Abstract
Purpose
To examine racial differences in gestational weight gain (GWG) and pregnancy-related hypertension.
Methods
Logistic regression models tested racial differences in adequacy of GWG and pregnancy-induced hypertension in all singleton live births from the South Carolina 2004-2006 birth certificates.
Results
Compared to white women, black and Hispanic women had 16%-46% lower odds of gaining weight above the recommendations. However, the odds of inadequate GWG was ~50% higher in black and Hispanic women with a pregnancy body mass index (BMI) <25kg/m2. Furthermore, compared to women with adequate GWG, women with excessive GWG had higher odds of pregnancy-related hypertension (underweight: 2.35, 95% CI(1.66, 3.32); normal: 2.05, 95% CI(1.84, 2.27); overweight: 1.93, 95% CI(1.64, 2.27); obese: 1.46, 95% CI(1.30, 1.63)). Among women with a BMI <25 kg/m2, black women had higher odds of pregnancy-related hypertension than white women (underweight: 1.64, 95% CI(1.14, 2.36); normal weight: 1.28, 95% CI(1.15, 1.42)), while among women with a BMI ≥25 kg/m2, Hispanic women had 40% lower odds.
Conclusion
Programs are needed to curb excessive GWG in all racial groups and to help some sub-groups ensure adequate GWG. Maternal obesity and GWG are two factors that should be used in combination to reduce racial differences in pregnancy-related hypertension.
Introduction
Pregnancy-related hypertension, including gestational hypertension, preeclampsia, and eclampsia, is the leading cause of maternal death in industrialized countries, accounting for 16% of deaths.[1] It is also a serious condition that may lead to maternal and offspring complications.[2] An estimated 3% of pregnancies are complicated by preeclampsia and 5 to 10% by hypertensive disorders including chronic hypertension,[1] and the prevalence of these disorders are increasing in the US.[2, 3]
Racial disparities exist for these disorders with the burden being the highest in black women.[2, 4, 5] Data from the 1998-1999 National Inpatient Sample found the prevalence of pregnancy-related hypertension to be 6.5% in black, 4.7% in white, and 3.8% in Hispanic women.[5] Following adjustment for age, gestational diabetes, preexisting diabetes and hypertension, black women had increased odds of pregnancy-related hypertension, while Hispanic women had a decreased odds of gestational hypertension but not preeclampsia compared to white women.[5] Another study found the age-adjusted prevalence of pregnancy-related hypertension increased significantly more among black women (4.8%) than that in white (2.6%) or Hispanic women (2.3%).[2]
The observed increase in pregnancy-related hypertension might be explained by the increase in both high prepregnancy body mass index (BMI) and excessive gestational weight gain (GWG), both risk factors for pregnancy-related hypertension[6-24]. Furthermore, because minority women are more likely to be overweight or obese before pregnancy [25] and overweight and obese women are more likely than normal weight women to exceed GWG recommendations[23], high prepregnancy BMI and GWG might also contribute to the increasing racial gap in pregnancy-related hypertension. Yet, some studies have found that black women gain less total weight gain during pregnancy than white women [15, 16, 26, 27]. Thus, it is important to examine the interactive effects of GWG and prepregnancy BMI in explaining the racial differences in pregnancy-related hypertension.
To date, the joint effect of GWG and prepregnancy BMI on the risk of pregnancy-related hypertension has received some attention in previous research.[11, 12, 14, 17-19] Half of these studies, however, have been underpowered.[11, 18, 19] Approximately half of studies have been conducted in the geographic areas with limited racial diversity such as China,[13] Canada[11] or predominantly Caucasian, European populations.[12, 14, 19, 20, 24] Studies conducted in US populations have controlled for race,[16, 17, 21] or have been restricted to a specific racial/ethnic group[18, 22] or to only normal weight[16] or obese women.[21] They have not investigated the role of race in pregnancy-related hypertension and how other modifiable risk factors may contribute to racial differences. Although DeLaTorre et al. examined racial disparities in gestational hypertension by prepregnancy BMI, they studied a group of high-risk pregnant women who received comprehensive home-based nursing services,[15] thus limiting its generalizability.
The aim of this paper was to examine the interactive roles of GWG and prepregnancy BMI in racial disparities in pregnancy-related hypertension in a large population of women residing in the state of South Carolina (SC) who delivered in 2004-2006. SC has poor maternal and child health indicators compared to the rest of the nation and women living the southern United States have the highest. prevalence of gestational hypertension.[3] Each year approximately one third of births in SC are to black women, which provide a unique opportunity to examine the proposed questions. Given that few population-based studies have examined racial differences in GWG, we first examined racial differences in GWG according to prepregnancy BMI. We then examined whether racial differences in pregnancy-related hypertension were explained by differences in GWG and prepregnancy BMI.
Data and Methods
Live, singleton births between 20-44 weeks with a birth weight greater than 500 grams to mothers without prepregnancy hypertension were included from the 2004-6 SC birth certificates (n=44,274 non-Hispanic black, 79,004 non-Hispanic white, 12,401 Hispanic women). Women whose race/ethnicity did not fit into one of these categories (3,287) or women with missing information on race/ethnicity (1,271) were excluded from the analysis due to small numbers and the difficulty in defining heterogeneity in this category. Births before 20 weeks were not included because preeclampsia and gestational hypertension are diagnosed after 20 weeks of pregnancy.[28] Additionally, exclusions were made for missing information for prepregnancy weight, height or BMI (2,975); a prepregnancy BMI less than 10 (7) or greater than 80 (2); missing GWG (641); gestational weight loss of more than 30 pounds (311) or GWG greater than 97 pounds (390), as well as missing information for other covariates (2,963) such as date of first prenatal care visit (2,197), maternal education (374), and marital status (288). Cut points of less than 10 and greater than 80 for BMI and less than -30 and greater than 97 pounds for GWG have been used previously to define improbable values.[26, 29]
Main variables
The SC birth certificate collected information on total GWG and clinical estimates of gestational age in weeks. Considering that total GWG varies by weeks of gestation at delivery, we used a measure of adequacy of GWG which takes into account gestational age at delivery. Table 1 summarizes the 2009 IOM guideline for each prepregnancy BMI group: underweight (<18.5 kg/m2), normal weight (18.5-24.9), overweight (25.0-29.9) and obese (≥30.0). It assumes that women with BMI <25 typically gain 4.4 lbs during the first 12 weeks of pregnancy compared to 2.2 lbs if they are overweight and 1.1 lbs if they are obese.[30] For each BMI group, we divided the lower and upper limits of recommended weight-gain range by expected mean weight gain at 40 weeks’ gestation to derive corresponding adequate ranges of expected weight gain based on the recommendation as shown in the last column of Table 1. We calculated the ratio of actual weight gain at delivery to the expected weight gain for that gestational week according to the 2009 Institute of Medicine (IOM)’s recommendations. If the ratio of actual to expected weight gain fell into the adequate ranges based on the recommendation shown in Table 1, then the woman was defined as gaining adequate weight during pregnancy. If the ratio fell above or below these ranges, then total GWG was considered to be above (excessive) or below (inadequate) the recommendation, respectively. Additional details are available in previous studies.[31, 32]
Table 1.
The 2009 Institute of Medicine’s recommendations on total weight gain and rate of weight gain for singleton pregnancy
| Prepregnancy weight category |
Body Mass Index (BMI) |
Recommended total weight gain |
Rate of weight gain in the second and third trimesters* |
Adequate ranges of expected weight gain based on the recommendation** |
||
|---|---|---|---|---|---|---|
| Range in kg | Range in lbs | Mean (range) in kg/week |
Mean (range) in lbs/week |
|||
| Underweight | < 18.5 | 12.5 – 18 | 28-40 | 0.51 (0.44-0.58) | 1.0 (1.0-1.3) | 0.79 – 1.14 |
| Normal weight | 18.5-24.9 | 11.5 – 16 | 25-35 | 0.42 (0.35-0.50) | 1.0 (0.8-1.0) | 0.86 – 1.20 |
| Overweight | 25.0-29.9 | 7.0 – 11.5 | 15-25 | 0.28 (0.23-0.33) | 0.6 (0.5-0.7) | 0.81 – 1.34 |
| Obese | ≥ 30.0 | 5.0 – 9.0 | 11-20 | 0.22 (0.17-0.27) | 0.5 (0.4-0.6) | 0.78 – 1.41 |
Calculations assume that total weight gain in the first trimester (<13 weeks of pregnancy) is 0.5 kg for obese women, 1 kg for overweight women, and 2 kg for normal weight or underweight women.
This is calculated by dividing the lower and upper limits of recommended weight gain range by expected weight gain at 40 weeks’ gestation. For example, for underweight women, the expected weight gain at 40 week’s gestation is 15.77 kg (2 kg+[(40-13)*0.51). Thus, the adequate range of expected weight gain based on recommendation for underweight women is (0.79-1.14), where 0.79 = (12.5/15.77).
The SC birth certificate collects information on hypertension status: 1) prepregnancy (chronic); 2) gestational (pregnancy-induced hypertension, preeclampsia); and 3) eclampsia. In this study, pregnancy-related hypertension includes pregnancy-induced hypertension, preeclampsia, and eclampsia. This definition is consistent with previous studies,[3-5] although Wallis et al.[3] examined preeclampsia/eclampsia as a separate category. Race/ethnicity was categorized as non-Hispanic black, Hispanic, and non-Hispanic white (hereafter, black, Hispanic and white).
Statistical analyses
For GWG, we estimated the prevalence of inadequate or excessive GWG by race/ethnicity and prepregnancy BMI categories. Multinomial logistic regression models were used to predict the outcome of excessive or inadequate GWG compared to adequate GWG. Independent variables were race, prepregnancy BMI, and race*prepregnancy BMI. Covariates, based on previous studies, were maternal age, race/ethnicity, marital status, smoking status, education level, month prenatal care began, and parity. Odds ratios (OR) and 95% confidence intervals (CI) from both the crude and adjusted models were presented.
We also estimated the prevalence of pregnancy-related hypertension by race, GWG, prepregnancy BMI category, and other characteristics. Cochran-Armitage trend tests were used to assess whether the prevalence of pregnancy-related hypertension increased with advancing BMI category. Multiple logistic regression models were used to predict the outcome of pregnancy-related hypertension. Independent variables were race, prepregnancy BMI, GWG, race*prepregnancy BMI, race*GWG, and prepregnancy BMI*GWG. Other covariates were the same as described above. In the case of significant interaction terms, stratified analyses were conducted. In addition to all covariates mentioned in GWG model, we further added categorical GWG as covariates in the models. All analyses were conducted using SAS 9.2 (Cary, NC). The study received approval from the local Institution Review Board.
Results
Sample characteristics
White women comprised nearly 60% of the pregnant population in SC followed by black women who made up about one third and Hispanic women who accounted for less than 10%. Approximately one quarter of mothers did not graduate from high school, but nearly half, had more than a high school education. Over half of women were multiparous and married. Nearly 15% of mothers reported smoking while pregnant and just over one quarter began prenatal care ≥13 weeks gestation. Before pregnancy, 4.7% of women were underweight, 46.7% normal weight, 20.7% overweight, and 27.9% obese (Table 2).
Table 2.
Sample characteristics for all women and women with pregnancy-related hypertension in South Carolina, 2004-2006.
| Total Sample | Pregnancy-related hypertension |
|||
|---|---|---|---|---|
|
|
||||
| % | n | % | p value* | |
| Total | 100.0 | 133,849 | 5.7 | |
| Race | ||||
| Non-Hispanic White | 58.3 | 77,980 | 5.8 | <.0001 |
| Non-Hispanic Black | 32.6 | 43,671 | 6.3 | |
| Hispanic | 9.1 | 12,198 | 3.5 | |
| Gestational weight gain (GWG)** | ||||
| Inadequate | 28.4 | 39,146 | 4.3 | <.0001 |
| Adequate | 22.8 | 35,425 | 3.6 | |
| Excessive | 48.8 | 59,278 | 7.6 | |
| Pre-pregnancy BMI | ||||
| Underweight | 4.7 | 6,251 | 2.9 | <.0001 |
| Normal weight | 46.7 | 62,520 | 3.9 | |
| Overweight | 20.7 | 27,722 | 5.8 | |
| Obese | 27.9 | 37,356 | 9.2 | |
| Parity | ||||
| None | 45.7 | 61,118 | 7.3 | <.0001 |
| 1 or more | 54.3 | 72,731 | 4.5 | |
| Maternal Education | ||||
| <12 years | 24.0 | 32,152 | 4.8 | <.0001 |
| 12 years | 26.4 | 35,131 | 5.9 | |
| >12 years | 49.7 | 66,566 | 6.1 | |
| Smoking during Pregnancy | ||||
| Yes | 14.2 | 18,994 | 5.4 | 0.0214 |
| No | 85.8 | 114,855 | 5.8 | |
| Prenatal Care Started | ||||
| <13 weeks | 73.1 | 97,903 | 6.1 | <.0001 |
| 13 weeks or later | 26.9 | 35,946 | 4.7 | |
| Maternal Age | ||||
| <20 years | 14.4 | 19,326 | 5.6 | <.0001 |
| 20-34 years | 75.5 | 100,993 | 5.6 | |
| 34+ years | 10.1 | 13,530 | 6.7 | |
| Marital Status | ||||
| Yes | 55.4 | 74,121 | 5.7 | 0.7458 |
| No | 44.6 | 59,728 | 5.8 | |
p-value was based on chi-square tests of independence.
Gestational age was accounted for in the determination of adequacy of gestational weight gain using the 2009 Institute of Medicine’s recommendations.
Gestational weight gain
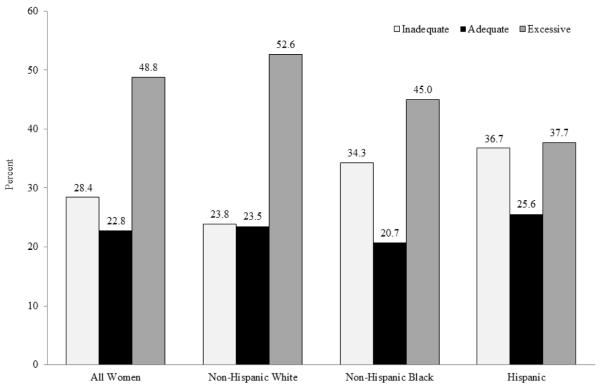
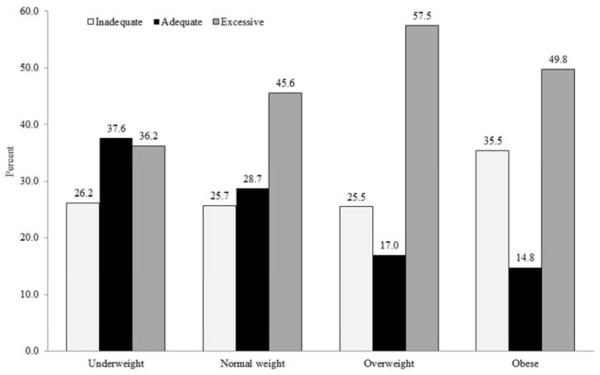
Only 22.8% of SC women gained weight within the 2009 recommended range during the pregnancy, 48.8% had excessive GWG and 28.4% had inadequate GWG. GWG varied by race/ethnicity (Figure 1a, p<0.001). Excessive GWG was most prevalent among white women (52.6%) and least among Hispanic women (37.7%). Inadequate GWG, on the other hand, was higher in black (34.3%) and Hispanic women (36.7%) than white women (23.8%). GWG also varied by prepregnancy BMI (Figure 1b, p<0.001). Overweight and obese women had a higher prevalence of excessive GWG. Obese women also had the highest proportion of inadequate GWG and the lowest proportion of gaining within the recommendation.
Figure 1a.
Gestational weight gain by race/ethnicity in South Carolina, 2004-6
Figure 1b.
Gestational weight gain by pre-pregnancy body mass index in South Carolina, 2004-6
A significant interaction between race and prepregnancy BMI on GWG was identified, so all models were stratified by prepregnancy BMI class (p <0.001). Black women had 16%-30% lower odds of excessive GWG than white women regardless of their prepregnancy BMI. Hispanic women had 29%-46% lower odds of excessive GWG than white women if their prepregnancy BMI was normal, overweight or obese. However, compared to white women in the same weight category, the odds of inadequate GWG was ~50% higher in black and Hispanic women whose prepregnancy BMI was <25. Overweight Hispanic women had 20% lower odds of inadequate GWG during pregnancy (95% CI: 0.70, 0.90)(Table 3). The results did not change when analyses were restricted to women who delivered full term infants (≥37 weeks).
Table 3.
Adjusted* odds of excessive or inadequate gestational weight gain (GWG) in non-Hispanic black women and Hispanic women compared to non-Hispanic white women by pre-pregnancy BMI category
| Underweight | Normal weight | Overweight | Obese | |
|---|---|---|---|---|
| Excessive GWG vs adequate GWG | ||||
| Non-Hispanic Black | 0.70 (0.60, 0.81) | 0.84 (0.80, 0.89) | 0.78 (0.72, 0.85) | 0.84 (0.78, 0.90) |
| Hispanic | 0.85 (0.66, 1.09) | 0.69 (0.65, 0.75) | 0.54 (0.48, 0.61) | 0.71 (0.62, 0.80) |
| Non-Hispanic White | 1.00 | 1.00 | 1.00 | 1.00 |
| Inadequate GWG vs adequate GWG | ||||
| Non-Hispanic Black | 1.53 (1.30, 1.79) | 1.49 (1.41, 1.58) | 0.96 (0.88, 1.06) | 1.04 (0.96, 1.12) |
| Hispanic | 1.60 (1.23, 2.07) | 1.44 (1.34, 1.56) | 0.80 (0.70, 0.90) | 0.88 (0.78, 1.01) |
| Non-Hispanic White | 1.00 | 1.00 | 1.00 | 1.00 |
Adjusted for parity, maternal education, smoking during pregnancy, month prenatal care started, maternal age and marital status.
Pregnancy-related hypertension
As shown in Table 2, pregnancy-related hypertension was least prevalent among Hispanic women (3.5%) and similar in black (6.3%) and white (5.8%) women. Pregnancy-related hypertension was most prevalent among women who gained excessive weight during pregnancy (7.6%), followed by those who gained under the guideline (4.3%) and within the guideline (3.6%). The prevalence of pregnancy-related hypertension increased with prepregnancy BMI category: 2.9%, 3.9%, 5.8%, and 9.2% for underweight, normal weight, overweight, and obese women, respectively.
Interactions between race and prepregnancy BMI (p <0.001) and prepregnancy BMI and GWG (p<0.001) were significant when all covariates were in the model. Thus, all stratified results by prepregnancy BMI category were presented below. As shown in Table 4, the prevalence of pregnancy-related hypertension was the highest among women who were obese before pregnancy and then gained excessive weight during pregnancy (10.4% among black women, 12.3% among white women, 7.3% among Hispanic women). The prevalence of hypertension was about the same for women with inadequate and adequate GWG within each BMI group regardless of race. There were significant increasing trends in the prevalence of pregnancy-related hypertension with increasing prepregnancy BMI in all joint categories by race and GWG except among Hispanic women who gained adequate weight during pregnancy (Table 4).
Table 4.
Prevalence of pregnancy-related hypertension by joint categories of pre-pregnancy weight status and gestational weight gain (GWG) among non-Hispanic black, non-Hispanic white, and Hispanic women in South Carolina, 2004-6.
| Pregnancy-related hypertension (%) |
|||||
|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | P for trend* | |
| Non-Hispanic Black | |||||
| Inadequate GWG | 2.3 | 3.4 | 3.4 | 7.0 | <0.001 |
| Adequate GWG | 3.9 | 3.3 | 3.6 | 7.4 | <0.001 |
| Excessive GWG | 5.5 | 6.3 | 7.5 | 10.4 | <0.001 |
| Non-Hispanic White | |||||
| Inadequate GWG | 1.5 | 2.1 | 3.8 | 7.9 | <0.001 |
| Adequate GWG | 1.6 | 2.4 | 4.2 | 8.4 | <0.001 |
| Excessive GWG | 4.6 | 5.3 | 8.0 | 12.3 | <0.001 |
| Hispanic | |||||
| Inadequate GWG | 0.7 | 1.8 | 2.2 | 5.2 | <0.001 |
| Adequate GWG | 0.7 | 2.6 | 2.0 | 3.1 | 0.3498 |
| Excessive GWG | 2.9 | 4.4 | 4.2 | 7.3 | <0.001 |
P-values were based on Cochran-Armitage Trend tests.
As shown in Table 5, excessive GWG was associated with 1.5-2.3 times higher odds of pregnancy-related hypertension. The odds ratios for the association between pregnancy-related hypertension and excessive GWG decreased as BMI increased from underweight (OR: 2.35, 95% CI: 1.66, 3.32) to obese category (OR: 1.46, 95% CI: 1.30, 1.63). Black women with a BMI < 25 had higher odds of pregnancy-related hypertension than white women in the same weight category (OR for underweight: 1.64, 95% CI: 1.14, 2.36; OR for normal weight: 1.28, 95% CI: 1.15, 1.42). Furthermore, Hispanic women with a BMI ≥ 25 had significantly lower odds of pregnancy-related hypertension (OR for overweight: 0.61, 95% CI: 0.48, 0.77; OR for obese: 0.66, 95% CI: 0.55, 0.79). Results did not differ when restricted to women with full-term births.
Table 5.
Joint effect of body mass index (BMI), race and gestational weight gain (GWG) on pregnancy-related hypertension
| Pregnancy-related hypertension: Adjusted* OR (95% CI) | ||||
|---|---|---|---|---|
|
| ||||
| Underweight | Normal weight | Overweight | Obese | |
| Gestational weight gain | ||||
| Inadequate | 0.83 (0.52, 1.33) | 0.93 (0.81, 1.07) | 0.91 (0.75, 1.11) | 0.96 (0.85, 1.08) |
| Excessive | 2.35 (1.66, 3.32) | 2.05 (1.84, 2.27) | 1.93 (1.64, 2.27) | 1.46 (1.30, 1.63) |
| Adequate | 1.00 | 1.00 | 1.00 | 1.00 |
| Race/Ethnicity | ||||
| Non-Hispanic Black | 1.64 (1.14, 2.36) | 1.28 (1.15, 1.42) | 0.97 (0.85, 1.10) | 0.96 (0.88, 1.05) |
| Hispanic | 0.53 (0.23, 1.24) | 0.86 (0.73, 1.02) | 0.61 (0.48, 0.77) | 0.66 (0.55, 0.79) |
| Non-Hispanic White | 1.00 | 1.00 | 1.00 | 1.00 |
Adjusted for maternal race/ethnicity, GWG, education, smoking during pregnancy, month prenatal care started, maternal age and marital status.
Discussion and Conclusions
Only about one in five South Carolina women gained weight within the 2009 recommended range during pregnancy, with an additional 48.8% gaining excessive weight and 28.4% gaining inadequate weight. Overweight and obese women were more likely to gain excessive weight during pregnancy. Gaining excessive weight was prevalent in both white (52.6%) and black women (45%) and was slightly lower among Hispanic women (37.7%). Normal weight and underweight black and Hispanic women (BMI<25) had about a 50% higher odds of gaining inadequate weight during pregnancy compared to white women, and overweight Hispanic women had modestly lower odds of gaining inadequate weight during pregnancy. To our knowledge few studies have examined the joint effect of race and prepregnancy BMI status on GWG.
These findings indicate a strong need for education and lifestyle intervention programs designed to help the majority of pregnant women gain weight within the recommended guidelines. Programs should include all racial groups as they all face weight gain challenges during pregnancy. For white women, programs designed to prevent excessive GWG is particularly important. In contrast, for normal weight black and Hispanic women, programs designed to ensure adequate weight gain during pregnancy are crucial. Existing intervention programs have placed more attention on excessive weight gain,[10, 33-35] but our findings suggest that attention should also be placed on inadequate weight gain during pregnancy in some sub-groups.
Another unique contribution of this study was its examination of the interactive effects of prepregnancy BMI, race, and GWG on the risk of pregnancy-related hypertension. Consistent with two previous large studies,[12, 15] we found that gaining excessive GWG was associated with an increased risk of pregnancy-related hypertension in all BMI categories. Smaller studies restricted to normal weight [16, 24] or obese women [11, 20, 21] further confirm this finding. Two studies found high GWG to be associated with gestational hypertension and preeclampsia, respectively, in normal weight, but not obese women [14, 17].
Stratifying our results allowed us to examine the relative importance of excessive GWG by BMI category. Our results suggest that although the odds of pregnancy-related hypertension increased with excessive GWG, the odds decreased as prepregnancy BMI increased. To our knowledge, we are only the second study to examine such an association. A similar trend was observed in the stratified results from Cedergren et al., whose results showed decreasing odds of preeclampsia with each increasing prepregnancy BMI category.[12]
Racial differences in the risk of pregnancy-related hypertension were clearly observed by prepregnancy BMI and remained after adjusting for GWG and other covariates. Among women with a BMI <25, black women had higher odds of pregnancy-related hypertension than white women. Among overweight and obese women (BMI≥25), Hispanic women had lower odds of pregnancy-related hypertension than white women. Three studies [2, 4, 5] examined the racial differences in pregnancy-related hypertension and all confirm our finding that pregnancy-related hypertension is highest in black women. Unlike our study, however, Fang et al.[4] and Baraban et al.[2], reported pregnancy-related hypertension to be lower among white women than Hispanic women. This may be due to differences in the Hispanic population, a term which encompasses a very heterogeneous group of people, in New York City[4] and Los Angeles[2] compared to SC. The older databases in other two studies may also explain these differences.
Only our study and Shen et al.’s [5] sought to determine reasons for racial disparities in pregnancy-related hypertension by adjusting the results for probable explanatory factors. Shen et al.[5] adjusted for maternal age, gestational diabetes, preexisting diabetes, and pre-existing hypertension and continued to see an increased odds of pregnancy-related hypertension in black women and a decreased odds of pregnancy-related hypertension in Hispanic women compared to white women. Shen et al.[5] did not stratify their results by prepregnancy BMI and could not detect whether racial differences varied by prepregnancy BMI. Our more detailed, BMI-specific results may help to better inform and tailor future interventions.
Our findings suggest that maternal obesity and GWG are two factors that should be used in combination in targeting, understanding and reducing the risk of pregnancy-related hypertension. Depending on prepregnancy BMI, the effect of race on pregnancy-related hypertension varied. To reduce racial differences in pregnancy-related hypertension, attention should be paid to black women who are underweight or normal weight before pregnancy. The increased risk of pregnancy-related hypertension among overweight and obese white women (compared to Hispanic women) should also be addressed in programs aiming to prevent pregnancy-related hypertension.
The main strength of this study is the use of a large, unselected multiethnic population including all pregnant women living in SC, which allowed us to carefully examine the interactive effects of race, prepregnancy BMI, and GWG on the risk of pregnancy-related hypertension. We also applied the latest 2009 recommendation for GWG. Our measure of adequacy of GWG represents an enhancement in methods for analyzing adequacy of GWG by considering the gestational age at delivery, a factor closely related to total GWG.
Our study also has limitations. First, our analysis would have benefitted from more detailed information on the risk factors related to pregnancy-related hypertension in order to better understand racial differences. For example, family history of hypertension and previous preterm deliveries are known to be higher among black women. Second, although we used a large unselected sample of all pregnant women in SC, some caution should be used in generalizing these findings to women living in other geographic regions. Because SC has a high prevalence of cardiovascular diseases and hypertension, the racial differences observed here may not be representative of other regions. Third, we were not able to differentiate between sub-types of hypertensive disorders in pregnancy. Finally, data from birth certificates may not be accurate and were not validated in SC. However, prior validation studies from other states show that compared with information from medical records, birth certificates have very high agreement on demographic variables such as race/ethnicity (Kappa =0.88) [36]. The validity for pregnancy-related hypertension, preeclampsia,[37] and prepregnancy BMI [38] measures are reasonably good.
In brief, this study found that excessive weight gain is prevalent in all racial groups. The higher prevalence of excessive than inadequate weight gain in all racial groups indicates that addressing excessive GWG may warrant more attention than addressing inadequate GWG. In terms of racial differences, we found black and Hispanic women were less likely to gain excessive weight than white women in all prepregnancy BMI categories and more likely to gain inadequate weight if their BMI was normal or underweight. These findings may help to better direct future intervention programs aimed at encouraging women to gain appropriate weight. Specifically, underweight and normal weight black and Hispanic women may benefit from programs that emphasize the hazards of inadequate GWG.
Regarding pregnancy-related hypertension, both underweight and normal weight black women had higher odds of pregnancy-related hypertension than white women while overweight and obese Hispanic women had lower odds. Due to the serious nature of pregnancy-related hypertension for both the mother and infant, understanding and addressing these disparities should be a top priority.
Acknowledgments
We thank the Division of Biostatistics, the South Carolina Department of Health and Environmental Control for making the data available for analysis. Drs. Liu and Wilcox’s efforts were partially supported by award number R21HD061885 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. The research was conducted in accord with prevailing ethical principles.
List of Abbreviations and Acronyms
- BMI
body mass index
- CI
confidence interval
- GWG
gestational weight gain
- IOM
Institute of Medicine
- OR
odds ratio
- SC
South Carolina
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011 Aug;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- [2].Baraban E, McCoy L, Simon P. Increasing prevalence of gestational diabetes and pregnancy-related hypertension in Los Angeles County, California, 1991-2003. Prev Chronic Dis. 2008 Jul;5(3):A77. [PMC free article] [PubMed] [Google Scholar]
- [3].Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008 May;21(5):521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- [4].Fang J, Madhavan S, Alderman MH. The influence of maternal hypertension on low birth weight: differences among ethnic populations. Ethn Dis. 1999 Autumn;9(3):369–76. [PubMed] [Google Scholar]
- [5].Shen JJ, Tymkow C, MacMullen N. Disparities in maternal outcomes among four ethnic populations. Ethn Dis. 2005 Summer;15(3):492–7. [PubMed] [Google Scholar]
- [6].Bianco AT, Smilen SW, Davis Y, Lopez S, Lapinski R, Lockwood CJ. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstet Gynecol. 1998 Jan;91(1):97–102. doi: 10.1016/s0029-7844(97)00578-4. [DOI] [PubMed] [Google Scholar]
- [7].Doherty DA, Magann EF, Francis J, Morrison JC, Newnham JP. Pre-pregnancy body mass index and pregnancy outcomes. Int J Gynaecol Obstet. 2006 Dec;95(3):242–7. doi: 10.1016/j.ijgo.2006.06.021. [DOI] [PubMed] [Google Scholar]
- [8].Linne Y. Effects of obesity on women’s reproduction and complications during pregnancy. Obes Rev. 2004 Aug;5(3):137–43. doi: 10.1111/j.1467-789X.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- [9].Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001 Aug;25(8):1175–82. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- [10].Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008 Mar;9(2):140–50. doi: 10.1111/j.1467-789X.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- [11].Brennand EA, Dannenbaum D, Willows ND. Pregnancy outcomes of First Nations women in relation to pregravid weight and pregnancy weight gain. J Obstet Gynaecol Can. 2005 Oct;27(10):936–44. doi: 10.1016/s1701-2163(16)30739-3. [DOI] [PubMed] [Google Scholar]
- [12].Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet. 2006 Jun;93(3):269–74. doi: 10.1016/j.ijgo.2006.03.002. [DOI] [PubMed] [Google Scholar]
- [13].Chen Z, Du J, Shao L, Zheng L, Wu M, Ai M, et al. Prepregnancy body mass index, gestational weight gain, and pregnancy outcomes in China. Int J Gynaecol Obstet. 2010 Apr;109(1):41–4. doi: 10.1016/j.ijgo.2009.10.015. [DOI] [PubMed] [Google Scholar]
- [14].Crane JM, White J, Murphy P, Burrage L, Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J Obstet Gynaecol Can. 2009 Jan;31(1):28–35. doi: 10.1016/s1701-2163(16)34050-6. [DOI] [PubMed] [Google Scholar]
- [15].de la Torre L, Flick AA, Istwan N, Rhea D, Cordova Y, Dieguez C, et al. The effect of new antepartum weight gain guidelines and prepregnancy body mass index on the development of pregnancy-related hypertension. Am J Perinatol. 2011 Apr;28(4):285–92. doi: 10.1055/s-0030-1271211. [DOI] [PubMed] [Google Scholar]
- [16].DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol. 2007 Oct;110(4):745–51. doi: 10.1097/01.AOG.0000284451.37882.85. [DOI] [PubMed] [Google Scholar]
- [17].Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal-weight women: effects of gestational weight change. Obstet Gynecol. 1996 Mar;87(3):389–94. doi: 10.1016/0029-7844(95)00446-7. [DOI] [PubMed] [Google Scholar]
- [18].Fortner RT, Pekow P, Solomon CG, Markenson G, Chasan-Taber L. Prepregnancy body mass index, gestational weight gain, and risk of hypertensive pregnancy among Latina women. Am J Obstet Gynecol. 2009 Feb;200(2):167 e1–7. doi: 10.1016/j.ajog.2008.08.021. [DOI] [PubMed] [Google Scholar]
- [19].Heude B, Thiebaugeorges O, Goua V, Forhan A, Kaminski M, Foliguet B, et al. Pre-Pregnancy Body Mass Index and Weight Gain During Pregnancy: Relations with Gestational Diabetes and Hypertension, and Birth Outcomes. Matern Child Health J. 2012 Feb;16(2):355–63. doi: 10.1007/s10995-011-0741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jensen DM, Ovesen P, Beck-Nielsen H, Molsted-Pedersen L, Sorensen B, Vinter C, et al. Gestational weight gain and pregnancy outcomes in 481 obese glucose-tolerant women. Diabetes Care. 2005 Sep;28(9):2118–22. doi: 10.2337/diacare.28.9.2118. [DOI] [PubMed] [Google Scholar]
- [21].Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol. 2007 Oct;110(4):752–8. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- [22].Ogunyemi D, Hullett S, Leeper J, Risk A. Prepregnancy body mass index, weight gain during pregnancy, and perinatal outcome in a rural black population. J Matern Fetal Med. 1998 Jul-Aug;7(4):190–3. doi: 10.1002/(SICI)1520-6661(199807/08)7:4<190::AID-MFM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [23].Olson CM. Achieving a healthy weight gain during pregnancy. Annu Rev Nutr. 2008;28:411–23. doi: 10.1146/annurev.nutr.28.061807.155322. [DOI] [PubMed] [Google Scholar]
- [24].Thorsdottir I, Torfadottir JE, Birgisdottir BE, Geirsson RT. Weight gain in women of normal weight before pregnancy: complications in pregnancy or delivery and birth outcome. Obstet Gynecol. 2002 May;99(5 Pt 1):799–806. doi: 10.1016/s0029-7844(02)01946-4. [DOI] [PubMed] [Google Scholar]
- [25].Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006 Apr 5;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- [26].Chu SY, Callaghan WM, Bish CL, D’Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004-2005: fueling future obesity. Am J Obstet Gynecol. 2009 Mar;200(3):271 e1–7. doi: 10.1016/j.ajog.2008.09.879. [DOI] [PubMed] [Google Scholar]
- [27].Caulfield LE, Witter FR, Stoltzfus RJ. Determinants of gestational weight gain outside the recommended ranges among black and white women. Obstet Gynecol. 1996 May;87:760–6. doi: 10.1016/0029-7844(96)00023-3. [DOI] [PubMed] [Google Scholar]
- [28].Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000 Jul;183(1):S1–S22. [PubMed] [Google Scholar]
- [29].CDC . Pediatric and Pregnancy Nutrition Surveillance System, Summary and Data Quality Sections, Biologically Implausible Values. CDC; [cited 2012 March 12]. 2009. Available from: http://www.cdc.gov/pednss/how_to/review_data_quality/periodic_data_quality_report/data_quality.htm. [Google Scholar]
- [30].Institute of Medicine Committee to Reexamine IOM Pregnancy Weight Guidelines . Weight Gain During Pregnancy: Reexamining the Guidelines. Institute of Medicine and National Research Council of the National Academies; Washington D.C.: 2009. [Google Scholar]
- [31].Bodnar LM, Siega-Riz AM, Arab L, Chantala K, McDonald T. Predictors of pregnancy and postpartum haemoglobin concentrations in low-income women. Public Health Nutr. 2004 Sep;7(6):701–11. doi: 10.1079/phn2004597. [DOI] [PubMed] [Google Scholar]
- [32].Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010 Jun;91(6):1642–8. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol. 2009 Feb;113:305–12. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- [34].Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes Relat Metab Disord. 2002 Nov;26(11):1494–502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- [35].Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol. 2004 Aug;191(2):530–6. doi: 10.1016/j.ajog.2004.01.027. [DOI] [PubMed] [Google Scholar]
- [36].Zollinger TW, Przybylski MJ, Gamache RE. Reliability of Indiana birth certificate data compared to medical records. Ann Epidemiol. 2006 Jan;16(1):1–10. doi: 10.1016/j.annepidem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [37].Roohan PJ, Josberger RE, Acar J, Dabir P, Feder HM, Gagliano PJ. Validation of birth certificate data in New York State. J Community Health. 2003 Oct;28(5):335–46. doi: 10.1023/a:1025492512915. [DOI] [PubMed] [Google Scholar]
- [38].Park S, Sappenfield WM, Bish C, Bensyl DM, Goodman D, Menges J. Reliability and validity of birth certificate prepregnancy weight and height among women enrolled in prenatal WIC program: Florida, 2005. Matern Child Health J. 2011 Oct;15(7):851–9. doi: 10.1007/s10995-009-0544-4. [DOI] [PubMed] [Google Scholar]