Abstract
Migration and mobility have had a profound influence on the global HIV epidemic. We propose a network-dyadic conceptual model to interpret previous literature and inform the development of future research with respect to study design, measurement methods, and analytic approach. In this model, HIV transmission is driven by risk behaviors of migrants that emerges and is enabled by mobility, the bridging of sub-epidemics across space and time, and the displacement effects on the primary residential sending community for migrants. To investigate these causal pathways, empirical study designs must measure the relative timing of migratory events, sexual risk behaviors, and incident HIV infections. Network-based mathematical models using empirical data on partnerships help gain insight into the dynamic disease transmission systems. Although the network-dyadic conceptual model and related network methods may not address all questions related to migration and HIV, they provide a unified approach for future research on this important topic.
Keywords: Mobility, sub-Saharan Africa, mathematical modeling, exponential random graph models
1. INTRODUCTION
The collapse of geographical space over the last 200 years has had profound effects on the circulation of human populations and thus has been a key driver of infectious diseases across time and space (1). Migration and migrants specifically have had a significant role in the history of the global HIV pandemic. In 2008, the Joint United Nations Program on HIV/AIDS identified migrants as one of the most vulnerable groups to HIV infection and its consequences (2).
HIV is one of the most devastating disease epidemics, with over 34 million people worldwide currently living with HIV, and AIDS has been responsible for more than 25 million deaths since first recognized in 1981 (3). This is a critical time for HIV prevention and treatment: incidence has declined in many countries, tools for clinical management of disease have improved, and the numbers of those on treatment have subsequently increased. Many new effective prevention tools are now available, including early initiation of antiretroviral therapy to suppress HIV viral load, oral and microbicidal prophylaxis, male circumcision, and even structural microfinance programs (4). Yet, there are many unresolved empirical questions concerning the drivers of HIV transmission within and across populations that may help explain disease disparities.
Migration and mobility have been foci of HIV disparities research since the beginning of the epidemic (5). Migrants not only exhibit higher risk for acquisition of HIV and other sexually transmitted infections than non-migrants, but disproportionately transmit those infections to others (5–12). Population migration was a significant determinant of the spread of HIV in countries with large generalized epidemics, including Kenya (6), South Africa (10), Uganda (13), and Zimbabwe (14). Circular migration in particular can catalyze HIV transmission rates in the home region, as migrants are more likely than non-migrants to engage in risky sexual behaviors, become infected with HIV while away, and return home to infect their at-home partners (15). This is a form of concurrency if the migrant had an ongoing partnership at home, a network phenomenon of having two or more overlapping partnerships (16), suggested by some (17, 18) but not all (19) to impact generalized HIV epidemics.
Migration and HIV studies are subject to many methodological and measurement challenges in part because there are many forms of migration, from short-term circular labor migration to lifetime rural to urban migratory patterns (20, 21). Consequently, our understanding of the etiologic impact of migration on HIV risk is mixed, has evolved with the changing epidemic stage, and depends on social context and measurement methods across studies (20, 22, 23).
Our aim is to present a framework to help organize a complex and varied research literature on migration and HIV. In this paper, we propose a network-dyadic model to frame the conceptualization, design, measurement, and analysis of the role of migration on HIV. This model theorizes a disease transmission process occurring within a series of potentially overlapping, concurrent sexual dyads drawn from a network of potential partners, and entrance and exit from that network through migration. This paper is not a systematic review of the empirical literature; two recent articles that qualitatively synthesized the literature (20, 24) find that measures have been too varied to provide a meta-analytic summary. In this review, we begin by outlining our model with application to causal pathways. Next, we discuss how study design and measurement methods can address model components, and finally, suggest how mathematical models may help gain insight into the complex relationships between migration and HIV at the levels of individuals, dyads, networks, and populations.
2. MODELS, PATHWAYS, AND HYPOTHESES
Migration can be associated with disease in many ways, but the aim of most empirical research is causal inference on that relationship. Underlying that is some hypothesized mechanism or pathway by which migration directly or indirectly causes infection. That pathway is often motivated by a larger conceptual model that incorporates units or levels for analysis. Here we propose a network-dyadic model unifying several commonly proposed causal pathways motivating research on migration and HIV.
Conceptual Models
Conceptual models link theories of causation to specific causal pathways of interest. Models can represent formal motivating theories (often in the social sciences), but may be informal heuristic tools for causal propositions (common in epidemiology). Models typically visualize the structural framework of causal pathways by depicting the different “levels” of analysis (25, 26). Because they facilitate complex multi-level processes in which individuals are embedded within geographic or other aggregate units, models play an important role in migration research.
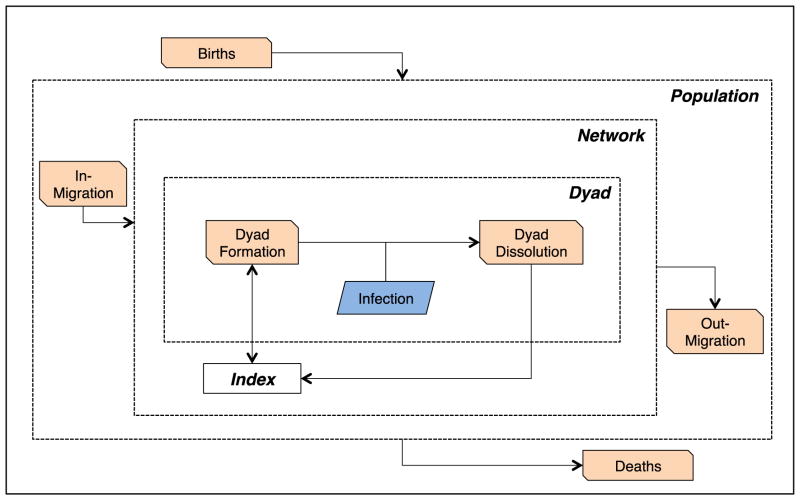
In Figure 1, we provide a conceptual model for the impact of migration on HIV infection in which infection occurs within a nested network-dyadic process. The four structural levels are the index individual, the sexual partner dyad comprised of two individuals, the sexual network from which dyads arise, and the population generating the network. The borders of these levels are dotted to indicate that they are porous. Demographic transition processes are indicated by slanted orange boxes, and the main epidemiological transition in blue.
Figure 1. Network-Dyadic Conceptual Model for Migration and HIV.
Demographic processes are shown in orange slanted boxes, and the main infection transmission process in blue. The four nested levels within the model (population, network, dyad, and index) are shown with dotted lines to show their porous borders. A partnership dyad is formed by the union of two index individuals; with concurrency, dyad formation can occur multiply before any dyad dissolution occurs. HIV infection may only occur within a dyad between the formation and dissolution processes.
Sexual network processes are structured and dynamic, with dyads forming and dissolving conditional on individual attributes (e.g., heterosexual preference) and higher-order network properties (e.g., availability of potential partners). In the Figure, an index enters into the dyad state through the dyad formation process; infection occurs only within that dyad, which may subsequently dissolve into two indexes again. For disease, dyads start in one of three states: both partners infected, both uninfected, or serodiscordant (only one partner infected). In the second state, a new infection of one or both members may occur only if one of the members forms a new dyad with an outside infected index before that first partnership dissolves (i.e., concurrency). The bidirectional arrow between the index and the dyad formation process signifies that new dyads may be formed with concurrent partners before the dissolution of any dyads established earlier. In the third, serodiscordant state, infection of the negative partner may occur either through concurrency or by direct transmission within the original dyad. An example of the latter is chained serial monogamy of one or both partners; if both partners are monogamous within a partnership then one of them must be infected at the dyad formation for any disease transmission to become possible. This therefore limits any transmission from partners established later to those earlier: this backward path of infection is a core transmission catalyst of concurrency (27). In either case of the starting dyad state, infection only occurs between dyad formation and dissolution.
Migration enters onto this process by adding new individuals into the network, and may also cause dyad dissolution when one partner leaves the network. Of significance are the traits of the in-migrants relative to the existing network: high-risk migrants may bring disease into a low-risk network, or a high-risk network with risk attrition through disease-related mortality may experience a reduction in the average risk level through migration (28). Mobility may expand the size of the network when an individual travels to another region and forms a new partnership that bridges previously unconnected components of the population (29). Thus, migration can refer both to demographic migration, in which people geographically change residences, and also network migration, which occurs when individuals enter into a network of available sexual partners from a larger global population. Often the two are intrinsically linked in network-based HIV research.
Causal Pathways
Given the general conceptual model, causal pathways may be visualized using directed acyclic graphs (DAGs) (30). In DAGs, nodes connected by a directed line represent a specific causal hypothesis that the sending node (exposure) causes the receiving node (infection). All factors that may bias the direct causal association must be included; confounders are represented as common causes of both exposure and outcome. Complex time-dependent causal pathways must be diagramed without feedback loops (hence, acyclic) by partitioning nodes into time-specific components.
Our conceptual model is consistent with any number of DAGs, and Figure 2 illustrates one etiologic pathway in which concurrency occurs within a stable, long-term dyad. In this DAG, both dyad members are uninfected at baseline. A standard study would recruit one member of the dyad, and that person is designated as the index. However, the dyadic infection risk is of interest under the conceptual model, so both index and partner must be represented. Thus, this particular DAG illustrates the following hypothesized chain of events. The partner’s travel causes him to form a concurrent partnership with another person, and both travel and that partnership formation are influenced by his general risk profile (e.g., a propensity to engage in risky behavior). The secondary partnership increases the risk of the partner’s infection, which in turn increases the infection risk to the index. Also, the partner’s mobility causes the index to adopt new risk behaviors, which then occurs by sexual activity with her own secondary partner. Thus, the partner’s travel may be both a direct and indirect source of infection risk to the at-home index.
Figure 2. Directed Acyclic Graph (DAG) for HIV Infection within Stable Couples with Concurrency.
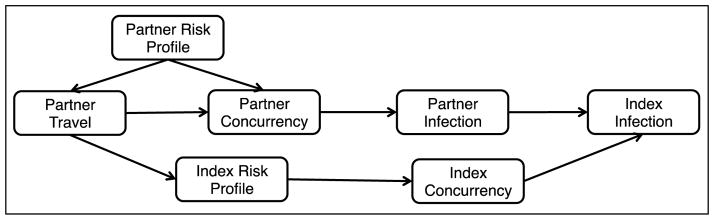
This DAG represent two possibilities for causal pathways from partner travel to index infection within a long-term stable couple in which both members are uninfected at baseline.
For simplicity, this DAG aggregates a large set of behavioral risk variables into profiles and does not refer to circular migration. Yet this basic structure highlights the complexities of causal inference under a network conceptual model. Needed are both exposure measurements in which the index is asked about some features of her partner’s behavior, and also sophisticated statistical analysis methods that distinguish the indirect effects of partner concurrency from the direct effects of index concurrency (31). The study design must differentiate prevalent from incident infection, determine the temporal relation between migration and concurrency, and distinguish the direct individual versus the indirect dyadic effects. Finally, another DAG entirely would be needed to represent the situation where a migrant is at high risk of infection through serial monogamy.
Network Pathways for Migration and HIV
Having considered this framework, we now suggest three pathways consistent with our conceptual model. First, the intrinsic risk pathway posits that mobile persons are at higher risk of infection due to some mechanism independently causing disease exposure and outcome. This was found in early HIV studies among truck drivers in East Africa: psychosocial properties (e.g., a risk profile) and demographic characteristics (e.g., ethnicity) influenced both labor migration and high-risk sexual relations (5). This may drive transmission both in the context of concurrency and serial monogamy with high partnership turnover. In some cases, this intrinsic risk may be a confounder causing both exposure and outcome, and controlling for risk would provide an unbiased causal effect of migration on disease. We call this the selection mechanism, and it is currently represented in Figure 2. In other cases, that risk may be a mediator: the act of travel facilitates that risk behavior by creating opportunities for it. We call this the enabling mechanism, and in Figure 2, would require placing the partner risk profile intermediate between travel and concurrency. Controlling for it would estimate the impact of travel on HIV above the effect of that mechanism. It should also be noted that reverse causation is possible, wherein HIV infection induces migration to seek medical care or social support, or because of marital dissolution (32).
Second, the bridging pathway asserts that migrants link otherwise distinct sub-populations. Whereas the intrinsic risk pathway concerns the risk to the migrant, this pathway addresses the downstream effects to the migrant’s contacts and can accommodate consequences of HIV-induced migration (32). This pathway may help explain the population-level changes in HIV prevalence in rural versus urban areas in South Africa through circular migration (33). The circular migration hypothesis more generally is that rural residents travel for work to an urban area with higher HIV prevalence, become infected there, and bring back infection to their lower prevalence village (34). Network bridging may also manifest in risk behaviors themselves when there is a diffusion of behavioral norms (35). Overall, the bridging pathway concerns a macro-level effect resulting from micro-level behaviors; these patterns are difficult to estimate through individual-level studies. Required is either a mathematical model or a longitudinal linked-dyad study (36).
Third, the community displacement pathway concerns the sexual network structure in a sending community with significant out-migration, wherein the temporary removal of circular migrants increases the risk behaviors of those remaining. The mediated pathway in Figure 2 illustrates this: the partner’s travel influences the risk behavior of the index. In Tanzania, the separation of couples through travel or bilocational housing increased the risk behavior of the stay-at-home spouse (21). Out-migration can also impact the broader network structure by creating a sex ratio imbalance (37).
These three pathways are commonly embedded in research implicitly motivated by the network conceptual model linking mobility to risk behavior to disease. As a starting point, it may be helpful to consider the reasons driving the migratory process: The intrinsic risk pathways may hold for many types of migration, but the mechanism behind it may differ by why migration occurs. The selection mechanism would be appropriate for voluntary migrants, while the enabling mechanism could explain heightened risk for “forced” or “pushed” migrants, such as those who migrate due to major socio-political or environmental forces. There are many other such models and different associated pathways. The point of this section was to highlight the process by which causal theory may be translated into testable hypotheses given alternative explanations for associations in the data.
3. STUDY DESIGNS
In order to test pathways linking migration and HIV, study designs must capture individual, dyadic, and network features of the population. Here we review how study designs are able to ascertain different components of the model, and how each design is limited.
Cross-Sectional Methods
Cross-sectional studies are the most common, and the main strength of these studies is their common use of population-based sampling in order to make generalizable inference that many longitudinal studies cannot. Yet they are limited in estimating causal pathways of migration and HIV. Timeframe of measurements must be precise in order to align exposure with outcome. They suffer from length-biased sampling, in which the exposure of interest may be related not to disease incidence but to disease duration (38). Yet many compelling cross-sectional studies highlight critical components of the network-dyadic conceptual model of migration and HIV, such as the role of sending and receiving population characteristics, differences by sex, and social support (14, 39–43).
Cross-sectional studies have identified key characteristics that moderate or mediate the relationship between migration and HIV risk, but the direction and size of these effects differ significantly across studies. For instance, a number of cross-sectional studies demonstrate that residential context matters – where migrants move from, where they move to, and why they move – but no clear patterns emerge. HIV risk varies for migrants originating from and traveling to rural or urban areas (40–42), from areas with high levels of poverty (39), with different reasons to move (14), and with different levels of social support (43). These are individual, dyadic, and network characteristics that influence the relationship between migration and HIV risk. Of course, many large scale migratory patterns are driven by higher level socio-political, economic, or environmental factors. The relationship between HIV risk and migrants that move for these reasons may be different and require unique consideration (44).
Many cross-sectional studies test how sex moderates the relationship between migration and HIV, although these findings have been far from harmonious as well. A number of studies find mobility associated with HIV risk for women but not men (45–47). On the other hand, the associations between mobility and risk may be greater for men than women in some settings (48), and may depend on additional factors such as rural or urban context (6). Thus cross-sectional studies are useful in identifying critical components of the migration and HIV conceptual framework, but conflicting results may arise due to omitted variable bias and endogeneity, or improper alignment of exposure and outcome variables.
A number of cross-sectional studies demonstrate that the timing, frequency, and duration of mobility influence the relationship between migration and HIV risk. Risk of HIV infection and risk behaviors increase with more time away from home (48) and number of times traveled (43), potentially due to risk behavior associated with a time-dependent acculturation or acclimation process to the receiving sexual network. Indeed, the relationship between mobility and HIV risk may be non-linear; among adult men in Zimbabwe, each additional overnight trip away from home was associated with a higher probability of concurrency, but with a diminishing rate of increase after a threshold of five trips per month (49). Timing of the study relative to the course of the epidemic will influence results as well (22). If the network bridging pathway holds then at some point there may be a state of equilibrium between HIV prevalence in the linked communities and migration would no longer be a risk factor from exposure to a high prevalence network.
Novel Cross-Sectional Methods
Testing causal pathways requires correctly assessing the timing of exposure and outcomes. Although cross-sectional studies may not be able to explicitly align exposure and outcome, there are a number of techniques that can directly or indirectly assess timing. Cross-sectional studies with retrospective measurement can assess timing, frequency, and duration of travel as well as sexual partnership characteristics, but with potential for recall bias. Event-history techniques can minimize recall bias by anchoring events in time and allow interviewers to check for and reconcile inconsistencies in reports (50). Egocentric sampling techniques can be used to measure data on recent sexual partnerships without recruiting partners. With this approach, subjects recall one or more recent sexual partners and describe partners’ attributes. Additional questions about sexual activity (date of first and last sex), partnership behavior, frequency of sex acts and condom use are asked (51).
Cross-sectional studies that recruit partners can test dyad-level processes such as the community displacement pathway (14, 21, 47, 49, 52). At a dyad level, migration can lead to increased HIV risk for the stay-at-home partner of a migrant directly through his or her own actions or indirectly through exposure to the returning migrant. Due to differences in populations, study design, and measurement, results range from no evidence of increased risk among partners of migrants (47), to increased risk for female partners of male migrants (10, 21), increased risk for male partners of female migrants (36), or even a protective effect associated with being separated from one’s spouse (14, 49).
Longitudinal Studies
Longitudinal studies can overcome many of the challenges of cross-sectional studies by more precisely measuring when exposures occurred relative to outcome infections. But these studies are subject to several limitations: selection bias, informative censoring due to migration, as well as high cost. For these reasons, there have not been many longitudinal studies of migration and HIV risk. One such longitudinal study in Tanzania, however, found increased risky sexual behavior as well as HIV prevalence among mobile women, and also among risk behaviors and HIV prevalence for husbands of long-term mobile women (36). This study used data from three survey rounds and thus was able to measure HIV incidence, but were still unable to align mobility episodes with HIV infection in order to explicitly test for causal pathways because the surveys were conducted two to three years apart. Therefore, their findings could be due to long-term female mobility among married women as a consequence of a troubled marriage or husband’s infidelity.
Longitudinal studies can test competing hypotheses under the intrinsic risk pathway: is increased risk associated with migration due to a selection or enabling effect, both, or neither? In a cohort study in Zimbabwe enrolling HIV-uninfected migrants and non-migrants at baseline that followed them over time to track incident infections (22), there was no association between out-migration and incident HIV infection. This implies no support for either the selection or the enabling hypotheses. However, the investigators were challenged by tracking out-migrants and experienced a high loss to follow-up (36% of out-migrants with known destinations were found and interviewed). This selection bias may be one reason for no association; risk-taking migrants may have been harder to find, or migrants living with relatives for support – and thus less likely to engage in high-risk behaviors (43) – were easier to retain in the study. In general, informative censoring with loss to follow-up due to migration-related study withdrawal is key consideration for longitudinal studies.
Longitudinal studies can also test whether HIV infection precedes migration. A recent study using panel data from Malawi first found that, indeed, migrants exhibited higher HIV risk behavior and were significantly more likely to be HIV-infected than non-migrants. However, in contrast to the common assumption that migration precedes HIV infection, HIV-infected individuals were significantly more likely to migrate over time than HIV-uninfected individuals, and marital dissolution may be fueling migration for HIV-infected individuals (32).
Other Designs
Qualitative and mixed-methods study designs could inform a deeper understanding of the context of migration and HIV, lead to further hypothesis development within the network-dyadic conceptual model. Few qualitative studies have addressed migration and HIV in sub-Saharan Africa (53–55). Qualitative studies will be very useful to discern qualities of sending and receiving networks that moderate or exacerbate relationship between mobility and HIV risk. Maasai migrants in Nairobi, Kenya, for example, did not exhibit increased sexual risk behavior despite very low knowledge of and access to condoms (55). In-depth qualitative analyses suggested that Maasai migrants regulated their behavior (e.g., partner selection) in a unique way that reflected knowledge of HIV. Overall, qualitative studies can provide useful insights into new causal pathways consistent with the network-dyadic model, as well as inform the interpretation with already studied pathways.
Ecological studies examine the health of populations, not individuals, and may potentially test the bridging pathway hypothesis. Indeed, in-migration at a population level was associated with HIV prevalence in a number of countries in sub-Saharan Africa, but mostly before the year 2000 (23). Migration appears to explain HIV variance across countries with expanding epidemics but less in countries with stabilized epidemics, which supports the bridging pathway. These types of analyses must be careful to avoid the ecological fallacy; two measures correlated at the population-level do not imply correlation at the individual level. Modelers have to be careful in selecting outcomes that avoid this fallacy. In a recent paper, Kenyon et al. demonstrate how spurious associations may be drawn, and existing associations missed, when outcome measures are incorrectly chosen; in particular, they argue that peak HIV prevalence as an outcome might be better in avoiding bias due to time of introduction of the virus, than HIV prevalence (56).
Moreover, the bias from such ecological fallacies may be substantial when the aggregate units are large, since there are so many potential confounding factors (57). A persistent limitation in all these study designs is their general micro-level focus at either the individual or dyadic level, whereas infectious disease transmission occurs within a dependent feedback system in which incidence rates are a function of current prevalence (58). Additionally, an atomistic fallacy, in which an individual’s disease risk is assumed to solely be a function of his own behaviors or traits, is more pervasive in this literature on macro-level processes like migration (59), and effects of these fallacies are possibly magnified in infection transmission systems with non-linear dynamics such as HIV (58).
4. MEASUREMENT METHODS
What is the best way to conceptualize and measure migration as it relates to HIV risk within a network framework? Challenges of measurement exist regardless of study design. No clear and consistent measures of migration have been used across studies, and contradictory findings are due in part to these inconsistencies (20). Nonetheless, calling for a strict definition would be counterproductive since migration manifests itself in numerous ways, and the type of movement is typically related to social and epidemiologic context and reasons for move. Rather, we need to acknowledge common challenges in measurement, identify intersecting components of migration measurement, and then build upon this knowledge to conceptualize and measure migration in specific contexts.
The three major methodological issues in trying to conceptualize and measure migration in relation to HIV risk are: what is the appropriate time frame, what is the appropriate spatial scale, and what is the appropriate definition of and way to capture sending and receiving network characteristics? We focus on the measurement of migration, but of course there are methodological challenges to measuring both HIV/STI (56) and sexual risk behavior (60). An in-depth discussion of these measures is beyond the scope of this paper.
Migration is typically a continuous, repeated process rather than a single event. Thus the timing of migration in relation to past migration is significant; the first time an individual migrates, for example leaving a family home, is very different than subsequent migrations and may be linked to different risk exposure. Timing of migration in relation to other social, demographic, and epidemiologic processes is important as well, and the effect of migration on HIV risk may be long-lasting or cumulative over time. People move continuously, so which spatial scale defines migration as a risk exposure to HIV? Many studies simply define migration as sleeping outside of one’s home (e.g., (36), but geographic distance from home may alter risk. In the U.S., intra-city mobility between different neighborhoods significantly impacts risk behaviors and HIV infection (61), thus greater geographic distance from home may not directly translate to greater HIV risk.
Most often, measures of migration are based on some measureable characteristic of movement in time and space (see Table 1). Migration has been conceptualized as a function of how often an individual traveled or moved residences in a set time frame (23, 36, 45, 47, 49), how long a migrant was away from home (14, 62), geographic space (63), or a combination of these concepts to identify categories of migrants (36, 46). These measures capture spatio-temporal movement, and the potential magnitude of exposure. Each method is subject to biases (mentioned in Table 1), but more importantly, these measures do not capture the quality of exposure. For instance, if an individual migrates to a particular destination and exhibits high sexual risk behavior, no association between migration and HIV infection would be found if there was no HIV at the destination.
Table 1.
Temporal, spatial, and network-based migration measurement features
| Measurement | Example | Strengths | Weaknesses |
|---|---|---|---|
| Temporal and spatial characteristics of migration | |||
| Frequency | Number of migrations since year X; number of overnight trips in the last month; number of nights present in household in last 6 months | Ability to construct continuous, categorical, or dichotomous variables; test for non- linear relationships | Recall bias, heaping potential, assumes each trip associated with equal exposure |
| Time frame | Slept outside household at least one night before one of interviews; living elsewhere at time of interview | Useful at population level, avoids recall bias | Selection bias, informative censoring; potential for recall bias without longitudinal study design |
| Duration | Lived outside community for >=1 month in last year, away for >=1 night in last month | Measures magnitude of exposure | Sampling bias (exclude current out-migrants); broad categories |
| Directionality | Moved to current town of residence within past 12 months (directed); visitor with permanent residence outside study site | Distinguish between circular and directed migrants | Sampling bias (exclude current out-migrants) |
| Geographic distance | Distance from home; distance to major city | Measure of potential separate (isolated) or overlapping sending network | Geographic distance may not correlate with risk |
| Individual, dyadic, and network characteristics of migration | |||
| Characteristics of migrant | Age, sex, race/ethnicity, religion, prior migration | Distinguish potential strata for analysis | Potential unmeasureable risk profiles; omitted variable bias |
| Reason or motivation | high risk (e.g. marriage) or low risk (e.g., looking for work) reason to move; type of work | Reason to move highly correlated with risk | Require categorization |
| Characteristics of dyad | Partner of migrant; living apart | Partner’s current or history of migration may influence index risk | Partner may not be source of risk (e.g., unmeasured concurrency) |
| Characteristics of sending community | Proportion of male (female) migrants | Useful for community displacement hypothesis | Time frame of out- migration not captured; level of community ‘social monitoring’ important |
| Characteristics of receiving community | HIV prevalence; socio- demographic composition; history of migration | Assess potential risk associated with linkage to different networks (bridging) | Amount of exposure not explicit; require prior knowledge or preliminary research |
Migration is not only the geographical movement of persons from one country or locality to another, but movement within and between social and sexual networks. Migration influences the formation and dissolution of ties in a network, and thus network structure. But the characteristics of those networks inform HIV risk as well. The process of conceptualizing and measuring migration must consider how to define the networks that a migrant is linked to, and what individual, dyadic, and network characteristics are the most salient regarding HIV risk. Fewer studies define mobility in these terms (20), listed in the second section of Table 1.
The network-dyadic conceptual model considers individuals in partnerships formed within networks. Measures of migration must address each of these levels. Individual socio-demographic characteristics of migrants that are hypothesized to influence the relationship between migration and HIV need to be measured, such as age and sex. Other social and behavioral characteristics such as previous migration or reason to move may moderate the relationship; for instance, risk associated with migration for marriage is most likely different than looking for a job (14, 22). Dyadic level measures originate from the same foundational characteristics, but expressing these terms as dyadic level variables can be challenging. One must now consider direct exposure (individual) and indirect exposure (partner), as well as the complex timing of direct and indirect exposure related to risk behavior or HIV infection. Lastly, risk is not always contained in a closed dyad and measures must account for exposure outside of primary partnerships (17).
It is important to consider three categories of sending and receiving network characteristics for the study of migration and HIV risk: compositional, contextual, and collective. Compositional explanations refer to characteristics of individuals in a network; contextual explanations refer to opportunity structures in the local physical and social environment; and collective explanations refer to socio-cultural and historical features of communities (64). HIV prevalence or sex ratios in a network are compositional characteristics that influence HIV risk associated with migration to that network. Ability to access health care is an example of a contextual characteristic. Lastly, collective explanations emphasize the importance of shared norms, traditions, and values as they may influence potential associations between migration and HIV, including issues such as availability of social support, history of migration, and potential for migrant assimilation and acculturation (or lack thereof) (20). Additionally, network characteristics are clearly tied with reason for migration. For example, networks of environmental refugees or other “forced” migrants will have different compositional, contextual, and collective characteristics than those of voluntary migrants. These characteristics will clarify the level of risk associated with migration and sexual risk behavior within a network.
5. ANALYSIS AND MATHEMATICAL MODELING
The network-dyadic model for migration and HIV implies complex causal pathways, measurement methods, and study designs. The same is true for analytic methods, since standard regression methods correlating individual-level exposures and outcomes may not estimate causal effects of interest. One issue is the dependent spatio-temporal data structure, in which there is clustering of observations within areas or time intervals. Another issue is complex mediated pathways, in which the act of migration is just one step in a series of events that ultimately leads to infection, or infection is just one of many events that lead to migration (65). Since many tools for statistical analysis of dependent observations and mediated pathways are available, we focus instead on a quantitative method that has received less attention: the network-based mathematical modeling of epidemic transmission dynamics.
Statistical analyses typically estimate summary measures and their uncertainty from the data given a modeling framework characterized by a probability distribution. The goal is inference to a broader data generating process. Mathematical models, in contrast, start with parameters (which may be estimated from data) that are fed into a dynamic mathematical system in which the disease incidence rates are a function of those parameters and evolving epidemic states. Populations are usually subdivided into groups based on their risk profile, demographic traits, and other features thought to play an important role in population-level transmission (66–68). For migration models, the spatial structure of the subpopulations is a key feature. Mathematical models of HIV transmission dynamics are most often used for two main purposes: 1) to form and reform hypotheses, such as what determinants of transmission lead to or sustain disparities, using a number of hypothetical model scenarios, and 2) for public health planning, such as evaluating the potential impact of a prevention intervention (69).
To date, mathematical models of migration and HIV transmission have been of two sorts: static models and deterministic compartmental models. Static models are time-invariant analytic estimations of disease effects from a set of equations representing transmission probabilities and contact structures. Results are often not forecasts, but estimates of a relative risk comparing exposure groups. Lurie et al., for example, used a static model to find that women partnered with migrant men are twice as likely to be infected from outside their main partnership as within (52). The main limitation of these models is that they treat incidence rates as time-invariant. This simplification may have profound effects for migration processes since the timeframe in which two previously unconnected subpopulations may mix and influence each other’s incidence can extend over decades.
A common solution is to use deterministic compartmental models. These models use differential equations to model groups as they transition from susceptible to infected states. For HIV, disease is typically modeled as a four-state process from uninfected, acute stage infection, chronic infection, and AIDS. Compartments are further subdivided by risk and demographics, including geography. For example, Coffee et al. modeled the effect of circular migration among South African labor migrants by including movement between residential and labor areas, and assortative mixing by risk levels (33), finding that migration impacted HIV transmission largely due to a change in the migrants’ behavior, rather than a bridging of two disconnected areas. This model tested the competing evidence for the intrinsic risk pathway versus the bridging pathway. Xiridou et al. investigated whether migration from high-prevalence areas could drive the epidemic potential in low-prevalence areas; they found little evidence for such effect between Africa and the Netherlands because the migrant population was small and the mixing between migrants and natives sparse (70). Finally, Walker et al. explored how the timing of migration and characteristics of migrants can influence epidemic properties after the peak epidemic prevalence has been reached. In-migration of high-risk persons before the peak prevalence can strongly influence the equilibrium prevalence, since prevalence is driven by the risk behavior of migrants, their influx into new areas, and the impact on existing network structures (28).
Network-based mathematical models can represent complex behavioral phenomena critical to disease transmission. A robust network modeling method recently has been developed with exponential random graph models (ERGM) (71–73) with applications to migration. ERGM models stochastically simulate partnership formation and dissolution, and then disease transmission on that partnership network. These models may be parameterized with cross-sectional egocentric data, in which persons are randomly sampled and asked characteristics about their partners. ERGM statistical theory allows for a simulation of the complete network based on this data, with dynamics introduced by estimation of average partnership durations. Relevant network predictors may include momentary degree distribution (number of current partnerships), assortative mixing (similarity of partners on certain traits), and higher-order network properties. Onto this, the HIV transmission simulation incorporates HIV features (e.g., stage-specific transmission probabilities) and key bio-behavioral parameters (e.g., coincident STI infections). Importantly, dependence between the partnership and the disease modules is possible, with network structure influencing whom gets infected and disease history determining how partnerships form or dissolve (e.g., through HIV-related mortality).
This ERGM epidemic modeling framework has huge potential for migration-related HIV research. Population bridging may be estimated from empirical data measuring rates of mobility between two areas and numbers of sexual contacts in each. This explicit spatio-temporal network structure allows for testing for threshold levels at which migratory bridging leads to epidemics. Migration-driven concurrency is the key network structure of interest, and may have a dramatic impact on HIV incidence in either of the sending or receiving regions. Khanna et al. have suggested that ERGM-derived network models are well-suited to represent complex mixing phenomena due to migration over time and space (74). Pairwise compartmental models have also addressed partnership concurrency and HIV (75); these models may be useful for migration and HIV research as well.
While ERGM-derived network models have their clear advantages, mathematical modeling generally can be a useful tool to investigate the complex dependent transmission systems that are invoked in migration-related HIV research. Theoretically, future modeling is needed to provide formal comparisons of modeling approaches discussed here, and substantively, network-based models should incorporate data-derived estimates of migration rates between areas, area-specific contact rates, and other relevant epidemiologic features.
5. CONCLUSION
A unified set of measures and study designs does not seem possible for a field as broad as migration and HV in sub-Saharan Africa. However, a shared understanding of conceptual issues, common hypotheses, and strengths and weaknesses of study designs and measures to test hypothesis will organize and advance the field. We largely concentrated on the generalized HIV epidemic in sub-Saharan Africa, and thus the general framework is most relevant to the African context. Nevertheless, migration is a dynamic process, and the role of migration in HIV transmission is a non-linear function of individual, dyadic, and network features regardless of context. Network-based epidemic models and other mathematical models are becoming more common in the field, and will be useful to test complex pathways between migration and HIV transmission at multiple scales.
There are many new and important research agendas yet to be explored in the field of migration and HIV. Today the role of migration in the HIV epidemic is qualitatively different than in the past, especially due to the move toward treatment as prevention. Migrants may be a significant population to target for prevention, such as interventions to improve linkage to care and ART adherence. Understanding patterns of migration is essential for HIV surveillance and calculating unbiased population metrics (63, 76, 77). Lastly, migration patterns may be essential in vaccine development considerations since they predict the distribution of HIV sub-types in sub-Saharan Africa (78). The tools we reviewed in this paper will help unify future research on migration and HIV risk in sub-Saharan Africa.
References
- 1.Cliff A, Haggett P. Time, travel and infection British Medical Bulletin. 2004;69:87–99. doi: 10.1093/bmb/ldh011. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. Policy brief: HIV and International Labour Migration. 2008. [Google Scholar]
- 3.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2012. 2012. [Google Scholar]
- 4.Padian NS, McCoy SI, Karim SSA, Hasen N, Kim J, Bartos M, et al. HIV prevention transformed: the new prevention research agenda. Lancet. 2011;378(9787):269–78. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn TC. Population Migration And The Spread Of Type-1 And Type-2 Human Immunodeficiency Viruses. Proc Natl Acad Sci U S A. 1994;91(7):2407–14. doi: 10.1073/pnas.91.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockerhoff M, Biddlecom AE. Migration, Sexual behavior and the risk of HIV in Kenya. International Migration Review. 1999;33(4):833–56. [Google Scholar]
- 7.Gras MJ, Weide JF, Langendam MW, Coutinho RA, van den Hoek A. HIV prevalence, sexual risk behaviour and sexual mixing patterns among migrants in Amsterdam, The Netherlands. Aids. 1999;13(14):1953–62. doi: 10.1097/00002030-199910010-00019. Epub 1999/10/08. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch JS, Higgins J, Bentley ME, Nathanson CA. The social constructions of sexuality: Marital infidelity and sexually transmitted disease - HIV risk in a Mexican migrant community. Am J Public Health. 2002;92(8):1227–37. doi: 10.2105/ajph.92.8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt CW. Migrant labor and sexually transmitted disease: AIDS in Africa. Journal of Health and Social Behavior. 1989;30(4):353–73. [PubMed] [Google Scholar]
- 10.Lurie MN, Williams BG, Zuma K, Mkaya-Mwamburi D, Garnett G, Sturm AW, et al. The Impact of Migration on HIV-1 Transmission in South Africa: a Study of Migrant and Nonmigrant Men and their Partners. Sex Transm Dis. 2003;30(2):149–56. doi: 10.1097/00007435-200302000-00011. Epub 2003/02/05. [DOI] [PubMed] [Google Scholar]
- 11.Potterat JJ, Rothenberg RB, Woodhouse DE, Muth JB, Pratts CI, Fogle JS. Gonorrhea as a Social Disease. Sex Transm Dis. 1985;12(1):25–32. doi: 10.1097/00007435-198501000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Zuma K, Gouws E, Williams B, Lurie M. Risk factors for HIV infection among women in Carletonville, South Africa: migration, demography and sexually transmitted diseases. Int J Std Aids. 2003;14(12):814–7. doi: 10.1258/095646203322556147. [DOI] [PubMed] [Google Scholar]
- 13.Nunn AJ, Wagner HU, Kamali A, Kengeya-Kayondo JF, Mulder DW. Migration and HIV-1 seroprevalence in a rural Ugandan population. Aids. 1995;9(5):503–6. [PubMed] [Google Scholar]
- 14.Coffee MP, Garnett GP, Mlilo M, Voeten H, Chandiwana S, Gregson S. Patterns of movement and risk of HIV infection in rural Zimbabwe. J Infect Dis. 2005;191:S159–S67. doi: 10.1086/425270. [DOI] [PubMed] [Google Scholar]
- 15.Lurie M. Migration and AIDS in southern Africa: a review. South African Journal Of Science. 2000;96(6):343–7. [Google Scholar]
- 16.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11(5):641–83. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Morris M, Epstein H. Direct empirical evidence for the role of concurrency in sub-Saharan African heterosexual epidemics: 10 studies, 9 countries and 20 years of data confirm the primary role of concurrent partnerships in HIV transmission. 19th International AIDS Conference; 22–27 July; Washington, D.C. 2012. [Google Scholar]
- 18.Goodreau SM, Cassels S, Kasprzyk D, Montano DE, Greek A, Morris M. Concurrent Partnerships, Acute Infection and HIV Epidemic Dynamics Among Young Adults in Zimbabwe. AIDS Behav. 2012;16(2):312–22. doi: 10.1007/s10461-010-9858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanser F, Barnighausen T, Hund L, Garnett GP, McGrath N, Newell ML. Effect of concurrent sexual partnerships on rate of new HIV infections in a high-prevalence, rural South African population: a cohort study. Lancet. 2011;378(9787):247–55. doi: 10.1016/S0140-6736(11)60779-4. Epub 2011/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deane KD, Parkhurst JO, Johnston D. Linking migration, mobility and HIV. Trop Med Int Health. 2010;15(12):1458–63. doi: 10.1111/j.1365-3156.2010.02647.x. [DOI] [PubMed] [Google Scholar]
- 21.Vissers DCJ, Voeten H, Urassa M, Isingo R, Ndege M, Kumogola Y, et al. Separation of spouses due to travel and living apart raises HIV risk in Tanzanian couples. Sex Transm Dis. 2008;35(8):714–20. doi: 10.1097/OLQ.0b013e3181723d93. [DOI] [PubMed] [Google Scholar]
- 22.Mundandi C, Vissers D, Voeten H, Habbema D, Gregson S. No difference in HIV incidence and sexual behaviour between out-migrants and residents in rural Manicaland, Zimbabwe. Trop Med Int Health. 2006;11(5):705–11. doi: 10.1111/j.1365-3156.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 23.Voeten HACM, Vissers DCJ, Gregson S, Zaba B, White RG, de Vlas SJ, et al. Strong Association Between In-Migration and HIV Prevalence in Urban Sub-Saharan Africa. Sex Transm Dis. 2010;37(4):240–3. doi: 10.1097/OLQ.0b013e3181c3f2d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weine SM, Kashuba AB. Labor migration and HIV risk: a systematic review of the literature. AIDS behav. 2012;16(6):1605–21. doi: 10.1007/s10461-012-0183-4. Epub 2012/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diez Roux AV. Next steps in understanding the multilevel determinants of health. J Epidemiol Community Health. 2008;62(11):957–9. doi: 10.1136/jech.2007.064311. Epub 2008/10/16. [DOI] [PubMed] [Google Scholar]
- 26.Diez Roux AV, Aiello AE. Multilevel analysis of infectious diseases. J Infect Dis. 2005;191 (Suppl 1):S25–33. doi: 10.1086/425288. Epub 2005/01/01. [DOI] [PubMed] [Google Scholar]
- 27.Morris M. Sexual networks and HIV. AIDS. 1997;11(Supplement A):S209–S16. [PubMed] [Google Scholar]
- 28.Walker PT, Hallett TB, White PJ, Garnett GP. Interpreting declines in HIV prevalence: impact of spatial aggregation and migration on expected declines in prevalence. Sexually transmitted infections. 2008;84:II42–II8. doi: 10.1136/sti.2008.029975. [DOI] [PubMed] [Google Scholar]
- 29.Morris M, Podhisita C, Wawer MJ, Handcock MS. Bridge populations in the spread of HIV/AIDS in Thailand. AIDS. 1996;10(11):1265–71. doi: 10.1097/00002030-199609000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 31.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. Epub 2000/08/24. [DOI] [PubMed] [Google Scholar]
- 32.Anglewicz P. Migration, Marital Change, and HIV Infection in Malawi. Demography. 2012;49(1):239–65. doi: 10.1007/s13524-011-0072-x. Epub 2011/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffee MP, Lurie MN, Garnett GP. Modelling the impact of migration on the HIV epidemic in South Africa. Aids. 2007;21(3):343–50. doi: 10.1097/QAD.0b013e328011dac9. [DOI] [PubMed] [Google Scholar]
- 34.Lurie MN, Harrison A, Wilkinson D, Abdool Karim SS. Circular migration and sexual networking in rural KwaZulu/Natal: implications for the spread of HIV and other sexually transmitted diseases. Health transition review : the cultural, social, and behavioural determinants of health. 1997;7:17–28. [PubMed] [Google Scholar]
- 35.Gelpi-Acosta C, Hagan H, Jenness SM, Wendel T, Neaigus A. Sexual and injection-related risks in Puerto Rican-born injection drug users living in New York City: A mixed-methods analysis. Harm Reduct J. 2011;8:28. doi: 10.1186/1477-7517-8-28. Epub 2011/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishamawe C, Vissers DCJ, Urassa M, Isingo R, Mwaluko G, Borsboom G, et al. Mobility and HIV in Tanzanian couples: both mobile persons and their partners show increased risk. Aids. 2006;20(4):601–8. doi: 10.1097/01.aids.0000210615.83330.b2. [DOI] [PubMed] [Google Scholar]
- 37.Pouget ER, Kershaw TS, Niccolai LM, Ickovics JR, Blankenship KM. Associations of sex ratios and male incarceration rates with multiple opposite-sex partners: potential social determinants of HIV/STI transmission. Public health reports. 2010;125 (Suppl 4):70–80. doi: 10.1177/00333549101250S411. Epub 2010/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon R. Length Biased Sampling in Etiologic Studies. American journal of epidemiology. 1980;111(4):444–52. doi: 10.1093/oxfordjournals.aje.a112920. [DOI] [PubMed] [Google Scholar]
- 39.Greif MJ, Dodoo FN-A. Internal migration to Nairobi’s slums: Linking migrant streams to sexual risk behavior. Health Place. 2011;17(1):86–93. doi: 10.1016/j.healthplace.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Lagarde E, van der Loeff MS, Enel C, Holmgren B, Dray-Spira R, Pison G, et al. Mobility and the spread of human immunodeficiency virus into rural areas of West Africa. International journal of epidemiology. 2003;32(5):744–52. doi: 10.1093/ije/dyg111. [DOI] [PubMed] [Google Scholar]
- 41.Pison G, Le Guenno B, Lagarde E, Enel C, Seck C. Seasonal migration: a risk factor for HIV infection in rural Senegal. J Acquir Immune Defic Syndr. 1993;6(2):196–200. [PubMed] [Google Scholar]
- 42.Sambisa W, Stokes CS. Rural/urban residence, migration, HIV/AIDS, and safe sex practices among men in Zimbabwe. Rural Sociology. 2006;71(2):183–211. [Google Scholar]
- 43.Weine S, Bahromov M, Loue S, Owens L. HIV sexual risk behavior and multilevel determinants among male labor migrants from Tajikistan. Journal of Immigrant and Minority Health. 2013;15(4):700–10. doi: 10.1007/s10903-012-9718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spiegel PB. HIV/AIDS among conflict-affected and displaced populations: Dispelling myths and taking action. Disasters. 2004;28(3):322–39. doi: 10.1111/j.0361-3666.2004.00261.x. [DOI] [PubMed] [Google Scholar]
- 45.Camlin CS, Hosegood V, Newell ML, McGrath N, Barnighausen T, Snow RC. Gender, migration and HIV in rural KwaZulu-Natal, South Africa. Plos One. 2010;5(7):e11539. doi: 10.1371/journal.pone.0011539. Epub 2010/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan MR, Patnaik P, Brown L, Nagot N, Salouka S, Weir SS. Mobility and HIV-related sexual behavior in Burkina Faso. AIDS behav. 2008;12(2):202–12. doi: 10.1007/s10461-007-9314-8. [DOI] [PubMed] [Google Scholar]
- 47.Kwena ZA, Camlin C, Shisanya CA, Mwanzo I, Bukusi EA. Short-Term Mobility and the Risk of HIV Infection among Married Couples in the Fishing Communities along Lake Victoria, Kenya. Plos One. 2013;8(1):e54523. doi: 10.1371/journal.pone.0054523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lydie N, Robinson NJ, Ferry B, Akam E, De Loenzien M, Abega S, et al. Mobility, sexual behavior, and HIV infection in an urban population in Cameroon. J Acquir Immune Defic Syndr. 2004;35(1):67–74. doi: 10.1097/00126334-200401010-00010. [DOI] [PubMed] [Google Scholar]
- 49.Cassels S, Manhart L, Jenness SM, Morris M. Short-term Mobility and Increased Partnership Concurrency among Men in Zimbabwe. Plos One. 2013;8(6):e66342. doi: 10.1371/journal.pone.0066342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luke N, Clark S, Zulu E. The Relationship History Calendar: Improving the Scope and Quality of Data on Youth Sexual Behavior. Demography. 2011;48:1151– 76. doi: 10.1007/s13524-011-0051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris M. Network Epidemiology: A handbook for survey design and data collection. Oxford: Oxford University Press; 2004. [Google Scholar]
- 52.Lurie MN, Williams BG, Zuma K, Mkaya-Mwamburi D, Garnett GP, Sweat MD, et al. Who infects whom? HIV-1 concordance and discordance among migrant and non-migrant couples in South Africa. Aids. 2003;17(15):2245–52. doi: 10.1097/00002030-200310170-00013. [DOI] [PubMed] [Google Scholar]
- 53.Camlin CS, Kwena ZK, Dworkin S, Cohen C, Bukusi EA. “She mixes her business”: HIV transmission and acquisition risks among migrant and highly mobile women in western Kenya. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camlin CS, Kwena ZK, Dworkin SL. Jaboya vs. Jakambi: Status, negotiation and HIV risk in the “sex for fish” economy in Nyanza Province, Kenya. Aids Educ Prev. 2013;25(3):216–31. doi: 10.1521/aeap.2013.25.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coast E. Local understandings of, and responses to, HIV: Rural-urban migrants in Tanzania. Social science & medicine. 2006;63(4):1000–10. doi: 10.1016/j.socscimed.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Kenyon C, Colebunders R, Voeten H, Lurie M. Peak HIV prevalence: a useful outcome variable for ecological studies. International Journal of Infectious Diseases. 2013;17(5):e286–8. doi: 10.1016/j.ijid.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wakefield J. Ecologic studies revisited. Annual review of public health. 2008;29:75. doi: 10.1146/annurev.publhealth.29.020907.090821. [DOI] [PubMed] [Google Scholar]
- 58.Koopman JS, Longini IM. The Ecological Effects of Individual Exposures and Nonlinear Disease Dynamics in Populations. Am J Public Health. 1994;84(5):836–42. doi: 10.2105/ajph.84.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auchincloss AH, Diez-Roux AV. A new tool for epidemiology: The usefulness of dynamic-agent models in understanding place effects on health. American Journal of Epidemiology. 2008;168(1):1–8. doi: 10.1093/aje/kwn118. [DOI] [PubMed] [Google Scholar]
- 60.Fenton KA, Johnson AM, McManus S, Erens B. Measuring sexual behaviour: methodological challenges in survey research. Sexually transmitted infections. 2001;77(2):84–92. doi: 10.1136/sti.77.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Egan JE, Frye V, Kurtz SP, Latkin C, Chen MX, Tobin K, et al. Migration, Neighborhoods, and Networks: Approaches to Understanding How Urban Environmental Conditions Affect Syndemic Adverse Health Outcomes Among Gay, Bisexual and Other Men Who Have Sex with Men. Aids Behav. 2011;15:S35–S50. doi: 10.1007/s10461-011-9902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuma K, Lurie MN, Williams BG, Mkaya-Mwamburi D, Garnett GP, Sturm AW. Risk factors of sexually transmitted infections among migrant and non-migrant sexual partnerships from rural South Africa. Epidemiology And Infection. 2005;133(3):421–8. doi: 10.1017/s0950268804003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feldacker C, Emch M, Ennett S. The who and where of HIV in rural Malawi: Exploring the effects of person and place on individual HIV status. Health Place. 2010;16(5):996–1006. doi: 10.1016/j.healthplace.2010.06.004. Epub 2010/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Social science & medicine. 2002;55(1):125–39. doi: 10.1016/s0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 65.VanderWeele TJ. Mediation and mechanism. European journal of epidemiology. 2009;24(5):217–24. doi: 10.1007/s10654-009-9331-1. Epub 2009/03/31. [DOI] [PubMed] [Google Scholar]
- 66.Baggaley RF, Fraser C. Modelling sexual transmission of HIV: testing the assumptions, validating the predictions. Current Opinion in HIV and AIDS. 2010;5(4):269–76. doi: 10.1097/COH.0b013e32833a51b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cassels S, Clark S, Morris M. Mathematical Models for HIV Transmission Dynamics: Tools for social and behavioral science research. J Acquir Immune Defic Syndr. 2008;47(Supplement 1):S34– S9. doi: 10.1097/QAI.0b013e3181605da3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet. 2011;378(9790):515–25. doi: 10.1016/S0140-6736(10)61505-X. Epub 2011/04/13. [DOI] [PubMed] [Google Scholar]
- 69.Cassels S, Goodreau SM. Interaction of mathematical modeling and social and behavioral HIV/AIDS research. Curr Opin HIV AIDS. 2011;6(2):119–23. doi: 10.1097/COH.0b013e328343acad. Epub 2011/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiridou M, van Veen M, Coutinho R, Prins M. Can migrants from high-endemic countries cause new HIV outbreaks among heterosexuals in low-endemic countries? Aids. 2010;24(13):2081–8. doi: 10.1097/QAD.0b013e32833a6071. [DOI] [PubMed] [Google Scholar]
- 71.Goodreau SM, Carnegie NB, Vittinghoff E, Lama JR, Sanchez J, Grinsztejn B, et al. What Drives the US and Peruvian HIV Epidemics in Men Who Have Sex with Men (MSM)? PLoS One. 2012;7(11):e50522. doi: 10.1371/journal.pone.0050522. Epub 2012/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Handcock MS, Hunter DR, Butts CT, Goodreau SM, Morris M. statnet: Software Tools for the Representation, Visualization, Analysis and Simulation of Network Data. J Stat Softw. 2008;24(1):1548–7660. doi: 10.18637/jss.v024.i01. Epub 2008/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S The Network Modeling Group. Concurrent partnerships and HIV prevalence disparities by race: Linking science and public health practice. Am J Public Health. 2009;99(6):1023–31. doi: 10.2105/AJPH.2008.147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khanna A, Dimitrov D, Goodreau SM. Circular migrations and HIV transmission: a comparison of compartmental and network modeling. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention; June 30 – July 3; Kuala Lumpur, Malaysia. 2013. [Google Scholar]
- 75.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378(9787):256–68. doi: 10.1016/S0140-6736(11)60842-8. Epub 2011/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verguet S, Lim SS, Murray CJ, Gakidou E, Salomon JA. Incorporating Loss to Follow-up in Estimates of Survival Among HIV-Infected Individuals in Sub-Saharan Africa Enrolled in Antiretroviral Therapy Programs. J Infect Dis. 2013;207(1):72–9. doi: 10.1093/infdis/jis635. Epub 2012/10/27. [DOI] [PubMed] [Google Scholar]
- 77.Dombrowski JC, Kent JB, Buskin SE, Stekler JD, Golden MR. Population-based metrics for the timing of HIV diagnosis, engagement in HIV care, and virologic suppression. Aids. 2012;26(1):77–86. doi: 10.1097/QAD.0b013e32834dcee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tatem AJ, Hemelaar J, Gray RR, Salemi M. Spatial accessibility and the spread of HIV-1 subtypes and recombinants. AIDS. 2012;26(18):2351–60. doi: 10.1097/QAD.0b013e328359a904. Epub 2012/09/07. [DOI] [PubMed] [Google Scholar]