Abstract
Background
In the Single Ventricle Reconstruction (SVR) trial, one-year transplant-free survival was better for the Norwood procedure with right ventricle-to-pulmonary artery shunt (RVPAS) compared with a modified Blalock-Taussig shunt (MBTS). At 3 years, we compared transplant-free survival, echocardiographic right ventricular ejection fraction (RVEF), and unplanned interventions in the treatment groups.
Methods and Results
Vital status and medical history were ascertained from annual medical record, death index and phone interviews. The cohort included 549 patients randomized and treated in the SVR trial. Transplant-free survival for the RVPAS vs. MBTS groups did not differ at 3 years (67% vs. 61%, P=.15) or with all available follow-up of 4.8±1.1 years (logrank P=.14). Pre-Fontan RVEF was lower in the RVPAS vs. MBTS group (41.7±5.1% vs. 44.7±6.0%, P=.007), and RVEF deteriorated in RVPAS (P=.004) but not MBTS subjects (P=.40) (pre-Fontan minus 14-month mean −3.25±8.24% vs. 0.99±8.80%, P=.009). The RVPAS vs. MBTS treatment effect had non-proportional hazards (P=.004); the hazard ratio (HR) favored the RVPAS before 5 months (HR=0.63; 95% CI 0.45–0.88), but the MBTS beyond one year (HR=2.22; 95% CI 1.07–4.62). By 3 years, RVPAS subjects had a higher incidence of catheter interventions (P<.001), with an increasing HR over time (P=0.005): <5 months HR= 1.14 (95% CI, 0.81–1.60); 5 months-1 year HR=1.94 (1.02–3.69), and >1 year HR=2.48 (1.28–4.80).
Conclusions
By 3 years, the Norwood procedure with RVPAS, compared with MBTS, was no longer associated with superior transplant-free survival. Moreover, RVPAS subjects had slightly worse RVEF and underwent more catheter interventions, with increasing hazard ratio over time.
Clinical Trial Registration Information
clinicaltrials.gov. Identifier: NCT00115934.
Keywords: congenital heart defect, congenital heart disease, cardiac surgery, single ventricle, Norwood procedure
Among congenital heart lesions, hypoplastic left heart syndrome (HLHS) and related single right ventricle (RV) anomalies are associated with the highest mortality and cost.1, 2 The first stage of palliation for patients with these defects is the Norwood procedure, in which pulmonary blood flow is provided by either a modified Blalock-Taussig shunt (MBTS) or a right ventricular-to-pulmonary artery shunt (RVPAS). The RVPAS has the theoretical advantage of reducing the aortic diastolic run-off and coronary arterial steal, but could have morbidities related to the conduit or right ventriculotomy.3, 4 In 2005, we began the Single Ventricle Reconstruction (SVR) Trial, a randomized trial that compared outcomes of the Norwood procedure with either a RVPAS or MBTS, in patients with HLHS and related anomalies.5 The SVR trial demonstrated significantly better transplant-free survival at one year post-randomization in the RVPAS, compared with the MBTS group (74% vs. 64%, respectively). The survival advantage with the RVPAS appeared to diminish, however, beyond the first year. Moreover, by age 12 months, the RVPAS group had undergone more unintended cardiovascular interventions. We hypothesized that trade-offs between transplant-free survival and adverse effects of the RVPAS vs. MBTS strategies might evolve over time.
To address the longer-term outlook after the Norwood procedure with RVPAS vs. MBTS in a multi-center study, we compared the composite endpoint of death or transplantation in SVR trial patients treated with the two surgical strategies, using all available follow-up, when the last enrolled patient had reached age 3 years. We also compared the shunt groups with respect to pre-Fontan echocardiographic right-ventricular ejection fraction (RVEF) and unplanned interventions to age 3 years.
METHODS
Subjects
The design of the SVR trial has previously been published.6 Our analytic cohort includes 549 subjects who underwent the Norwood procedure, after exclusion of one subject who withdrew in week 1. The data include a minimum of 3 years follow-up on all survivors. The Institutional Review Board of each participating center approved this study, and parents/guardians of enrolled subjects provided informed consent.
Data Obtained
We collected data annually, including vital status and surgical and catheter-based interventions, both by review of medical records and the death index, and through a phone interview with parents or guardians. Echocardiograms were reviewed in a core laboratory, and RVEF was calculated using the biplane pyramidal method.7 The primary causes of death after 1 year were adjudicated.8
Statistical Analysis
We analyzed the MBTS and RVPAS groups according to their treatment assignment (intention-to-treat) unless otherwise specified. We used the logrank test to determine whether the distributions of time to the earliest occurrence of death or transplantation using all available follow-up differed by assigned shunt (with a Wald test for comparison of 3-year event rates). Three subjects had their follow-up time censored at biventricular repair. Cox regression with a time-dependent treatment indicator was used to estimate how the size of the treatment difference varied by time. The time-dependent results are reported as <5 months, 5 months-1 year, and > 1 year. These time points were chosen a priori: 5 months was the average age for performance of the Stage II procedure, and one year was the primary trial endpoint, with later times capturing longer-term outcomes. The test of proportional hazards is calculated using SAS 9.3 and is a supremum test based on the pattern of cumulative martingale residuals or the score component process vs. follow-up time. We also used Cox regression with the time of the 14-month echocardiogram as time zero to examine the association between RVEF and late death or transplant.
To determine whether the treatment effect differed across pre-specified subgroups, a treatment group by subgroup interaction test from Cox regression was used for: birth weight: <2500 vs. ≥2500 g; pre-Norwood tricuspid regurgitation with proximal jet width <2.5 vs. ≥2.5 mm; use of deep hypothermic circulatory arrest vs. regional cerebral perfusion during the Norwood procedure; annual surgeon Norwood volume; and annual center single ventricle volume based on the patients screened for the trial.
Secondary outcomes, such as total number of catheterization interventions occurring by age 3 years, were compared by shunt type using Poisson regression and time to first catheterization intervention was estimated using the Kaplan-Meier method. Only the birth weight and annual surgeon Norwood volume subgroup factors were examined for the catheterization outcomes, and only birth weight was examined for the other morbidity outcomes.
We used logistic regression to determine additional pre- and intraoperative risk factors for transplant-free survival at age 3 years in 542 subjects (7 excluded due to loss to follow-up, withdrawal or biventricular repair prior to 3 years). Nonlinearly associated variables identified by generalized additive modeling were appropriately transformed prior to use in stepwise selection for the multivariable model. A one-sample t-test was used to assess whether mean within-person change in RVEF differed from zero. For all analyses, including tests of interaction, a p-value of 0.05 was considered significant. All analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC) and R version 2.14.1.
RESULTS
Among 342 subjects alive without cardiac transplantation at the time of this report, the mean follow-up was 4.8±1.1 years (median 5.0, interquartile range 4.0–5.7 years). Time to Fontan procedure using all available follow-up was similar in the two treatment groups (logrank P=0.90). By 3 years, 58% had undergone the Fontan procedure (62% in the MBTS group and 54% in the RVPAS group). Mean age at the Fontan procedure was 2.8±0.8 years (range 1.2–5.6 years).
Transplant-free survival
By 3 years after randomization, the transplant-free survival rate was 61% for the MBTS group and 67% for the RVPAS group (P=.15; Table 1); by 5 years, the rates were 60% and 64%, respectively, an absolute difference that was less than half of the 10% difference seen at 1 year.
Table 1.
Clinical Events from Norwood to Age 3 Years by Shunt Type
| Outcome | All (n=549) | MBTS* (n=275) | RVPAS† (n=274) | P-value‡ |
|---|---|---|---|---|
| Death or cardiac transplant | 197 | 107 | 90 | 0.15 |
| Death (prior to transplant) | 177 | 97 | 80 | |
| Cardiac transplant | 20 | 10 | 10 | |
| Death/transplant ≤1 year | 172 | 100 | 72 | |
| Death/transplant >1 to ≤3 years | 25 | 7 | 18 | |
| Incidence per 100 patient-years | ||||
| Cardiac surgeries | 164.3 | 167.9 | 161.0 | 0.37 |
| Catheter intervention | 43.1 | 30.2 | 54.7 | <0.001 |
| Interventional catheterizations | 31.2 | 23.9 | 37.7 | <0.001 |
| Complications | 289.7 | 296.1 | 284.0 | 0.23 |
| Number of Patients (%) | ||||
| Pacemaker placed | 12/549 (2.2%) | 5/275 (1.8%) | 7/274 (2.6%) | 0.58 |
| Thrombotic event | 77/549 (14.0%) | 39/275 (14.2%) | 38/274 (13.9%) | 1.000 |
| Stroke | 34/549 (6.2%) | 12/275 (4.4%) | 22/274 (8.0%) | 0.08 |
| Seizure | 64/549 (11.7%) | 29/275 (10.5%) | 35/274 (12.8%) | 0.43 |
| PLE§ | 7/549 (1.3%) | 2/275 (0.7%) | 5/274 (1.8%) | 0.29 |
| Cirrhosis | 0/549 (0.0%) | 0/275 (0.0%) | 0/274 (0.0%) | --- |
| Plastic bronchitis | 1/549 (0.2%) | 0/275 (0.0%) | 1/274 (0.4%) | 0.499 |
MBTS = modified Blalock-Taussig shunt
RVPAS = right-ventricular-to-pulmonary-artery shunt
P-values for incidence rate and proportion comparisons are based on Poisson regression and Fisher exact test, respectively. P-value for comparison of 3-year death/transplant is based on Wald test of the pointwise Kaplan-Meier event rate estimates at 3 years.
PLE = protein-losing enteropathy
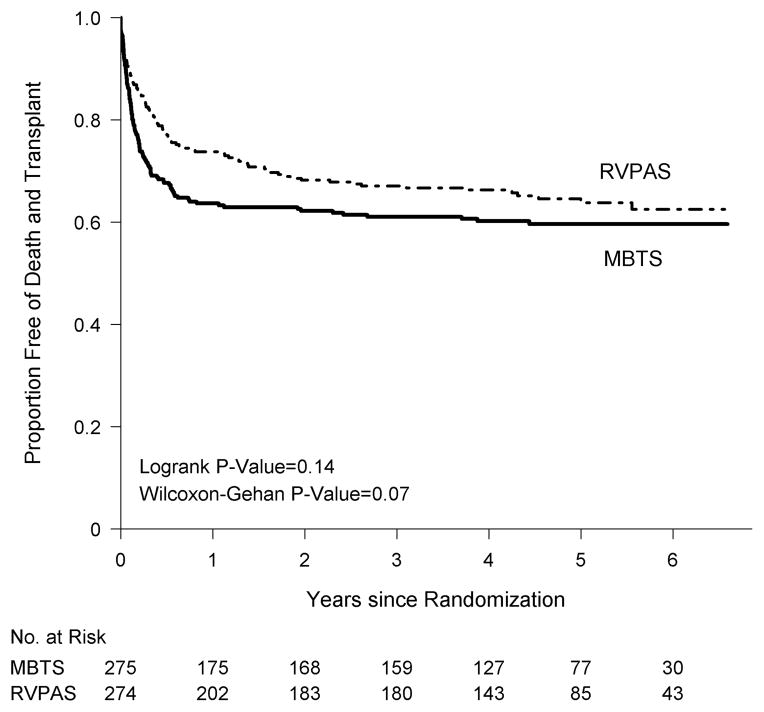
Using all available follow-up data, transplant-free survival did not differ by assigned shunt (logrank P=.14, Figure 1); death or transplant had occurred in 110 MBTS subjects (100 deaths, 10 transplants) and 97 RVPAS subjects (86 deaths, 11 transplants). After Year 1, there were 10 events (9 deaths, 1 transplant) in the MBTS group and 25 events (18 deaths, 7 transplants) in the RVPAS group. Altogether, in the MBTS vs. RVPAS groups, respectively, death or transplant occurred in 100 vs. 72 subjects by one year post randomization, in 4 vs. 15 subjects between 1 and 2 years, and in 6 vs. 10 subjects after two years. Neither all-cause mortality nor cardiac transplantation differed by shunt group.
Figure 1.
Comparison of the shunt types by intention-to-treat analysis in their freedom from the composite endpoint of death or cardiac transplant (i.e., transplant-free survival).
We found non-proportional hazards for the magnitude of the treatment effect on transplant-free survival (Proportional Hazards [PH] P=.004). Specifically, the RVPAS group had a lower hazard prior to age 5 months (the mean age for Stage II surgery), a similar hazard from 5 months to one year, and a higher hazard after one year compared to that of the MBTS group: RVPAS vs. MBTS hazard ratio (HR) at <5 months, HR=0.63 (95% Confidence Interval [CI] 0.45–0.88); 5 months-1 year, HR=0.94 (95% CI 0.44–2.01); >1 year, HR=2.22 (95% CI 1.07–4.62). Correspondingly, transplant-free survival among survivors to 1 year was worse in the RVPAS group (logrank P=.03, Figure 2), despite comparable Stage II surgery morbidity and growth, neurodevelopmental status, RVEF and tricuspid regurgitation grade at 14 months of subjects in the two treatment groups. Primary causes of death after 1 year did not differ between the shunt groups (Supplementary Appendix, Table 1).
Figure 2.
Kaplan-Meier curve of the shunt types by intention-to-treat analysis in freedom from the composite endpoint of death or cardiac transplant, conditional on transplant-free survival to one year.
Right ventricular function
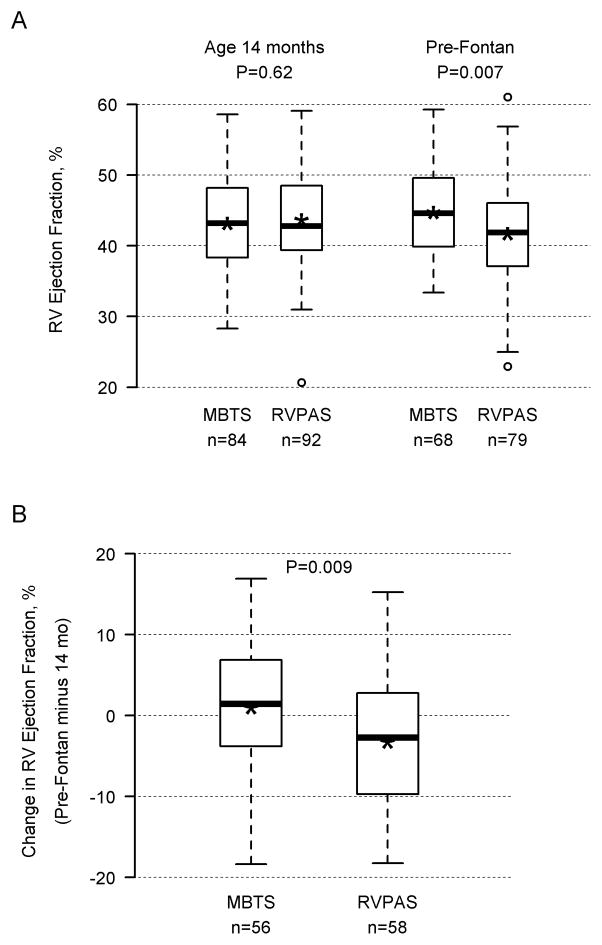
Patients who died or underwent transplant after the 14-month echocardiogram, but before Fontan surgery, had a lower mean RVEF at 14 months than transplant-free survivors (33.7±9.4%, n=12 vs. 43.2±7.3%, n=224). Moreover, lower RVEF at 14 months was associated with increased risk of later death/transplant (N=236; HR 1.92 per 5-unit decrease, 95% CI 1.45–2.50; P<0.001). There was no significant interaction between shunt group and RVEF, indicating that the association of RVEF with later death/transplant did not depend on shunt type. Pre-Fontan echocardiograms from which RVEF could be calculated were available in 258 patients (123 MBTS, 135 RVPAS) at mean age 2.7±0.8 years. Mean RVEF measured before the Fontan procedure was lower in the RVPAS vs. MBTS group (41.7±7.1% vs. 44.7±6.0%, respectively, P=.007). In paired analysis of 114 subjects with calculable RVEF on both 14-month and pre-Fontan echocardiograms, RVPAS subjects had a significant decline in RVEF over a period of 18.7±9.5 months (−3.25±8.24, P=0.004), while the MBTS subjects did not (0.99±8.80, P=0.40) (group difference in mean change, P=0.009 Figure 3). This decline is likely a conservative estimate, since pre-Fontan RVEF was not available for many subjects who died or were transplanted after one year.
Figure 3.
Panel A shows right ventricular ejection fraction, assessed by 2-dimensional echocardiography, at 14 months (age 14.1±1.0 months) and before Fontan surgery (age 2.7±0.8 years) according to shunt type; Panel B shows change in right ventricular ejection fraction (pre-Fontan minus 14 months) according to shunt type. There was a significant decline in the RVPAS group (P=0.004), but not in the MBTS group (R=0.40).
Subgroup Analyses of Transplant-Free Survival
In pre-specified subgroup analysis, transplant-free survival was better for infants with birth weight of at least 2500 g, those with Norwood surgery performed by a higher-volume surgeon, and infants without pre-Norwood tricuspid regurgitation. A significant interaction of shunt by subgroup was present only for annual Norwood surgeon volume; in the highest volume stratum, MBTS was beneficial, whereas in lower volume strata, the RVPAS was beneficial or no shunt difference could be detected. However, the highest-volume stratum included only 2 surgeons, so we could not distinguish between surgeon and volume effects.
Independent predictors for worse transplant-free survival at 3 years, evaluating only covariates measured prior to and during the Norwood procedure, included diagnoses other than aortic stenosis/mitral stenosis, obstructed PV drainage, a genetic syndrome, moderate-to-severe tricuspid regurgitation prior to the Norwood procedure, open sternum postoperatively on the day that the Norwood procedure was performed, use of ECMO during the Norwood procedure, annual surgeon Norwood volume of ≤5/year, and lower birth weight when birth weight was <2.5 kg (Supplementary Appendix, Tables 2 and 3). Of note, neither shunt assignment nor type of shunt in place at the end of the Norwood procedure independently predicted three-year transplant-free survival.
Cardiac catheterization
By 3 years, subjects in the RVPAS group, compared with the MBTS group, had a higher incidence of catheter interventions between the Norwood procedure and 3 years (P<.001; Table 1). Catheter intervention incidence rates and their variation with time and shunt type are summarized in Supplementary Appendix Tables 4–7.
The RVPAS group, compared with MBTS patients, had an earlier time to first catheter intervention of any type (P=.01), as well as to first stent placement (P=.03) and coiling of aortopulmonary (P<.001) and veno-venous collaterals (P=.01), but had similar time to first balloon angioplasty, including pulmonary artery angioplasty (Supplementary Figure 1). Non-proportional hazards existed for catheter intervention of any type (PH P=.005) and for aortopulmonary collateral (APC) coil insertion (PH P=.001). The hazard ratio for catheter intervention of any type increased over time (Table 2): <5 months RVPAS vs. MBTS HR=1.14 (95% CI, 0.81–1.60); 5 months-1 year HR=1.94 (95% CI 1.02–3.69), and >1 year HR=2.48 (95% CI 1.28–4.80). In contrast, the effect of shunt type on coil placement in APCs decreased with time: RVPAS vs. MBTS <5 months, 18 events vs. 0 events; 5 months-1 year, HR=3.76, and >1 year, HR=1.48. There were no significant interactions of shunt type and subgroup factors in the analysis of time to catheter procedures.
Table 2.
| Hazard Ratio (95% CI), RVPAS vs. MBTS
| ||||||
|---|---|---|---|---|---|---|
| Catheter Intervention (RVPAS vs. MBTS) | <5 Months | 5 Months-1 Yr | >1 Yr | Overall | Overall Wald P | Proportional Hazard testing p-value |
| All intervention types | 1.14 (0.81, 1.60) | 1.94 (1.02, 3.69) | 2.48 (1.28, 4.80) | 1.44 (1.10, 1.89) | 0.008 | 0.005 |
| Number of Events | 77 vs. 61 | 27 vs. 14 | 27 vs.13 | 131 vs. 88 | ||
| Balloon angioplasty | 0.89 (0.59, 1.35) | 1.59 (0.73, 3.44) | 1.47 (0.57, 3.80) | 1.07 (0.76, 1.50) | 0.70 | 0.10 |
| Number of Events | 45 vs. 44 | 18 vs. 10 | 11 vs. 7 | 74 vs. 61 | ||
| Stent | 1.65 (0.87, 3.11) | 5.58 (0.67, 46.31) | 1.48 (0.42, 5.23) | 1.81 (1.05, 3.12) | 0.03 | 0.09 |
| Number of Events | 26 vs. 15 | 6 vs.1 | 6 vs. 4 | 38 vs. 20 | ||
| Any type of coil | 8.49 (1.98, 36.46) | 2.97 (1.33, 6.61) | 1.60 (0.83, 3.09) | 2.62 (1.65, 4.16) | <0.001 | 0.01 |
| Number of Events | 19 vs. 2 | 24 vs. 8 | 22 vs. 15 | 65 vs. 25 | ||
| Aortopulmonary collateral coils | — | 3.76 (1.40, 10.07) | 1.48 (0.73, 3.03) | 3.06 (1.80, 5.21) | <0.001 | 0.001 |
|
| ||||||
| Number of Events | 18 vs. 0 | 19 vs. 5 | 18 vs.13 | 55 vs.18 | ||
RVPAS = right-ventricular-to-pulmonary-artery shunt;
MBTS = modified Blalock-Taussig shunt
Center was a significant predictor of time to first catheter intervention of any type and of the specific procedures of balloon angioplasty and APC coil placement (all P<.001), with a trend toward significance for stenting (P=.06). However, the effect of shunt assignment on the hazard of stent, coil, APC coil and any catheter intervention type remained significant after adjusting for center (Supplementary Appendix, Table 8). Furthermore, the higher rate of catheter interventions in the RVPAS group was not explained by a greater prevalence of early cyanosis in the RVPAS group, as indicated by use of supplemental oxygen, worsening cyanosis as an indication for the Stage II procedure, or lower aortic saturation or pulmonary-to-systemic artery blood flow ratios at cardiac catheterization before Stage II.
To determine whether lower transplant-free survival beyond one year in the RVPAS group could be related to the greater number of catheter interventions in this group, we compared the treatment groups with respect to transplant-free survival after adjusting for the number of interventions. However, the difference in transplant-free survival between the two shunt groups beyond one year (i.e., among one-year transplant-free survivors) remained significant (adjusted P=.03, HR= 2.2), and the effect of catheter interventions was not significant (P=0.85).
Pulmonary artery interventions at surgery
Patients in the RVPAS group, compared with the MBTS group, had a higher incidence of surgical pulmonary artery (PA) interventions that were not part of the surgeons’ usual Stage II or Fontan procedures (19 vs. 12 interventions per 100 patient-years, respectively, P=.007). When these surgical interventions were combined with catheter PA interventions, the incidence rates were 28 vs. 18 interventions per 100 patient-years, respectively (P<.001).
Relationship of pulmonary artery interventions to aortopulmonary collaterals
The incidence of surgical pulmonary artery (PA) interventions that were not part of the surgeon’s usual Stage II or Fontan procedure was approximately two-fold higher in patients who underwent coiling of APCs compared with those who did not: 26 vs. 14 interventions per patient-year, respectively, P<.001. Moreover, the incidence of any unplanned PA intervention (surgical or catheter) was three-fold higher among those who received APC coils, compared with those without APC coils (47 vs. 18 events per 100 patient-years, P<.001). However, the difference in PA intervention incidence rates by APC status did not depend on shunt type.
Aortic Arch Interventions
Because increased systemic afterload may cause ventricular dysfunction, we explored whether the RVPAS group, compared with those assigned to the MBTS group, had more interventions on the aortic arch. Between the postoperative Norwood period to age 3 years, the incidence of catheter and surgical interventions on the aortic arch in the MBTS vs. RVPAS groups was 8.7 vs. 11.6 events per 100 patient-years (p=0.12). The majority of these procedures occurred during the interstage period (27 of 46 procedures in the MBTS group and 39 of 69 interventions in the RVPAS group). Almost all aortic arch interventions after the Norwood procedure were performed at interventional catheterization, with aortic arch reoperation performed in only 13 patients (7 MBTS, 6 RVPAS).
Other clinical events
The shunt groups did not differ significantly with respect to any other clinical outcomes, including the incidences of cardiac surgeries, complications, thrombosis, stroke, seizure, protein-losing enteropathy, cirrhosis or plastic bronchitis (Table 1).
DISCUSSION
In the SVR trial, the Norwood procedure with RVPAS, compared with the MBTS, was associated with better transplant-free survival at 12 months after randomization.5 By 3 years, however, we found that transplant-free survival no longer differed significantly between the shunt groups. Indeed, the relative benefits of the RVPAS and MBTS varied with the time since the Norwood procedure. The hazard ratio for transplant-free survival favored the RVPAS strategy before 5 months, the average age at Stage II surgery, but it favored the MBTS group after one year. The disproportionate number of later events in the RVPAS group eliminated its early survival advantage. Furthermore, the RVPAS vs. MBTS hazard ratio for catheter intervention increased over time: beyond 1 year after randomization, children assigned to the RVPAS were more than twice as likely to have undergone a catheter intervention as those assigned to the MBTS group. These data are consistent with those of smaller single-center retrospective studies that demonstrated a greater rate of interventions in those whose Norwood procedure was performed with a RVPAS than with an MBTS.9, 10 Finally, patients whose Norwood procedure was performed with the RVPAS, compared with the MBTS, had a 1.5-fold higher incidence of PA surgical procedures not part of usual Stage II or Fontan procedures. Our findings highlight the adverse longer-term effects of the RVPAS.
Our finding of more late deaths or transplants in the RVPAS group is consistent with the hypothesis that a right ventriculotomy has longer-term adverse effects on systemic RV myocardial function. Immediately after the Norwood procedure and, to a lesser extent, before the Stage II procedure, RVEF was better in the RVPAS than MBTS group. By 14 months, the shunt groups had similar RVEF.5 In this follow-up analysis, diminished RV function at 14 months was a highly significant determinant of lower transplant-free survival. Moreover, between 14-month and pre-Fontan echocardiography, RVEF declined in the RVPAS group but not in the MBTS group. Because the majority of patients who died or underwent transplant did not undergo a pre-Fontan echocardiogram, and a disproportionately higher number of the late events occurred in the RVPAS group, the results are likely to underestimate the degree of decline in RV dysfunction in the RVPAS group over the second and third years of life. These data, in combination, suggest that late deterioration in RV function may explain our finding of worse transplant-free survival among RVPAS subjects who were alive without transplant at age one year.
An interesting finding in our study was the greater incidence of APC coiling among subjects in the RVPAS group. In patients with deficient antegrade flow through the native pulmonary arteries, APCs can improve oxygenation.11–13 However, they also have adverse effects, increasing the volume load in the vulnerable single ventricle and thereby producing cardiac dysfunction and atrioventricular valve regurgitation. Moreover, higher preoperative APC flow lengthens the duration of chest tube drainage and hospital stay at the Fontan procedure.14, 15 We found a highly significant relationship between APC coiling and interventions to correct PA distortion or stenosis, independent of shunt type. These data suggest that the higher prevalence of APCs requiring coiling in the RVPAS group may be related to greater disturbances in pulmonary artery flow produced by conduit placement. However, our study design did not allow us to analyze the fundamental pathophysiologic mechanisms for greater collateral growth, such as the biophysics of pulmonary blood flow or angiogenic cytokine production.16–19
This manuscript should be viewed in light of additional limitations. Subgroup sizes were small, limiting power to identify some associations. In testing the proportional hazards assumption for catheterization interventions, a small number of events occurred within each time period, so the estimated hazard ratio within each period had a large confidence interval. We could not assess regional RV wall motion and the impact of focal scarring/dyskinesis.20 Our analyses do not permit us to define patient factors associated with better outcomes of the RVPAS vs. MBTS, a topic addressed in an earlier publication.21 We did not collect information on diagnostic cardiac catheterizations (i.e., without interventions), other than those performed before the Stage II and Fontan procedures. We also did not collect information on the surgical techniques that were used to create or to repair the right ventriculotomy in RVPAS subjects and their potential impact on RVEF decline. It is possible that some methods for management of the proximal RVPAS could mitigate the observed mid-term decline in RVEF. Finally, our study cannot determine with certainty the reason for loss of survival advantage in the RVPAS group beyond one year. Although the loss of the survival advantage could be attributable to a consequence of the RVPAS, such as the right ventriculotomy, it is also possible that the RVPAS allowed some marginal candidates to survive longer in the short term compared with the MBTS, without improving mid-term transplant-free survival.
In summary, in contrast to findings in the first year after the Norwood procedure, transplant-free survival at 3 years did not differ in patients randomized to receive the RVPAS, compared with the MBTS. Moreover, the RVPAS group had slightly worse RVEF prior to the Fontan procedure and a higher rate of unplanned interventions. Follow-up of this cohort is underway to delineate the evolving natural history after the Norwood procedure using the RVPAS compared with the MBTS strategy.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by grants (HL068269, HL068270, HL068279, HL068281, HL068285, HL068288, HL068290, HL068292, and HL085057) from the National Heart, Lung, and Blood Institute (NHLBI). This work is solely the responsibility of the authors and do not necessarily represent the official views of NHLBI or NIH.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects--United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;562:25–29. [PubMed] [Google Scholar]
- 2.Dean PN, Hillman DG, McHugh KE, Gutgesell HP. Inpatient costs and charges for surgical treatment of hypoplastic left heart syndrome. Pediatr. 2011;1285:e1181–e1186. doi: 10.1542/peds.2010-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohye RG, Ludomirsky A, Devaney EJ, Bove EL. Comparison of right ventricle to pulmonary artery conduit and modified Blalock-Taussig shunt hemodynamics after the Norwood operation. Ann Thorac Surg. 2004;783:1090–1093. doi: 10.1016/S0003-4975(03)01386-9. [DOI] [PubMed] [Google Scholar]
- 4.Pizarro C, Mroczek T, Malec E, Norwood WI. Right ventricle to pulmonary artery conduit reduces interim mortality after stage 1 Norwood for hypoplastic left heart syndrome. Ann Thorac Surg. 2004;78:1959–1963. doi: 10.1016/j.athoracsur.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, Newburger JW, Pearson GD, Tabbutt S, Wernovsky G, Wruck LM, Atz AM, Colan SD, Jaggers J, McCrindle BW, Prakash A, Puchalski MD, Sleeper LA, Stylianou MP, Mahony L. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atallah-Yunes NH, Kavey RE, Bove EL, Smith FC, Kveselis DA, Byrum CJ, Gaum WE. Postoperative assessment of a modified surgical approach to repair of tetralogy of Fallot. Long-term follow-up. Circulation. 1996;94(9 Suppl):II22–II26. [PubMed] [Google Scholar]
- 8.Ohye RG, Schonbeck JV, Eghtesady P, Laussen PC, Pizarro C, Shrader P, Frank DU, Graham EM, Hill KD, Jacobs JP, Kanter KR, Kirsh JA, Lambert LM, Lewis AB, Ravishankar C, Tweddell JS, Williams IA, Pearson GD. Cause, timing, and location of death in the Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:907–914. doi: 10.1016/j.jtcvs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischbach J, Sinzobahamvya N, Haun, Schindler E, Zartner P, Schneider M, Hrasks V, Asfour B, Photiadis J. Interventions After Norwood Procedure: Comparison of Sano and Modified Blalock-Taussig Shunt. Pediatr Cardiol. 2013;34:112–118. doi: 10.1007/s00246-012-0396-3. [DOI] [PubMed] [Google Scholar]
- 10.Photiadis J, Sinzobahamvya N, Haun C, Schneider M, Zartner P, Schlinder E, Asfour B, Hraska V. Does the shunt type determine mid-term outcome after Norwood operation? Eur J Cardiothorac Surg. 2012;42:209–216. doi: 10.1093/ejcts/ezr299. [DOI] [PubMed] [Google Scholar]
- 11.Triedman JK, Bridges ND, Mayer JE, Jr, Lock JE. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993;22:207–215. doi: 10.1016/0735-1097(93)90836-p. [DOI] [PubMed] [Google Scholar]
- 12.McElhinney DB, Reddy VM, Tworetzky W, Petrossian E, Hanley FL, Moore P. Incidence and implications of systemic to pulmonary collaterals after bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2000;69:1222–1228. doi: 10.1016/s0003-4975(99)01088-7. [DOI] [PubMed] [Google Scholar]
- 13.Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion: quantification with MRI. Circ Cardiovasc Imaging. 2009;2:219–225. doi: 10.1161/CIRCIMAGING.108.834192. [DOI] [PubMed] [Google Scholar]
- 14.Glatz AC, Rome JJ, Small AJ, Gillespie MJ, Dori Y, Harris MA, Keller MS, Fogel MA, Whitehead KK. Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging. 2012;5:218–225. doi: 10.1161/CIRCIMAGING.111.966986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geva T. Quantification of systemic-to-pulmonary artery collateral flow: challenges and opportunities. Circ Cardiovasc Imaging. 2012;5:175–177. doi: 10.1161/CIRCIMAGING.111.972182. [DOI] [PubMed] [Google Scholar]
- 16.Heil M, Schaper W. Pathophysiology of collateral development. Coron Artery Dis. 2004;15:373–378. doi: 10.1097/00019501-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 18.Persson AB, Buschmann IR. Vascular growth in health and disease. Front Mol Neurosci. 2011;4:14. doi: 10.3389/fnmol.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori Y, Shoji M, Nakanishi T, Fujii T, Nakazawa M. Elevated vascular endothelial growth factor levels are associated with aortopulmonary collateral vessels in patients before and after the Fontan procedure. Am Heart J. 2007;153:987–994. doi: 10.1016/j.ahj.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Helbing WA, Bosch HG, Maliepaard C, Rebergen SA, van der Geest RJ, Hansen B, Ottenkamp J, Reiber JH, de Roos A. Comparison of echocardiographic methods with magnetic resonance imaging for assessment of right ventricular function in children. Am J Cardiol. 1995;76:589–594. doi: 10.1016/s0002-9149(99)80161-1. [DOI] [PubMed] [Google Scholar]
- 21.Tweddell JS, Sleeper LA, Ohye RG, Williams IA, Mahony L, Pizarro C, Pemberton VL, Frommelt PC, Bradley SM, Cnota JF, Hirsch J, Kirshbom PM, Li JS, Pike N, Puchalski M, Ravishankar C, Jacobs JP, Laussen PC, McCrindle BW. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–159. doi: 10.1016/j.jtcvs.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.