathalie Perez-Staub, Email: nathalie.perez@sat.aphp.fr.
Alexandra Hadengue, Email: alexandra.hadengue@gercor.com.fr.
Aimery de Gramont, Email: aimery.de-gramont@sat.aphp.fr.
Franck Bonnetain, Email: franck.bonnetain@univ-fcomte.fr.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Field K, Lipton L. Metastatic colorectal cancer-past, progress and future. World J Gastroenterol. 2007;13(28):3806–3815. doi: 10.3748/wjg.v13.i28.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- Jessup JM, Gunderson LL, Greene FL, Washington MK, Compton CC, Sobin LH, Minsky B, Goldbert RM, Hamilton SR. Staging System for Colon and Rectal Carcinoma. Ann Surg Oncol. 2010;2011(18):1513–1517. [Google Scholar]
- Köhne CH, Cunningham D, Di Constanzo F, Glimelius B, Blijham G, Aranda E, Scheithauer W, Rougier P, Palmer M, Wils J, Baron B, Pignatti F, Schöffski P, Micheel S, Hecker H. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13(2):308–317. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- Chibaudel B, Bonnetain F, Tournigand C, Bengrine-Lefevre L, Teixeira L, Artru P, Desramé J, Larsen AK, André T, Louvet C, de Gramont A. Simplified prognostic model in patients with oxaliplatin-based or irinoecan-based first-line chemotherapy for metastatic colorectal cancer: A GERCOR study. Oncologist. 2011;16(9):1228–1238. doi: 10.1634/theoncologist.2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE, Lee KL, Mark DB. Tutorial in biostatistics. Multivariable prognostic models issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, Osoba D, Bjordal K, Bottomley A. EORTC Clinical Groups. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- Diouf M, Filleron T, Barbare JC, Fin L, Picard C, Bouché O, Dahan L, Paoletti X, Bonnetain F. The added value of quality of life (QoL) for prognoses of overall survival in patients with palliative hepatocellular carcinoma. J Hepatol. 2013;58:509–521. doi: 10.1016/j.jhep.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Braun DP, Gupta D, Grutsch JF, Staren ED. Can changes in health related quality of life scores predict survival in stage III and IV colorectal cancer? Qual Life Res. 2011;9:62. doi: 10.1186/1477-7525-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efficace F, Innominato PF, Bjarnason G, Coens C, Humblet Y, Tumolo S, Genet D, Tampellini M, Bottomley A, Garufi C, Focan C, Giacchetti S, Lévi F. Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. Validation of patient’s self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: results of an international study by the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2008;26:2020–2026. doi: 10.1200/JCO.2007.12.3117. [DOI] [PubMed] [Google Scholar]
- Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, Chirivella I, Perez-Staub N, Louvet C, André T, Tabah-Fisch I, de Gramont A. OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- Group EQ. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Schemper M. Predictive accuracy and explained variation. Stat Med. 2003;22:2299–2308. doi: 10.1002/sim.1486. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK TG, Altman DG. Developing a prognostic model in presence of missing data: an ovarian cancer study. J Clin Epidemiol. 2003;56:28–37. doi: 10.1016/S0895-4356(02)00539-5. [DOI] [PubMed] [Google Scholar]
- Marshall M, Altman DG, Royston P, Holder RL. Comparaison of techniques for handling missing covariate data within prognostic modelling studies: a simulation study. BMC Med Res Methodol. 2010;10:7. doi: 10.1186/1471-2288-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Marshall M, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. doi: 10.1186/1471-2288-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournoux-Facon C, Paoletti X, Barbare JC, Bouché O, Rougier P, Dahan L, Lombard-Bohas C, Faroux R, Raoul JL, Bedenne L, Bonnetain F. Development and validation of a new prognostic score of death for patients with hepatocellular carcinoma in palliative setting. J Hepatol. 2011;54(1):108–114. doi: 10.1016/j.jhep.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- Mauer M, Bottomley A, Coens C, Gotay C. Prognostic factor analysis of health-related quality of life data in cancer: statistical methodological evaluation. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):179–196. doi: 10.1586/14737167.8.2.179. [DOI] [PubMed] [Google Scholar]
- Vach W, Blettner M. Encyclopedia of Biostatistics. New York: John Wiley & Sons; 1998. Missing data in epidemiologic studies; pp. 2641–2654. [Google Scholar]
- Moons KGM, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? Br Med J. 2009;338:b375. doi: 10.1136/bmj.b375. 1317-1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of the multivariate analysis after QoL imputation.