Abstract
Experimental animal and adult human data suggest that stress exposure is associated with alterations in immune system function that may underlie increased susceptibility to disease and behavioral disorders. The implications of these data for child psychology and psychiatry are not yet clear. The current review seeks to distil and translate the relevant animal and adult human work to children in order to advance a developmental model of psychoneuroimmunology. In addition to reviewing key specific findings, we consider biological/conceptual models and technical aspects of psychoneuroimmunology work in pediatric populations, and outline the rationales and advantages of integrating hypotheses concerning neuroinflammation in developmental studies of psychopathology.
Keywords: immunology, psychoneuroimmunology, neuroinflammation, stress, developmental psychopathology
Introduction
The notion that stress may be bad for children’s health is not new. A half-century ago, Meyer & Haggerty (Meyer, 1962) showed a dose-response pattern between stress in the family and streptococcal infection in the child. Nevertheless, many decades on, we are still far from understanding how robust the links are between stress and immunity, by what mechanisms stress disrupts the developing immune system in the child, and how these mechanisms may alter brain and behavioral development in childhood. That is in contrast to a wealth of compiled research findings in these areas in animals and, to a lesser extent, in adults. The current research review considers what is known about stress exposure and immunity and behavioral development and psychopathology in children. We review basic biology, and consider explanatory models, candidate mechanisms, practical issues concerning existing and future studies, and the near- and medium-term clinical implications of research findings.
This review is organized in the following manner. We first provide several arguments for the importance of neuroimmunology for child psychology and psychiatry. Our aim is to raise the awareness of the relevance of immunology for normal and pathological development and to inspire interest in this area. Second, we offer a primer on immune function; the aim here is to provide a brief introduction to key concepts and molecules that will be needed to interpret the concepts and findings that follow. It would not be reasonable to attempt a comprehensive review (and there remains important unknowns in this area in any event), and so some degree of simplifying is inevitable. The third section focuses on psychoneuroimmunology (PNI), a paradigm that links basic immunological mechanisms to brain and psychological and social factors, such as stress. It is in this section that we offer mini-reviews of research on immune dysregulation in specific mental disorders, and highlight some of the leading alternative developmental models for mechanisms linking psychosocial experiences such as stress to immune function, brain, and behavioral health. In the fourth section we focus on specific mechanisms by which stress exposure and stress physiology – factors now common in studies of child behavioral health – alter immune function. Stress and stress physiology receive particular attention because they provide a natural bridge from current research approaches into studies of the developing immune system. Fifth, we raise some methodological questions and comments that we derive from published research, with attention to research strategy as well as, in a more limited way, technical issues. The sixth and final section seeks to translate research findings and questions to clinical problems and settings. Although we are some distance from immune-targeted treatments for child mental disorders, there are clear benefits of seeking to integrate neuroinflammation hypotheses in children's mental health, and some of these are highlighted.
For practical reasons such as space, there are some areas that do not get substantial exposure in this review. For example, we do not focus much attention on genetic factors and epigenetic mechanisms of immune function, an area that is sizable and growing (see, for example, Lim, Li, Holloway, & Rao, 2013). We do note, however, that studies demonstrating the role of early social experiences such as caregiving on epigenetic changes in the brain have now been extended to immune markers such as T cells (Provencal et al., 2012). Also noteworthy in this regard is genetic evidence suggesting that genes associated with immune function may influence depression and treatment response (Bufalino, Hepgul, Aguglia, & Pariante, 2013). Additionally, it is naturally important to consider psycho-biological and immune mechanisms within a broader evolutionary framework. We refer readers to other treatments of this issue, including the ‘infection-defense hypothesis’ (Anders, Tanaka, & Kinney, 2013), which offers one example of how connections between immune function and mental disorders may have evolutionary significance.
The relevance of immunology and neuroinflammation hypotheses for child mental health
We begin with an account of why those working the area of child mental health would consider immune function. One reason is that immunological hypotheses offer important and compelling alternative explanations for the etiology of behavioral and neurodevelopmental disorders within psychology and psychiatry. The Maternal Immune Activation (MIA) hypothesis for schizophrenia and neurodevelopmental disorders, exemplified by influenza during pregnancy, is a well-known example (A. S. Brown, Begg, et al., 2004; A. S. Brown, Hooton, et al., 2004; A. S. Brown, Susser, Lin, & Gorman, 1995). For example, Brown et al. (A. S. Brown, Begg, et al., 2004) reported in their case control study that 25% of cases diagnosed with schizophrenia spectrum disorder had mothers exposed to influenza in the first trimester of pregnancy based on an index of antibody titer, compared with 11% in controls. Other evidence, including data from in vitro and animal studies (Bilbo et al., 2005; Kohman & Rhodes, 2013; Monje, Toda, & Palmer, 2003) and non-human primate models (Short et al., 2010), shows that immunological activation in the prenatal or early postnatal period predicts brain volume and neurogenesis that may underlie behavioral and psychological outcomes. Similarly, favored biological mechanisms for childhood psychopathology, such as the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system, are intricately and bi-directionally linked with the immune system (see below). Still other developmental indicators that attract attention in developmental psychopathology, such as sleep (Dahl & Lewin, 2002), are linked with and confounded by immune system function. This short but diverse set of examples illustrates how pervasive immune factors are likely to be in understanding causes or effects in child mental health. Failing to consider immunological mechanisms may lead to mis-specifying etiological models, with consequent problems for assessment and treatment.
Second, there is now a substantial body of adult human work – alongside extensive animal work – on the links between psychological well-being and immunity that need translation to pediatric samples. Specifically, we need to consider if, as is shown in adults, inflammation is reliably linked with affective symptoms and may account for some of the attendant adverse health risks attributed to psychopathology, such as cardiovascular disease. We return to this issue in section 3.1. Engaging in research of this type could have sizable benefits for improving the psychological and somatic health of the child, and further improve the public health standing of child mental health in the broader debates on health and healthcare.
Third, as accepted concepts such as the biopsychosocial medical model (Engel, 1977) and psychoneuroimmunology (Ader, Cohen, & Felten, 1995) make clear, disciplinary distinctions for understanding and improving health are artificial. Considering immune system responses alongside behavioral response to stress in children is merely recognizing biological realities of how the body operates. Fourth, there may be valuable treatment implications for understanding immune system function in childhood psychological and psychiatric disorders. If, for example, parenting interventions reduce stress exposure and behavioral symptoms of the child (Scott & Dadds, 2009), then it is natural to wonder (based on adult work) if there are consequent improvements to be expected in inflammatory markers that may signal long-term benefits on metabolic, cardiovascular and immune function. A recent study (Brotman et al., 2012) showing that conventional parenting interventions (which did not target eating) predicted lower BMI in at-risk children several years later is interesting in this regard because BMI is one of the more notable risks for metabolic and cardiovascular disease in adulthood, and obesity is a robust cause of inflammation.
Fifth, expertise in child development and behavior is needed to advance the ongoing research on stress and immune function. Research findings reviewed below demonstrate that one or other measure of immune function is associated with socio-economic status, but these findings do not provide clear direction for mechanistic or intervention research. That is because socio economic status is not a particularly useful risk index because it is too encompassing to identify mechanisms; neither is it a plausible intervention target. Work in this area needs behavioral development and clinical expertise to assist in identifying specific risk factors that mediate the social class effect on health, and to identify targets for intervention. Robust sources of stress for children, such as parenting and family conflict, are natural candidates to link with specific measures of immune function in children; these studies are now needed.
Brief primer on the immune system and neuroimmunology
We avoid a detailed review of the immune system, but offer a primer on key ideas to help build an organizational framework for collating the findings reviewed.
The human immune system is a well-orchestrated network of tissues, cells, and molecules dispersed throughout the body and charged with protecting the host from foreign invasion. Although often conceptually divided into innate and adaptive arms, they share similarities, and there is distinct cross-talk and interplay between the two complementary systems. For example, both the innate and adaptive systems execute three main functions: detecting threat, responding with appropriate effector functions, and regulating the ensuing response. Unique to the adaptive system is the generation of protective immune memory responses that can be quickly activated upon subsequent encounters with specific pathogens (Chaplin, 2006).
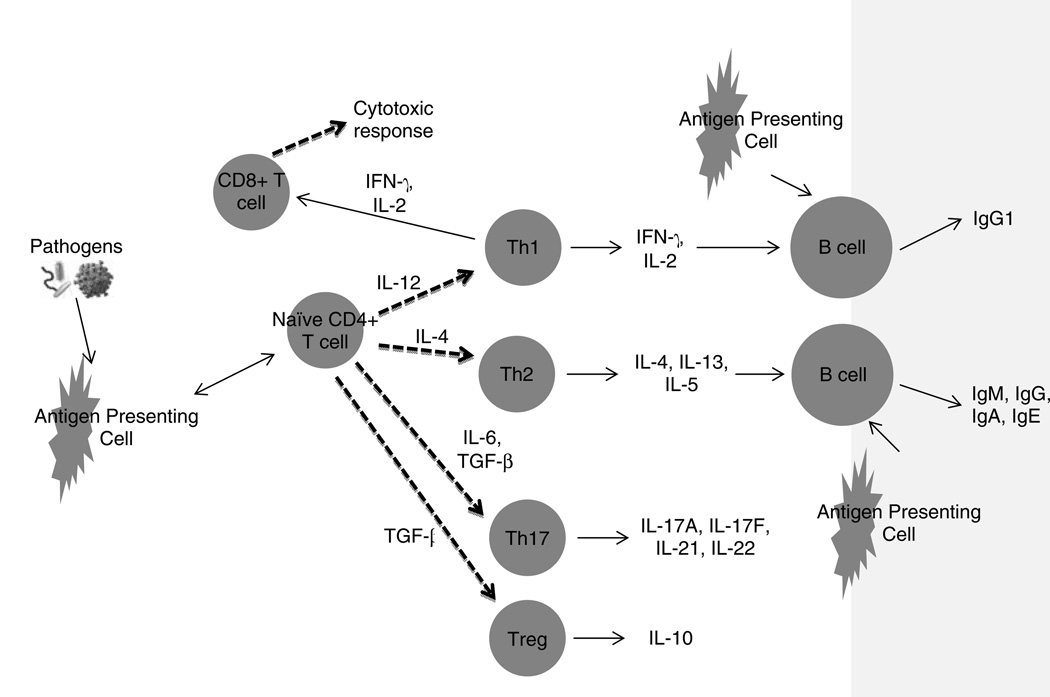
The innate immune system is the host’s first line of defense and consists of several cell types that detect invaders by means of non-specific pattern recognition receptors (Kollmann, Levy, Montgomery, & Goriely, 2012). Macrophages, dendritic cells, neutrophils, and epithelial cells are members of this group. Recognition triggers the production of effector molecules, including reactive oxygen species, as well as the release of chemokines and cytokines, both proinflammatory and immunosuppressive, that recruit cells and shape the ensuing response. Antigen presenting cells (APC), such as dendritic cells, are activated, take up antigen for processing, and travel to local lymphoid organs where they interact with T cells and B cells of the adaptive immune system to initiate an antigen specific response (Figure 1). CD4+ T helper cells (Th) are generated and differentiate into four main types, Th1, Th2, Th17, and T regulatory cells based upon the cytokine milieu and the presence of co-stimulatory molecules on the APCs (Miossec, Korn, & Kuchroo, 2009). Antigen specific B cells and CD8+ T cells are also stimulated during this process in the secondary lymphoid organs.
Figure 1. Key features and functions of the adaptive immune response.
Some key features of the adaptive immune response; see text for description. Circles represent cell types. Arrows indicate direct (one-headed) or bi-directional (double-headed) effects. Dashed lines indicate cell differentiation. Molecules above lines indicate catalyst for effect.
(Abbreviations: IL: interleukin; IFN-γ: interferon-γ; TGF: transforming growth factor; Ig: immunoglobulin; Th: T helper cells; Treg: T regulatory cell.)
Interleukin(IL)-12 and interferon-γ (IFN-γ) produced by the innate system direct development along the Type 1 axis, and the CD4+ Th1 response is dominated by the production of interferon-γ (IFN-γ) and interleukin(IL)-2. These cytokines function to activate macrophages and antigen specific cytotoxic CD8+ T lymphocytes to destroy host cells infected with intracellular pathogens (Chaplin, 2006). Type 2 responses are generated when IL-4 is the predominant cytokine present and CD4+ Th2 cells participate in humoral, anti-parasitic, and allergic responses. The presence of transforming growth factor beta (TGF-β), IL-6, IL-21 and IL-23 induce the differentiation of CD4+ Th17 cells which direct a strong proinflammatory response predominated by neutrophil activation that functions in the elimination of extracellular pathogens (Miossec, et al., 2009). CD4+ Th17 cells are also thought to play a role in inflammatory disorders and autoimmunity. In addition to these three types of effector cells, CD4+ T cells exposed to TGF-β can also be induced to differentiate into T regulatory cells with immunosuppressive and anti-inflammatory properties. B lymphocytes are an additional component of the adaptive immune system and a critical participant in the humoral response directed against extracellular pathogens. Through cytokine influences and interactions with APCs and T cells in secondary lymphoid organs, B cells develop into antibody secreting plasmablasts that travel to the bone marrow where they complete their differentiation into long-lived plasma cells responsible for prolonged antibody production (Chevrier et al., 2009; Pihlgren et al., 2006).
There are normative developmental changes in the immune system which may have relevance for studying stress and potential developmental changes in the impact of stress on the immune system. Changes in the immune response with age are best detected at the extremes, with the newborn immune system notable for having several distinct differences from that of older children and adults. Although innate pathogen detection via pattern recognition receptors is intact in newborn cells, cytokine secretion is characterized by decreased production of type 1 interferons and IL-12, with relative increased production of the immunosuppressive cytokine IL-10. These differences are thought to underlie the well-described CD4+ Th2 bias characteristic of the infant adaptive immune response (Lee et al., 2008). Additionally, newborns and infants have robust Th17 responses compared to adults but decreased production of the proinflammatory cytokines TNF-α and IL-1β (Kollmann, et al., 2012; PrabhuDas et al., 2011). The antibody response of newborns and infants is also notable for having a longer lead-time with a shorter maintained in the bone marrow (Pihlgren, et al., 2006).
Older adults, by contrast, appear to have alterations in the relative frequencies of pattern recognition receptors as well as decreased innate effector functions. These changes are accompanied by elevated levels of the pro-inflammatory cytokines IL-6 and TNF-α, referred to as “inflammaging”(Kollmann, et al., 2012). Additionally, later adulthood is notable for the process of immune-senescence characterized by the accumulation of terminally differentiated memory T cells combined with a paucity of naïve T cells able to respond to new threats (Bauer et al., 2013). There is also a relative shift from Type 1 to Type 2 responses and diminished cell mediated immunity (Bauer, et al., 2013). Immuno-senescence markers are linked with normal aging and it has been suggested that this process may be hastened in stress-exposed individuals.
In contrast to developmental changes into old age, less is known about normative changes in the immune system from childhood through young adulthood, and how these changes may modulate the stress-sensitivity of the immune system. Nonetheless, significant windows of vulnerability to immune system perturbations from prenatal life through adolescence have emerged, with evidence pointing to clinically relevant long-term responses from early exposures (Dietert et al., 2000; Kollmann, et al., 2012). For example, recent work from our lab indicates that prenatal maternal anxiety may alter adaptive immune function in the infant (O'Connor et al., 2013), and data from other fields demonstrate that nutrition, environmental exposures to drugs and chemicals, and exposures to microbes exert substantial effects on immune system development from conception through early childhood (Dietert, DeWitt, Germolec, & Zelikoff, 2010; Marques, O'Connor, Roth, Susser, & Bjorke-Monsen, 2013). A further period of vulnerability is postulated from early childhood through adolescence when immune memory is being established, although evidence of this is inconclusive.
Psychoneuroimmunology
Knowledge of some of the basic building blocks of the immune system now need to be placed in the context of neural development and in a psychological context. Psychoneuroimmunology (PNI), broadly defined as the study of the linkages between psychological processes, the nervous system, and the immune system, formally emerged in the mid-1970’s with the seminal work of Ader and Cohen (Ader & Cohen, 1975). A key observation undergirding the basis of PNI was that changes in immune function could be behaviorally conditioned in a manner analogous to the classical conditioning studies of Pavlov and his canine subjects. In this early study, rats were exposed to the novel taste of saccharin (the conditioned stimulus or CS) and then injected with the immunosuppressive drug cyclophosphamide (the unconditioned stimulus or UCS); cyclophosphamide produces sickness in the rats and subsequent conditioned taste aversion. Three days following this pairing, rats were injected with sheep red blood cells (SRBC) and subjected to various stimuli. Rats that were re-exposed to saccharin at the time of immunization had lower agglutinating antibody titers to SRBC compared to rats that were not re-exposed to saccharin. Several other stringent control groups included in the protocol provided solid evidence of the effects of behavioral conditioning leading to suppression of antibody responses. Ader and colleagues later established that antibody responses could not only be suppressed, but could also be enhanced, by behavioral conditioning protocols, using antigen itself (keyhole limpet hemocyanin) as the UCS and a chocolate milk solution as the CS (Ader, Kelly, Moynihan, Grota, & Cohen, 1993).
Several additional lines of evidence converged to establish as incontrovertible the connections between the immune system and the brain. First, a variety of central nervous system (CNS) manipulations in rats, including lesioning the pituitary, suppressed antibody production to SRBC, decreased skin contact hypersensitivity to dinitrochlorobenzene, and decreased the severity of adjuvant-induced arthritis. Skin graft survival was also prolonged in hypophysectomized rats compared with controls (Nagy & Berczi, 1978). Reciprocally, antigenic challenge with SRBC was shown to decrease hypothalamic noradrenergic activity in rodents, one of the first suggestions that the communication between the brain and the immune system is bidirectional (Besedovsky et al., 1983). Second, Felten and colleagues observed sympathetic innervation of the spleen and other lymphoid organs, and that peripheral catecholamine levels modulate a variety of immune responses; in particular, loss of splenic catecholamines resulted in impaired T and B cell immunity and proliferative responses (Felten et al., 1987; Madden, Felten, Felten, Sundaresan, & Livnat, 1989). Third, changes in numerous hormone or neurotransmitter levels produce a range of changes in immune function, and vice versa, at least in part because lymphoid cells express receptors for a wide variety of hormones and transmitters, including glucocorticoid receptors (Wira & Munck, 1970), β-adrenergic receptors (Fuchs, Albright, & Albright, 1988), and prolactin and growth hormone receptors (Kelley, Arkins, & Li, 1992). Thus, a variety of receptors with essential roles in child behavior and psychopathology also play a role in controlling immune responses.
It is now abundantly clear that the CNS regulates the immune system and that the immune system helps shape CNS development. In addition to the early studies of Besedovsky and colleagues suggesting that antigen exposure could alter noradrenergic activity in the brain (Besedovsky et al., 1983), Watkins and colleagues observed that cytokines (reviewed in (Watkins, Maier, & Goehler, 1995)) could signal the brain indirectly via stimulation of the vagus nerve. At about the same time, Banks and colleagues showed that proinflammatory cytokines, including interleukin (IL)-6, IL-1 and tumor necrosis factor (TNF)-α can be transported across the blood brain barrier, thus directly altering CNS function (Banks, Kastin, & Broadwell, 1995) and activating astrocytes and microglia (see below), which then make more of these cytokines.
Finally, although it was once thought that the CNS should be devoid of lymphoid cells for optimum function, it is now known that immune cells traffic in and out of the brain. T lymphocytes that are CD4+ but not restricted to Th1 or Th2 phenotypes reside in the CNS and, at least in rodent models, are imperative for many aspects of brain and cognitive function – such as the case of hippocampal neurogeneis (Wolf et al., 2009) – a function termed “protective autoimmunity” by Kipnis and colleagues (Schwartz & Kipnis, 2011). Indeed, a newly emerging dogma in neuroimmunology is that these T cells and microglia (see below) are critical for maintaining brain plasticity. These findings provide the necessary cellular basis for connecting the immune system and the brain, and demonstrate that the brain can no longer be considered an immune privileged site. In addition, these data suggest a rich interdependence of the immune system and the nervous system for normal health and psychological function.
Accumulating evidence indicates that microglia, the resident macrophages of the brain, provide surveillance and maintenance functions and, importantly, play an essential role in the non-injured brain to promote healthy normal neurodevelopment. Microglia are also rapidly gaining attention because of their possible role in the development of psychopathology. For example, a recent study using a knock-out mouse model (Paolicelli et al., 2011) identified microglia as the critical cell type performing synaptic pruning, an essential function for normal development; mice with experimentally reduced microglia displayed immature brain circuitry. Results such as these underscore the immune system's role in neural plasticity, or the ongoing cellular changes in the brain by which synapses are formed, modified and pruned to create efficient neural circuits (Luo & O'Leary, 2005). Animal studies have also demonstrated that microglia can directly phagocytose cellular debris and neural precursor cells and thereby shape early brain development (Cunningham, Martinez-Cerdeno, & Noctor, 2013; Stevens et al., 2007). Several other features of microglia are notable. One is that rodent studies show that microglia activation is increased with stress exposure, and that this effect can be decreased by drugs such as minocycline, which inhibits microglia activation; moreover, a specific deficiency in microglia has been associated with abnormal grooming in mice that appears to model OCD like behaviors in humans (Beumer et al., 2012). Equally important is the observation that there is a change in microglia form and function (e.g., what they release, including IL-1β) in development, which may be especially evident in certain brain regions such as the hippocampus, cortex and amygdala; these changes likely underlie the role that microglia may play in developmental programming of the brain from early immune activation (Bilbo, 2013), a topic addressed below. The evidence on microglia is based largely on the rodent model. Direct human evidence for the role of microglia is not possible except in post-mortem studies; however, positron emission tomography (PET) scan studies that assess radiotracers that specifically bind to microglia have been used to infer activated microglia activity in the brain in several disorders, e.g. (Suzuki et al., 2013).
Although the evidence for immune activity in brain development is very strong, the precise nature of these connections is complicated, and not yet fully explicated. Thus, for example, experimental animal manipulations demonstrate that IL-1 can both facilitate and impair learning and memory (see, for example, the review of Yirmiya & Goshen, 2011). Similarly, microglia have been shown to have neuroprotective as well as destructive influence on brain development and function. The complex nature of these findings underscores the multifaceted nature of the interactions between the immune system and neural development and the likely involvement of many factors; developmental programming is certainly one of these.
Clinical findings on psychoneuroimmunology
Our research review and synthesis focuses on human rather than animal work because of our translational research aims. A starting point to illustrate psychoneuroimmunology (PNI) in humans is the seminal work of Kiecolt-Glaser and colleagues. They have shown that variation in stress, either experimentally induced or a product of circumstance, is associated with compromised immune function and physical health in adults: stress predicts higher rates of illness following pathogen exposure, protracted wound healing, poorer response to immunization, and alterations in several specific indicators of immune function at tissue and cell levels (Bonneau, Kiecolt-Glaser, & Glaser, 1990; Christian, Graham, Padgett, Glaser, & Kiecolt-Glaser, 2006; Ebrecht et al., 2004; Fagundes, Glaser, & Kiecolt-Glaser, 2013; Glaser & Kiecolt-Glaser, 2005; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002; Powell, Allen, Hufnagle, Sheridan, & Bailey, 2011). Equally compelling is the work of Cohen and colleagues, who showed, via a powerful design, that stress is associated with susceptibility to illness (Cohen, Tyrrell, & Smith, 1991). Other cornerstone findings include the demonstration that negative affect predicts response to immunization. One example of this was reported by Marsland and colleagues (Marsland, Cohen, Rabin, & Manuck, 2001) in the case of hepatitis B antibody response. The wealth of studies in adults that is now available means that it is no longer sensible to research if there are stress-brain/behavior-immune links; it is no longer a plausible null hypothesis that there are not links. Rather, contemporary research focuses on mechanisms and consideration of particular phenotypes that may be of interest.
Evidence of immune dysregulation in specific childhood mental disorders
Of particular relevance to this review is research assessing parallels between immunology and psychopathology in adult and pediatric samples; these findings may contribute to a developmental model of PNI (see next section) and provide a broader context for assessment and treatment of child mental health problems.
An abundance of research in adults associates multiple markers of neuro-inflammation with psychiatric symptoms (Dantzer, O'Connor, Lawson, & Kelley, 2011; Howren, Lamkin, & Suls, 2009; J. C. (O'Connor et al., 2009; O'Donovan et al., 2010). Tremendous growth in our understanding of a possible role of proinflammatory cytokines, for example, extends to multiple psychiatric and psychological disorders, including anxiety, depression and cognitive function (Capuron & Miller, 2011). Moreover, at least some types of depression may be causally mediated by chronic increases in proinflammatory cytokines (Raison & Miller, 2011), which affect many brain regions and, in particular, instruct the hypothalamus to initiate a robust constellation of depressive-like symptoms, including fever, fatigue, anhedonia, and decreased activity, originally known as “sickness behavior.” These associations are now being scrutinized for what they may tell us about mechanisms and treatment options.
Evidence linking altered immune function and pediatric behavioral or emotional symptoms is far more limited (Birmaher et al., 1994; Keller, El-Sheikh, Vaughn, & Granger, 2010) and dominated by studies of medically ill children (Mackner, Pajer, Glaser, & Crandell, 2010; Sokal et al., 1998). A recent review of the pediatric evidence of cytokines and depression (Mills, Scott, Wray, Cohen-Woods, & Baune, 2013) is noteworthy for several reasons. One is that a number of studies were identified, and were shaped by an array of plausible biological models. It was not clear from these studies that there was generally greater inflammation (e.g., higher levels of pro-inflammatory cytokines) in depressed children and adolescents – the hypothesis borrowed from the adult literature. On the other hand, an important caveat is that the range of methods in the studies was too broad to permit formal meta-analysis; that is in contrast to the existence of several meta-analyses of the adult literature (Howren, et al., 2009). Difficulties in summarizing pediatric research findings on depression and immune function mimic difficulties in summarizing findings on stress exposure and immune function in children, e.g., (Slopen, Koenen, & Kubzansky, 2012). Inevitably, further studies in this area may clarify where the signals exist, but more attention is needed to methodological detail and operationalizing stress, symptoms, and immune function and the mediating mechanisms; a consideration of methodological issues for research is given later in the review. In any event, it is clear from the Mills et al. (2013) review and other reports that a number of specific and plausible mechanisms may explain why childhood psychopathology may be connected with dysregulation in the immune system. That is the broader significant point and the reason for stimulating further research in this field.
Abnormalities in immune function have been widely-reported in neurodevelopmental disorders, notably autism, in both pediatric and adult samples (Ashwood et al., 2011; Croonenberghs, Bosmans, Deboutte, Kenis, & Maes, 2002; Suzuki, et al., 2013). In the case of autism, there are many plausible mechanisms for an association. The role of microglia and T cells in brain development are natural candidates, and it is no coincidence that these immune targets have attracted particular attention. However, although the neuroimmunology of autism is probably more advanced than other childhood (onset) psychiatric disorders, basic questions remain about the relevance of these findings for etiology and treatment. That is partly because so many kinds of immune system abnormalities have been reported (including in animal models); understanding how these abnormalities relate to each other and to the neurodevelopmental abnormalities underlying autism will require further clinical and experimental investigation. A role for the immune system in other neurodevelopmental disorders is also suspected. One notable example is Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS), which describes a sudden onset of symptoms of obsessive-compulsive and tic disorders/Tourette Syndrome (and perhaps other symptoms, including mood) following streptococcal infection (Garvey, Giedd, & Swedo, 1998; Swedo, Garvey, Snider, Hamilton, & Leonard, 2001; Swedo & Grant, 2005). It should be anticipated that future research findings will expose further examples of immune system involvement in neuropsychiatric symptoms and syndromes, and this may provide additional opportunities for investigating mechanisms of action.
Alongside the accumulating evidence of associations between immune function and childhood psychopathology, it must be remembered that the immune system has essential, neuroprotective roles in healthy brain development, as reviewed in the prior section. That means that immune-mediated effects of psychopathology may derive from a range of processes, from exaggerated or abnormal stimulation to a lack of normal immune function activity to support neuronal development.
Developmental models of psychoneuroimmunology
As described above in the discussion of basic immunology mechanisms, there are many acknowledged kinds of developmental changes within the immune system that span the life-course; equally dramatic developmental changes have been described in neuroendocrine functions that mediate stress perception and stress physiology. That implies that a single model to account for linkages between psychological factors, brain, and immune system communication will not be invariant over the life-span (Ader, 1983; Coe, 1996; Moynihan, Heffner, Caserta, & O'Connor, 2013). Accordingly, models are needed to account for why, for example, stress-immune associations may vary across development and by what mechanisms there may be a carrying-forward of effects of early immune activation on subsequent brain, behavioral, and health outcomes.
One major research focus relevant to a developmental PNI model connects stress exposures in childhood with immune function and health outcomes in adulthood, e.g. (Fagundes, et al., 2013; Felitti et al., 1998; Miller, Chen, & Parker, 2011; Taylor, Lehman, Kiefe, & Seeman, 2006; Wegman & Stetler, 2009). These studies extend a long history of experimental animal work (Coe, 1993; Coe & Laudenslager, 2007; Coe, Lubach, Schneider, Dierschke, & Ershler, 1992). The most common study design to demonstrate associations uses retrospective reports of childhood stress to predict adult health and biomarkers of disease. These investigations show that childhood stress exposure such as low socio-economic status or more specific risks such as adverse rearing and trauma predict disorders of multiple systems, including autoimmune disease, cardiovascular, periodontal, and respiratory diseases, cancer, and other ailments (Almeida et al., 2010; D. W. Brown et al., 2010; Chen, Miller, Kobor, & Cole, 2010; Dube et al., 2009; Felitti, et al., 1998; Jackson, Kubzansky, Cohen, Weiss, & Wright, 2004; Lemieux, Coe, & Carnes, 2008; Wegman & Stetler, 2009). A stronger methodological case – with largely comparable effects – comes from a smaller number of prospective longitudinal studies in which childhood stress is assessed directly and then linked with subsequent biomarkers of health e.g.(Caspi, Harrington, Moffitt, Milne, & Poulton, 2006; Danese et al., 2009; Miller & Chen, 2010; Miller et al., 2009). Importantly, this substantial evidence base does not indicate if childhood risk exposure has more immediate impact on biomarkers of health in childhood. That is, it is not yet clear when in development the health effects (in terms of end stage disease or biomarker risks) of early stress exposure are reliably detectable.
A critical question for research is whether or not the child's immune system is susceptible to the effects of stress and, if so, whether the mechanisms responsible parallel what has been reported in animal and adult work. As we noted earlier, there is comparatively little research on individual differences in the normative changes in immune system function in the first decade of life, and even less research considering what this variation means for susceptibility to stress. The lack of relevant pediatric research means that contrary predictions might be made concerning stress exposure and immune function in children, namely: a) the effects of stress exposure on immune function have a childhood onset; b) the effects of stress exposure on immunity are not detectable in childhood because there has not been an appreciable or cumulative load and/or children’s “immunological reserves” are greater than adults’ – or that immune systems in adults are simply more vulnerable; c) children’s immune systems, because they are still in development, are especially vulnerable to the effects of stress. Differentiating among these contrasting developmental hypotheses is essential because they have sizably different implications for clinical and public health.
One germane line of investigation has adopted a cumulative stress hypothesis. Explicit in the “allostatic load” model, this approach focuses on the wear and tear of stress on the body over a prolonged period of time (Juster, McEwen, & Lupien, 2010; McEwen & Wingfield, 2003; Seeman, Epel, Gruenewald, Karlamangla, & McEwen, 2010). The notion here is that, for example, adaptive neuroendocrine responses to short-term stress exposure will, if activated persistently, have deleterious effects on the body and hasten the onset of disease in the long term. What is not clear is how prolonged the exposure needs to be in order to observe effects, but it is notable that the majority of these studies, which largely focus on neuroendocrine responses, focus on adult outcomes (for exception, see (Evans, 2003; Evans, Kim, Ting, Tesher, & Shannis, 2007; Flaherty et al., 2009)). By extension, it may be that stress exposure – and the attendant adaptive short-term neuroendocrine and immune responses – will have deleterious effects on the immune system only if stress exposure is prolonged and the adaptive short-term responses are transformed into maladaptive chronic disease processes.
Although still too few in number to differentiate among alternative hypotheses, PNI studies in children have appeared in recent years and raise further questions and clinical directions. One study (Caserta et al., 2008) of an ambulatory, generally well sample of 5–10 year-olds assessed on 7 occasions, reported significant links between family stress and “early onset” alteration of immune function. Specifically, a major index of caregiving and family stress, parental psychiatric symptoms, predicted increased levels of febrile illness as defined by detailed health diary data. Less anticipated, and more striking, was the finding that parental psychiatric symptoms predicted enhanced natural killer (NK) cell functioning independent of recent illnesses. That latter finding hints that there may be, in early development, activation of the immune system in response to early stress that may later take the form of down-regulation (as childhood stress is linked with weaker immune response in adults); see also (Mills, et al., 2013). Other immune function analyses from the same study indicated that an index of family stress was associated with poorer immune control of latent cytomegalovirus (CMV). Interestingly, parallel findings have been reported on the effects of early severe stress on poor control of latent virus in both normative stress-exposed children (Slopen, McLaughlin, Dunn, & Koenen, 2013) and children who experienced early institutional rearing (Shirtcliff, Coe, & Pollak, 2009). A subsequent report (Caserta, Wyman, Wang, Moynihan, & O'Connor, 2011) indicated that the proinflammatory cytokine IL-6 was lower among those who expressed high self-efficacy; a lack of link between IL-6 and standard depressive symptoms, which appears robust in adults (Howren, et al., 2009), was not found, but a trend for its emergence among the oldest children (in early adolescence) was suggested.
The list of potentially important immune markers is too long (see below), the variation in how and when stress is operationalized is too diverse, and the number of published studies is too few to yield robust replications on the links between stress and immune function in children, e.g., see (Mills, et al., 2013; Slopen, et al., 2012). What the empirical studies do suggest, however, is a basis for further clinical study. It is too soon to tell if the links between stress and immune markers in children are as robust as they appear to be in adults, but the pediatric studies have at the least begun to clarify why the SES-health gradient extends to children (Chen, Martin, & Matthews, 2007; Chen, Matthews, & Boyce, 2002).
An alternative developmental model of PNI derives from a range of early experience models, such as “developmental programming” that underlies research on the developmental origins of health and disease (DOHaD) (Glover, O'Connor, & O'Donnell, 2010; Hanson & Gluckman, 2011; Swanson, Entringer, Buss, & Wadhwa, 2009). A core feature of the programming model is that exposures during particularly sensitive periods in ontogeny “program” or “set” biological systems (e.g., glucose metabolism, microglia activation), with the effects carried forward into adulthood (Beumer, et al., 2012; Gluckman, Hanson, Cooper, & Thornburg, 2008). Importantly, the bulk of DOHaD-related research examines adult health outcomes; few assess childhood onset effects (Ibanez, Ong, Dunger, & de Zegher, 2006).
The degree to which immune function may be programmed is an area of considerable interest and importance. One particular application of the programming model uses prenatal maternal anxiety or stress as an initiator of a possible immune programming effect. Human evidence is not yet conclusive, but certainly suggestive. Several reports link prenatal maternal anxiety or stress to asthma (Khashan et al., 2012; Lefevre et al., 2011) and infectious diseases (Nielsen, Hansen, Simonsen, & Hviid, 2011) in children. Studies that assess specific immune mechanisms are far less common, but equally suggestive. For example, infants whose mothers were characterized as anxious in pregnancy, according to self-report and clinical evaluation, exhibited poorer adaptive immune response at 6 months of age according to both in vitro and in vivo methods (O'Connor, et al., 2013). Those findings are broadly consistent with a retrospective study of prenatal maternal stress and cytokine production in adulthood (Entringer et al., 2008), and with other reports linking immune markers in cord blood with prenatal stress (Duijts et al., 2008; Marques, et al., 2013; Sternthal et al., 2009; Wright et al., 2010). These results do not establish a programming mechanism, but they do highlight how the human immune system is vulnerable to and responds to exposures from the prenatal period. Furthermore, these human findings complement the now substantial animal evidence for long-term effects of pre- or neonatal infection on brain development and later immune response that substantiate a programming hypothesis, perhaps operating in part through microglia (Bilbo, 2013). It is an interesting possibility that immune mechanisms may be at play to explain other potential programming effects. For example, the association between maternal (pre-pregnancy) BMI and symptoms of ADHD in children has been reported in several studies and is inferred as a possible programming effect (Rodriguez et al., 2008). The underlying mechanisms may be immunological insofar as experimental animal data show that a maternal high fat diet (admittedly not identical to BMI) is associated with a wide range of immunological effects in the brain (see, for example, Bilbo & Tsang, 2010).
A further conceptual point is that there is substantial variability in virtually all known markers of immune function, including susceptibility to infectious disease and antibody titer response to immunization. These individual differences provide the starting point for developmental PNI hypotheses about vulnerability and resilience. We know that there is wide variation in children's psychological and behavior responses to stress, even stress as severe as institutional rearing (Gunnar & van Dulmen, 2007; O'Connor, Marvin, Rutter, Olrick, & Britner, 2003; Smyke et al., 2007). Models and analyses examining stress-immune function links in children must also attend to the possibility of protective and moderating factors; the general shape of these is unknown, but examples have been provided (Fuligni et al., 2009). Similarly, there is a need to consider specific psychological processes such as cognitive coping strategies, and how these are also shaped by development and experience. One of the few reported examples indicated that the cognitive style of self-efficacy was associated with lower levels of the pro-inflammatory cytokine IL-6 (Caserta, et al., 2011).
Forming developmental models will also require integrating findings from the life – course. One possible illustration is T cell function in advanced cognitive decline (Vescovini et al., 2010), but there are others. Developmental concepts such as immune-senescence and “inflammaging,” derived from studies of aging, may provide some hints for where to look for effects of stress exposure in pediatric samples.
A final conceptual point for a developmental or any other kind of model of PNI is that it would not make much evolutionary sense for a species if the immune system, which is essential for survival and (reproductive) fitness, could be significantly compromised from an exposure as common in the natural (pre-historic and contemporary) environment as stress. The implication is that it may not be profitable to define immune outcomes in terms of “deficits” but rather to conceptualize how the immune system adapts to stress exposures. This is the notion behind the predictive adaptive response (Gluckman, Hanson, & Spencer, 2005), and highlights the evolutionary context that is required for understanding human development.
Stress mechanisms and PNI
Developmental studies of risk, resilience, and psychopathology now commonly incorporate biological markers; probably the most popular of these is cortisol, a downstream product of the hypothalamic-pituitary-adrenal (HPA) axis (M. Gunnar & Quevedo, 2007; O'Donnell et al., 2013). We highlight this because cortisol, and glucocorticoids more generally, have clear, demonstrated effects on the immune system. Moreover, bi-directional associations between the stress (e.g., HPA) and immune systems imply a natural confounding in research. Thus, studies examining cortisol and stress system physiology will also be assessing changes in or induced by immune function mechanisms. So, for example, studies that assess the role of early stress exposure on HPA axis function must also consider the role of immune system changes induced by stress and in concert with HPA axis disturbance. This is a re-emerging theme in this review: immune function mechanisms provide an important alternative hypothesis for many studies of child development and psychopathology, and require direct testing. Opportunities to expand pediatric studies of stress and stress physiology to immune system function are plentiful; we discuss these in the next section.
One fundamental task for research is to document how stress alters immune function. Sheridan (Avitsur, Stark, & Sheridan, 2001) was one of the first to demonstrate, in an animal model, that glucocorticoid resistance is one likely mechanism linking prolonged stress exposure to altered immune function. More specifically, decreased receptor sensitivity to glucocorticoids that may result from prolonged stress and glucocorticoid exposure may reduce the anti-inflammatory effect of glucocorticoids; consequently, stressed individuals exhibit (persistent) inflammation, leading to a variety of disease states.
The prediction that glucocorticoid receptor insensitivity is associated with excessive innate immune responses and increased inflammation is straightforward. Indeed, Miller et al. (Miller & Chen, 2010) have shown that, over a period of 1.5 years, adolescent girls living in an environment marked by chronic family stress showed increasing production of IL-6 and associated glucocorticoid resistance that mirrored the inflammatory profile. Recent work (Cole, Hawkley, Arevalo, & Cacioppo, 2011) suggests that dysregulated gene expression—favoring glucocorticoid receptor down-regulation and elevated inflammatory cytokines—occurs in specific immune cell subtypes, plasmacytoid dendritic cells, and monocytes (antigen presenting cell, APC) and B lymphocytes. Application to adaptive immunity is less clear (Hunzeker et al., 2011; Kelly, Juern, Grossman, Schauer, & Drolet, 2010). In an animal model, Sheridan and colleagues (Avitsur et al., 2007; Avitsur, Powell, Padgett, & Sheridan, 2009) reported that mice in disrupted social settings develop glucocorticoid insensitivity and have increased markers of adaptive immune responses to influenza virus A infection. However, it remains to be determined if prolonged exposure to social disruption would result in a sustained increase in adaptive responses or eventual immune suppression. In support of the latter, the work of Cohen et al. shows that low SES during early childhood results in poorer immune response to infection with common cold viruses in adulthood (Cohen, Doyle, Turner, Alper, & Skoner, 2004).
The pattern of elevated inflammatory cytokine responses to chronic stress mimics the process of “inflammaging” or immunosenescence in older adults, characterized by declining adaptive responses, expanded late effector CD8+ T cell pools and decreased naïve T cell numbers (Franceschi et al., 2007; McElhaney & Effros, 2009; Pawelec, Larbi, & Derhovanessian, 2010). One possible model is that chronic (or perhaps early) stress accelerates the typical process of immune-senescence via exaggerated immune responses in childhood. Other mechanisms are also likely, but have not yet attracted robust evidence.
Practical scientific considerations for developmental PNI research
There are many practical and technical issues to consider in studying the interactions among psychological exposures and experiences, the brain, and the immune system, and how these may change across development. The most obvious is how best to obtain immune-informative data from children. Although some studies have employed saliva samples, and this may be adequate for some purposes in some conditions, the vast majority of research questions will require blood collection. That may seem a good deal more intrusive than saliva, but anecdotal data on the acceptability of blood collection from venipuncture is generally positive among those in the field, and institutional review boards should also provide little resistance to blood draw (although these are notoriously varied across site, it is deemed “minimal risk” in many settings). The key message is that sampling blood is not yet conventional, but should not pose substantial impediments. More specific questions, such as how much volume can be collected, are determined by established safety guidelines. For example, one convention for obtaining blood from healthy children is that the amount drawn may not exceed the lesser of 50 ml or 3 ml per kg in an 8-week period, and collection may not occur more frequently than 2 times per week. The amount of blood needed will also of course depend on the questions being posed, but the maximum allowable is certainly adequate for multiple kinds of analyses. Research utilizing multiplexing (e.g., luminex) technology is gaining momentum; its principle advantage is that it requires remarkably little sample, perhaps as little as a pinprick or heel stick. However, technical questions remain about the comparability with traditional high sensitivity assays, the “gold standard” in the field.
A further major consideration is storage and processing of the blood. Assays requiring analyses only on fresh (i.e., not frozen) samples do create practical constraints and demands, such as the need for swift transportation and substantial coordination with the lab technicians. Although, in this regard, there is a wealth of research demonstrating the feasibility of blood collection, processing, and storage in the field. There is then the time demands on processing samples, which may take hours or even days, which will set limits on when data are collected (e.g., to avoid lab staffing through evenings and the week-end).
Probably the most challenging practical and scientific question is which aspects of immune function should be explored. There is no consensus that some markers are more sensitive or predictive than others. Experimental animal work reviewed above shows that stress alters both innate and adaptive immunity, but it is not clear from available data if the innate or adaptive immune system is more susceptible to stress. Of course, such a distinction is partly artificial, as the interplay between the two suggests that an impact on one may at least indirectly alter the other (Franceschi, et al., 2007; McElhaney & Effros, 2009). More fundamentally, the complexity of the immune system means that, inevitably, no single study would be equipped to examine each of the multiple aspects of the functioning immune system, let alone the dynamic, systemic nature of its actions. For example, among those studies assessing variation in cytokines, the vast majority focus on just a few of the plausible many candidates. That could lead to misspecification of effects given that cytokines are pleiotropic and can act in antagonistic or synergistic or redundant manner with other cytokines. Guidance for developing protocols for researching PNI in children is suggested from adult studies – although much of the work is still in the development/exploratory phase. What is clear is that general statements about prenatal anxiety or childhood stress influencing the “immune system” are too broad and vague to be very illuminating. Going forward, it will be important to clarify which specific aspects of immune function are targeted, and the methods by which an association is demonstrated.
Several markers and paradigms that may be incorporated into a developmental PNI program of study are offered below (Table 1). Measuring circulating cytokines is among the most common approach, with a limited handful of proinflammatory (IL-6, TNF-alpha) and anti-inflammatory (IL-4, IL-10) cytokines typically included. It is important to distinguish the assessment of circulating (unstimulated) cytokines from an assessment of stimulated cytokines, that is, examining cellular response to an antigen or mitogen. Results will differ substantially between the stimulated and unstimulated methods, and this could lead to problems in interpretation of effects. There is then the question of in vivo (e.g., assessing antibody titer following inoculation) and in vitro (e.g., assessing cell responder frequencies under various conditions) immune measures. Here again, the point is not that there is a “best” index but rather that the particular measure of immune system function be centered in a clear psychobiological model. There is, at present, too little consideration of why certain biomarkers of immune system function were measured but others were not.
Table 1.
Example markers of immune function for pediatric studies
| Biological construct | Data (sample) source | Treatment | Example markers |
|---|---|---|---|
| Immune outcomes/markers | |||
| Disease states, illness history, illness susceptibility | Parent Report, Medical record | N/A | Autoimmune diseases, (febrile) illnesses, allergic diseases/asthma |
| Circulating Inflammation/ pro-inflammatory cytokines | Serum | N/A | CRP, IL-6, TNF-alpha |
| Stimulated Inflammation/ pro-inflammatory cytokines | PBMCs | ± LPS or other antigens or mitogens | CRP, IL-6, TNF-alpha |
| Antibody titer to vaccine | Serum | N/A | Antigen-specific antibodies |
| T cell frequencies to vaccine | PBMC | Re-stimulation with vaccine antigen | Responder cell frequencies |
| Viral recrudescence Late effector CD8+ populations specific for CMV | PBMC | N/A | CD8+CD28-CD57+ population |
| Natural killer cell function | PBMC | Incubate cells with labeled tumor cell targets | |
| Possible mechanisms | |||
| Glucocorticoid resistance and innate immunity | Whole blood | ± LPS ±-hydrocortisone |
IL-6 |
Note: CRP: C-reactive protein; IL: interleukin; LPS: lipopolysaccharides; PBMC: peripheral blood mononuclear cells; N/A: not applicable. Under the “Treatment” column, we give examples of the kinds of laboratory manipulations that have been made to evoke the immune response in in vitro assessments.
Another paradigm for studying stress-immune-behavior links is to examine viral recrudescence, that is, the emergence of an otherwise latent virus, or a “breaking through” of the virus. Examples in the literature of this include the Epstein-Barr virus and a number of herpes viruses. A final set of immune measures to consider are functional health outcomes. Gathering actual data on illness proclivity is a time-intensive assessment, but has distinct advantages over global ratings of health. An example of using illness diary data to assess functional health outcomes was recently reported (Caserta, et al., 2008). Empirically, illness data are quite distinct from specific measures of immune functioning, and the reality is that specific measures of immune processes (pro-inflammatory cytokines) are not stand-ins for disease states or other functional outcomes such as febrile illness.
Finally, studies of cytokines and other immune biomarkers must account for a host of confounds and complexities, from diurnal variation and sleep quality, e.g. (M. F. O'Connor et al., 2009) to recent illnesses (Caserta, et al., 2008). And, efforts to incorporate psychological factors in studies of immune function should not ignore lessons from basic immunology, such as the critical role of history of exposure to infectious agents. An example of how early exposure to infectious agents may moderate the impact of stress on immune function was recently reported (McDade, Hoke, Borja, Adair, & Kuzawa, 2013). Additionally, investigations must attend to the developmental changes because, as noted elsewhere in this review, there are substantive normative changes in the immune markers as a function of age (although what it is about age that might influence these outcomes in not yet clear). For example, the dramatic degree of changes in the normally functioning immune system was demonstrated for lymphocyte subsets (Shearer et al., 2003). A table of research paradigms for studying PNI and some associated evidence is provided in Table 1.
Clinical implications for child psychology and psychiatry
Public health attention is sharply focused on the adverse effects of stress on children’s current and long-term health and how these findings may be translated to practice (Marie-Mitchell & O'Connor, 2013; WHO, 2007; Wise & Blair, 2007). Indeed, many of those conditions associated with greatest disease burden or disability adjusted life years (DALYs) are thought to be products of stress exposure and seem likely to be at the intersection of inflammation and behavioral/psychiatric disorders - the particular focus of the pediatric neuroinflammation hypothesis.
Several lines of clinically-oriented research will be informed by ongoing programs of research in developmental PNI. One involves the integration of health and immune markers in behaviorally-oriented family and parenting interventions. Evidence that these programs improve behavioral adjustment is substantial (see, for example, Scott et al., 2010). It is now time to consider if these behavioral improvements (and the stress physiology and other biological mechanisms that are altered in tandem with behavior change) have carry-over salutary effects on somatic health and might explain individual differences to susceptibility to infectious disease. One interesting recent example (Brotman, et al., 2012) was the report that a standard parenting intervention (“The Incredible Years”) predicted lower BMI and clinical obesity several years later in two samples of high-risk youth, despite not targeting eating and activity in the intervention. The lesson here is that positive changes induced by positive parenting may have salutary health effects on the child, and that a broader biobehavioral outcome assessment is needed.
In addition, as most child mental health practitioners are now well aware, there is an “epidemic” of adult-like diseases in children, including metabolic/inflammatory diseases that may be linked with early stress and social disparities (Wise, 2007, 2009). Child mental health scientists and clinicians need to be equipped to manage and contribute to these changing health concerns. A developmental PNI perspective is equipped to integrate behavioral science within a broader health context, and stimulate mechanistic research that could reveal novel intervention options. Moreover, this model offers clear and practical guidance for where to “look for” the effects of stress exposure throughout the body.
There is not yet sufficient evidence to support immune-targeted treatment for child mental health and behavioral disorders. However, some notable findings have been reported, including the addition of minocycline, an anti-inflammatory drug that reduces microglial activation (it has other actions as well), to anti-psychotic drugs for adult schizophrenia (Chaudhry et al., 2012; Miyaoka et al., 2008). Other promising examples of immune-targeted treatments, as a primary or adjunctive therapy, for adult psychopathology have been reported, such as a recent report using a TNF-alpha antagonist to treat depression (Raison et al., 2013). An example of immune factors altering treatment using conventional psychopharmacological agents was also recently reported (Warner-Schmidt, Vanover, Chen, Marshall, & Greengard, 2011); results demonstrated that anti-inflammatory agents had an antagonistic effect on the impact of anti-depressants. Perhaps somewhat parallel is research on the impact of standard psychopharmacology treatments on immune parameters. In one of the few studies in this area relevant to child mental health, Henje Blom and colleagues (Henje Blom et al., 2012) reported that IL-6 was elevated in adolescent girls not treated with SSRIs for emotional disorders. Many other studies suggest that psychopharmacological treatments alter immune parameters (De Berardis et al., 2010), but so far only the experimental animal studies provide causal evidence that that immune system changes induced by psychopharmacology treatments may be relevant to their effects on brain and behavior.
At present there simply is not enough of an evidence base to introduce PNI in the child mental health clinic for treatment purposes. On the other hand, the potential advantages of integrating the models and methods of PNI in the clinic for research purposes are substantial, and essential to circumvent the inability of experimental animal research findings to inform practice. Applied PNI research may well yield medium-term gains in improving the shape of biopsychosocial interventions for promoting lasting positive health outcomes in children.
Conclusion
Translating experimental animal work on the links between stress, behavior, and immunity to human health has occupied basic and clinical scientists for many years. We now have a strong evidence base indicating that acute and chronic stress likely has, at least in some circumstances, direct and indirect influence on immune function; in more functional terms, prolonged stress exposure can diminish response to immunization, increase susceptibility to illness, and exacerbate inflammation and existing conditions. Furthermore, these connections between psychological, brain, and immune function are bi-directional rather than uni-directional. To a considerable degree, these findings are based on adults. The transition from a model that focused on pathology (e.g., stress exposure reduces immune response) to one that also emphasizes immune mechanisms in the normal and healthy brain provides one impetus for devising developmental models of PNI and to examine PNI in pediatric samples. What is clear at this stage is that these ongoing efforts may substantially alter how child (mental) health is conceptualized and promoted.
Key points.
Research findings are beginning to outline the immune system's essential roles in normal neurodevelopment, and show how psychological stress may impair somatic health in children.
The inter-disciplinary field of psychoneuroimmunology may have practical application to child psychology and psychiatry.
Psychoneuroimmunology research findings provide a cellular basis for connecting the immune system and the brain, demonstrating that the brain is not an immune-privileged site.
Developmental models are needed to describe the connections between stress exposure, the brain, and the immune system; findings so far reported in adults may not extend to (young) children.
Acknowledgements
The authors' work is partly supported by National Institutes of Health grants MH073019, MH073842, MH097293, and HD038938. This review article was invited by the journal, for which the authors have been offered a small honorarium payment towards personal expenses; it has undergone full, external peer review.
Footnotes
The authors have declared that they have no competing or potential conflicts of interest.
References
- Ader R. Developmental psychoneuroimmunology. Developmental Psychobiology. 1983;16:251–267. doi: 10.1002/dev.420160402. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosomatic Medicine. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N, Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet. 1995;345:99–103. doi: 10.1016/s0140-6736(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Ader R, Kelly K, Moynihan JA, Grota LJ, Cohen N. Conditioned enhancement of antibody production using antigen as the unconditioned stimulus. Brain, Behavior, and Immunity. 1993;7:334–343. doi: 10.1006/brbi.1993.1033. [DOI] [PubMed] [Google Scholar]
- Almeida ND, Loucks EB, Kubzansky L, Pruessner J, Maselko J, Meaney MJ, et al. Quality of parental emotional care and calculated risk for coronary heart disease. Psychosomatic Medicine. 2010;72:148–155. doi: 10.1097/PSY.0b013e3181c925cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Tanaka M, Kinney DK. Depression as an evolutionary strategy for defense against infection. Brain, Behavior, Immunity. 2013;31:9–22. doi: 10.1016/j.bbi.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain, Behavior, and Immunity. 2011;25:840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Kinsey SG, Bidor K, Bailey MT, Padgett DA, Sheridan JF. Subordinate social status modulates the vulnerability to the immunological effects of social stress. Psychoneuroendocrinology. 2007;32:1097–1105. doi: 10.1016/j.psyneuen.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Powell N, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Immunolology Allergy Clinics of North America. 2009;29:285–293. doi: 10.1016/j.iac.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Hormones and Behavior. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Muller GC, Correa BL, Vianna P, Turner JE, Bosch JA. Psychoneuroendocrine interventions aimed at attenuating immunosenescence: a review. Biogerontology. 2013;14:9–20. doi: 10.1007/s10522-012-9412-5. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Da Prada M, Burri R, Honegger C. The immune response evokes changes in brain noradrenergic neurons. Science. 1983;221:564–566. doi: 10.1126/science.6867729. [DOI] [PubMed] [Google Scholar]
- Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. Journal of Leukocyte Biology. 2012;92:959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Frank A. Beach award: programming of neuroendocrine function by early-life experience: a critical role for the immune system. Hormones and behavior. 2013;63:684–691. doi: 10.1016/j.yhbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behavioral neuroscience. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Rabin BS, Garcia MR, Jain U, Whiteside TL, Williamson DE, et al. Cellular immunity in depressed, conduct disorder, and normal adolescents: role of adverse life events. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:671–678. doi: 10.1097/00004583-199406000-00008. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Kiecolt-Glaser JK, Glaser R. Stress-induced modulation of the immune response. Annals of the New York Academy of Sciences. 1990;594:253–269. doi: 10.1111/j.1749-6632.1990.tb40485.x. [DOI] [PubMed] [Google Scholar]
- Brotman LM, Dawson-McClure S, Huang KY, Theise R, Kamboukos D, Wang J, et al. Early childhood family intervention and long-term obesity prevention among high-risk minority youth. Pediatrics. 2012;129:e621–e628. doi: 10.1542/peds.2011-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Archives of General Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Gorman JM. Affective disorders in Holland after prenatal exposure to the 1957 A2 influenza epidemic. Biological Psychiatry. 1995;38:270–273. doi: 10.1016/0006-3223(95)00241-8. [DOI] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Felitti VJ, Edwards VJ, Malarcher AM, Croft JB, et al. Adverse childhood experiences are associated with the risk of lung cancer: a prospective cohort study. BMC Public Health. 2010;10:20. doi: 10.1186/1471-2458-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain, Behavior, and Immunity. 2013;31:31–47. doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacological Therapy. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, O'Connor TG, Wyman PA, Wang H, Moynihan J, Cross W, et al. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain, Behavior, and Immunity. 2008;22:933–940. doi: 10.1016/j.bbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Wyman PA, Wang H, Moynihan J, O'Connor TG. Associations among depression, perceived self-efficacy, and immune function and health in preadolescent children. [Research Support, N.I.H., Extramural] Development and Psychopathology. 2011;23:1139–1147. doi: 10.1017/S0954579411000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: risk of cardiovascular disease. Archives of Pediatric Adolescent Medicine. 2006;160:805–811. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- Chaplin DD. 1. Overview of the human immune response. The Journal of Allergy and Clinical Immunology. 2006;117(2 Suppl Mini-Primer):S430–S435. doi: 10.1016/j.jaci.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Chaudhry IB, Hallak J, Husain N, Minhas F, Stirling J, Richardson P, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. Journal of Psychopharmacology. 2012;26:1185–1193. doi: 10.1177/0269881112444941. [DOI] [PubMed] [Google Scholar]
- Chen E, Martin AD, Matthews KA. Trajectories of socioeconomic status across children's lifetime predict health. Pediatrics. 2007;120:e297–e303. doi: 10.1542/peds.2006-3098. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: how and why do these relationships change with age? Psychology Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molular Psychiatry. 2010;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier S, Genton C, Kallies A, Karnowski A, Otten LA, Malissen B, et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3895–3900. doi: 10.1073/pnas.0809736106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL. Psychosocial factors and immunity in nonhuman primates: a review. Psychosomatic Medicine. 1993;55:298–308. doi: 10.1097/00006842-199305000-00007. [DOI] [PubMed] [Google Scholar]
- Coe CL. Developmental psychoneuroimmunology revisited. Brain, Behavior, and Immunity. 1996;10:185–187. doi: 10.1006/brbi.1996.0017. [DOI] [PubMed] [Google Scholar]
- Coe CL, Laudenslager ML. Psychosocial influences on immunity, including effects on immune maturation and senescence. Brain, Behavior, and Immunity. 2007;21:1000–1008. doi: 10.1016/j.bbi.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Schneider ML, Dierschke DJ, Ershler WB. Early rearing conditions alter immune responses in the developing infant primate. Pediatrics. 1992;90:505–509. [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosomatic Medicine. 2004;66:553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. New England Journal of Medicine. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. Journal of Neuroscience : the official journal of the Society for Neuroscience. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. The Journal of Adolescent Health : official publication of the Society for Adolescent Medicine. 2002;31(6 Suppl):175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatric Adolescent Medicine. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardis D, Conti CM, Serroni N, Moschetta FS, Olivieri L, Carano A, et al. The effect of newer serotonin-noradrenalin antidepressants on cytokine production: a review of the current literature. [Editorial Review] International Journal of Immunopathology and Pharmacology. 2010;23:417–422. doi: 10.1177/039463201002300204. [DOI] [PubMed] [Google Scholar]
- Dietert RR, DeWitt JC, Germolec DR, Zelikoff JT. Breaking patterns of environmentally influenced disease for health risk reduction: immune perspectives. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Review] Environmental Health Perspectives. 2010;118:1091–1099. doi: 10.1289/ehp.1001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert RR, Etzel RA, Chen D, Halonen M, Holladay SD, Jarabek AM, et al. Workshop to identify critical windows of exposure for children's health: immune and respiratory systems work group summary. Environmental Health Perspectives. 2000;108(Suppl 3):483–490. doi: 10.1289/ehp.00108s3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijts L, Bakker-Jonges LE, Labout JA, Jaddoe VW, Hofman A, Steegers EA, et al. Perinatal stress influences lymphocyte subset counts in neonates. The generation R study. Pediatric Research. 2008;63:292–298. doi: 10.1203/PDR.0b013e318163a29f. [DOI] [PubMed] [Google Scholar]
- Ebrecht M, Hextall J, Kirtley LG, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology. 2004;29:798–809. doi: 10.1016/S0306-4530(03)00144-6. [DOI] [PubMed] [Google Scholar]
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Nelson EL, Hellhammer DH, Wadhwa PD, Wust S. Influence of prenatal psychosocial stress on cytokine production in adult women. Developmental Psychobiology. 2008;50:579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43:341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain, Behavior, and Immunity. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventative Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, et al. Noradrenergic sympathetic neural interactions with the immune system: Structure and function. Immunological. Review. 1987;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Flaherty EG, Thompson R, Litrownik AJ, Zolotor AJ, Dubowitz H, Runyan DK, et al. Adverse childhood exposures and reported child health at age 12. Academy of Pediatrics. 2009;9:150–156. doi: 10.1016/j.acap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Fuchs BA, Albright JW, Albright JF. B-adrenergic receptors on murine lymphocytes: Density varies with cell maturity and lymphocyte subtype and is decreased after antigen administration. Cellular Immunolology. 1988;114:231–245. doi: 10.1016/0008-8749(88)90318-8. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Irwin MR, Kiang L, Cole SW. Daily family assistance and inflammation among adolescents from Latin American and European backgrounds. Brain Behahavior and Immunology. 2009;23:803–809. doi: 10.1016/j.bbi.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Giedd J, Swedo SE. PANDAS: the search for environmental triggers of pediatric neuropsychiatric disorders. Lessons from rheumatic fever. [Review] Journal of Child Neurology. 1998;13:413–423. doi: 10.1177/088307389801300901. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Reviews of Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glover V, O'Connor TG, O'Donnell K. Prenatal stress and the programming of the HPA axis. Neuroscience Biobehavioral Review. 2010;35:17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecolological Evolution. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, van Dulmen MH. Behavior problems in postinstitutionalized internationally adopted children. Developmental Psychopathology. 2007;19:129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. American Journal of Clinical Nutrition. 2011;94(6 Suppl):1754S–1758S. doi: 10.3945/ajcn.110.001206. [DOI] [PubMed] [Google Scholar]
- Henje Blom E, Lekander M, Ingvar M, Asberg M, Mobarrez F, Serlachius E. Pro-inflammatory cytokines are elevated in adolescent females with emotional disorders not treated with SSRIs. [Research Support, Non-U.S. Gov't] Journal of affective disorders. 2012;136:716–723. doi: 10.1016/j.jad.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. [Meta-AnalysisReview] Psychosomatic medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hunzeker JT, Elftman MD, Mellinger JC, Princiotta MF, Bonneau RH, Truckenmiller ME, et al. A marked reduction in priming of cytotoxic CD8+ T cells mediated by stress-induced glucocorticoids involves multiple deficiencies in cross-presentation by dendritic cells. Journal of Immunology. 2011;186:183–194. doi: 10.4049/jimmunol.1001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. Journal of Clinical Endocrinology and Metabolism. 2006;91:2153–2158. doi: 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- Jackson B, Kubzansky LD, Cohen S, Weiss S, Wright RJ. A matter of life and breath: childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. International Jpurnal of Epidemiology. 2004;33:271–278. doi: 10.1093/ije/dyh003. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Review. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Keller PS, El-Sheikh M, Vaughn B, Granger DA. Relations between mucosal immunity and children's mental health: the role of child sex. Physiology and Behavior. 2010;101:705–712. doi: 10.1016/j.physbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Arkins S, Li YM. Growth hormone, prolactin, and insulin-like growth factors: new jobs for old players. Brain Behavior and Immunity. 1992;6:317–326. [PubMed] [Google Scholar]
- Kelly ME, Juern AM, Grossman WJ, Schauer DW, Drolet BA. Immunosuppressive effects in infants treated with corticosteroids for infantile hemangiomas. Archives of Dermatoogy. 2010;146:767–774. doi: 10.1001/archdermatol.2010.90. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Wicks S, Dalman C, Henriksen TB, Li J, Mortensen PB. Prenatal stress and risk of asthma hospitalization in the offspring: a Swedish population-based study. Psychosomatic Medicine. 2012;74:635–641. doi: 10.1097/PSY.0b013e31825ac5e7. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R, et al. Psychoneuroimmunology and psychosomatic medicine: back to the future. Psychosomatic Medicine. 2002;64:15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain, Behavior, and Immunity. 2013;27:22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, et al. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. Journal of Experimental Medicine. 2008;205:2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre F, Moreau D, Semon E, Kalaboka S, Annesi-Maesano I, Just J. Maternal depression related to infant's wheezing. Pediatric Allergy and Immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2011;22:608–613. doi: 10.1111/j.1399-3038.2011.01155.x. [DOI] [PubMed] [Google Scholar]
- Lemieux A, Coe CL, Carnes M. Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain, Behavior, and Immunity. 2008;22:994–1003. doi: 10.1016/j.bbi.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PS, Li J, Holloway AF, Rao S. Epigenetic regulation of inducible gene expression in the immune system. Immunology. 2013;139:285–293. doi: 10.1111/imm.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annual Review of Neuroscience. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Mackner L, Pajer K, Glaser R, Crandell W. Relationships between depression, cytokines, and cortisol in pediatric inflammatory bowel disease. Gastroenterology. 2010;138:A212–A213. [Google Scholar]
- Madden KS, Felten SY, Felten DL, Sundaresan PR, Livnat S. Sympathetic neural modulation of the immune system. I. Depression of T cell immunity in vivo and in vitro following chemical sympathectomy. Brain, Behavior, and Immunity. 1989;3:72–89. doi: 10.1016/0889-1591(89)90007-x. [DOI] [PubMed] [Google Scholar]
- Marie-Mitchell A, O'Connor TG. Adverse childhood experiences: translating knowledge into identification of children at risk for poor outcomes. Academic Pediatrics. 2013;13:14–19. doi: 10.1016/j.acap.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Marques AH, O'Connor TG, Roth C, Susser E, Bjorke-Monsen AL. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Frontiers in Neuroscience. 2013;7:120. doi: 10.3389/fnins.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Cohen S, Rabin BS, Manuck SB. Associations between stress, trait negative affect, acute immune reactivity, and antibody response to hepatitis B injection in healthy young adults. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2001;20:4–11. [PubMed] [Google Scholar]
- McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa C. Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain, Behavior, and Immunity. 2013;31:23–30. doi: 10.1016/j.bbi.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Current Opinion in Immunology. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behaior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Meyer RJ, Haggerty RJ. Streptococcal infections in families. Pediatrics. 1962;29:539–549. [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Science USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research Review: The role of cytokines in depression in adolescents: a systematic review. Journal of Child Psychology and Psychiatry. 2013;54:816–835. doi: 10.1111/jcpp.12080. [DOI] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. The New England Journal of Medicine. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clinical Neuropharmacology. 2008;31:287–292. doi: 10.1097/WNF.0b013e3181593d45. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moynihan JA, Heffner KL, Caserta MT, O'Connor TG. Stress and immune function in humans: a life-course perspective. In: Kusnecov AV, Anisman H, editors. The Wiley-Blackwell Handbook of Psychoneuroimmunology. Oxford: John Wiley and Sons; 2013. [Google Scholar]
- Nagy E, Berczi I. Immunodeficiency in hypophysectomized rats. Acta Endocrinology (Copenhagen) 1978;89:530–537. doi: 10.1530/acta.0.0890530. [DOI] [PubMed] [Google Scholar]
- Nielsen NM, Hansen AV, Simonsen J, Hviid A. Prenatal stress and risk of infectious diseases in offspring. American Journal of Epidemiology. 2011;173:990–997. doi: 10.1093/aje/kwq492. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. MolecularPsychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Marvin RS, Rutter M, Olrick JT, Britner PA. Child-parent attachment following early institutional deprivation. Developmental Psychopathology. 2003;15:19–38. doi: 10.1017/s0954579403000026. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Winter MA, Hunn J, Carnahan J, Pressman EK, Glover V, et al. Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain, Behavior, and Immunity. 2013;32:21–28. doi: 10.1016/j.bbi.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KJ, Glover V, Jenkins J, Browne D, Ben-Shlomo Y, Golding J, et al. Prenatal maternal mood is associated with altered diurnal cortisol in adolescence. Psychoneuroendocrinology. 2013;38:1630–1638. doi: 10.1016/j.psyneuen.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O'Farrelly C. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain, Behavior, and Immunity. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. Journal of Comparative Pathology. 2010;142(Suppl 1):S39–S44. doi: 10.1016/j.jcpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Pihlgren M, Friedli M, Tougne C, Rochat AF, Lambert PH, Siegrist CA. Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. Journal of Immunology. 2006;176:165–172. doi: 10.4049/jimmunol.176.1.165. [DOI] [PubMed] [Google Scholar]
- Powell ND, Allen RG, Hufnagle AR, Sheridan JF, Bailey MT. Stressor-induced alterations of adaptive immunity to vaccination and viral pathogens. Immunology and Allergy Clinics of North America. 2011;31:69–79. doi: 10.1016/j.iac.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nature Immunology. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- Provencal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, et al. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15626–15642. doi: 10.1523/JNEUROSCI.1470-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is Depression an Inflammatory Disorder? CurrentPsychiatry Reports. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. Journal of the American Medical Association Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Obel C, Taanila A. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. International Journal of Obesity. 2008;32:550–557. doi: 10.1038/sj.ijo.0803741. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, et al. A conceptual revolution in the relationships between the brain and immunity. Brain, Behavior, and Immunity. 2011;25:817–819. doi: 10.1016/j.bbi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S, Dadds MR. Practitioner review: When parent training doesn't work: theory-driven clinical strategies. Journal of Child Psychology and Psychiatry. 2009;50:1441–1450. doi: 10.1111/j.1469-7610.2009.02161.x. [DOI] [PubMed] [Google Scholar]
- Scott S, Sylva K, Doolan M, Price J, Jacobs B, Crook C, et al. Randomised controlled trial of parent groups for child antisocial behaviour targeting multiple risk factors: the SPOKES project. Journal of Child Psychology and Psychiatry. 2010;51:48–57. doi: 10.1111/j.1469-7610.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Annals of the New York Academy of Science. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. Journal of Allergy and Clinical Immunology. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc Natl Acad Sci U S A. 2009;106(8):2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, et al. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biological Psychiatry. 2010;67:965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain, Behavior, and Immunity. 2012;26:239–250. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Slopen N, McLaughlin KA, Dunn EC, Koenen KC. Childhood adversity and cell-mediated immunity in young adulthood: does type and timing matter. Brain, Behavior, and Immunity. 2013;28:63–71. doi: 10.1016/j.bbi.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyke AT, Koga SF, Johnson DE, Fox NA, Marshall PJ, Nelson CA, et al. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry. 2007;48:210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- Sokal EM, Conjeevaram HS, Roberts EA, Alvarez F, Bern EM, Goyens P, et al. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology. 1998;114:988–995. doi: 10.1016/s0016-5085(98)70318-x. [DOI] [PubMed] [Google Scholar]
- Sternthal MJ, Enlow MB, Cohen S, Canner MJ, Staudenmayer J, Tsang K, et al. Maternal interpersonal trauma and cord blood IgE levels in an inner-city cohort: a life-course perspective. Journal of Allergy and Clinical Immunology. 2009;124:954–960. doi: 10.1016/j.jaci.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, et al. Microglial activation in young adults with autism spectrum disorder. Journal of the American Medical Academy Psychiatry. 2013;70:49–58. doi: 10.1001/jamapsychiatry.2013.272. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Seminars of Reproductive Medicine. 2009;27:391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedo SE, Garvey M, Snider L, Hamilton C, Leonard HL. The PANDAS subgroup: recognition and treatment. CNS Spectrums. 2001;6:419–422. doi: 10.1017/s1092852900021799. 425–426. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Grant PJ. Annotation: PANDAS: a model for human autoimmune disease. Journal of Child Psychology and Psychiatry. 2005;46:227–234. doi: 10.1111/j.1469-7610.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Vescovini R, Biasini C, Telera AR, Basaglia M, Stella A, Magalini F, et al. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. Journal of immunology. 2010;184:3242–3249. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Minireview: Cytokine-to-brain communication: A review and analysis of alternative mechanisms. Life Sciences. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine. 2009;71:805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- WHO. Early child development: a powerful equalizer. Geneva: Wolrd Health Organization; 2007. Available from: http://www.who.int/maternal_child_adolescent/documents/ecd_final_m30/en/ [Google Scholar]
- Wira C, Munck A. Specific glucocorticoid receptors in thymus cells. Localization in the nucleus and extraction of the cortisol-receptor complex. Journal of Biological Chemistry. 1970;245:3436–3438. [PubMed] [Google Scholar]
- Wise PH. The future pediatrician: the challenge of chronic illness. Journal of Pediatrics. 2007;151(5 Suppl):S6–S10. doi: 10.1016/j.jpeds.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Wise PH. Confronting social disparities in child health: a critical appraisal of life-course science and research. Pediatrics. 2009;124(Suppl 3):S203–S211. doi: 10.1542/peds.2009-1100H. [DOI] [PubMed] [Google Scholar]
- Wise PH, Blair ME. The UNICEF report on child well-being. Ambulatory Pediatrics. 2007;7:265–266. doi: 10.1016/j.ambp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. Journal of Immunology. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. American Journal of Respiratory and Critical Care Medicine. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]