Abstract
Previous research has shown a wide array of age-related declines in vision. The current study examined the effects of perceptual learning (PL), external noise, and task difficulty in fine orientation discrimination with older individuals (mean age 71.73, range 65–91). Thirty-two older subjects participated in seven 1.5-hour sessions conducted on separate days over a three-week period. A two-alternative forced choice procedure was used in discriminating the orientation of Gabor patches. Four training groups were examined in which the standard orientations for training were either easy or difficult and included either external noise (additive Gaussian noise) or no external noise. In addition, the transfer to an untrained orientation and noise levels were examined. An analysis of the four groups prior to training indicated no significant differences between the groups. An analysis of the change in performance post-training indicated that the degree of learning was related to task difficulty and the presence of external noise during training. In addition, measurements of pupil diameter indicated that changes in orientation discrimination were not associated with changes in retinal illuminance. These results suggest that task difficulty and training in noise are factors important for optimizing the effects of training among older individuals.
Keywords: Perceptual Learning, Aging, External Noise, Task Difficulty, Orientation Discrimination
1. Introduction
Research has demonstrated a wide range of age-related declines in vision (for a review see Andersen, 2012). These declines include, but are not limited to, decrements in visual acuity (Weale, 1975), contrast sensitivity (Crassini, Brown, & Bowman, 1988), motion perception (Andersen & Atchley, 1995; Bennett, Sekuler, & Sekuler, 2007; Betts, Taylor, Sekuler, & Bennett, 2005; Roudaia, Bennett, Sekuler, & Pilz, 2010; Trick & Silverman, 1991), sensitivity to optical flow (Andersen & Enriquez, 2006; Atchley & Andersen, 1998), masking (Andersen, Ni, Bower, & Watanabe, 2010), and orientation discrimination (Betts, Sekuler, & Bennett, 2007). Studies have also found age-dependent reductions in photoreceptors (Curcio, Millican, Allen, & Kalina, 1993), loss of retinal ganglion cells (Curcio & Drucker, 1993) and decreases in retinal illuminance (Weale, 1988) that are likely to be underlying factors in age-related declines in vision and visual perception. In addition, neurophysiological studies have shown increased spontaneous neural firing and decreased orientation selectivity in single-cell recordings in visual cortex in senescent cats and rhesus monkeys (Schmolesky, Wang, Pu, & Leventhal, 2000), suggesting that increased noise in cortical processing may be a contributing factor to age-related declines in older humans.
Age-related declines among human observers can have a profound effect on the health and well-being of older individuals. Epidemiological studies have shown that age related declines in vision are associated with increased crash risk during driving and is a contributing factor to the increased likelihood of falls that occur with advanced age (Ivers, Cumming, Mitchell, & Attebo, 1998; Owsley et al., 1998). In addition, age-related declines in vision have been found to be associated with overall declines in quality of life and mobility (Whal et al., 1999; Charlton et al., 2006).
Given these broad declines in visual function with age, an important issue is whether any procedures exist to improve vision. One promising procedure is perceptual learning (PL) – the improvement in performance due to practice or repeated exposure in perceptual tasks. Research with younger (college age) individuals conducted in the past few decades has demonstrated improvement following practice of low-level perceptual tasks (Ahissar & Hochstein, 1996; Fahle & Edelman, 1993; Fiorentini & Berardi, 1981; Karni, Sagi, & Science, 1991), suggesting that PL might be a useful intervention for improving age related declines in vision. For example, recent research in clinical populations (Levi & Polat, 1996; Polat, 2009) has shown that training using PL methods can improve performance and in some cases transfer to real world tasks. Polat (2009) found that some participants, after PL training, reported that they were able to read without the use of their glasses. Thus, a potential benefit of PL research is that it may lead to interventions that could improve visual function for older individuals.
While PL has been examined in numerous studies with healthy college age individuals, a relatively small number of studies have examined perceptual learning and aging. Early research found evidence of PL in a motion discrimination task for both younger and older observers (Ball & Sekuler, 1986). The improved performance due to training was retained by participants for at least one month. In addition, learning was found when the displays were defocussed for younger individuals suggesting that the age-related differences in learning were unlikely to have been caused by differences in optical focus. More recent research examined perceptual learning in a texture discrimination task in older and younger observers (Andersen et al., 2010). Participants were presented with stimuli that contained a centrally presented letter and a peripherally presented texture pattern in noisy texture and were required to identify the central letter and orientation of the peripheral texture pattern. An older experimental group, who trained for three days with near threshold stimuli, showed significant improvements in performance that were similar to performance of untrained younger individuals. The improved performance was retained when assessed 3 months following training. An older control group that performed the same number of trials but were presented supra-threshold stimuli showed no significant improvement demonstrating that the learning was not likely to be due to task learning. To determine whether improved performance was due to changes in divided attention a second experiment also assessed participant’s performance in a divided attention task pre and post-training. While older participants again exhibited significant learning in the texture discrimination task, no evidence of learning in the divided attention task was found. This finding suggests that the improved performance from training was unlikely to be due to changes in divided attention. A third experiment showed that learning was location specific and did not transfer to an untrained region of the visual field. The results of the study suggest that PL can result in considerable improvements in performance that is maintained.
In addition to these studies, Bower & Andersen (2011) examined age-related changes in efficiency as a result of PL in discriminating motion. Participants performed a motion discrimination task using drifting sine-wave gratings and random dot cinematograms (RDCs) that included six levels of external noise (Gaussian contrast noise). The Perceptual Template Model (Dosher & Lu, 1998, 1999; Lu & Dosher, 2009) was used to identify three possible mechanisms of perceptual learning---stimulus enhancement (a reduction in additive internal noise), external noise exclusion (an improved ability to filter noise in the environment), and internal multiplicative noise reduction (a reduction in noise that increases in amplitude with the strength of the stimulus). In their first experiment, Bower & Andersen (2011) found that older and younger individuals showed significant improvement in motion discrimination with drifting sine-wave gratings following 5 days of training. For younger individuals, this improvement was associated with an increase in external noise exclusion. Older individuals also showed evidence of greater external noise exclusion as well as increased stimulus enhancement suggesting a reduction in internal noise. A second experiment examined motion training using random dot cinematograms. The results indicated significant learning for both age groups, with both groups showing evidence of improve external noise exclusion and stimulus enhancement. Transfer of training was examined by assessing motion discrimination performance with the untrained motion stimuli (e.g., transfer to RDCs when trained with sine-wave gratings). Transfer of training occurred when training with either type of motion stimuli, but greater transfer occurred when training with RDCs. The study demonstrates that not only can PL serve to reduce internal noise in older individuals and improve their ability to filter external noise, but can also transfer to untrained stimuli.
Training has also been used to examine whether PL can alter center-surround antagonism or spatial suppression in motion processing. Contrary to many other aging studies that found age-related declines in vision, older individuals have been found to have better performance in discriminating large high-contrast motion targets (Betts et al., 2005). Betts and colleagues suggested that this finding was due to a decline in center-surround inhibition for older individuals. Given this result Bower, Watanabe & Andersen (2013) examined whether perceptual learning could be used to improve motion discrimination in older individuals and examine if any changes in center-surround antagonism would occur as a result of training. Older and younger participants discriminated the motion direction of large (5°) or small (0.7°) diameter sine-wave gratings at 92%, 22%, or 2.8% contrast. Following training both groups showed improved duration thresholds with older individuals demonstrating improvements at all size and contrast levels. However, training did not result in changes in spatial suppression. In summary, the finding of perceptual learning in older individuals, across several different types of visual information, suggests that PL may be a useful intervention to improve vision among the elderly.
While previous research on perceptual learning has shown that older individuals can benefit from these training interventions, an important question to consider is what factors optimize learning in older individuals? If perceptual learning methods are used as an intervention then transfer of training to a wide array of untrained stimuli would be optimal. Studies have shown that increased performance from PL training is specific to the location in the visual field (Karni & Sagi, 1991; Fahle & Poggio, 1994; Schoups, Vogels & Orban, 1995), task (Fahle & Poggio, 1994; Levi, Polat & Hu, 1997), orientation (Karni & Sagi, 1991; Schoups, Vogels & Orban, 1995, Ahissar & Hochstein, 1996), as well as the eye of training (Karni & Sagi, 1991). Research has also shown that training with stimuli that do not contain external noise may be optimal and transfer to stimuli embedded in noise, possibly due to increased difficulty in extracting a stimulus template in noisy displays (Dosher & Lu, 2005). Training in an easy task has also shown to be less specific than training in a difficult task (Ahissar & Hochstein, 1997). Though, it has been argued that it may not be the difficulty of training but precision of the transfer task (tasks that require a high degree of precision during tests of transfer may show high specificity as compared to low-precision tasks; (Jeter, Dosher, Petrov, & Lu, 2009).
The goal of the present study was to examine factors that might optimize learning for older observers. The previous studies on perceptual learning and aging have either trained using stimuli that did not include external noise (Andersen et al., 2010; Ball & Sekuler, 1986; Bower, Watanabe, & Andersen, 2013; Richards et al., 2006), or that included external noise (Bower & Andersen, 2012). However, to our knowledge no study has examined whether training with external noise is a critical factor for optimal learning in older individuals. In addition, no previous study, including those with control groups performing the task in an easy supra-threshold condition, has examined the relationship between task difficulty and training in noise on learning and specificity. In the present study, we examined the effects of training difficulty and training in noise on learning in older individuals. In addition, we also assessed the importance of these factors for specificity of learning by exploring transfer to an untrained orientation as well as to higher noise levels.
Participants were assigned to one of four training groups: a group that was trained in an easy condition in the absence of external noise, a group that was trained in an easy condition that included external noise, a group that was trained in a difficult condition in the absence of external noise, and lastly a group that was trained in a difficult condition that included external noise. Before and after five days of training older individual’s performance in a fine orientation discrimination task at 5 levels of external noise was examined for a trained and an untrained standard. An additional issue examined was whether changes in retinal illuminance may be an underlying factor in perceptual learning in older individuals. Research has shown age-related declines in retinal illuminance (Weale, 1988), or a reduction in the amount of light reaching the retina. One possible way in which performance might improve, but be due to a peripheral (non-cortical) factor, is that observers dilate their pupils during training and thus increase the amount of light reaching the retina. To examine this issue, pupil diameter measurements were recorded using an eye-tracker and retinal illuminance values derived pre and post training. If performance improvements due to training are due to retinal illuminance then the change in performance due to training should be correlated with the change in retinal illuminance.
2. Methods
2.1 Subjects
Thirty-two older individuals from the surrounding community (16 male and 16 female) participated in the experiment. All observers were paid for their participation in the experiment, were naïve concerning the experimental purpose and had normal or corrected-to-normal visual acuity. All subjects were screened using an array of cognitive and perceptual tests. Demographic information of the participants is presented in Table 1. One-way analyses of variance were conducted and no significant differences were found on any measure prior to training (F(3, 31) <= 2.5, p > 0.05). Participants were also pre-screened for eye disease and neurological disorders.
Table 1.
Means and standard deviations of participant demographics and results from cognitive and perceptual tests.
| Variable | Group 1 | Group 2 | Group 3 | Group 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 74.00 | 7.69 | 72.50 | 5.68 | 70.38 | 3.20 | 69.50 | 3.89 |
| Education (years) | 17.50 | 2.78 | 16.25 | 4.71 | 15.25 | 3.88 | 15.75 | 2.60 |
| Log Contrast Sensitivity | 1.35 | 0.08 | 1.31 | 0.11 | 1.35 | 0.08 | 1.37 | 0.05 |
| LogMAR Visual Acuity | 0.09 | 0.08 | 0.08 | 0.10 | 0.07 | 0.06 | 0.08 | 0.08 |
| Digit Span Forward | 11.63 | 2.45 | 11.25 | 2.19 | 9.88 | 1.73 | 10.13 | 1.81 |
| Digit Span Backward | 8.00 | 1.41 | 7.63 | 1.19 | 6.25 | 1.67 | 6.38 | 1.92 |
| WAIS – Matrix Reasoning | 19.00 | 3.55 | 15.13 | 6.88 | 13.75 | 2.66 | 16.75 | 2.76 |
Note:
Contrast sensitivity measured using the Pelli Robson Test (Pelli, Robson & Wilkins, 1988).
2.2 Apparatus
Stimuli were presented on a 21″ CRT monitor (Viewsonic P225F) at a resolution of 1024×768 with a refresh rate of 100Hz (non-interlaced). The monitor was driven by a Dell Vostro 430 equipped with an Intel Core i5 750 processor using the Windows XP (Service Pack 3) operating system. The mean luminance value of the monitor was 53.82 cd/m2. An NVIDIA GeForce GTS 240 graphics card was used along with a Bits ++ system (Cambridge Research Systems). This allowed the system to achieve 14-bit grayscale (16,384 grayscale levels). Custom experimental software was written in MATLAB (The Mathworks, Inc., version 7.8.0.347); the Psychophysics Toolbox extensions were also utilized (Brainard, 1997; Pelli, 1997). The monitor was calibrated using a ColorCal2 colorimeter (Cambridge Research Systems). Gamma correction was performed through linearization of the color lookup table.
2.3 Stimuli and Procedures
The experiment consisted of 1.5 hours per day of testing/training over seven days. Participants were required to complete the study within three weeks of their first testing session. The monitor was viewed at a distance of 94 centimeters. Head position was stabilized with the use of an EyeLink 1000 Tower Mount (SR Research) and stimuli were viewed binocularly. Any corrective lenses or contacts normally worn by the participants were allowed during the experiment. All stimuli were viewed through a plano-convex glass collimation lens (45.7 cm diameter) with a 19% magnification factor to minimize accommodative focus. The size of the stimuli was corrected to account for this magnification factor. The experiment was run in a darkened room and the only light source in the room during the experiment was the monitor. Stimuli during the experiment were Gabor patches presented at 40% Michelson contrast, 1.5 cycles/deg visual angle, with 0.65 deg standard deviation of the Gaussian mask. The phase of the Gabor was randomized up to +/−180 degrees on each trial.
2.3.1 Task Practice
Before the beginning of testing on the first day all participants were given a 40 trial practice session to familiarize them with the task. These practice trials were presented without noise. Participants completed 20 trials using a standard that was 45 degrees clockwise off vertical and 20 trials using a standard that was 45 degree counter-clockwise off vertical. At the beginning of each trial participants were shown a fixation point in the center of the display that alternated from black to white every 400 milliseconds (ms) for 1600 ms (Betts, 2005). Participants were then presented the standard orientation for 100 ms. A second fixation point then alternated black and white every 250 ms for 1000 ms. Participants were then shown the target stimulus for 100 ms. During task familiarization the target was rotated either 25 degrees clockwise or 25 degrees counter-clockwise way from the standard orientation. The screen was then changed to a uniform image of mid-gray value of the display indicating that they should make their response. The participant’s task was to judge whether the target stimulus was rotated clockwise or counter-clockwise compared to the previously presented standard orientation. Responses were made using the left and right arrow keys on the keyboard. Audio feedback was provided on each trial indicating whether the participant was correct. Participants were then prompted to “Press any key to continue” to begin the next trial. During the familiarization task participants were instructed to get at least one trial incorrect to familiarize them with the auditory feedback provided on incorrect trials.
2.3.2 Testing
Testing of orientation discrimination thresholds occurred during the first and last day (day 7) of the experiment. Participants were tested at five external noise levels. The Gabors were embedded in additive Gaussian noise in four of the five testing blocks. Block one consisted of a no-noise condition with the standard deviation of the additive Gaussian noise set to zero, the standard deviation of the Gaussian distribution was then increased by 0.084375 in each successive block for a maximum standard deviation of the Gaussian distribution of 0.3375 in block 5.1 Standard orientations were either clockwise 25 degrees or counterclockwise 25 degrees off vertical. These standards were counterbalanced across subjects for testing order as well as for their trained orientation. These two standards were chosen based on previous research (Matthews, Liu, & Qian, 2001) that found that improvements in orientation sensitivity degrade approximately 40 degrees away from the trained orientation. These two specific standard orientations were chosen, as they are 50 degrees offset from one another. During days 1 and 7, pre-training orientation thresholds for the trained and untrained orientation standards were assessed at the five noise levels using QUEST (Watson & Pelli, 1983). QUEST was initialized with a criterion level of 0.75, β = 1.4,2 δ = 0.025, and γ = 0.5. Participants completed 50 trials for each block during testing. All stimuli were viewed through a circular annulus with a radius of 8 degrees visual angle that was placed against the surface of the monitor. The annulus was used to remove the use of edge cues that may be used in the orientation discrimination task. The background between trials and during inter-stimulus-intervals (ISI) consisted of the mid-gray value of the monitor (for no noise blocks) or additive Gaussian noise with the mean set at the mid-gray level of the monitor and a standard deviation that matched the additive Gaussian noise (for blocks with external noise). Trials progressed in the same fashion as described in the familiarization task, though the standard orientations were 25 degrees clockwise or 25 counterclockwise off vertical as previously described, and the orientation offset on each trial was determined by QUEST. The maximum allowable orientation offset was set at 25 degrees and the minimum was set to 0.1 degrees. Post-training thresholds for the trained and untrained orientations were measured on day 7 using the same procedure as that used on day 1. Pupil size was also measured on each trial during testing days using an Eyelink 1000 configured with a tower mount and was used to derive retinal illuminance values.
2.3.3 Training
Training occurred on days 2 through 6 using the same stimuli as that used in the testing phase with two modifications. Participants were randomly assigned to one of four training groups (group 1: easy training without noise; group 2: easy training with noise; group 3: difficult training without noise; or group 4: difficult training with noise). Prior to each training day, each participant completed 50 trials to assess any changes in threshold for the trained orientation. These thresholds were assessed at the noise level for which they would be trained. During training, all participants completed five blocks consisting of 150 trials per block. Participants were allowed to take a short break after each block. Group 1 (easy condition with no noise) completed all trials at a 153 degree orientation offset. Group 2 was trained in an easy condition (15 degree orientation offset) that also contained additive Gaussian noise (the noise level used in block three of the testing days; 0.16875 standard deviation of the Gaussian distribution). Group 3 was trained in a difficult no noise condition. For these participants the orientation offset was determined by each participant’s psychometric function. During each training day, the first block was set at their 80% correct orientation threshold and subsequent blocks increased in difficulty by 5% to a maximum difficulty in block five of 60% correct. Group 4 was trained at their 60–80% correct orientation thresholds. However, they also were trained in noise (the noise level used in block three of the testing days; 0.16875 standard deviation of the Gaussian distribution). Training for all subjects resulted in a total of 750 training trials per day, for a total of 3750 training trials over the course of the experiment.
3. Results
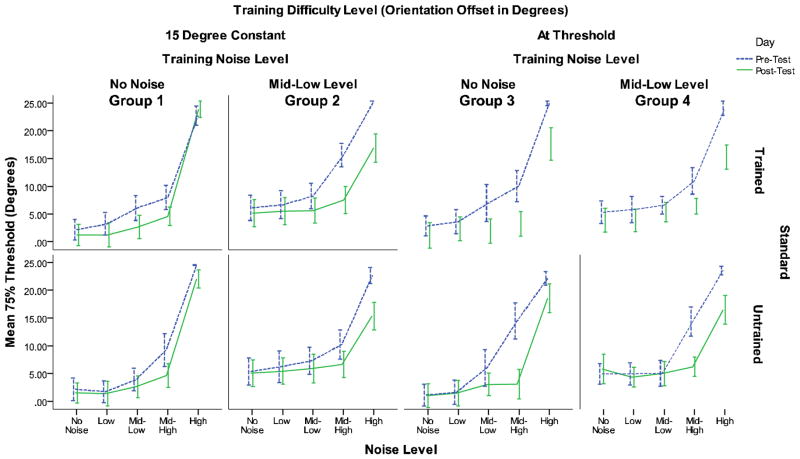
An analysis conducted on pre-training thresholds showed no significant differences between groups (F(3, 31) = 0.430, p = .733) indicating that all groups had similar performance levels prior to training. Thresholds from the testing days were analyzed using a linear mixed-effects regression using the lme4 package (Bates, Maechler, & Bolker, 2013) in R (R Foundation for Statistical Computing, 2013). These models have been used previously in research on aging and vision (Ball et al., 2013; Bennett et al., 2007; Salthouse, 2009). While all groups showed significant learning using a standard repeated-measures mixed analysis of variance, the use of mixed-effects models allows for a reduction of noise at the participant level by fitting a random intercept to each participant, and providing a better estimate of the fixed effects at the population level (Laird & Ware, 1982; Raudenbush & Bruk, 2002). For cases in which the predicted threshold from QUEST was greater than the maximum allowable orientation offset of 25 degrees, the threshold was rounded down to the maximum allowable orientation offset. The optimal model was selected by back-fitting all possible model parameters using log-likelihood ratio tests with a cutoff of p < 0.05. Random-effects were then forward fitted using the same criterion, and the fixed effects were again back-fitted after choosing the optimal random effects structure. P-values were calculated with Markov chain Monte Carlo sampling to estimate the posterior distributions of the fixed effects using pvals.fnc from the languageR package (Baayen, 2011). A second-degree polynomial for noise was also added to the model to account for nonlinearity of noise on thresholds particularly at higher levels of noise (see Figure 1). A standard variable coding scheme was used.4 The optimal random effects structure required random intercepts for subjects and random slopes for noise by subject and the second-order polynomial of noise by subject. To test whether the correlated random effects were a concern for the final model, an additional model was fit that allowed for correlated random effects. No significant changes in the parameters of interest were found so the simpler model was chosen.
Figure 1.
Pre-test and post-test training thresholds for the trained and untrained standard for the four training groups based on training difficulty and training noise level.
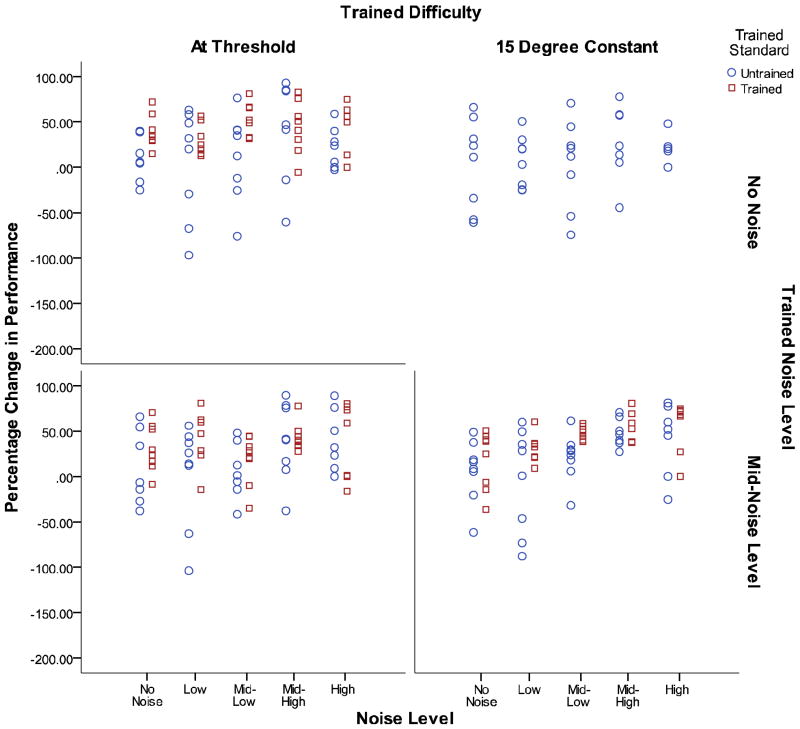
Parameter estimates and p-values for the full optimal model are presented in Table 2. While the raw data suggests specificity, the optimal model for the full analysis indicated that the inclusion of a parameter for the trained versus untrained standard did not improve fit. The optimal model included no parameters for orientation standard, which suggests that there was no significant degree of specificity for the trained orientation found in any training condition. This lack of specificity is somewhat surprising and will be discussed later in this analysis. A plot of the magnitude of learning from pre-test to post-test for the trained and untrained standards are displayed in Figure 3. Each point reflects the change in threshold from pre-test to post-test, and is indicated for each participant for their trained and untrained standards at each of the five noise levels. Magnitude of learning values greater than two standard deviations from the mean were trimmed. There was a significant effect of day (p < 0.001) indicating that all groups exhibited significant learning. Significant linear (p < 0.001) and second-degree polynomial (p < 0.001) effects of noise were found indicating that increased noise caused a significant increase in thresholds.
Table 2.
Parameter Estimates and p-values for the full mixed-effects regression
| Estimate | t | SE | p | |
|---|---|---|---|---|
| (Intercept) | 4.6272 | 0.3583 | 12.9161 | 0.0001*** |
| Day | −2.7471 | 0.4432 | 6.1990 | 0.0001*** |
| Noise | 3.6042 | 0.2164 | 16.6573 | 0.0001*** |
| Trained Difficulty | −0.1114 | 0.6809 | 0.1636 | 0.8592 |
| Trained Noise Level | −0.3302 | 0.7165 | 0.4609 | 0.6178 |
| Noise^2 | 1.6766 | 0.1294 | 12.9578 | 0.0001*** |
| Day x Noise | −1.5296 | 0.2011 | 7.6070 | 0.0001*** |
| Day x Trained Difficulty | 0.7973 | 0.5687 | 1.4019 | 0.1686 |
| Day x Trained Noise Level | 1.3930 | 0.8863 | 1.5716 | 0.1188 |
| Noise x Trained Noise Level | −0.8937 | 0.4327 | −2.0651 | 0.0366 |
| Day x Noise^2 | −0.1709 | 0.1699 | 1.0059 | 0.3142 |
| Trained Noise Level x Noise^2 | −0.2952 | 0.2588 | 1.1409 | 0.2604 |
| Day x Noise x Trained Noise Level | −1.2096 | 0.4022 | 3.0079 | 0.0024** |
| Day x Trained Difficulty x Trained Noise Level | −2.5281 | 1.1375 | 2.2226 | 0.0294* |
| Day x Trained Noise Level x Noise^2 | −1.0651 | 0.3399 | 3.1338 | 0.0024** |
Note:
p < .05
p < .01
p < .001; p-values are based on MCMC estimates
Figure 3.
The magnitude of learning from pre-test to post-test for the trained and untrained standards split by training group, changes in performance as plotted range from −200% to 100%.
A significant day by noise interaction (p < 0.001) was also found demonstrating that the degree of learning was greater in higher noise conditions. A significant day by noise by trained noise effect was also found (p = .002). Significant effects of day by noise (p < 0.001) and a noise by trained noise level (p = .037) were also found which are qualified by the three-way (day by noise by trained noise level) interaction. This result indicates that learning was greater at higher levels of external noise for groups trained with noise (Groups 2 and 4) compared to the groups that were not trained with noise (Groups 1 and 3). A significant day by trained difficulty by trained noise level effect was also found (p = .029), indicating that the degree of learning was greater for low levels of external noise for those in the difficult training condition that were trained in the absence of external noise (Group 3). However, learning was greatest in the highest levels of external noise for those trained in the easy condition in the presence of external noise (Group 2). A significant interaction of day by noise by trained noise level was found (p = .002). According to this result, learning was greater at higher levels of external noise for Groups 2 and 4 (individuals trained in the condition including external noise) as compared to Groups 1 and 3 (individuals trained in the absence of external noise). A day by noise^2 by trained noise level was also found (p = .002) demonstrating that the benefit of training in noise also altered the non- linear component.
Overall, training for Group 1 (easy difficulty condition in the absence of external noise) showed the lowest amount of learning. Training for Group 3 (difficult training in the absence of external noise) showed the greatest amount of learning in the no-noise to mid-level noise conditions. Learning for Group 4 (difficult training in the presence of external noise) showed little learning at lower levels of external noise with significant learning at the mid to highest levels of external noise. Lastly, training for Group 2 (easy condition in the presence of external noise) showed small amounts of learning in the absence of noise and at the second lowest noise level, but a higher degree of learning at the mid to highest levels of external noise. These results suggest that learning to filter external noise was not influenced by the difficulty of training. For optimal learning at low levels of external noise, a difficult task in the absence of external noise was most effective. However, surprisingly, if learning to filter external noise is of greater importance, then training difficulty is of little consequence unless conducted in the presence of external noise.
While the results demonstrate the potential benefits of training in noise and training difficulty, the results did not indicate specificity of learning, which is common to studies of perceptual learning. A close examination of the data suggests that this may be due to a greater influence of the highest noise condition on performance as compared to other noise levels. While learning occurred at all noise levels, the learning at the highest noise level was quite large. Prior to training, 30 participants reached the maximum possible orientation offset at the highest noise level on at least one of the tested standard orientations– a level suggesting that subjects had considerable difficulty with discriminating orientation at this level of noise. However, following training, 19 of these 30 participants continued to have a high degree of difficulty in discriminating orientation at this noise level. The remaining 11 individuals had a considerable decrease in thresholds suggesting a considerable improvement in performance. The following additional analyses were conducted to determine whether the highest noise level may be the primary factor in the previous results. Two additional models were fitted, one model that excluded the highest noise level, and a second model examining learning only at the highest noise level. Optimal models were selected using the same procedure as that used in the analysis of the full dataset. In addition, the p-values were also calculated using the same method.
The results of the linear mixed-effects regression, when excluding the highest level of noise, are presented in Table 3. A standard variable coding approach was used.5 We found a significant effect of day (p < .001) indicating significant learning across the training groups. Significant effects of noise (p <.001) and noise^2 (p <.001) were also found suggesting that noise caused a significant increase in thresholds and that the effect of noise also included a nonlinear component. In this model, no effect of trained noise level was found, indicating that when noise is not the limiting factor on performance that the inclusion of noise in training may not be necessary. A four-way interaction of day by noise by trained difficulty by standard was found (p =.028). Learning at lower levels of external noise transferred in the easy training condition to a higher degree between standards compared to individuals in the difficult training condition. However, learning at higher levels of external noise transferred more between standards in the difficult training group. All training conditions showed significant learning for the trained orientation, with a greater degree of learning at lower levels of noise in the difficult training condition. The interactions of day by noise (p <.001), day by noise^2 (p =.002) and noise^2 by standard (p <.022) were significant, though these interactions can only be interpreted at the average of the parameters that are not present in each particular interaction. The removal of the fifth noise level in the analysis suggests that the effect of training in external noise (Groups 2 and 4) did not significantly influence learning or specificity when external noise was not the primary limiting factor. Consistent with previous research, specificity of learning at low-levels of external noise showed a smaller degree of transfer in the difficult training conditions (Groups 3 and 4) than in the easy training conditions (Groups 1 and 2; see Ahissar & Hochstein, 1997). Task precision at the time of testing was equal for all training conditions so it is unlikely that precision influenced the results of the study (Jeter et al., 2009).
Table 3.
Parameter Estimates and p-values for the restricted mixed-effects regression
| Estimate | t | SE | p | |
|---|---|---|---|---|
| (Intercept) | 4.2850 | 0.3100 | 13.8240 | 0.0001*** |
| Day | −1.4093 | 0.3990 | −3.5323 | 0.0004*** |
| Noise | 1.5311 | 0.1889 | 8.1069 | 0.0001*** |
| Trained Difficulty | −0.0769 | 0.5654 | −0.1359 | 0.8998 |
| Noise^2 | 0.6854 | 0.1527 | 4.4875 | 0.0001*** |
| Day x Noise | −1.5932 | 0.2229 | −7.1465 | 0.0001*** |
| Day x Trained Difficulty | 0.5126 | 0.4985 | 1.0283 | 0.3048 |
| Noise x Trained Difficulty | −0.1144 | 0.3777 | −0.3028 | 0.7506 |
| Day x Standard | −0.6949 | 0.4985 | −1.3940 | 0.1692 |
| Noise x Standard | −0.1270 | 0.2229 | −0.5695 | 0.5764 |
| Trained Difficulty x Standard | 0.6916 | 0.4985 | 1.3875 | 0.1730 |
| Day x Noise^2 | −0.7573 | 0.2492 | −3.0384 | 0.0022* |
| Noise^2 x Standard | −0.3641 | 0.1557 | −2.3386 | 0.0218* |
| Day x Noise x Trained Difficulty | 0.7415 | 0.4459 | 1.6631 | 0.1050 |
| Day x Noise x Standard | 0.6627 | 0.4459 | 1.4862 | 0.1552 |
| Day x Trained Difficulty x Standard | −0.8370 | 0.9970 | −0.8396 | 0.3988 |
| Noise x Trained Difficulty x Standard | 0.8855 | 0.4459 | 1.9861 | 0.0500 |
| Day x Noise x Trained Difficulty x Standard | −1.9868 | 0.8917 | −2.2280 | 0.0282* |
Note:
p < .05
p < .01
p < .001; p-values are based on MCMC estimates
The results of the linear mixed-effects regression, for the highest level of external noise, are presented in Table 4. The optimal random effects structure only included subject random intercepts as noise and noise2 were dropped from the analysis (as this analysis only included a single noise level). A significant effect of day was found (p < .001) indicating significant learning for all groups. A significant effect of trained noise level was also found (p = .014). A significant day by trained noise level was also found (p =.004) indicating that learning at the highest noise level was greater for individuals trained in external noise (Groups 2 and 4). No significant effect of standard or trained difficulty was found. Participants in the groups that trained with external noise showed significantly greater learning for the highest noise level that transferred to the untrained orientation. This suggests that learning to filter external noise may not be specific to the trained orientation.
Table 4.
Parameter Estimates and p-values for the mixed-effects regression for the highest noise level
| Estimate | t | SE | p | |
|---|---|---|---|---|
| (Intercept) | 19.3347 | 0.9273 | 20.8503 | 0.0001*** |
| Day | −6.0209 | 0.8690 | −6.9283 | 0.0001*** |
| Trained Noise Level | −3.6841 | 1.8546 | −1.9864 | 0.0138* |
| Day x Trained Noise Level | −5.9975 | 1.7381 | −3.4506 | 0.0042** |
Note:
p < .05
p < .01
p < .001; p-values are based on MCMC estimates
Retinal Illuminance
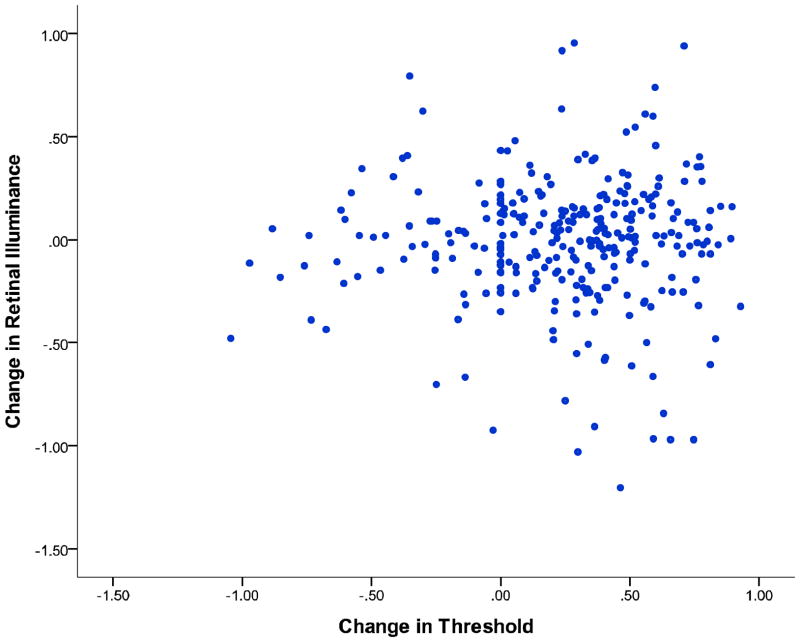
Another important issue is whether changes in retinal illuminance may be a factor in perceptual learning in the present study. To examine retinal illuminance we recorded pupil diameter measurements using an Eyelink 1000 eye-tracker. Pupil diameter measurements taken during presentation of the stimulus on each trial were combined across blocks for each subject and a mean pupil diameter was calculated. To examine the effects of retinal illuminance on training the percentage change in retinal illuminance (in Trolands) from pre-test to post-test was correlated with the percentage change in orientation discrimination thresholds (pre-test to post-test). Any block in which the eye tracker could not obtain a valid pupil measurement was dropped. This occurred in 2.8% of the total number of blocks. No significant relationship between percentage change in retinal illuminance and percentage change in performance in the orientation discrimination task was found, r(297) = 0.08, p = .889. Though a significant difference from pre-test retinal illuminance measured in Trolands (M = 3100.26, SD = 1306.82), and post-test retinal illuminance was found (M = 2975.09, SD = 1226.51), t(297) = 2.228, p = .027. The lack of a significant correlation between the change in retinal illuminance and task improvement, and a small decrease in retinal illuminance after training, as shown in Figure 2, suggests that a change in retinal illuminance is unlikely to be the source of improvement in the present study.
Figure 2.
A scatterplot of the correlation between percentage change in threshold and percentage change in retinal illuminance from pre-test to post-test.
4. Discussion
The present study provides evidence that training difficulty and the presence of external noise are important factors to improve PL in older individuals. Overall, orientation discrimination thresholds for older individuals improved as the result of five days of training. Training at higher levels of external noise showed transfer to a non-trained orientation standard suggesting that the improved performance was general. Training in the absence of external noise produced learning that showed transfer at all but the highest levels of external noise. However, training in the presence of external noise also produced learning at lower noise levels. The difficulty of training also influenced learning, with difficult training resulting in greater improvements in the absence of external noise. The amount of transfer was found to be smaller in the difficult training condition at lower noise levels. Individuals in the easy training condition that did not include external noise, and who had the same number of trials as participants in the other training groups, showed the smallest amount of learning. This suggests that the results of the present study are unlikely to be due to task familiarity in the other training conditions.
An important question is what aspect of visual processing in older individuals might change as a result of PL? Several different hypotheses have been proposed to account for PL. One hypothesis is that the improvements are due to reweighting of the inputs to visual cortex (Petrov, Dosher & Lu, 2005). A second and possibly related proposal is that improvements could be due to changes in internal noise in the system, external noise exclusion or changes in multiplicative noise (Lu and Dosher, 2009). The finding in the present study of transfer to an untrained orientation is consistent with the hypothesis that reweighting of inputs may account for the effects of PL training. Indeed, previous studies have found a high degree of orientation specificity in perceptual learning tasks (Fiorentini & Berardi, 1981; Karni et al., 1991; Matthews & Welch, 1997; Schoups, Vogels, & Orban, 1995; Vogels & Orban, 1985). However, studies have also found near perfect transfer to untrained orientations (Fine & Jacobs, 2000). Research has suggested that the degree of transfer may be high in easy low-precision tasks (Ahissar & Hochstein, 1997; Jeter et al., 2009). The present study examined learning for a difficult high-precision fine-orientation discrimination task, and the high degree of transfer obtained in the present study suggests that the processes for learning may be different for older and younger adults. However, many older participants showed a decrease in performance for the untrained orientation after training, as can be seen in Figure 3. An important issue for future research will be to examine these age-related differences in specificity and the possibility of different mechanisms of learning. Our results for older individuals do not provide evidence of changes in internal noise as no significant change in performance occurred in the no-noise conditions. We did find consistent evidence of changes in external noise exclusion, as indicated by improved performance after training for higher noise levels.
What possible mechanism might account for the findings observed in this study? A well-known finding in electrophysiological studies of aging and vision is that inhibition in neuronal systems decrease with age. Declines in inhibition have two possible outcomes in visual processing. One outcome is that inhibitory declines result in overall elevated random firing of neurons in vision cortex. Studies with senescent monkeys and cats show clear evidence of elevated random neuronal firing and are consistent with the hypothesis (Hua et al., 2006; Schmolesky et al., 2000). It is well documented in the literature (e.g., Marr & Heldreth, 1980) that inhibition is also important in filtering images and identifying luminance boundaries (including the luminance pattern in a Gabor patch) in noisy stimuli. Declines in inhibition may thus reduce the degree to which such boundaries are recovered when noise is present. The results of the present study, which found differential learning for older subjects based on difficulty and the presence or absence of noise in training, suggests that changes in inhibition that influence noise filtering may account for the present results. An important issue for future research will be to more directly test and evaluate this hypothesis.
In summary, the results of the present study suggest that PL can be used to improve orientation discrimination among older individuals. These results, considered together with previous research on training with texture (Andersen et al., 2010) and motion (Ball & Sekuler, 1986; Bower & Andersen, 2012; Bower et al., 2013), suggest that PL is a useful procedure for improving visual function among older populations, but that training may be more efficient when noise is present or when task difficulty is high.
Perceptual learning training improves orientation discrimination in older adults
Magnitude of learning is dependent on task difficulty and external noise
Results indicate optimization of training should consider these factors
Improved performance was not due to changes in retinal illuminance
This study is the first to show improved orientation discrimination in older adults
Acknowledgments
Support for this research was provided by NIH grants EY018334 and AG031941.
Footnotes
These noise levels were chosen based on a preliminary study examining older individuals’ tolerance to external noise in the task.
This β value is based on a preliminary study designed to find the optimal β value for the task.
The magnitude of the orientation offset was determined from pilot studies indicating that this value was well above threshold, and which found thresholds ranging from 2 to 3 deg in the absence of external noise for older participants.
Variable coding was as follows: day was effects coded with −0.5 for pre-test and 0.5 for post-test, noise was effects coded with −2, −1, 0, 1, 2 for each noise block respectively, orientation standards were effects coded with −0.5 for the untrained standard, and 0.5 for the trained standard; trained difficulty was effects coded with −0.5 for the difficult training condition and 0.5 for the easy training condition, and noise training condition was effects coded with −0.5 for the no noise condition and 0.5 for the training condition that included external noise.
All variables were coded in the same way, except for noise. Noise was again effects coded, this time the first four noise levels were coded as −1.5, −0.5, 0.5, 1.5 respectively, the fifth noise level was removed from this analysis. The optimal random effects for this model required intercepts for subjects and slopes for noise by subject and second-degree polynomial noise by subject were found to significantly improve the model.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahissar M, Hochstein S. Learning Pop-out Detection: Specificities to Stimulus Characteristics. Vision Research. 1996;36(21):3487–500. doi: 10.1016/0042-6989(96)00036-3. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387(6631):401–6. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Andersen GJ. Aging and vision: changes in function and performance from optics to perception. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(3):403–10. doi: 10.1002/wcs.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen GJ, Atchley P. Age-Related Differences in the Detection of Three-Dimensional Surfaces From Optic Flow. Psychology and Aging. 1995;10(4):650–8. doi: 10.1037//0882-7974.10.4.650. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Enriquez A. Aging and the Detection of Observer and Moving Object Collisions. 2006;21(1):74–85. doi: 10.1037/0882-7974.21.1.74. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Ni R, Bower JD, Watanabe T. Perceptual learning, aging, and improved visual performance in early stages of visual processing. Journal of Vision. 2010;10(13):4. doi: 10.1167/10.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley P, Andersen GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychology and aging. 1998;13(2):297–308. doi: 10.1037//0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Baayen RH. R package version 1.4. 2011. LanguageR: Data sets and functions with “Analyzing Linguistic Data: A practical introduction to statistics”. [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Willis SL. Effects of Cognitive Training Interventions With Older Adults. 2013;288(18):2271–81. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Improving Visual Perception in Older Observers. Journal of Gerontology. 1986;41(2):176–82. doi: 10.1093/geronj/41.2.176. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. R package version 0.999999-2. 2013. Lme4: Linear mixed-effects models using S4 classes. [Google Scholar]
- Bennett PJ, Sekuler R, Sekuler AB. The effects of aging on motion detection and direction identification. Vision research. 2007;47(6):799–809. doi: 10.1016/j.visres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision research. 2007;47(13):1769–80. doi: 10.1016/j.visres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging Reduces Center-Surround Antagonism in Visual Motion Processing. Neuron. 2005;45(3):361–6. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Bower JD, Andersen GJ. Aging, perceptual learning, and changes in efficiency of motion processing. Vision research. 2012;61:144–56. doi: 10.1016/j.visres.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JD, Watanabe T, Andersen GJ. Perceptual learning and aging: improved performance for low-contrast motion discrimination. Frontiers in psychology. 2013;4(66):1–6. doi: 10.3389/fpsyg.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crassini B, Brown B, Bowman K. Age-related changes in contrast sensitivity in central and peripheral retina. Perception. 1988;17(3):315–32. doi: 10.1068/p170315. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Annals of Neurology. 1993;33(3):248–57. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Investigative Ophthalmology & Visual Science. 1993;34(12):3278–3296. [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13988–93. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Mechanisms of perceptual learning. Vision Research. 1999;39(19):3197–221. doi: 10.1016/S0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning in clear displays optimizes perceptual expertise: learning the limiting process. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(14):5286–90. doi: 10.1073/pnas.0500492102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M, Edelman S. Long-term learning in vernier acuity: Effects of stimulus orientation, range and of feedback. Vision Research. 1993;33(3):397–412. doi: 10.1016/0042-6989(93)90094-D. [DOI] [PubMed] [Google Scholar]
- Fine I, Jacobs Ra. Perceptual learning for a pattern discrimination task. Vision Research. 2000;40(23):3209–30. doi: 10.1016/S0042-6989(00)00163-2. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Learning in grating waveform discrimination: Specificity for orientation and spatial frequency. Vision Research. 1981;21(7):1149–58. doi: 10.1016/0042-6989(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. Journal of the American Geriatrics Society. 1998;46(1):58–64. doi: 10.1111/j.1532-5415.1998.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Jeter PE, Dosher BA, Petrov A, Lu Z-L. Task precision at transfer determines specificity of perceptual learning. Journal of Vision. 2009;9(3) doi: 10.1167/9.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D, Science C. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(11):4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird N, Ware J. Random-Effects Models for Longitudinal Data. Biometrics. 1982;38(4):963–74. [PubMed] [Google Scholar]
- Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(13):6830–4. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. Mechanisms of perceptual learning. Learning & perception. 2009;1(1):19–36. doi: 10.1556/LP.1.2009.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N, Liu Z, Qian N. The effect of orientation learning on contrast sensitivity. Vision Research. 2001;41(4):463–71. doi: 10.1016/S0042-6989(00)00269-8. [DOI] [PubMed] [Google Scholar]
- Matthews N, Welch L. Velocity-dependent improvements in single-dot direction discrimination. Perception & psychophysics. 1997;59(1):60–72. doi: 10.3758/bf03206848. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, Sloane ME, Roenker DL, White MF, Overley ET. Visual Processing Impairment and Risk of Motor Vehicle Crash Among Older Adults. JAMA: The Journal of the American Medical Association. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Polat U. Making perceptual learning practical to improve visual functions. Vision Research. 2009;49(21):2566–73. doi: 10.1016/j.visres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- R Foundation for Statistical Computing. R: A language and environment for statistical computing. Vienna, Austria: 2013. [Google Scholar]
- Raudenbush S, Bruk A. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- Richards E, Bennett PJ, Sekuler AB. Age related differences in learning with the useful field of view. Vision research. 2006;46(25):4217–31. doi: 10.1016/j.visres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Roudaia E, Bennett PJ, Sekuler AB, Pilz KS. Spatiotemporal properties of apparent motion perception and aging. 2010;10:1–15. doi: 10.1167/10.14.5.Introduction. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30(4):507–14. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature neuroscience. 2000;3(4):384–90. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Schoups AA, Vogels R, Orban GA. Human perceptual learning in identifying the oblique orientation: retinotopy, orientation specificity and monocularity. The Journal of physiology. 1995;483:797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick GLGL, Silverman SESE. Visual sensitivity to motion. Neurology. 1991;41(9):1437. doi: 10.1212/wnl.41.9.1437. [DOI] [PubMed] [Google Scholar]
- Vogels R, Orban G. Vision research. 1985. The effect of practice on the oblique effect in line orientation judgments. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: A Bayesian adaptive psychometric method. Attention, Perception, & Psychophysics. 1983;33(2):113–20. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Weale RA. Senile changes in visual acuity. Transactions of the ophthalmological societies of the United Kingdom. 1975;95(1):36–8. [PubMed] [Google Scholar]
- Weale RA. Age and the transmittance of the human crystalline lens. The Journal of physiology. 1988;395(1):577–87. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]