Abstract
Children who are burned > 40% total body surface area lose significant quantities of both bone and muscle mass due to acute bone resorption, inflammation and endogenous glucocorticoid production, which result in negative nitrogen balance. Because administration of the bisphosphonate pamidronate within 10d of the burn injury completely prevents the bone loss we asked whether muscle protein balance was altered by the preservation of bone. We reviewed the results from 17 burned pediatric subjects previously enrolled in a double-blind randomized controlled study of pamidronate in the prevention of post-burn bone loss and who were concurrently evaluated for muscle protein synthesis and breakdown by stable isotope infusion studies during the acute hospitalization. We found a significantly lower fractional protein synthesis rate (FSR) in the pamidronate group and a correspondingly lower rate of appearance of the amino acid tracer in venous blood suggesting lower muscle protein turnover. Moreover, net protein balance(synthesis minus breakdown) was positive in the subjects receiving pamidronate and negative in those receiving placebo. Muscle fiber diameter was significantly greater in the pamidronate subjects and leg strength at 9 months post-burn was not different between subjects who received pamidronate and normal physically fit age-matched children studied in our lab. Leg strength in burned subjects who served as controls tended to be weaker, although not quite significantly so. If substantiated by a larger study, these results suggest that bone may have a paracrine mechanism to preserve muscle and this finding may have implications for the treatment of sarcopenia in the elderly.
Keywords: Corticosteroids < CELL/TISSUE SIGNALING - Endocrine Pathways, Sarcopenia < SKELETAL MUSCLE, Bone-muscle interactions < SYSTEMS BIOLOGY, BONE INTERACTORS, Antiresorptives < THERAPEUTICS, EXERCISE
INTRODUCTION
Burn injury in children is associated with resorptive bone loss (1) likely due to the acute systemic inflammatory response (2) and the stress-associated production of endogenous glucocorticoids (2,3). We have previously shown that a single dose of the bisphosphonate pamidronate within the first ten days of burn injury followed by a second dose a week later completely blocks the resorptive bone loss (4) and that the effect is maintained for at least two years post-burn (5).
Muscle wasting also occurs post-burn along with negative nitrogen balance and this effect may be attributable to either the endogenous glucocorticoid production or the systemic inflammatory response (2,3).
Because the mechanism of bisphosphonate action involves interference with cholesterol biosynthesis in the osteoclast (6), it is possible that these drugs could act to interfere with steroid production during the stress response and attenuate muscle breakdown.
The aim of our study was to review data previously obtained from patients enrolled in the completed randomized controlled double blinded trial of pamidronate who were concurrently enrolled in stable isotope infusion studies of muscle protein synthesis and breakdown to determine whether the parameters of muscle protein kinetics, synthesis, breakdown and net balance between synthesis and breakdown, differed between subjects receiving pamidronate and those receiving placebo.
METHODS
We reviewed the database of burned children who enrolled in the randomized controlled double-blinded study of pamidronate and placebo between 2000 and 2004 (4) who were simultaneously enrolled in infusion studies of stable isotopes in order to evaluate muscle protein synthesis, breakdown, and net balance in the first 30-60d post-burn. The infusion protocol is well-established (7,8) and consisted of an 8 hr primed (2µmol/kg) continuous (0.08 µmol/kg/min intravenous infusion of L[-ring-2H5] phenylalanine with an additional infusion of unlabelled amino acids during the last 3h. Arterial and venous blood samples were drawn at baseline, 2h, and every 15 min between hours 4–5 and 7–8. Vastus lateralis muscle biopsies were obtained at 2,5, and 8h. Leg blood flow was measured by the indocyanine green dilution technique (7) between hours 3–4 and 6–7. Muscle protein fractional synthesis rate (FSR) was determined by the direct incorporation method. FSR 1 is the synthesis rate during the basal period (incorporation of tracer into the muscle in the period between the first two muscle biopsies. FSR 2 is the synthesis rate during the infusion of unlabeled amino acids (incorporation of tracer into the muscle in the period between the second and third muscle biopsies). Changes in muscle phenylalanine enrichment over time was divided by plasma enrichment and expressed as %/hr. A two-compartment model was used in addition to calculate muscle protein synthesis and breakdown rates. Because phenylalanine is neither synthesized nor oxidized in the muscle, its rate of appearance (Ra) in venous blood is taken as the rate of muscle protein breakdown provided there are no changes in intracellular enrichment. The net balance (NB) of phenylalanine is calculated from the arterio-venous difference in phenylalanine multiplied by the leg blood flow (7) and represents the difference between muscle protein synthesis and breakdown as calculated from the initial 5 hr infusion period.
Additional muscle biopsy tissue was frozen and later thawed in warm saline, embedded in paraffin, sectioned, and examined by light microscopy for determination of muscle fiber diameter).
In addition, a functional cognate of these studies, muscle strength, was estimated by leg strength as peak torque corrected for body weight at 9 m post-burn utilizing isokinetic dynamometry (9) Briefly, assessment of isokinetic strength was made on the patient’s dominant leg extensors at an angular velocity of 150°/second using the Biodex System 3 dynamometer (Biodex Medical System, Shirley NY). All patients performed a warm-up session of three sub-maximal repetitions without load. Following this warm-up, the patient was asked to perform 10 maximal full leg extensions and full leg flexions. The Biodex system calculated and provided the peak torque (PKT) measurement corrected for gravitational movements of the lower leg and the lever arm. The highest measurement of the 10 repetitions was selected.
Finally, this PKT was corrected for each subject’s body weight.
Urine for determination of free cortisol excretion expressed as micrograms (µg)/24h was analyzed by previously published methods (2,3).
Statistical analyses were carried out by use of one-way ANOVA, unpaired t tests, or Welch t tests in cases of unequal variances between groups.
RESULTS
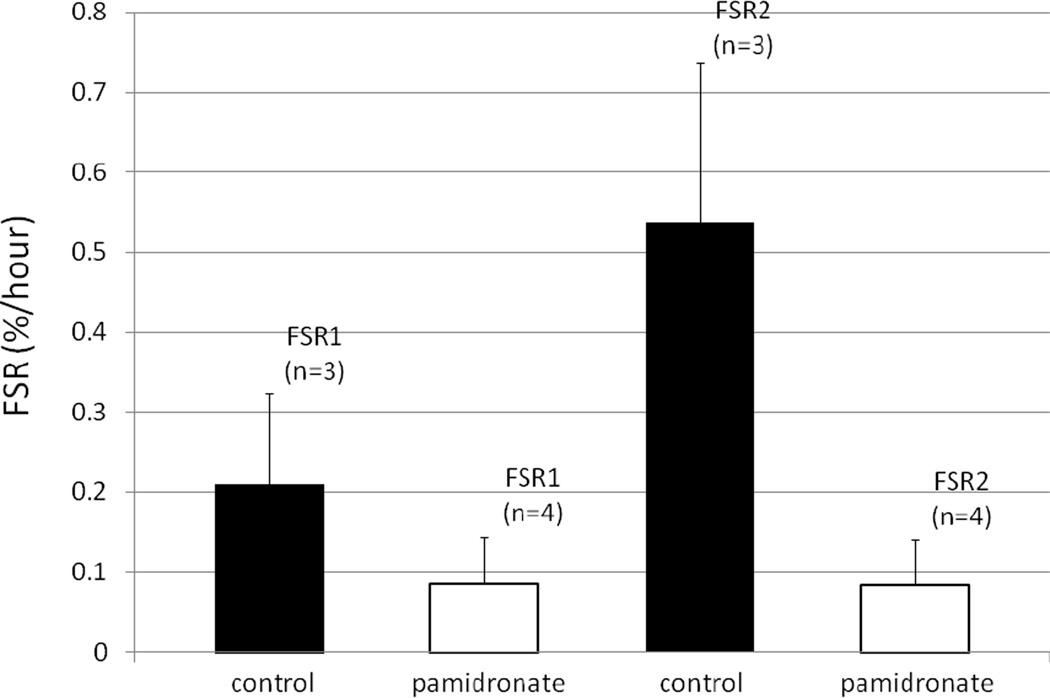
Post hoc analysis of our patient database identified 17 subjects who had participated in the pamidronate study, 10 receiving pamidronate, 7 receiving placebo (4,5), who underwent concurrent stable isotope infusion studies at our institution. There were no differences in age between pamidronate and placebo groups, 13.4+ 4.0 (SD) vs 12.5 + 3.6 yr respectively. Total body surface area burns 65.7 + 18.1% for the pamidronate group and 58.4 + 17.8% for the placebo subjects. The differences in age and burn size between the two groups were not significant. In the control group FSR2 was significantly higher than FSR1, reflecting the increased supply of building blocks for muscle protein synthesis. In the pamidronate group, however, FSR2 was not different from FSR1 (Figure 1).
1.
Fractional synthetic rate (FSR) of muscle protein during a basal period (FSR 1) and during an unlabelled amino acid infusion period (FSR2) in pamidronate and control subjects. Results are expressed as percent per hour.
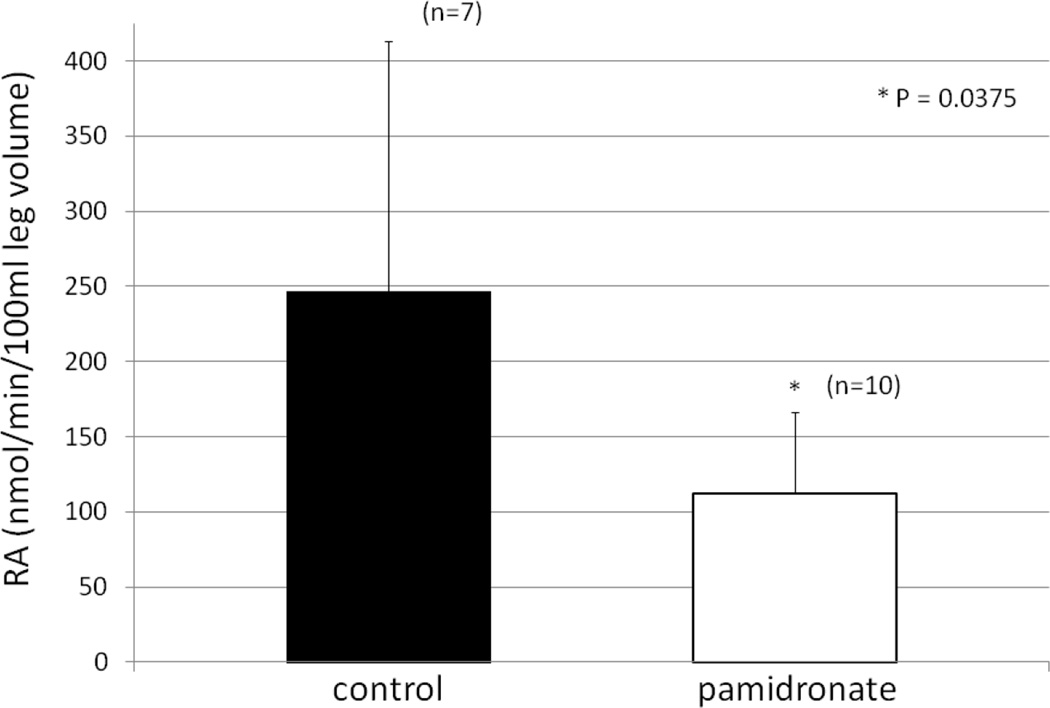
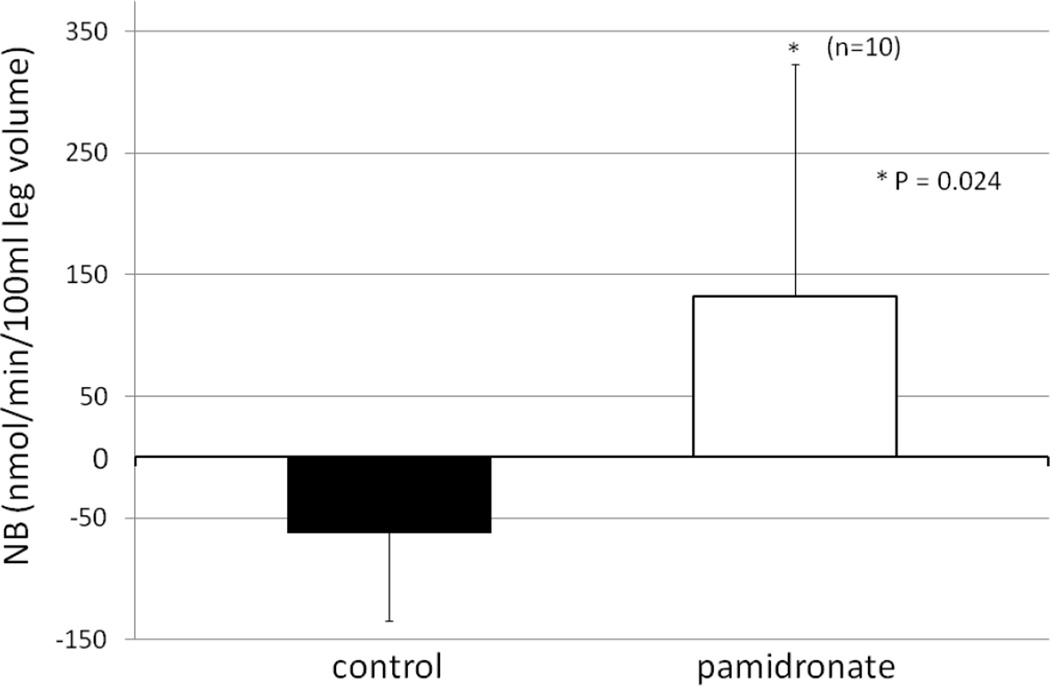
Similarly, Ra, or muscle breakdown rate, was significantly higher in the control group than in the group receiving pamidronate (Figure 2). Moreover, NB was negative in the control group, a usual finding in the burned patient with muscle protein breakdown exceeding the synthesis, and negative nitrogen balance. However, in the subjects receiving pamidronate NB was positive, and there was a significant difference between the pamidronate and placebo groups (Figure 3).
2.
Rate of appearance (Ra) of phenylalanine in venous blood in pamidronate and control subjects expressed as nmol/min/100 ml leg volume.
3.
Net balance (NB, synthesis minus breakdown) of phenylalanine as calculated from the arterio-venous difference in phenylalanine concentration multiplied by the blood flow and expressed as nmol/min/100 ml leg volume.
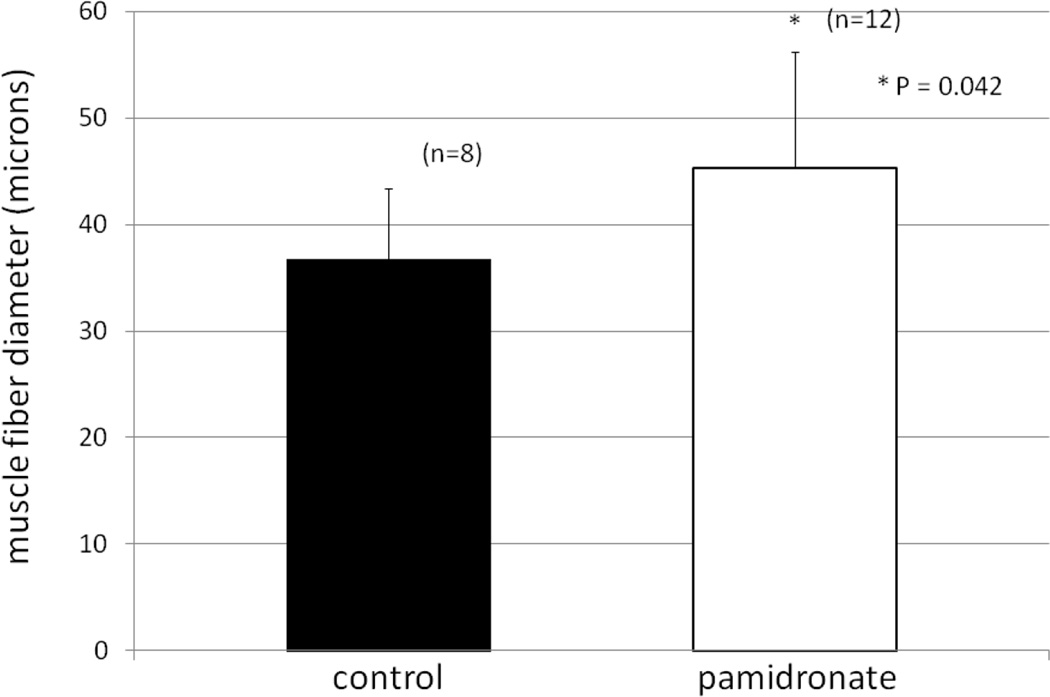
With regard to the additional muscle biopsies, 12 were found for the group receiving pamidronate and 8 were found for patients in the control group. A mean of 30 + 8 (SD) muscle fibers were counted per biopsy of the subjects receiving pamidronate and 28 + 8 fibers were counted in biopsies for the control subjects. As shown in Figure 4, mean muscle fiber diameter in the pamidronate group was significantly greater than that in the controls, substantiating the stable isotope findings by different means.
4.
Muscle fiber diameter from m. vastus lateralis biopsies of subjects receiving either pamidronate or placebo and expressed in microns.
Furthermore, subjects receiving pamidronate had a peak torque of 149.5 + 62% (n=5) of their body weight on the Biodex machine at 9 months post-burn. This compared to 153.4 + 29.9 % for normal, age-matched, physically fit children (n=22), and contrasts to 112.2 + 51.7 % (n=6) for the control subjects, p=0.052 by ANOVA. There were no significant differences in burn size between groups of children undergoing the exercise program.
Urine free cortisol excretion in subjects receiving pamidronate was 319.9 + 292.6 µg/24h (n=12) compared to 399 + 183.5 µg/24h in the controls (n=9) during the corresponding time period. There was no significant difference between the groups, p=0.486. The upper limit of normal for pediatric cortisol values ranges from 50–125 µg/24h.
DISCUSSION
This post-hoc analysis of a completed clinical trial demonstrates that in the burned patients administration of pamidronate within the first 10d following burn injury attenuated muscle loss as indicated by decreased muscle synthesis and breakdown, a positive net balance of muscle protein, and larger muscle fiber diameter. Muscle strength exhibited a tendency to be greater than in controls at 9 m post-burn but the difference was not quite significant between controls and unburned physically fit children of similar age. The question raised by these data is how did this happen?
There are at least three possible explanations. The first is that pamidronate acts by inhibiting farnesyl pyrophosphate synthase, an enzyme active in cholesterol biosynthesis (6) and therefore might interfere with the biosynthesis of glucocorticoid precursors or other intracellular signaling proteins. For pamidronate to protect muscle by reduction of steroid biosynthesis reduction of free cortisol excretion should be seen. Inasmuch as urine free cortisol excretion is not significantly reduced by pamidronate, this possibility is remote.
A second scenario is that pamidronate attenuates the post-burn inflammatory response, which also can contribute to muscle wasting (10). We think this is unlikely. The inflammatory response results in high levels of circulating cytokines, especially interleukins (IL)-1β and IL-6 (2). These cytokines are known to cause up-regulation of the parathyroid calcium-sensing receptor (CaSR) (11–13) , a condition documented in a sheep model of burn injury (14) that may be an adaptive response to the acute resorption of bone (1). Following pamidronate administration there was no amelioration of the hypocalcemic hypoparathyroidism resulting from CaSR up-regulation (4) suggesting that the inflammatory response had not been significantly altered.
A third possibility, and one that would appear to be most likely, is that bone produces myogenic factors that reach muscle by means of an as yet unidentified paracrine mechanism or mechanisms. It is known that during development bone produces Indian hedgehog (15) and vascular endothelial growth factor (16) that can both aid in muscle development. Other osteocyte products may also affect muscle. It has previously been shown that ketoconazole, an inhibitor of glucocorticoid production, can reduce endogenous steroid synthesis to normal in burned patients but fails to prevent muscle breakdown following burn injury (17). Thus reducing glucocorticoid production does not halt the catabolic response. Moreover, pamidronate administration appears to overcome the combined effects of inflammatory and glucocorticoid-mediated muscle breakdown. This speaks to the possibility that the anti-apoptotic properties of bisphosphonates (18) permit the development of healthy bone following the burn injury and that healthy bone is capable of producing substances that either protect the muscle from breakdown or support rapid regeneration of muscle tissue.
However, there also remains the possibility that these results represent an artifact of the retrospective nature of the study and the attendant possibility of inadvertent selection bias along with admittedly low numbers of subjects. A prospective study is needed to substantiate these findings.
If, however, these data are confirmed the necessity to treat osteoporosis would become greater as treatment may prevent or delay the onset of frailty.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grant P50GM60338 (4) and Shriners Hospitals for Children grant# 84090. GLK discloses that he sat on the Bone Toxicity Advisory Board for Novartis Pharmaceuticals, manufacturer of pamidronate (2012). Author’s Roles: EB carried out the isotope infusion studies and interpreted the data, reviewed and approved the manuscript. DNH provided the database and participated in data interpretation and manuscript review and approval. HKH analyzed the muscle fiber diameters, interpreted the data, and reviewed and approved the manuscript. OES carried out and interpreted the exercise studies, reviewed and approved the manuscript. MC assisted with data analysis, review and approval of the manuscript. GLK conceived of the idea, interpreted the data provided by each aspect of the study, and wrote and revised the manuscript. This work was presented in part at the 35th Annual Meeting of the American Society for Bone and Mineral Research, Baltimore MD 4–7 October 2013.
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jbmr.2162]
REFERENCES
- 1.Klein GL, Xie Y, Qin YX, Lin L, Hu M, Enkhbaatar P, Bonewald LF. Preliminary evidence of acute bone resorption in a sheep model of burn injury: an observational study. J Bone Miner Metab. 2013 doi: 10.1007/s00774-013-0483-4. epub ahead of print June 20. PMID: 23784552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein GL, Herndon DN, Goodman WG, Langman CB, Phillips WA, Dickson IR, Eastell R, Naylor KE, Maloney NA, Desai M, Benjamin D, Alfrey AC. Histomorphometric and biochemical characterization of bone following acute severe burns in children. Bone. 1995;17:455–460. doi: 10.1016/8756-3282(95)00279-1. [DOI] [PubMed] [Google Scholar]
- 3.Klein GL, Bi LX, Sherrard DJ, Beavan SR, Ireland D, Compston JE, Williams WG, Herndon DN. Evidence supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int. 2004;15:468–474. doi: 10.1007/s00198-003-1572-3. [DOI] [PubMed] [Google Scholar]
- 4.Klein GL, Wimalawansa SJ, Kulkarni G, Sherrard DJ, Sanford AP, Herndon DN. The efficacy of acute administration of pamidronate on the conservation of bone mass following severe burn injury in children: a double-blind, randomized, controlled study. Osteoporos Int. 2005;16:631–635. doi: 10.1007/s00198-004-1731-1. [DOI] [PubMed] [Google Scholar]
- 5.Przkora R, Herndon DN, Sherrard DJ, Chinkes DL, Klein GL. Pamidronate preserves bone mass for at least 2 years following acute administration for pediatric burn injury. Bone. 2007;41:297–302. doi: 10.1016/j.bone.2007.04.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research. Pp 51–76. New York: Wiley; 2004. [Google Scholar]
- 8.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzi PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-week resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 10.Tang K, Murano G, Wagner H, Nogueira L, Wagner PD, Tang A, Dalton MD, Gu Y, Peterson KL, Breen EC. Impaired exercise capacity and skeletal muscle function in a mouse model of pulmonary inflammation. J Appl Physiol. 2013;114:1340–1350. doi: 10.1152/japplphysiol.00607.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen PK, Rasmussen AK, Butters R, Feldt-Rasmussen U, Bendtzen K, Diaz R, Brown EM, Olgaard K. Inhibition of PTH secretion by interleukin-1 beta in bovine parathyroid glands in vitro is associated with an up-regulation of the calcium-sensing receptor mRNA. Biochem Biophys Res Comm. 1997;238:880–885. doi: 10.1006/bbrc.1997.7207. [DOI] [PubMed] [Google Scholar]
- 12.Toribio RE, Kohn CW, Capen CC, Rosol TJ. Parathyroid hormone (PTH) secretion, PTH mRNA, and calcium-sensing receptor mRNA expression in bovine parathyroid cells, and effects of interleukin (IL)-1, IL-6, and tumor necrosis factor alpha on equine parathyroid cell function. J Mol Endocrinol. 2003;31:609–620. doi: 10.1677/jme.0.0310609. [DOI] [PubMed] [Google Scholar]
- 13.Cannaff L, Zhou X, Hendy GN. The pro-inflammatory cytokine, interleukin-t, up-regulates calcium-sensing receptor gene transcription via Stat 1/3 and Sp 1/3. J Biol Chem. 2008;283:13586–13600. doi: 10.1074/jbc.M708087200. [DOI] [PubMed] [Google Scholar]
- 14.Murphey ED, Chattopadhyay N, Bai M, Kifor O, Harper D, Traber DL, Hawkins HK, Brown EM, Klein GL. Up-regulation of the parathyroid calcium-sensing receptor after burn injury in sheep: a potential contributory factor to post-burn hypocalcemia. Crit Care Med. 2000;28:3885–3890. doi: 10.1097/00003246-200012000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Bren-Mattison Y, Hausburg M, Olwin BB. Growth of limb muscle is dependent on skeletal derived Indian hedgehog. Dev Biol. 2011;356:486–495. doi: 10.1016/j.ydbio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sassoli C, Pini A, Chellini F, Mazzanti B, Nistri S, Nosi D, Saccardi R, Quercioli F, Zecchi- Orlandi S, Fromigli L. Bone marrow mesenchymal stromal cells stimulate skeletal myoblast proliferation through the paracrine release of VEGF. PLoS One. 2012;7:e37512. doi: 10.1371/journal.pone.0037512. Epub 2012 July 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeschke MG, Williams FN, Finnerty CC, Rodriguez NA, Kulp GA, Ferrando A, Norbury WB, Suman OE, Kraft R, Branski LK, Al-mousawi AM, Herndon DN. The effects of ketoconazole on post-burn inflammation, hypermetabolism, and clinical outcomes. PLoS One. 2012;7:e35465. doi: 10.1371/journal.pone.0035465. Epub 2012 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effects of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res. 2008;23:1712–1721. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]