Abstract
Spinal muscular atrophy (SMA) is an early-onset motor neuron disease characterized by loss of spinal motor neurons which leads to skeletal muscle atrophy. Proximal SMA results from the loss or mutation of the survival motor neuron (SMN) gene. In humans, the SMN gene is duplicated to produce two nearly identical genes, SMN1 and SMN2. SMN1 is lost in SMA but SMN2 is retained; in fact, the number of SMN2 copies correlates with disease severity. The SMN2 inducer D156844 increases the survival and improves phenotype of SMNΔ7 SMA mice. Maternal diet also modifies the survival and phenotype of these mice. In this study, we show the effect of maternal diet on the protective effects of D156844 in SMNΔ7 SMA mice. SMA mice maintained on the PicoLab20 Mouse diet survived longer when treated with D156844; the effect of diet was additive to the effect of D156844 on these mice. Brain levels of D156844 were higher in neonatal mice maintained on the PicoLab20 diet than those on the Harlan-Teklad 22/5 diet. SMN protein levels in the spinal cord were modestly elevated in D156844-treated, PicoLab20-maintained SMA mice. These data show that maternal diet does influence the responsiveness of D156844 in neonatal SMNΔ7 SMA mice.
Keywords: motor neuron disease; 2,4-diaminoquinazoline; maternal diet; spinal muscular atrophy; preclinical drug trial; neonatal mouse
INTRODUCTION
Proximal spinal muscular atrophy (SMA) is an autosomal recessive degenerative disease that is one of the leading genetic causes of infant death in the world. SMA is characterized by selective loss of α motor neurons of the anterior horn of the spinal cord (Crawford and Pardo, 1996) which leads to atrophy of limb and trunk muscles. SMA results from the loss or mutation of the SMN (survival motor neuron) gene (Lefebvre et al., 1995). In humans, there are two SMN genes (SMN1 and SMN2) which arose from gene duplication. SMN1 and SMN2 differ by a single nucleotide (C→T) within an exon splice enhancer of exon 7 (Lorson et al., 1999; Monani et al., 1999). Most of the transcript from SMN2, therefore, lacks exon 7; the SMN1 transcript, on the other hand, contains exon 7.
Like most other animals aside from humans, mice carry only one SMN gene (mSmn = SMN1) (DiDonato et al., 1997; Viollet et al., 1997). Loss of mSmn results in embryonic lethality (Schrank et al., 1997). The introduction of SMN2 into mSmn null mice by transgenesis rescues the embryonic lethality phenotype (Hsieh-Li et al., 2000; Monani et al., 2000). Those mice with low SMN2 copy numbers (i.e. 2) develop a severe SMA phenotype and die at 6–8 days (Hsieh-Li et al., 2000; Monani et al., 2000). Introduction of 3 SMN2 copies into mSmn nullizygous mice results in a milder SMA phenotype (Michaud et al., 2010). Mice with higher copy numbers (i.e. 8) of SMN2, on the other hand, are normal when compared to nontransgenic littermates (Monani et al., 2000) demonstrating that the SMN2 gene product can correct the SMA phenotype.
Screens of cell lines have be instrumental in identifying many compounds that can increase the expression of SMN2 and the inclusion of exon 7 in SMN2 transcripts. In fact, high-throughput screening of compounds which induce SMN2 expression from identified indoprofen (Lunn et al., 2004) and a family of quinazolines (Jarecki et al., 2005) as potential therapeutic agents for SMA. These are the first compounds identified from screens and, in most cases, the hits identified from the high-throughput screens have subopitmal therapeutic properties, i.e. they are toxic, require high doses or are rapidly metabolized. In fact, a series of quinazoline derivatives have been designed so as to more potently induce SMN2 promoter activity, increase SMN protein levels, are orally bioavailable and more easily penetrate the blood-brain barrier and possess drug-like properties. One such compound, a piperidine 2,4-diaminoquinazoline known as D156844, is very potent (EC50=4 nM) at activating SMN2 promoter activity with a maximal response of 2.3-fold (Thurmond et al., 2008). This lead compound also increases SMN protein levels in SMA patient fibroblast cultures as well as the number of intranuclear, SMN-containing gems to levels observed in SMA carrier fibroblasts. Oral administration of D156844 increases SMN protein levels in the spinal cord and significantly increases the mean lifespan of SMNΔ7 SMA mice by ~21–30% when given prior to motor neuron loss (Butchbach et al., 2010b). D156844 binds to the mRNA decapping enzyme DcpS (Singh et al., 2008). Another C5-substituted 2,4-diaminoquinazoline RG3039 also improves phenotype and increases lifespan of SMA mouse models (Gogliotti et al., 2013; Van Meerbeke et al., 2013).
SMNΔ7 SMA mice from dams fed the PicoLab20 diet survive on average 21% longer than those SMA mice from dams fed the Harlan-Teklad 22/5 diet (Butchbach et al., 2010a). One noticeable difference between the PicoLab20 and Harlan-Teklad 22/5 diets is the fat content with the PicoLab20 diet having a higher dietary fat content (9%) than the Harlan-Teklad 22/5 diet (5%). Higher dietary fat content may help to improve the phenotype and survival of neonatal SMA mice. Elevated dietary fat has also been shown to improve the phenotype in amyotrophic lateral sclerosis (ALS) mouse models (Dupuis et al., 2004; Mattson et al., 2007). Narver et al. (Narver et al., 2008) show that environmental enrichment and enhanced nutritional support significantly ameliorates the protective effects of trichostatin A (TSA) on SMNΔ7 SMA mice. Based on these observations, we wanted to determine the effect of diet on the responsiveness of SMNΔ7 SMA mice to the C5-substituted 2,4-diaminoquinazoline D156844.
EXPERIMENTAL PROCEDURES
Drug Formulation
D156844 ([5-(1-(2-fluorobenzyl)piperidin-4-ylmethoxy]quinazoline-2,4-diamine dihydrochloride) was synthesized by deCODE chemistry as described previously (Thurmond et al., 2008). D156844 was dissolved in ddH2O at a concentration of 3 mg/mL.
Animals and Drug Administration
SMNΔ7 SMA mice (SMN2+/+;SMNΔ7+/+;mSmn−/−) were generated from male and female carrier mice (SMN2+/+;SMNΔ7+/+;mSmn+/−) (Le et al., 2005). Breeder mice were provided with ad libitum water and rodent chow; these mice received either the Harlan-Teklad 22/5 rodent diet (#8640; Teklad) or the PicoLab20 Mouse diet (#5058; Purina). Only SMA and carrier pups were used in these experiments. The litter size was not controlled by culling additional pups because there is no correlation between litter size and survival of SMNΔ7 SMA mice (Butchbach et al., 2007a). Furthermore, culling the litters put unneeded stress on the dam which would adversely affect the results of this study (M.E.R.B., personal observation). Carrier and SMA littermate mice were treated with either D156844 (3 mg/kg/d) or vehicle (ddH2O) via oral administration as described previously (Butchbach et al., 2007b). Treatment began at postnatal day 4 (PND04) and continued for the lifetime of each SMA mouse. The treatment groups were not stratified based on sex because there is no significant difference in lifespan between male and female SMNΔ7 SMA mice (Butchbach et al., 2007a) and there are no sex-related differences in the responsiveness of SMNΔ7 SMA mice to D156844 (Butchbach et al., 2010b) or to the diets used in this study (Butchbach et al., 2010a). All experiments were conducted in accordance with the protocols described in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Detection of Drug Levels
Neonatal mice were treated orally with 3 mg/kg/d D156844 or vehicle for 5 days beginning at PND04. Forebrains were rapidly dissected from these mice 60 minutes after the final dosing and D156844 levels were measured by LC-MS/MS as described previously (Butchbach et al., 2010b).
Immunoblot
Immunoblot was used to measure protein expression in spinal cord extracts from SMNΔ7 SMA mice treated with D156844 or vehicle for 5 days starting at PND04. Spinal cords were rapidly dissected from euthanized PND08 pups at 1 hour after final dosing, frozen in liquid nitrogen and stored at −80°C until use. Immunoblots were completed as described in (Butchbach et al., 2010b) except that 10 μg spinal cord samples were loaded onto 12% polyacrylamide gels containing 0.1% SDS using the miniProtean system (BioRad). The resultant blots were probed with monoclonal antibodies directed against SMN (clone 8; BD Biosciences, 1:2000) or β-actin (AC-15; Sigma-Aldrich, 1:10000).
SMN Enzyme-linked Immunosorbent Assay (ELISA)
SMNΔ7 SMA mice were treated with 3 mg/kg/d D156844 or vehicle for 5 days beginning at PND04. SMN protein levels in spinal cord extracts was measured using the (human) SMN Enzyme Immunometric Assay from Assay Designs as described previously (Nguyen thi Man et al., 2008) except that 40 μg of spinal cord extract were used for each sample.
Statistical Analysis
Data are expressed as means ± standard errors. Kaplan-Meier curves were generated from the survival and onset of body mass loss data and tested using the Mantel-Cox log rank test. All statistical analyses were performed with SPSS v.20.0.
RESULTS
Effect of D156844 on the Survival and Disease Progression of SMNΔ7 SMA Mice Maintained on the PicoLab20 Diet
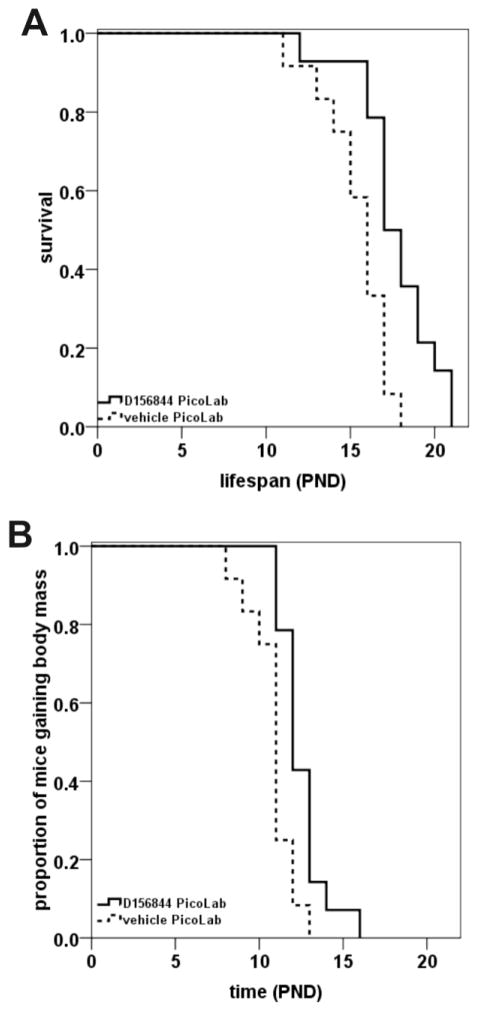
When maintained on a Harlan-Teklad 22/5 rodent diet, D156844-treated SMNΔ7 SMA live, on average, 21% longer than vehicle-treated SMNΔ7 SMA mice (Butchbach et al., 2010b). Treatment of SMNΔ7 SMA mice maintained on the PicoLab20 diet with 3 mg/kg/d D156844 (n=14) resulted in a 15% improvement in survival relative to vehicle-treated SMNΔ7 SMA mice (n=12) maintained on the same diet (Figure 1A; 17.7 ± 0.6 d vs. 15.4 ± 0.6 d; χ2=8.654, p=0.003). If we compare the average lifespan of PicoLab20-maintained, D156844-treated SMNΔ7 SMA mice to that of Harlan-Teklad 22/5-maintained, vehicle-treated SMNΔ7 SMA mice, then the diet/drug combination increased survival by 26.4% (17.7 ± 0.6 d vs. 14.0 ± 0.4 d; χ2=20.914, p<0.001). It is valid to compare the treated mice in this study (fed on the PicoLab20 diet) with those from the previously published study (Butchbach et al., 2010b) where they were maintained on the Harlan-Teklad 22/5 diet because the experiments in the PicoLab20 study (this study) were completed with the same mouse colony and the same housing conditions as the Harlan-Teklad 22/5 study (Butchbach et al., 2010b). The environmental conditions between the PicoLab20 and Harlan-Teklad 22/5 studies were, therefore, nearly identical.
Figure 1.
Oral administration of D156844 improved the survival of and delayed the onset of body mass loss in SMNΔ7 SMA mice maintained on the PicoLab20 diet. (A) Kaplan-Meier survival plot for SMNΔ7 SMA mice maintained on the PicoLab20 diet receiving either vehicle (dashed line; n=12) or 3 mg/kg/d D156844 (solid line; n=14) beginning at PND04. (B) Kaplan-Meier onset of body mass loss plot for SMNΔ7 SMA mice maintained on the PicoLab20 diet receiving either vehicle (dashed line; n=12) or 3 mg/kg/d D156844 (solid line; n=14) beginning at PND04.
As the SMNΔ7 SMA mice develop, they gain body mass until around PND11 after which their mass plateaus or decreases with age as motor neuron degeneration progresses (Butchbach et al., 2007a; Le et al., 2005). The onset of body mass loss can be used as an indicator of the end-stage of disease in these mice. The onset of loss of body mass was delayed by 15% in D156844-treated SMNΔ7 SMA mice maintained on the PicoLab20 diet relative to vehicle- treated SMNΔ7 SMA mice maintained on the same diet (Figure 1B; 12.5 ± 0.4 d vs. 10.8 ± 0.4 d; χ2=8.318, p=0.004). In SMNΔ7 SMA mice maintained on the Harlan-Teklad 22/5 diet, D156844 delays the onset of body mass loss modestly by 8% (Butchbach et al., 2010b). PicoLab20-maintained, D156844-treated SMNΔ7 SMA mice was delayed by 13% when compared to Harlan-Teklad 22/5-maintained, vehicle-treated SMNΔ7 SMA mice (12.5 ± 0.4 d vs. 11.1 ± 0.3 d; χ2=7.100, p=0.008).
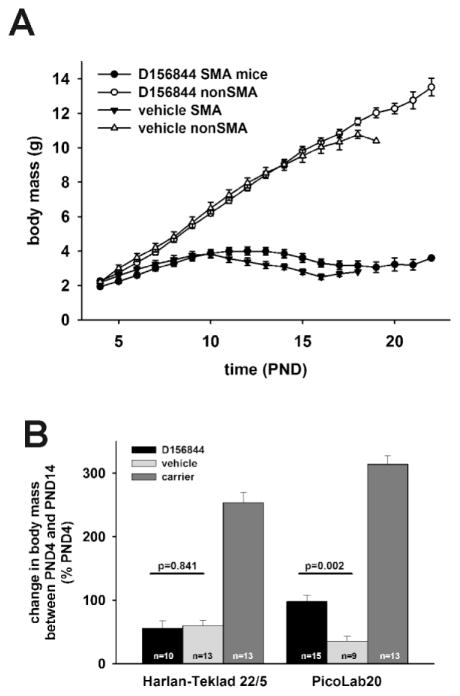
The body mass curve of SMNΔ7 SMA maintained on the PicoLab20 diet and treated with D156844 (solid circles in Figure 2A) had a similar shape to that for SMNΔ7 SMA mice maintained on the PicoLab20 diet and treated with vehicle (solid triangles in Figure 2A). The body masses for D156844-treated SMNΔ7 SMA mice were higher than vehicle-treated SMNΔ7 SMA mice between PND12 and PND16 but the differences were not statistically significant. Another way to compare the effect of D156844 on body mass is to measure the magnitude of change in body mass between PND14 and PND04—the onset of treatment. SMNΔ7 SMA mice maintained on the PicoLab20 diet and treated with D156844 exhibited a nearly 100% increase in body mass at PND14 relative to PND04 while SMNΔ7 SMA mice maintained on the PicoLab20 diet and treated with vehicle showed only a 35% increase in body mass at PND14 relative to PND04 (p=0.002; Figure 2B). On the other hand, there was no significant difference between the change in the body mass of Harlan-Teklad 22/5-maintained, D156844-treated SMNΔ7 SMA mice and the change in the body mass of SMNΔ7 SMA mice maintained on the Harlan-Teklad 22/5 diet and treated with vehicle (p=0.841).
Figure 2.
The effect of maternal diet on changes in body mass in response to D156844 treatment. A) Body mass curves of D156844- or vehicle-treated SMA and non-SMA mice maintained on the PicoLab20 diet. D) Body mass of SMA mice at PND14 maintained on either Harlan-Teklad 22/5 or PicoLab20 diets and treated with either D156844 or vehicle.
Effect of Diet on D156844 Levels in the CNS
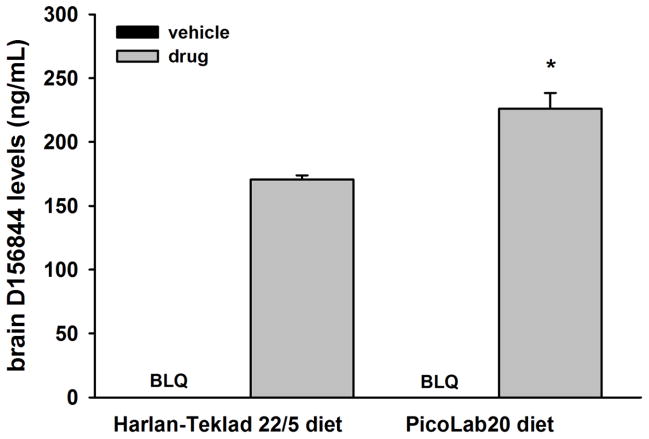
What is the basis for this differential response? To begin to address this question, we determined the effect of diet on CNS levels of D156844. Neonatal mice from dams maintained on either the Harlan-Teklad 22/5 diet or the PicoLab20 diet were treated with either D156844 (3 mg/kg/d) or vehicle for 5 days after which the brains were dissected and analyzed for D156844 levels by LC-MS/MS. D156844 levels were 32% higher in PicoLab20-fed neonatal mice when compared to Harlan-Teklad 22/5-fed neonatal mice (Figure 3; p=0.013). Diet may affect D156844 responsiveness in part by increasing CNS levels of the drug.
Figure 3.
The effect of maternal diet on brain D156844 levels in neonatal mice. Neonatal mice maintained on either the Harlan-Teklad 22/5 or PicoLab20 diets were treated with D156844 (3 mg/kg/d) or vehicle for 5 days beginning at postnatal 04 (PND04). Brain samples were analyzed for D156844 levels by liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). BLQ=below limit of quantitation (1 ng/mL). *p=0.013 when comparing drug-treated mice of different diets.
Effect of Diet on SMN Protein Levels in the Spinal Cord
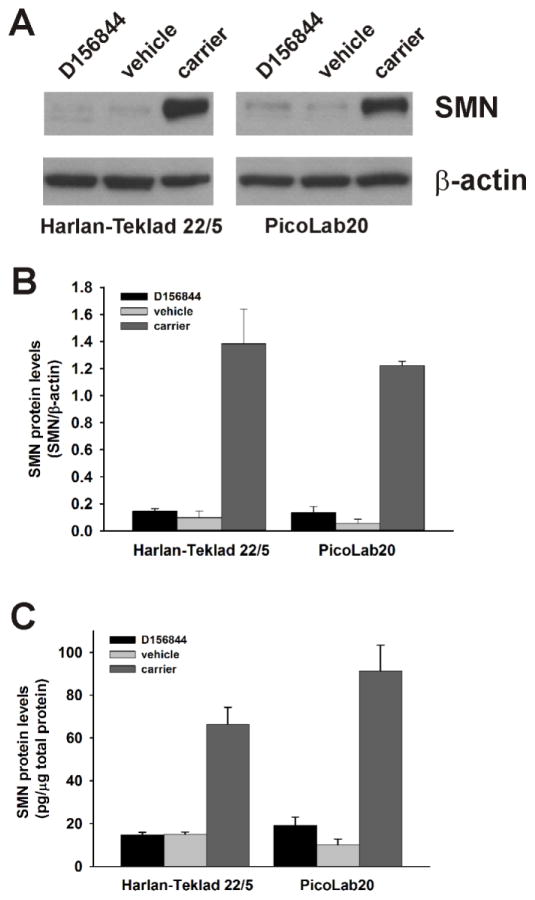
We have previously shown that D156844 increases SMN protein levels in SMNΔ7 SMA mouse spinal cord extracts (Butchbach et al., 2010b). Even though the PicoLab20 diet on its own does not affect SMN protein expression (Butchbach et al., 2010a), we examined the effect of maternal diet on D156844-mediated induction of SMN protein levels in spinal cord extracts from SMNΔ7 SMA mice. D156844 had a modest effect on spinal cord SMN protein levels in Harlan-Teklad 22/5- and PicoLab20-maintained SMNΔ7 SMA mice (n=3/group; Figure 4A). Relative quantitation of SMN protein levels—normalized to β-actin protein—levels showed a small but statistically insignificant increase in D156844-treated SMNΔ7 SMA mouse spinal cord extracts (Figure 4B). The lack of a statistically significant change in SMN protein expression could be partially due to the high interindividual variability amongst the samples. Interestingly, Van Meerbeke et al. (Van Meerbeke et al., 2013) recently showed a similar lack of significant change in SMN protein levels in SMNΔ7 SMA mice treated with the structurally related compound RG3039.
Figure 4.
The effect of maternal diet on the expression of SMN protein levels in the spinal cord of D156844-treated SMNΔ7 SMA mice. A) Representation SMN and β-actin immunoblots of spinal cords from D156844- or vehicle-treated SMNΔ7 SMA mice maintained on either the Harlan-Teklad 22/5 or PicoLab20 diets. C) Quantitation of SMN protein levels by ELISA in spinal cord extracts from D156844- or vehicle-treated SMNΔ7 SMA mice maintained on either the Harlan-Teklad 22/5 or PicoLab20 diets (n=5/group).
To quantitate the effect of diet on D156844 induction of SMN protein expression, we took advantage of a recently developed sandwich ELISA for detecting human SMN protein (Nguyen thi Man et al., 2008). SMNΔ7 SMA mice maintained on either the Harlan-Teklad 22/5 or PicoLab20 diets were treated for 5 days with either 3 mg/kg/d D156844 or vehicle (n=5/group). SMN protein levels were then quantified in spinal cord extracts from these treated mice. In SMNΔ7 SMA mice maintained on the PicoLab20 diet, D156844 increased SMN protein levels by 91% (19.2 ± 3.8 pg SMN/μg total protein vs. 10.1 ± 2.8 pg SMN/μg total protein; Figure 4C). The differences in SMN protein levels, however, were not statistically significant and the SMN protein levels were still small when compared to age-matched carrier mice (91.4 ± 11.9 pg SMN/μg protein). Contrary to previous observations (Butchbach et al., 2010b), SMN protein levels were unchanged in SMNΔ7 SMA mice maintained on the Harlan-Teklad 22/5 diet and treated with D156844 (14.8 ± 1.4 pg SMN/μg protein vs. 15.0 ± 1.1 pg SMN/μg protein). This discrepancy could be partially accounted for by the use of different techniques used to measure SMN protein levels (immunoblot vs. ELISA) in these studies and/or by the interindividual variability within the treatment groups.
DISCUSSION
In this study, we show that maternal diet has an effect on the responsiveness of neonatal SMA mice to the C5-substituted 2,4-diaminoquinazoline D156844. SMNΔ7 SMA mice maintained on the PicoLab20 Mouse diet survive 26% longer upon treatment with D156844 when compared to vehicle-treated, Harlan-Teklad 22/5-maintained SMNΔ7 SMA mice. For comparison, treatment of SMNΔ7 SMA mice maintained on the Harlan-Teklad 22/5 diet with D156844 increases the average lifespan by 21% (Butchbach et al., 2010b). Diet also augments the effect of D156844 on the onset of the end-stage of disease in these mice—as measured by the onset to the loss of body mass (Butchbach et al., 2007a).
The effect of diet on drug responsiveness in SMA mice is not unique to D156844. Treatment of SMNΔ7 SMA mice with the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) increases the median lifespan by about 20% (Avila et al., 2007). Environmental enrichment and enhanced nutritional support significantly ameliorates the protective effects of TSA on SMNΔ7 SMA mice (Narver et al., 2008). This study does not distinguish the effects of environmental enrichment and enhanced nutritional support on the responsiveness of these mice to TSA. Supplementation of neonatal SMNΔ7 SMA mice with a lipid-enriched formula does not affect the survival of these mice (Narver et al., 2008; Rose Jr et al., 2009) suggesting the environmental and dietary enhancements in the Narver et al. study increase the responsiveness to TSA. Dietary factors can, in a general sense, improve the protective effects of therapeutic agents having different mechanisms of action, i.e. inhibition of HDAC activity in the case of TSA and inhibition of the mRNA decapping enzyme DcpS (Singh et al., 2008) in the case of D156844.
One of the main differences between the PicoLab20 Mouse diet and the Harlan-Teklad 22/5 diet is the fat content (9.0% vs. 5.2% according to the product inserts for these diets provided by their manufacturers) although there are other differences between these diets. Increasing dietary fat content significantly improves the motor neuron disease phenotypes in mouse models for SMA (Butchbach et al., 2010a) and ALS (Dupuis et al., 2004; Mattson et al., 2007). Diet can have a profound effect on the pharmacokinetics and bioavailability of drugs especially on orally delivered drugs (Ruggiero et al., 2012; Welling, 1977). Central nervous system (CNS) tissues from neonatal mice maintained on the PicoLab20 diet have higher levels of D156844 than tissues from Harlan-Teklad 22/5-maintained mice. These data suggest that a higher fat diet improves the bioavailability of D156844 in mice but further studies are warranted to determine the importance of dietary fat content on drug responsiveness in SMA. It is equally possible that there may be another factor in the PicoLab20 diet which accounts for its ameliorative effects on SMNΔ7 SMA mice. Future studies wherein a single factor, i.e. dietary fat content, would be altered in the diets of SMNΔ7 SMA mice would help understand the mechanisms by which the PicoLab20 diet exerts its effect on survival and drug responsiveness of these mice.
In this study, we use survival and onset of body mass loss as primary indices of therapeutic efficacy in the SMNΔ7 SMA mouse. Based on power analysis, these two indices are the most powerful indicators of drug responsiveness in these mice (Butchbach et al., 2007a). The SMNΔ7 SMA mice also exhibit measurable changes in motor behavior (Butchbach et al., 2007a; El-Khodor et al., 2008) and respiratory function (El-Khodor et al., 2012) but a large cohort would be needed in order to observe statistically significant changes in response to treatment (Butchbach et al., 2007a). D156844 (Butchbach et al., 2010b) and the related DcpS inhibitor RG3039 (Van Meerbeke et al., 2013) do improve motor function in SMNΔ7 SMA mice.
SMA is a disease that primarily affects motor neurons. SMNΔ7 SMA mice display hypoglycemia (Butchbach et al., 2010a) which could result from metabolic abnormalities and possibly pancreatic dysfunction (Bowerman et al., 2012). In SMA mouse models, congenital heart defects and arrhythmias have been observed (Bevan et al., 2010; Biondi et al., 2012; Heier et al., 2010; Shababi et al., 2012; Shababi et al., 2010). These abnormalities in heart function are suggestive of autonomic dysfunction—in addition to motor dysfunction—in SMA mouse models. Studies have shown that the cardiac abnormalities can be ameliorated by SMN replacement via adeno-associated virus 9 (AAV9)-based gene delivery (Bevan et al., 2010; Shababi et al., 2012), TSA (Heier et al., 2010) or exercise (Biondi et al., 2012). Future studies will determine the effects of DcpS inhibitor-induced changes in SMN expression on pancreatic as well as cardiac dysfunction observed in SMA mice.
One unexpected observation was the minimal effect of D156844 on SMN protein levels in the spinal cords of PicoLab20-fed SMNΔ7 SMA mice. D156844 treatment of Harlan-Teklad 22/5-fed SMNΔ7 SMA mice results in a reproducible but non-statistically significant increase in SMN protein levels in the spinal cord (Butchbach et al., 2010b). The chemically related C5-substituted 2,4-diaminoquinazoline RG3039 does not result in a detectable increase in SMN protein levels in the spinal cords of treated SMNΔ7 SMA mice even though this compound significantly improved the phenotype and survival of these mice (Van Meerbeke et al., 2013). D156844 and RG3039 may increase SMN protein expression to an extent undetectable by immunoblot or ELISA or may selectively increase SMN protein levels in motor neurons, which account for a small proportion of cells in the spinal cord. Even though changes in SMN expression are either not statistically significant or undetectable in the central nervous system of SMNΔ7 SMA mice, DcpS inhibitors like D156844 and RG3039 have a marked effect on the phenotype and survival of severe SMA mice. These observations underscore the importance of using survival and onset of body mass loss as primary indices of therapeutic efficacy in preclinical drug trials for severe SMA. Small changes in SMN protein expression may provide a stronger therapeutic benefit in SMA patients with a milder disease severity, i.e. SMA type III. It would, therefore, be interesting to determine the effects of DcpS inhibitors on the phenotype of milder SMA mouse models like the SMN(A2G) mild SMA mouse (Monani et al., 2003) or the so-called Taiwanese SMA mouse model (4 copies of SMN2 on a mSmn(Δ7)−/− background; (Hsieh-Li et al., 2000)).
In summary, we show that maternal diet can have a significant effect on the responsiveness of neonatal SMNΔ7 SMA mice to the C5-substituted 2,4-diaminoquinazoline derivative D156844. The slightly higher fat diet increases CNS levels of D156844 in neonatal mice suggesting an important role of dietary fat in the bioavailability of this drug. The interaction between diet and D165844 may act to further increase SMN protein levels in the spinal cord of treated mice. This study also highlights the importance of maternal diet in the design of preclinical drug/therapeutics trials using neonatal mice.
HIGHLIGHTS.
treatment of SMNΔ7 SMA mice maintained on the PicoLab20 Mouse diet with D156844 delays onset of the end-stage of disease and improves survival
neonatal mice maintained on the PicoLab20 diet have elevated levels of D156844 in the CNS
SMN protein levels are elevated in SMNΔ7 SMA mice treated with D156844 and maintained on the PicoLab20 diet
Acknowledgments
We would like to thank Gisli Bragason, Bergros Gudmundsdottir and Margrét Thorsteindóttir from deCODE genetics for completing the drug level measurements. The study was supported in part by funds from Families of SMA (MERB and MEG) and the NINDS (R01NS38650; AHMB). MERB was also supported by the Nemours Foundation and the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM103464). The SMN EIA kits were generously provided by Assay Designs through their Kits for Charity program. Families of SMA financially supported and directed the identification and generation of the quinazoline series of compounds, including D156844.
ABBREVIATIONS
- ELISA
enzyme-linked immunosorbent assay
- LC-MS/MS
liquid chromatography-mass spectrometry/mass spectrometry
- PND
postnatal day
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
Footnotes
AUTHORS CONFLICTS OF INTEREST
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avila AM, Burnett BG, Taye AA, Gabanella F, Knight MA, Hartenstein P, Cizman Z, DiProspero NA, Pellizzoni L, Fischbeck KH, Sumner CJ. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J Clin Invest. 2007;117:659–671. doi: 10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan AK, Hutchinson KR, Foust KD, Braun L, McGovern VL, Schmelzer L, Ward JG, Petruska JC, Lucchesi PA, Burghes AHM, Kaspar BK. Early heart failure in the SMNΔ7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum Mol Genet. 2010;19:3895–3905. doi: 10.1093/hmg/ddq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi O, Lopes P, Desseille C, Branchu J, Chali F, Ben Salah A, Pariset C, Chanoine C, Charbonnier F. Physical exercise reduces cardiac defects in type 2 spinal muscular atrophy-like mice. J Physiol. 2012;590:5907–5925. doi: 10.1113/jphysiol.2012.238196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman M, Swoboda KJ, Michalski JP, Wang GS, Reeks C, Beauvais A, Murphy K, Woulfe J, Screaton RA, Scott FW, Kothary R. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol. 2012;72:256–268. doi: 10.1002/ana.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Edwards JD, Burghes AHM. Abnormal motor phenotype in the SMNΔ7 mouse model of spinal muscular atrophy. Neurobiol Dis. 2007a;27:207–219. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Edwards JD, Schussler KR, Burghes AHM. A novel method for oral delivery of compounds to the neonatal SMNΔ7 model of spinal muscular atrophy. J Neurosci Methods. 2007b;161:285–290. doi: 10.1016/j.jneumeth.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Rose FF, Jr, Rhoades S, Marston J, McCrone JT, Sinnott R, Lorson CL. Effect of diet on the survival and phenotype of a mouse model for spinal muscular atrophy. Biochem Biophys Res Commun. 2010a;391:835–840. doi: 10.1016/j.bbrc.2009.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Singh J, Thorsteindóttir M, Saieva L, Slominski E, Thurmond J, Andrésson T, Zhang J, Edwards JD, Simard LR, Pellizzoni L, Jarecki J, Burghes AHM, Gurney ME. Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy. Hum Mol Genet. 2010b;19:454–467. doi: 10.1093/hmg/ddp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis. 1996;3:97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- DiDonato CJ, Chen XN, Noya D, Korenberg JR, Nadeau JH, Simard LR. Cloning, characterization and copy number of the murine survival motor neuron gene: homolog of the spinal muscular atrophy-determining gene. Genome Res. 1997;7:339–352. doi: 10.1101/gr.7.4.339. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Oudart H, René F, Gonzalez de Aquilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci U S A. 2004;101:11159–11164. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khodor BF, Cirillo K, Beltran JA, Mushlin R, Winberg ML, Charney R, Chomicova O, Marino T, Ramboz S. Prediction of death in the SMND7 mouse model of spinal muscular atrophy: insight into disease stage and progression. J Neurosci Methods. 2012;209:259–268. doi: 10.1016/j.jneumeth.2012.06.020. [DOI] [PubMed] [Google Scholar]
- El-Khodor BF, Edgar N, Chen A, Winberg ML, Joyce C, Brunner D, Suárez-Fariñas M, Heyes MP. Identification of a battery of tests for drug candidate evaluation in the SMNΔ7 neonate model of spinal muscular atrophy. Exp Neurol. 2008;212:29–43. doi: 10.1016/j.expneurol.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Gogliotti RG, Cardona H, Singh J, Bail S, Emery C, Kuntz N, Jorgensen M, Durens M, Xia B, Barlow C, Heier C, Plasterer HL, Jacques V, Kiledjian M, Jarecki J, Rusche J, DiDonato CJ. The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models. Hum Mol Genet. 2013;22:4084–4101. doi: 10.1093/hmg/ddt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier CR, Satta R, Lutz C, DiDonato CJ. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Hum Mol Genet. 2010;19:3906–3918. doi: 10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh-Li HM, Chang JG, Jong YJ, Wu MH, Wang NM, Tsai CH, Li H. A mouse model for spinal muscular atrophy. Nat Genet. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- Jarecki J, Chen X, Bernardino A, Coovert DD, Whitney M, Burghes AHM, Stack J, Pollok B. Diverse small-molecule modulators of SMN expression found by high-throughput compound screening: early leads towards a therapeutic for spinal muscular atrophy. Hum Mol Genet. 2005;14:2003–2018. doi: 10.1093/hmg/ddi205. [DOI] [PubMed] [Google Scholar]
- Le TT, Pham LT, Butchbach MER, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AHM. SMNΔ7, the major product of the centromeric survival motor neuron gene (SMN2), extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing an is responsible for spinal muscular atrophy. Proc Natl Acad Sci U S A. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn MR, Root DE, Martino AM, Flaherty SP, Kelley BP, Coovert DD, Burghes AHM, Nguyen thi Man, Morris GE, Zhou J, Androphy EJ, Sumner CJ, Stockwell BR. Indoprofen upregulates the survival motor neuron protein through a cyclooxygenase-independent mechanism. Chem Biol. 2004;11:1489–1493. doi: 10.1016/j.chembiol.2004.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cutler RG, Camandola S. Energy intake and amyotrophic lateral sclerosis. Neuromol Med. 2007;9:17–20. doi: 10.1385/nmm:9:1:17. [DOI] [PubMed] [Google Scholar]
- Michaud M, Arnoux T, Bielli S, Durand E, Rotrou Y, Jablonka S, Robert F, Giraudon-Paoli M, Riessland M, Mattei MG, Andriambeloson E, Wirth B, Sendtner M, Gallego J, Pruss RM, Bordet T. Neuromuscular defects and breathing disorders in a new mouse model of spinal muscular atrophy. Neurobiol Dis. 2010;38:125–135. doi: 10.1016/j.nbd.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Monani UR, Lorson CL, Parsons DW, Prior TW, Androphy EJ, Burghes AHM, McPherson JD. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- Monani UR, Pastore MT, Gavrilina TO, Jablonka S, Le TT, Andreassi C, DiCocco JM, Lorson C, Androphy EJ, Sendtner M, Podell M, Burghes AHM. A transgene carrying an A2G missense mutation in the SMN gene modulates phenotypic severity in mice with severe (type I) spinal muscular atrophy. J Cell Biol. 2003;160:41–52. doi: 10.1083/jcb.200208079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monani UR, Sendtner M, Coovert DD, Parsons DW, Andreassi C, Le TT, Jablonka S, Schrank B, Rossoll W, Prior TW, Morris GE, Burghes AHM. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn−/− mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- Narver HL, Kong L, Burnett BG, Choe DK, Bosch-Marcé M, Taye AA, Eckhaus MA, Sumner CJ. Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition. Ann Neurol. 2008;64:465–470. doi: 10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyenthi Man, Humphrey E, Lam LT, Fuller JR, Lynch TA, Sewry CA, Goodwin PR, MacKenzie AE, Morris GE. A two-site ELISA can quantify upregulation of SMN protein by drugs for spinal muscular atrophy. Neurology. 2008;71:1757–1763. doi: 10.1212/01.wnl.0000313038.34337.b1. [DOI] [PubMed] [Google Scholar]
- Rose FF, Jr, Mattis VB, Rindt H, Lorson CL. Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Hum Mol Genet. 2009;18:997–1005. doi: 10.1093/hmg/ddn426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero A, Cefalo MG, Coccia P, Mastrangelo S, Maurizi P, Riccardi R. The role of diet on the clinical pharmacology of oral antineoplastic agents. Eur J Clin Pharmacol. 2012;68:115–122. doi: 10.1007/s00228-011-1102-8. [DOI] [PubMed] [Google Scholar]
- Schrank B, Götz R, Gunnersen JM, Ure JM, Toyka KV, Smith AG, Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci U S A. 1997;94:9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shababi M, Habibi J, Ma L, Glascock JJ, Sowers JR, Lorson CL. Partial restoration of cardio-vascular defects in a rescued severe model of spinal muscular atrophy. J Mol Cell Cardiol. 2012;52:1074–1082. doi: 10.1016/j.yjmcc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shababi M, Habibi J, Yang HT, Vale SM, Sewell WA, Lorson CL. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum Mol Genet. 2010;19:4059–4071. doi: 10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]
- Singh J, Salcius M, Liu SW, Staker BL, Mishra R, Thurmond J, Michaud G, Mattoon DR, Printen J, Christensen J, Bjornsson JM, Pollok BA, Kiledjian M, Stewart L, Jarecki J, Gurney ME. DcpS as a therapeutic target for spinal muscular atrophy. ACS Chem Biol. 2008;3:711–722. doi: 10.1021/cb800120t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond J, Butchbach MER, Palomo M, Pease B, Rao M, Bedell L, Keyvan M, Pai G, Mishra R, Haraldsson M, Andresson T, Bragason G, Thosteinsdottir M, Bjornsson JM, Coovert DD, Burghes AHM, Gurney ME, Singh J. Synthesis and biological evaluation of novel 2,4-diaminoquinazoline derivatives as SMN2 promoter activators for the potential treatment of spinal muscular atrophy. J Med Chem. 2008;51:449–469. doi: 10.1021/jm061475p. [DOI] [PubMed] [Google Scholar]
- Van Meerbeke JP, Gibbs RM, Plasterer HL, Miao W, Feng Z, Lin MY, Rucki AA, Wee CD, Xia B, Sharma S, Jacques V, Li DK, Pellizzoni L, Rusche JR, Ko CP, Sumner CJ. The DcpS inhibitor RG3039 improves motor function in SMA mice. Hum Mol Genet. 2013;22:4074–4083. doi: 10.1093/hmg/ddt257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet L, Bertrandy S, Beuno Brunialti AL, Lefebvre S, Burlet P, Clermont O, Cruaud C, Guénet JL, Munnich A, Melki J. cDNA isolation, expression and chromosomal localization of the mouse survival motor neuron gene (Smn) Genomics. 1997;40:185–188. doi: 10.1006/geno.1996.4551. [DOI] [PubMed] [Google Scholar]
- Welling PG. Influence of food and diet on gastrointestinal drug absorption: a review. J Pharmacokinet Biopharm. 1977;5:291–334. doi: 10.1007/BF01061694. [DOI] [PubMed] [Google Scholar]