Abstract
Objectives
To determine the effect of early childhood protein-energy malnutrition (ECPEM) on decayed, missing, filled tooth (DMFT) scores in the permanent dentition of rural Haitian adolescents aged 11–19 years (n=1,006).
Methods
We used data from a retrospective cohort that was developed from the Haitian Health Foundation database and merged records on weight-for-age covering the birth through 5-year-old period for all enrolled participants. Dental examinations and interviewer-administered structured questionnaires on demographic, socioeconomic status and relative sugar consumption were completed in 1,058 participants aged 11 to 19 years. The ECPEM was defined based on weight-for-age of the subjects during their first five years of life that were converted to Z-scores based on the National Center for Health Statistics referent database. Descriptive statistics were calculated. DMFT was regressed on ECPEM adjusting for age, sex, current BMI Z-score, SES, relative sugar consumption and number of permanent teeth present assuming a Poison distribution.
Results
Questionable malnutrition (RR =0.72; 95%CI, 0.61–0.86) and malnutrition (RR =0.58; 95%CI, 0.49–0.69) were associated with a statistically significant lower DMFT in Haitian adolescents.
Conclusions
ECPEM status is inversely associated with DMFT in Haitian participants. Further follow-up of these same participants will be recommended to evaluate the potential caries catch-up effect.
Keywords: early childhood malnutrition, permanent dentition, dental caries, enamel formation, Poisson regression
INTRODUCTION
Protein-energy malnutrition is the result of deficiencies in the intake of protein, energy foods or both, relative to a body’s needs[1], and manifests clinically as stunting or wasting. This type of malnutrition is the most common nutritional disorder in developing countries [2]; according to the United Nations International Children's Emergency Fund (UNICEF) more than 30% of children under 5 years of age suffered from moderate to severe protein-energy malnutrition (PEM) in 1995 [3]. Early childhood protein-energy malnutrition (ECPEM) may lead to death by starvation if the exposure is severe and frequent [4]; if the exposure is less severe or frequent it may lead lower resistance to infections with a potential for increased mortality [4]. Overall malnutrition contributes to about one third of the 9.7 million child deaths annually [5, 6].
It has been reported that as ECPEM worsens there is a reduction of salivary flow [2, 7, 8]. Psoter [8] suggested that exocrine glandular systems may be compromised for extended periods following ECPEM, which may have important implications for the body’s systemic antimicrobial defenses.
ECPEM has also been associated with enamel hypoplasia in the primary dentition [9, 10]. There are numerous reports of an association between ECPEM and delayed tooth emergence [9, 11, 12]. Psoter [9, 12] reported a delayed tooth exfoliation and emergence of the permanent teeth between 11 and 13 years of age when malnutrition was experienced between birth and 5 years of age.
The effect of ECPEM on dental caries has been investigated in the primary dentition, with most studies reporting a positive correlation [11, 13, 14], although there are still a few inconclusive reports [9, 15]. Alvarez [16] reported that the pattern of caries development as a function of age is significantly altered as a result of a delayed emergence and exfoliation of the deciduous teeth. However, these findings were based on a small sample (94 six year-old children). Alvarez [11] also reported that among children aged 3–9 years nutritional deficits that led to chronic malnutrition not only delayed tooth exfoliation and emergence but also appear to make the primary teeth more susceptible to caries later in life.
The permanent dentition forms and develops in the maxilla and mandible during early childhood (i.e., first molars are initiated during the fourth month in utero and calcification starts at birth and is completed about 3-years of age). The enamel of the permanent teeth starts to form around the 3rd-4th month after birth (with the maxillary central incisors); by age three all teeth but the third molars are at some stage of enamel formation and most crowns are fully formed but unerupted by age 5 [17]. Therefore, exposure to ECPEM during developmentally critical periods of early childhood (birth to five years) may affect caries risk in the permanent dentition during adolescence. This retrospective cohort study aimed to examine the association between early childhood protein-energy malnutrition (ECPEM) and permanent dentition dental caries.
The present study was conducted in rural Haiti. Haiti is one of the most densely populated countries in the Western hemisphere. Around 80% of the rural population lives in poverty and their life expectancy is only 54 years. The poverty level is also reflected in the prevalence of malnutrition; approximately half of children under 5 years of age experience some level of malnutrition [18]
METHODS
DATA SOURCE
The data for this report comes from a retrospective cohort study conducted in Jeremie, Haiti, in 2005 of the effects of ECPEM on the permanent dentition. The retrospective cohort was developed from the Haitian Health Foundation (HHF) database and merged records on weight-for-age covering the birth through 5-year-old period for all subjects. The HHF is a non-governmental organization that by 2003 was providing maternal and child health to over 200,000 individuals in the Jeremie region. In our sample 45.8% of the subjects were exposed to ECPEM and 37% were categorized as being exposed to questionable malnutrition during early childhood.
STUDY SAMPLE
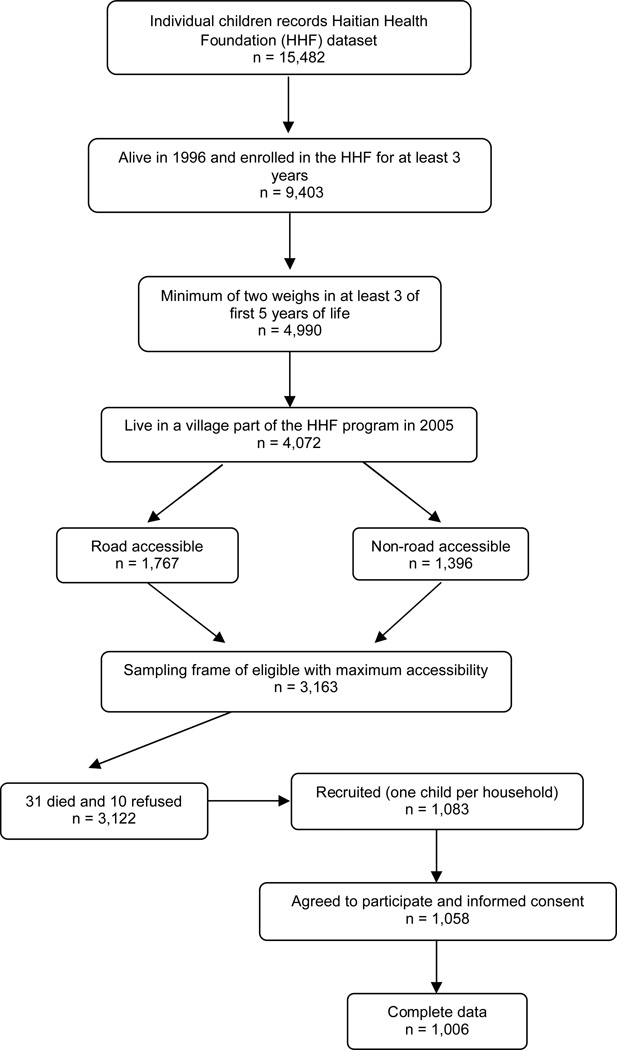
The 1988–1996 HHF database contains data on 15,482 maternal and child health participants. Exclusion criteria for the 2005 retrospective cohort study included individuals who died during the 1988–1996 period (n=541), and those enrolled in the HHF system for less than 3 years (n=5,538) as of 1996. Of the remaining 9,403 subjects, 53% (n= 4,990) met the inclusion criteria of at least two recorded weights per year for at least three of the first 5 years of life. Several villages were no longer part of the HHF system in 2005 with 918 subjects from these villages being excluded leaving 4,072 eligible subjects. The current study’s sampling frame was designed to include all respondents who lived in road accessible villages (n = 1,767 subjects, 13 villages) and those respondents who lived in non-road accessible villages that provided the largest sample size (n = 1,396 subjects, 6 villages). Thus, 3,163 potential respondents were included in the 2005 sampling frame. Of these eligible subjects, 31 had died since 1996 and 10 mothers refused to participate. Of the remaining 3,122 potential respondents, the oldest child between the ages of 11 and 19 from each household was selected. These exclusions yielded a total of 1,183 eligible respondents.
Of the total of 1,183 eligible respondents, 90.2% (n = 1,058) received a dental examination. One village was not surveyed due to security concerns. Subjects with missing data on any of the variables of interest (n= 52) were excluded from the analysis. Thus, the analysis was limited to 1,006 respondents aged 11 to 19 years (Figure 1). Prior to the study enrollment, two recruiters visited the 19 study villages between November 2004 and April 2005 to familiarize the villages with the study in order to maximize enrollment. Once the study sample was selected, informed consent from mother, and assent from child was obtained. The study was approved by the New York University Medical School and the State University of Haiti Dental School Institutional Review Boards.
Figure 1.
Study sample selection flow chart.
FIELD OPERATIONS
The field data collection was conducted between May and August of 2005. Intra-oral clinical examinations were conducted under natural daylight supplemented by portable head lamps and using mirrors and #23 explorers. Diagnosis was based upon visual-tactile examination. All examinations were conducted by examiners who were blinded as to which malnutrition group the subject belongs. Data collection included the administration of a socioeconomic status and relative sugar consumption questionnaires. Fluoride exposure as well as height/weight measurements were also assessed. Daily, 10% of subjects were reexamined for inter- and intra- examiner reliability testing. Data was double entered daily into Epi Info. Inter- and intra-examiner reliability for DMFS had Kappa of 0.63 and 0.84 respectively.
STUDY VARIABLES
Outcome
The study utilized the World Health Organization (WHO) diagnosis criteria [19] for dental caries with the subjects’ DMFT (decayed, missing, filled, permanent teeth) index computed from the individual tooth caries data.
Exposure
In the HHF database, early childhood nutritional status definition was based on weight-for-age of the subjects during their first five years of life, where they had at least 2 weightings per year. The aggregated data on weight-for-age was then converted to Z-scores based on the National Center for Health Statistics (NCHS) reference database to allow international comparisons as recommended by the WHO [20] and to allow comparisons with previous results reported from this database [8]. We used the three level aggregate score variable produced from the HHF database; based on the calculated Z-score the subjects were defined as having malnutrition if they had any Z-score ≤ −2.0; questionable malnutrition, if they had any Z-score ≤ −1.0; and no malnutrition for those having all Z-scores >−1.
Covariates
Covariates identified as potential confounders in previous studies [8, 10, 15, 21, 22] included age (years), sex, current BMI Z-score, relative sugar consumption, number of permanent teeth and socioeconomic status (SES). Age at dental exam was based on the HHF database and was recorded as a continuous variable in years. Sex was ascertained at the time of examination.
Current height and weight measurements were assessed during the 2005 field examinations. Current BMI scores were calculated from these data and later converted to an age and sex standardized Z-scores based on the NCHS 1978 data. Childs’ relative sugar consumption was assessed using a structured, interviewer-administrated questionnaire initially developed for rural Haiti in 1993 [23]. This questionnaire captures the child’s frequency of consumption of common sugar products on a weekly basis. An aggregate sugar score was computed reflecting the consumption frequency of sugar and sugar products over a one week period. Number of permanent teeth present at time of examination was used to account for possible delayed tooth emergence; any permanent tooth element visible through the mucosa was considered as present.
Socioeconomic status at time of interview was ascertained by administering a questionnaire developed for rural Haiti [24–26]. The questionnaire consisted of six questions: 1) does the house the child lives in have a working radio? 2) Does the house the child lives in have latrine? 3) How many rooms are there in the house the child lives in? 4) What is the floor made of? (three choices), 5) What is the roof made of? (three choices), 6) What is the water source of the house? (four choices). The responses to these questions were added to calculate a score and three categories (poor, poorer and poorest) based in the tertile distribution were created.
STATISTICAL ANALYSIS
Descriptive statistics were produced and included bivariate analysis by ECPEM categories and by caries status. Statistically significant differences across ECPEM and caries categories were determined using chi-square, t-tests and ANOVA.
Regression models assuming a Poisson distribution and utilizing a log link were fitted to estimate the effect of ECPEM on DMFT before and after controlling for covariates. Overall, three models were fitted, the unadjusted model evaluated the crude effect of ECPEM on DMFT; a model adjusted for age, gender, SES, current BMI Z-score, relative sugar consumption and number of permanent teeth; and a third model, the parsimonious model, was obtained using the log likelihood ratio test. We used previous studies and the associations observed in our data to inform the final selection of covariates.
The power calculation related to the outcome (dental caries) is based on the probability of having ECPEM and the prevalence of dental caries in the study population. All power calculations were carried out for a two-sided alpha value of 0.05. The data were organized and analyzed using the STATA 12 statistical software package.
RESULTS
Table 1 presents the distribution of selected characteristics for the subjects stratified by ECPEM status. The mean age of the subjects was 13.90 (SD=1.73), with a range from 11 to 19 years old; 54% were males and about 29% were classified as poorer and 31% as poorest. Current BMI Z-score (p<0.001), relative sugar frequency consumption (p=0.003) and number of permanent teeth (p=0.02) decreased as ECPEM severity increased. Likewise, dental caries prevalence and DMFT score also decreases as ECPEM severity increases (p=0.03 and p<0.001 respectively). The rate ratios from the Poisson regression analyses of the association of ECPEM with DMFT are presented on Table 2. An inverse association was found between levels of ECPEM and risk for caries. When compared to those with no exposure to ECPEM, the risk of having one or each additional carious lesion in the permanent teeth decreases 28%, (RR =0.72; 95% CI, 0.61–0.86) for those with questionable malnutrition, and 42% (RR =0.58;95%CI, 0.49–0.69) for those with severe malnutrition. After adjusting for all covariates the associations are essentially identical (RR = 0.72 for questionable malnutrition and RR= 0.61 for malnutrition). The risk of having at least one or each additional carious tooth increased 12% per year from 11 to 19 years of age (p<0.001) and the poorest subjects had a 27% reduced risk of having at least one or each additional carious tooth (0.001).
Table 1.
Distribution of selected characteristics according to early childhood nutritional status among Haitian adolescents in 2005.
| No malnutrition | Questionable | Malnutrition | p-value a | Total | ||
|---|---|---|---|---|---|---|
| N=173 | N=372 | N=461 | ||||
| Prevalence | 17.2% | 37% | 45.8% | < 0.001 | 1006 | |
| Age | 13.85 (1.8) | 14.01 (1.8) | 13.82 (1.7) | 0.26 | 13.90 (1.7) | |
| Sex | ||||||
| Female | 87(50.3) | 164 (44.0) | 215 (46.6) | 0.35 | 466 (46.3) | |
| Male | 86 (49.7) | 209 (56.2) | 246 (53.4) | 540 (53.8) | ||
| SES | ||||||
| Poor | 85 (49.4) | 169 (45.3) | 150 (32.5) | < 0.001 | 404 (40.2) | |
| Poorer | 50 (29.1) | 104 (27.9) | 157 (34.1) | 311 (30.9) | ||
| Poorest | 37 (21.5) | 100 (26.8) | 154 (33.4) | 291 (28.9) | ||
| BMI Z-score | −0.59 (0.78) | −1.10 (1.0) | −1.56 (1.1) | < 0.001 | −1.22 (1.1) | |
| Sugar b | 7.18 (5.1) | 7.16 (5.6) | 6.05 (4.7) | 0.003 | 6.65 (5.2) | |
| No. Permanent teeth | 26.18 (3.3) | 26.21 (3.2) | 25.55 (4.1) | 0.02 | 25.90 (3.6) | |
| Dental Caries Prevalence | 37.8 | 35.1 | 28.6 | 0.04 | 32.6 | |
| DMFT | 1.18 (2.2) | 0.85 (1.7) | 0.68 (1.4) | 0.004 | 0.83 (1.7) | |
n (%) with the exception of age (years), BMI-Z, No of permanent teeth and DMFT for which means (SD) are presented
P-values for chi-square (categorical variables) and ANOVA (continuous variables)
Child Relative weekly Sugar Consumption.
Table 2.
Unadjusted and adjusted rate ratio (IRR) and their 95% confidence intervals (CI) for DMFT by nutritional status during early childhood in Haitian adolescents: Jeremie, Haiti 2005.
| Unadjusted Model | Adjusted Model | Reduced Model a | ||
|---|---|---|---|---|
| Malnutrition | ||||
| No malnutrition | 1 | 1 | 1 | |
| Questionable malnutrition | 0.72 (0.61- 0.86) | 0.72 (0.60- 0.86) | 0.72 (0.60- 0.86) | |
| Malnutrition | 0.58 (0.49- 0.69) | 0.62 (0.52- 0.74) | 0.61 (0.51- 0.73) | |
| Age | --- | 1.12 (1.08–1.17) | 1.14 (1.10–1.18) | |
| Sex (Ref: Female) | --- | 1.08 (0.94–1.24) | --- | |
| SES | ||||
| Poor | --- | 1 | 1 | |
| Poorer | --- | 0.91 (0.78–1.07) | 0.91 (0.78–1.07) | |
| Poorest | --- | 0.73 (0.61–0.87) | 0.72 (0.60–0.86) | |
| Current BMI Z-score | --- | 0.99(0.92–1.06) | --- | |
| Sugar Consumption | --- | 1.01 (1.00–1.02) | --- | |
| No. Permanent teeth | --- | 1.02 (1.00–1.05) | --- | |
Poisson regression models.
n=1006
Dependent variable = DMFT. Caries as a continuous variable.
P-value for the log likelihood ratio test = 0.14.
The parsimonious model, produced by comparing the log likelihood ratio test after dropping non-significant variables, included ECPEM, age and SES with all variables having virtually identical rate ratios as the full model. The log likelihood ratio test confirmed that the parsimonious model fits best.
Dental caries is not normally distributed and even thought Poisson regression analysis is recommended we conducted the statistical analysis fitting both Poisson and Log-binomial regression analysis; both analyses attained the same overall result.
DISCUSSION
This retrospective study found an inverse effect of early childhood protein-energy malnutrition on dental caries where dental caries decreases as early childhood nutritional status severity increased.
Previous reports have suggested that the effect of malnutrition on the oral health depends on the time when the insult occurs [9, 11, 13, 14], but none of these reports tested for time of exposure to malnutrition and its effect on the dentition. We tried to isolate the effect of early childhood nutritional status by adjusting for current BMI Z-score (to rule out the effect of current nutritional status on dental caries) and found that current BMI Z-score has no effect on the statistical association between early childhood nutritional status and dental caries in the permanent dentition (data not shown).
Alvarez [11] reported that children aged 7–9 years with stunted growth showed a significantly higher percentage of carious lesions in the primary dentition than did well-nourished children of the same age. But in the age specific analysis he shows that delayed tooth eruption was associated with delayed caries acquisition; the bell shaped caries-prevalence curve as a function of age for the chronically malnourished children was shifted to the right by approximately 15 months when compared with the curve from the well-nourished control subjects; this indicated a delayed caries acquisition on the primary dentition in this population. It is possible that the age group in our population does not allow us to see a positive effect of ECPEM on dental caries because the malnourished population has not yet catch-up. Notable in this regard is that the number of permanent teeth as a covariate in the models approached statistical significance; there was an increased RR for each additional tooth.
Our findings, with a large sample, unexpectedly suggest that the long term effect of ECPEM on dental caries in the permanent dentition is inversed, and caries risk decreases as early childhood malnutrition severity increases. Even though our results do not support our hypothesis, it is biologically plausible to find a lower dental caries prevalence in those affected by early childhood protein-energy malnutrition if we consider delayed tooth exfoliation and eruption as a protective factor. One possible explanation is delayed tooth eruption but this study cannot completely accommodate the measurement of emergence timing in the permanent dentition. It is possible that if examined at a later age, the children malnourished in early childhood could catch up and surpass the caries levels of the healthier children
One limitation in this study is the possibility that extremely malnourished children may present with a stunted height for age unmasking the examiners to their exposure. However, generally survivors of early childhood malnutrition catch up to or surpass the stature of unexposed children [27]. Therefore, this risk is expected to be minimal.
One of the strengths of this study is its longitudinal design and the considerably large sample size, which allows the assessment of temporality. In addition, enamel hypoplasia can be misdiagnosed as fluorosis, which is a defect that occurs in enamel after tooth formation, leading to an overestimate diagnosis of enamel hypoplasia. In this population there is no fluoride in the water and no fluoride supplementation therefore the diagnosis of enamel hypoplasia is reliable. An additional strength of this study is that all subjects had similar data on malnutrition both during early childhood as well as current anthropomorphic measurements. Even though malnutrition is more prevalent in developing countries, it is not exclusive to these countries; therefore our results are generalizable to any population where malnutrition is an issue no matter the severity.
A subsequent follow-up study of these same subjects will be necessary to finally answer the questions regarding a catch-up effect and to rule out the role of other oral ecological effects.
ACKNOWLEDGEMENTS
We would like to thank the residents of Jeremie, Haiti for their participation in this study. This work was supported by NIDCR grants [R01-DE014708, and T32-DE007255].
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Shils ME, OJ, Moshe S. Modern Nutrition in Health and Disease. Philadelphia: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 2.Johansson I, S.A., Rajan BP, Parameswaran A. Salivary flow in dental caries in Indian children suffering from chronic malnutrition. Caries Res. 1992;(26):38–43. doi: 10.1159/000261425. [DOI] [PubMed] [Google Scholar]
- 3.UNICEF. Unmasking malnutrition. 1995 Available from: www.unicef.org/pon95/nutr0007.
- 4.Semba RD, B.M. Nutrition in Health in Developing Countries. Humana Press; 2001. [Google Scholar]
- 5.Vesel L, B.R., Martines J, Penny M, Bhandari N, Kirkwood BR The WHO Immunization-linked Vitamin A supplementation Study Group. Use of new World Health Organization child growth standards to assess how infant malnutrition relates to breastfeeding and mortality. Bulletin World Health Organization. 2010;88:39–48. doi: 10.2471/BLT.08.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNICEF. The state of the world's children 2008, in United Nations Childrens Fund. New York: 2007. [Google Scholar]
- 7.Johansson I, L.-L.M., Saellstrom AK. Saliva composition in Indian children with chronic proteinenergy malnutrition. J Dent Res. 1994;(73):11–19. doi: 10.1177/00220345940730010101. [DOI] [PubMed] [Google Scholar]
- 8.Psoter WP, S.A.L., Gebrian B, St. Jean Rodolph. Effect of childhood malnutrition on salivary flow and pH. Archieves of Oral Biology. 2007;53:231–237. doi: 10.1016/j.archoralbio.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Psoter WJ, B.C.R., Katz RV. Malnutrition and Dental Caries: A Review of the Literature. Caries Research. 2005;(39):441–447. doi: 10.1159/000088178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, N.J., Bian JY. Caries experience in deciduous dentition of rural Chinese children 3–5 years old in relation to the presence or absence of enamel hypoplasia. Caries Res. 1996;(30):8–15. doi: 10.1159/000262130. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez JO, L.C., Saman C, Caceda J, Montalvo J, Figueroa ML. Chronic malnutrition, dental caries, and tooth exfoliation in Peruvian children aged 3–9 years. Am.J.Clin.Nutr. 1988;(48):368–372. doi: 10.1093/ajcn/48.2.368. [DOI] [PubMed] [Google Scholar]
- 12.Psoter W, G.B., Prophete S, Reid B, Katz R. Effect of early childhood malnutrition on tooth eruption in Haitian adolescents. Community Dent Oral Epidemiol. 2008;36:179–189. doi: 10.1111/j.1600-0528.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 13.Nikiforuk G, F.D. The etiology of enamel hypoplasia: A unifying concept. The Journal of Pediatrics. 1981;98(6):888–893. doi: 10.1016/s0022-3476(81)80580-x. [DOI] [PubMed] [Google Scholar]
- 14.Maria CP, Saraiva SC, Bettiol Heloisa, Silva Antônio A, Barbieri Marco A. Is low birthweight associated with dental caries in permanent dentition? Paediatric and Perinatal Epidemiology. 2007;(21):49–56. doi: 10.1111/j.1365-3016.2007.00782_1.x. [DOI] [PubMed] [Google Scholar]
- 15.Scheutz F, M.M., Poulsen S, Frydenberg M. Caries risk factors in the permanent dentition of Tanzanian children: a cohort study (1997–2003) Community Dental Oral Epidemiology. 2007;35:500–506. doi: 10.1111/j.1600-0528.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez JO. Nutrition, tooth development, and dental caries. Am. J. Clin. Nutr. 1995;(61):410S–416S. doi: 10.1093/ajcn/61.2.410S. [DOI] [PubMed] [Google Scholar]
- 17.Woefel Julian B, R.C.S. Its Relevance to Dentistry. Sixth ed. Philadelphia, PA: Lippincot, Williams and Wilkins; 2002. Dental Anatomy; p. 422. [Google Scholar]
- 18.Reid BC, P.W., Gebrian B, Wang MQ. The Effect of an International Embargo on Malnutrition and Childhood Mortality in Rural Haiti. International Journal of Health Services. 2007;37(3):501–513. doi: 10.2190/MR65-2605-1285-0406. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Oral Health Surveys. Geneva: 1997. [Google Scholar]
- 20.WHO. Bulletin. Vol. 64. WHO; 1986. Use and interpretation of anthropometric indicators of nutritional status; pp. 929–941. [PMC free article] [PubMed] [Google Scholar]
- 21.Infante PF, G.G. Dental caries experience in the deciduous dentition of rural Guatemalan children ages 6months to 7 years. J.Dent.Res. 1976;(55):951–957. doi: 10.1177/00220345760550064501. [DOI] [PubMed] [Google Scholar]
- 22.Selwitz RH, I.A., Pitts NB. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 23.Psoter WP, G.B., Katz RV. Reliability of a sugar consumption questionnaire for rural Haiti. Puerto Rico Health Sciences Journal. 2008;27(1):69–74. [PubMed] [Google Scholar]
- 24.Milgrom P, R.C.A., et al. Dental Caries and its Relationship to Bacterial Infection, Hypoplasia, Diet and Oral Hygiene in 6- to 36- month old children. Community Dentistry and Oral Epidemiology. 2000;28:295–306. doi: 10.1034/j.1600-0528.2000.280408.x. [DOI] [PubMed] [Google Scholar]
- 25.Ellwood RP, O.M.D.M. Association Between Dental Enamel Opacities and Dental Caries in a North Wales Population. Caries Research. 1994;28:383–387. doi: 10.1159/000262006. [DOI] [PubMed] [Google Scholar]
- 26.Peres MA, L.M., Sheiham A, Peres KG, Barros FC, Hernandez FC. Social and biological early life influences on severity of dental caries in children aged 6 years. Community Dentistry and Oral Epidemiology. 2005;33:53–63. doi: 10.1111/j.1600-0528.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 27.Cameron N, P.M., Cole TJ. Catch-up Growth or Regression to the Mean? Recovery from Stunting Revisited. American Journal of Human Biology. 2005;17(4):412–417. doi: 10.1002/ajhb.20408. [DOI] [PubMed] [Google Scholar]