Abstract
Objectives
To determine the incidence of and predictors for recovery of normal echocardiographic function among children with idiopathic dilated cardiomyopathy (DCM).
Background
Most children with idiopathic DCM have poor outcomes; however, some improve.
Methods
We studied children less than 18 years old in the Pediatric Cardiomyopathy Registry who had both depressed left ventricular (LV) function (fractional shortening [FS] or ejection fraction [EF] z-score <−2) and LV dilation (end-diastolic dimension [LVEDD] z-score >2) at diagnosis and who had at least one follow-up echocardiogram 30 days to 2 years from the initial echocardiogram. We estimated the cumulative incidence and predictors of normalization.
Results
Among 868 children who met inclusion criteria, 741 (85%) had both echocardiograms. At 2 years, 22% had recovered normal LV function and size; 51% had died or undergone heart transplant (median, 3.2 months), and 27% had persistently abnormal echocardiograms. Younger age (hazard ratio, 0.90; 95% CI, 0.86 to 0.95) and lower LVEDD z-score (0.75; 95% CI, 0.68 to 0.84) independently predicted normalization. Nine children (9%) with normal LV function and size within 2 years of diagnosis later underwent heart transplant or died.
Conclusions
Despite marked LV dilation and depressed function initially, children with idiopathic DCM can recover normal LV size and function, particularly those younger and with less LV dilation at diagnosis. Investigations related to predictors for recovery, such as gene associations, serum markers, and the impact of medical therapy or ventricular unloading with assist devices are important next steps. Longer follow-up after normalization is warranted as cardiac failure can recur.
Clinical Trials Registration # NCT00005391
Keywords: cardiomyopathy, pediatrics, heart failure, echocardiography
INTRODUCTION
Idiopathic dilated cardiomyopathy (DCM) is a disease of the heart muscle characterized by ventricular chamber enlargement and systolic dysfunction (1, 2). The prognosis is usually poor, but some patients do recover normal cardiac function. Studies in both adults and children have reported recovery of cardiac function in 21% to 37% of patients, as indicated by serial echocardiograms (3–5). Survival of adults with idiopathic DCM has improved in recent decades, with more than half surviving for 10 years (5,6). Although risk factors for death or transplant in children with idiopathic DCM are well studied, predictors for normalization are largely unknown (7–10). With additional medical therapies being used to treat heart failure in children, as well as a marked increase in the use of ventricular assist devices, identifying predictors of recovering cardiac function are of particular importance.
We studied children with idiopathic DCM who had sufficient echocardiogram data to assess recovery of normal LV function and size. We identified the proportion of these children who regained normal echocardiographic measurements in the large, multicenter, NHLBI-funded Pediatric Cardiomyopathy Registry (PCMR) (11, 12). We sought to identify the clinical characteristics at presentation that predict echocardiographic normalization within 2 years. Additionally, we report longer-term follow-up data in children whose LV function and size returned to normal within 2 years of diagnosis to examine the permanence of recovery.
METHODS
Study Design
The design and conduct of the PCMR are detailed elsewhere (13, 14). Institutional Review Board approval for the PCMR was obtained at all participating centers. From 1990 to 2012, the PCMR enrolled more than 3,000 children less than 18 years old in whom cardiomyopathy was diagnosed at any of 98 pediatric cardiac centers in the United States and Canada. The data in the PCMR database current as of January 14, 2013 were analyzed.
Patient Classification
The PCMR diagnostic criteria for pure DCM (13, 14) are based on strict echocardiographic measurements related to left ventricular (LV) enlargement and depressed function; pathologic findings at autopsy or by endomyocardial biopsy; or clinical evidence from the diagnosing physician. We studied only children with idiopathic DCM, defined as having an unknown cause for DCM at the time of diagnosis (1, 6, 9). Excluded were children who were classified at presentation by the clinical investigators as having neuromuscular disease, familial cardiomyopathy, metabolic or mitochondrial disorder, or myocarditis based on the presentation of new-onset cardiac symptoms and/or echocardiographic abnormalities developing after a history of recent infection with or without endomyocardial biopsy evidence of myocarditis (4). Additionally, to examine recovery of normal LV function and size in a homogeneous group, the sample was restricted to children who had both LV dilation (i.e., LV end-diastolic dimension > 2 SD above normal for body-surface area) and depressed LV systolic function (LV fractional shortening or LVEF > 2 SD below normal for age) at diagnosis. Echocardiographic outcomes were determined only from children with echocardiographic data on function and size both at diagnosis and a follow-up within 2 years. A minimum interval of 30 days between the first and subsequent echocardiograms was used to classify children into two groups: those with persistently abnormal echocardiograms and those who recovered normal LV size and function. Figure 1 details the composition of the analytic cohort. Echocardiographic data beyond 2 years was not sufficiently complete to uniformly assess a longer follow-up period for recovering normal echocardiographic function and size. However, long-term follow-up data regarding death or heart transplant outside the 2-year window, where available, are reported for each group.
Figure 1. Sample selection to identify echocardiographic normalization in children with idiopathic dilated cardiomyopathy.
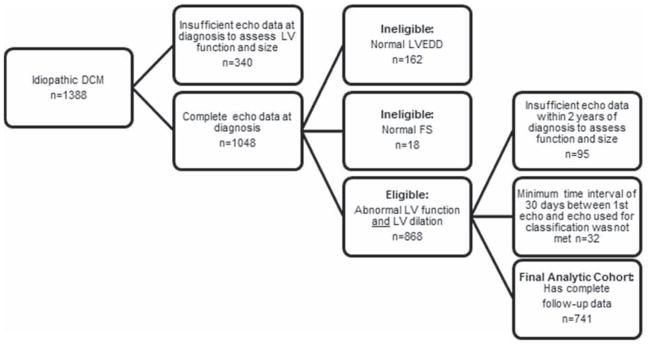
Sample selection to identify echocardiographic normalization in children with idiopathic dilated cardiomyopathy.
Data Collection
Patient age, race, sex, weight, height, and body surface area (BSA), were collected at diagnosis. Clinical evidence of congestive heart failure and echocardiographic measurements of LVEDD, LV end-systolic dimension (LVESD), LV FS, LV septal and posterior wall thicknesses, and LV mass were also collected at diagnosis and annually thereafter. Values for LV measurements are expressed as z-scores to adjust for the effect of body size and age. The z-score is the number of standard deviations from the mean value at a given BSA in a distribution of a large population of normal children. The z-score for the mean of this population distribution is 0, and the normal range is typically defined as −2 to +2 standard deviations (10).
Statistical Methods
The Data Coordinating Center at the New England Research Institutes, Watertown, MA, performed all data analyses. Summary statistics are presented as means and standard deviations or as medians and interquartile ranges for continuous variables and as percentages for categorical variables. Changes in echocardiographic measures obtained at diagnosis and follow-up were compared using a paired t-test. Patient characteristics among the 3 groups were compared with analysis of variance or the Kruskal-Wallis test for continuous variables and a Fisher exact test for categorical variables.
We used nonparametric competing-risks methodology to estimate the cumulative incidence rates of echocardiographic normalization vs. death or transplant vs. persistent abnormal LV size or function (15). Risk factors for the outcome of echocardiographic normalization in the presence of death or transplant as a competing risk were also identified using this methodology. Candidate predictors for the multivariable model were variables with a univariate p value less than 0.2 and had few missing data. A p value less than 0.05 was considered to be statistically significant. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC) or R version 2.14.1, including the cmprsk library.
RESULTS
Among 1388 children with idiopathic DCM diagnosed between 1990 and 2012, 868 met the echocardiographic inclusion criteria of both depressed LV systolic function and LV dilation (Figure 1). Of these, 741 (85%) had sufficient follow-up data 30 days to 2 years following diagnosis and were included in the analysis. The 127 with insufficient follow-up data were slightly older at diagnosis (median 2.0, interquartile range 0.6 to 8.4 years, vs. median 1.0, interquartile range 0.3 to 8.1 years; p = 0.01). Of the 741 in the analytic cohort, 96 were classified as achieving normalization of LV size and function based on echocardiographic data, 317 died or underwent heart transplant, and 328 were classified as persistently abnormal LV size and/or function.
Patient Characteristics
Children with normal echocardiograms within 2 years after diagnosis were younger at diagnosis (Table 1). The normalized group had less LV dilation at diagnosis and a higher mean fractional shortening z-score than did the persistently abnormal group and the death-or-heart transplant group (Table 2).
Table 1.
Characteristics of 741 Children with Idiopathic Dilated Cardiomyopathy at Diagnosis, by Echocardiographic Status or Clinical Outcome within 2 Years after Diagnosis.
| Characteristic | Status 2 years after diagnosis | |||
|---|---|---|---|---|
|
| ||||
| Normalized, n=96 | Death or Transplant, n=317 | Persistently Abnormal, n=328 | P | |
| Follow up since diagnosis, median, months | 9.5 | 3.2 | 17.7 | |
| Age at diagnosis, mean (SD), years | 2.4 (4.0) | 5.4 (6.0) | 3.7 (5.3) | <0.001 |
| Median age at diagnosis, years | 0.93 | 1.67 | 0.91 | <0.001 |
| <1 years, % | 55.2 | 42.9 | 53.4 | <0.001 |
| 1- to 10 years, % | 35.4 | 26.8 | 29.3 | |
| > 10 years, % | 9.4 | 30.3 | 17.4 | |
| Height-for-age, mean (SD), z-score | −0.64 (1.74) | −0.52 (1.69) | −0.32 (1.48) | 0.30 |
| (n=47) | (n=193) | (n=208) | ||
| Race, % | 0.07 | |||
| White | 53.8 | 55.6 | 53.7 | |
| Black | 16.1 | 23.8 | 22.2 | |
| Hispanic | 25.8 | 15.2 | 15.1 | |
| Other | 4.3 | 5.4 | 9.0 | |
| Male, % | 43.8 | 49.2 | 45.4 | 0.50 |
| Era of diagnosis, % | 0.50 | |||
| 1990–1999 | 60.4 | 62.5 | 57.9 | |
| 2000–2010 | 39.6 | 37.5 | 42.1 | |
| Congestive heart failure at diagnosis, % | 74.0 | 88.3 | 69.4 | <0.001 |
Table 2.
Left Ventricular Echocardiographic Profile at Diagnosis, by Echocardiographic Status or Clinical Outcome within 2 Years after Diagnosis.
| Status 2 years after diagnosis | ||||
|---|---|---|---|---|
|
| ||||
| Normalized, n=96 | Death or Transplant, n=317 | Persistently Abnormal, n=328 | P | |
| EDD, mean (SD), z-score | 4.56 (1.87) | 5.70 (2.06) | 5.41 (2.06) | <0.001 |
| ESD, mean (SD), z-score | 6.39 (2.03) | 7.69 (1.95) | 7.20 (2.09) | <0.001 |
| (n=87) | (n=287) | (n=293) | ||
| FS, mean (SD), z-score | −9.21 (2.86) | −10.28 (2.28) | −9.34 (2.81) | <0.001 |
| (n=90) | (n=297) | (n=313) | ||
| FS, mean (SD), % | 15.4 (6.9) | 11.4 (4.9 | 14.7 (6.3) | <0.001 |
| PWT, mean (SD), z-score | −0.16 (2.29) | −0.39 (2.57) | −0.45 (2.56) | 0.65 |
| (n=81) | (n=238) | (n=257) | ||
| SWT, mean (SD), z-score | −0.77 (1.46) | −0.94 (1.73) | −0.88 (1.88) | 0.77 |
| (n=79) | (n=217) | (n=240) | ||
| mass, mean (SD), z-score | 2.71 (1.85) | 3.42 (2.53) | 3.11 (2.23) | 0.046 |
| (n=81) | (n=238) | (n=257) | ||
| PWT:EDD ratio, mean (SD) | 0.13 (0.04) | 0.12 (0.07) | 0.12 (0.04) | 0.18 |
| (n=81) | (n=238) | (n=257) | ||
EDD, end diastolic dimension; ESD, end systolic dimension; FS, fractional shortening; PWT, posterior wall thickness; SWT, septal wall thickness.
In the normalized group, mean LV size, which was more than 4 standard deviations above normal at diagnosis, had a mean z-score of −0.06 on follow-up echocardiograms, and the fractional shortening z-score improved from a mean of −9 to close to zero (−0.1). In absolute terms, mean LV fractional shortening increased from 15% to 37%. In the death-or-transplant group, baseline and follow-up echocardiographic measurements for LV size and function did not change. In the persistently abnormal group, LV function improved slightly and LV size and mass decreased, but not enough to be considered normal (Table 3).
Table 3.
Left Ventricular Echocardiographic Profile from Time of Cardiomyopathy Diagnosis to 2 Years, by Echocardiographic Status or Clinical Outcome within 2 Years after Diagnosis. Mean and standard deviation are shown.
| Status at 2 years Post-Diagnosis
| ||||||
|---|---|---|---|---|---|---|
| Echo Characteristic |
Normalized | Death or Transplant | Persistently Abnormal | |||
|
| ||||||
| At Diagnosis, n=96 |
Follow-up Echocardiogram* n=96 |
At Diagnosis, n=317 |
Follow-up Echocardiogram* n=202 |
At Diagnosis, n=328 |
Follow-up Echocardiogram* n=328 |
|
| EDD, z-score | 4.56 (1.87) | −0.06 (4.25) | 5.70 (2.06) | 6.05 (2.34) | 5.41 (2.06) | 3.43 (2.63) |
| ESD, z-score | 6.39 (2.03) | −0.17 (3.56) | 7.69 (1.95) | 7.79 (2.37) | 7.20 (2.09) | 4.40 (3.06) |
| FS, z-score | −9.21 (2.86) | −0.08 (1.74) | −10.28 (2.28) | −9.79 (3.14) | −9.34 (2.81) | −5.49 (3.67) |
| FS, % | 15.40 (6.88) | 37.12 (4.76) | 11.44 (4.92) | 12.60 (7.19) | 14.65 (6.33) | 23.16 (9.14) |
| PWT, z-score | −0.16 (2.29) | −0.58 (1.84) | −0.39 (2.57) | 0.15 (2.29) | −0.45 (2.56) | −0.74 (2.64) |
| SWT, z-score | −0.77 (1.46) | −0.53 (1.40) | −0.94 (1.73) | −0.75 (1.49) | −0.88 (1.88) | −0.81 (1.90) |
| Mass, z-score | 2.71 (1.85) | −0.35 (1.83) | 3.42 (2.53) | 3.89 (2.44) | 3.11 (2.23) | 1.55 (2.60) |
| PWT:EDD | 0.13 (0.04) | 0.18 (0.13) | 0.12 (0.07) | 0.13 (0.05) | 0.12 (0.04) | 0.14 (0.08) |
| PWT:EDD, z-score | −1.80 (1.48) | −0.26 (4.66) | −2.23 (2.31) | −1.94 (1.88) | −2.17 (1.48 | −1.62 (2.63) |
EDD, end diastolic dimension; ESD, end systolic dimension; FS, fractional shortening; PWT, posterior wall thickness; SWT, septal wall thickness
Follow-up echocardiogram defined as: the first occurrence of normalization for the Normalized group; the latest echocardiogram before death/transplant for the Death/Transplant group; and the latest echocardiogram within 2 years following diagnosis for the Persistently Abnormal group.
Outcomes
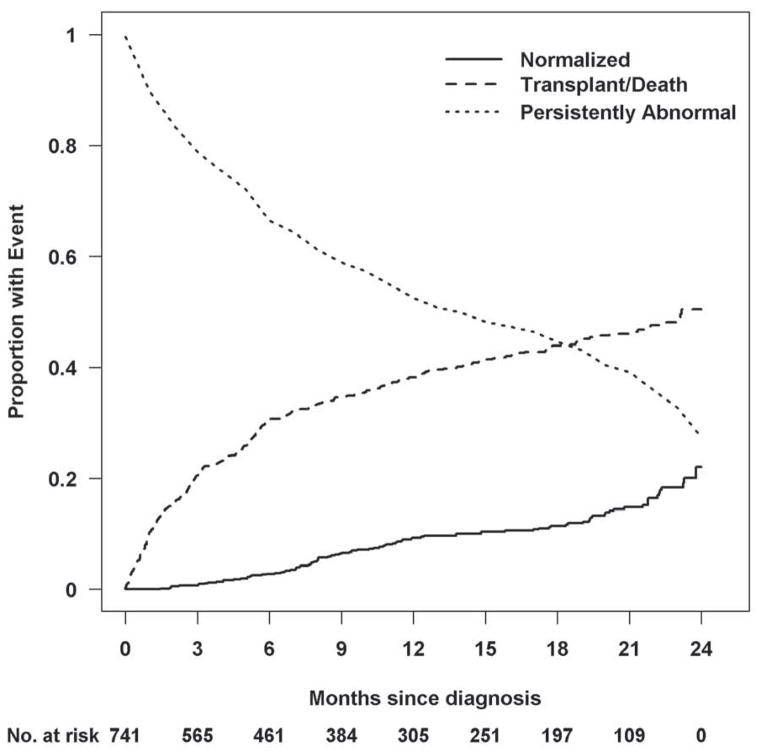
During the 2-year follow-up period, 22% of children had echocardiographic evidence of both normal LV function and size. The cumulative incidence of the three competing outcomes (echocardiographic normalization, death or heart transplant, and persistently abnormal LV function or LV dilation) is shown in Figure 2. In the 96 children with normal LV function and size during the 2-year period, the median time to recovering normal echocardiographic function and size was 9.5 months. Seven of these children subsequently underwent heart transplant. The time interval between the echocardiogram showing normal LV size and function and the heart transplant was between 2 weeks and 10 months in 6 children and 1.75 years in 1 child. Two additional children died at 3.4 and at 10.8 years after diagnosis. A subset (51 patients) of the remaining normalized cases had echocardiographic data later than 2 years. Ten (20%) had follow-up measurements that indicated only abnormal LV size or function but not both.
Figure 2. Estimated cumulative incidence rates for recovering normal echocardiographic function in the presence of the competing risk for death or transplant.
At any given time point, the probabilities associated with the 3 states totals to 1.0. At 2 years, 22% of children had normal echocardiographic values, 51% had died or undergone transplant, and 27% remained abnormal with respect to LV size and function.
For the 328 children who had persistently abnormal echocardiograms within 2 years of diagnosis, the mean (SD) time from diagnosis to the latest echocardiogram within the 2-year period was 16 (±7) months. In this group, 45 children underwent heart transplant or died outside the 2-year analysis window, during a median follow-up time of 3.3 years from diagnosis.
In the 317 children in the death-or-transplant group, 104 died and 213 underwent heart transplant within 2 years after diagnosis of idiopathic DCM. In these 317 children, the median time to death or transplant was 3 months after diagnosis.
Predictors at Diagnosis for Echocardiographic Normalization
On univariate analysis, z-scores for LVEDD, LVSD, LVFS, and LV mass were associated with normalization (Table 4). Multivariable analysis identified younger age at diagnosis and less LV dilation (a lower LVEDD z-score; i.e. closer to normal) as the two independent factors associated with a higher likelihood of recovering normal LV size and function by echocardiogram within 2 years after diagnosis. For each 1-year decrease in age at diagnosis, children were 1.1 times more likely to recover normal LV size and function within 2 years of diagnosis (hazard ratio [HR], 95% CI, 1.05 to 1.16, p < 0.001). For every 1-unit decrease in LVEDD z-score, the likelihood of recovering normal LV size and function within 2 years of diagnosis was 1.3 times higher (HR; 95% CI, 1.19 to 1.47, p < 0.001).
Table 4.
Hazards Ratios from Univariate Cox Regression Analysis of Possible Predictors of Echocardiographic Normalization in 741 Children with Idiopathic Dilated Cardiomyopathy and Abnormal Left Ventricular Function and Dilation at Diagnosis.
| Characteristic at diagnosis | N | Hazard ratio (95% CI) | p |
|---|---|---|---|
| Age, year* | 741 | 0.92 (0.88, 0.97) | <0.001 |
| Age group, years | 741 | 0.008 | |
| <1 | 2.77 (1.38, 5.57) | ||
| 1 to 10 | 3.05 (1.48, 6.28) | ||
| >10 | Reference | ||
| Male | 773 | 0.86 (0.58, 1.27) | 0.44 |
| 741 | 0.88 (0.59, 1.31) | 0.52 | |
| Body surface area z-score | 715 | 1.11 (0.97, 1.27) | 0.12 |
| Height-for-age z-score | 448 | 0.92 (0.78, 1.09) | 0.35 |
| Race | 741 | 0.07 | |
| White | 0.94 (0.42, 2.10) | ||
| Black | 0.65 (0.27, 1.61) | ||
| Hispanic | 1.49 (0.64, 3.49) | ||
| Other | Reference | ||
| Era of diagnosis | 741 | 0.93 | |
| 1990–1999 | 1.02 (0.65, 1.47) | ||
| 2000–2010 | Reference | ||
| Congestive heart failure | 740 | 0.84 (0.53, 1.31) | 0.44 |
| Family history of cardiomyopathy | 454 | 0.79 (0.39, 1.60) | 0.51 |
| Family history of sudden death | 462 | 0.62 (0.19, 2.03) | 0.43 |
| LVEDD z-score* | 741 | 0.78 (0.70, 0.87) | <0.001 |
| Tertile group: | |||
| < 4.29 | 2.67 (1.58, 4.50) | <0.001 | |
| 4.29 to < 6.29 | 1.56 (0.89, 2.76) | ||
| ≥ 6.29 | Reference | ||
| LVESD z-score | 667 | 0.78 (0.70, 0.87) | <0.001 |
| Tertile group: | |||
| < 6.26 | 3.48 (1.98, 6.12) | <0.001 | |
| 6.26 to < 8.17 | 1.59 (0.85, 3.00) | ||
| ≥ 8.17 | Reference | ||
| LV fractional shortening z-score | 700 | 1.08 (1.00, 1.17) | 0.041 |
| Tertile group: | |||
| < −11.1 | 0.72 (0.44, 1.19) | 0.31 | |
| −11.1 to < −8.99 | 0.73 (0.44, 1.18) | ||
| ≥ −8.99 | Reference | ||
| LV posterior wall thickness z-score | 576 | 1.04 (0.96, 1.12) | 0.37 |
| LV septal thickness z-score | 536 | 1.04 (0.94, 1.16) | 0.42 |
| LV mass z-score | 576 | 0.90 (0.83, 0.98) | 0.019 |
| Tertile group: | |||
| < 2.04 | 1.57 (0.90, 2.74) | 0.23 | |
| 2.04 to <4.00 | 1.50 (0.87, 2.61) | ||
| ≥ 4.00 | Reference | ||
| Log (PWT:EDD ratio) | 576 | 2.09 (1.24, 3.53) | 0.006 |
| Tertile group: | |||
| < −2.30 | 0.40 (0.22, 0.72) | 0.009 | |
| −2.30 to <−2.04 | 0.79 (0.49, 1.29) | ||
| ≥ −2.04 | Reference | ||
LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; PWT, posterior wall thickness
Age at diagnosis and LVEDD z-score at diagnosis comprised the final multivariable model for time to echo normalization (both terms p<0.001).
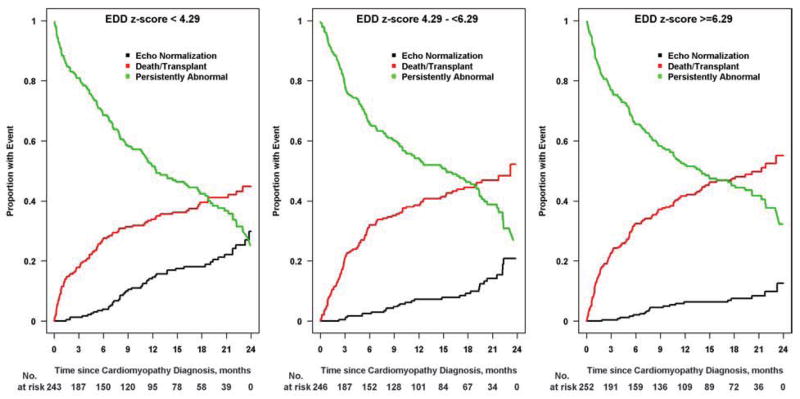
Cumulative incidence of recovering normal LV size and function by age is shown in Figure 3. Younger age at diagnosis, both as a continuous and a categorical variable (<1 year, 1 to 10 years, and >10 years), was associated with a higher incidence of normalization (p < 0.001 and p = 0.008, respectively). Normalization by 2 years was achieved in 24% of infants and in 26% of children aged 1 to 10 years, but in only 10% of those diagnosed over 10 years of age. Figure 4 shows the cumulative incidence of having a normal echo within 2 years of diagnosis based upon LVEDD z-score at diagnosis. Normalization with respect to LV size and function was achieved in 30% of children with LVEDD z-scores in the lowest tertile (<4.29), in 21% of those in the middle tertile, and in 13% of those with LVEDD z-score in the highest tertile (≥6.29). Event probabilities were similar when displayed according to LVESD z-score.
Figure 3. Estimated cumulative incidence rates for echocardiographic normalization by age at diagnosis.
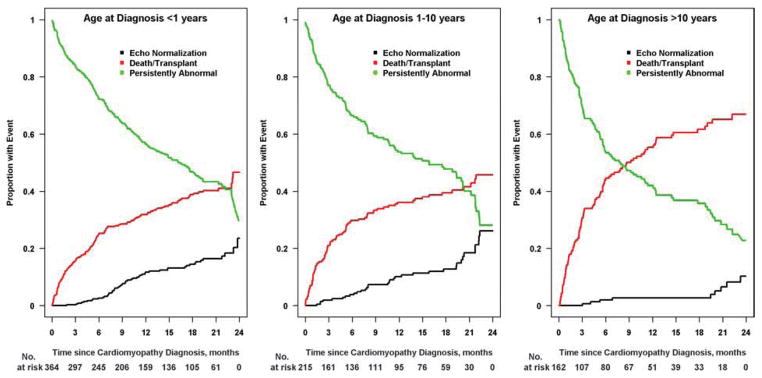
The cumulative incidence for recovery of normal echocardiographic function and size is lowest in children diagnosed after 10 years old (10% by 2 years; Figure 3C) and similar for the two younger age groups: 24% for those diagnosed before age 1 year (Figure 3A) and 26% for those diagnosis before between 1 and 10 years (Figure 3B). Black lines, echocardiographic normalization; red lines, death-or-transplant; green lines, persistently abnormal echocardiogram.
Figure 4. Cumulative incidence rates for echocardiographic normalization by end-diastolic dimensions (EDD) z-score tertile from the time of diagnosis.
Children with lower EDD z-scores at diagnosis were more likely to have recovered normal cardiac function by 2 years: 30% for EDD z-scores less than 4.29 (Figure 4A), 21% for the middle tertile (Figure 4B), and 13% for EDD z-scores 6.29 or higher (Figure 4C). Black lines, echocardiographic normalization; red lines, death-or-transplant; green lines, persistently abnormal echocardiogram.
We conducted a sensitivity analysis to assess whether potential misclassification arising from the use of follow-up echocardiograms obtained before but not precisely at the 2-year time target affected the robustness of the multivariable model. Of the 328 children classified as having persistently abnormal echocardiograms, 204 had an echocardiogram beyond the 2-year time period of interest. No subsequent echocardiogram was available for 124 children classified as persistently abnormal. As a worst-case scenario with regard to misclassification, we reclassified these 156 children (32+124) as having normalized by 2 years after diagnosis, resulting in 317 with death or heart transplant, 252 with normalization, and 172 with persistently abnormal echocardiograms. We obtained nearly the same multivariable model: it included age at diagnosis (HR, 1.0 per year; 95% CI, 1.04 to 1.1, p < 0.001) and LVESD z-score (HR, 1.16 per SD unit; 95% CI, 1.10 to 1.25, p < 0.001).
Medical Therapy
Only the presence or absence of anti-congestive therapy was collected throughout the study period. The proportion of children receiving anti-congestive medications at the time of diagnosis did not differ between groups: 85 of 96 in the normalized group (89%), 284 of 317 in the death-or-transplant group (90%), and 293 of 328 in the persistently abnormal group (89%). However, the proportion of children on anti-congestive therapy at the time of the last echocardiogram within 2 years did differ significantly between groups (p < 0.001), with the highest usage in the death-or-heart transplant group: 210 of 213 (99%) children in the death-or-transplant group, 83 of 96 (87%) in the normalized group, and 284 of 328 (87%) in the persistently abnormal group.
DISCUSSION
We report the 2-year cumulative incidence of recovery of normal LV size and function in children with idiopathic DCM from a large multi-center pediatric cardiomyopathy registry. Although half of the nearly 800 children died or underwent heart transplant within 2 years of presentation, 9% did recover normal LV size and function by 1 year and 22% by 2 years, with a median time to normalization of 9 months. Younger age and less LV dilation at diagnosis independently predicted normalization within 2 years of presentation. However, 9% of the children who recovered normal echocardiographic function within 2 years later died or underwent heart transplant.
Several studies have reported improved cardiac function in some children with DCM. The incidence of cardiac improvement ranges from 16% to 63%, depending on the definition of improvement and the cause of DCM (3, 16–19). In the prospective multicenter Carvedilol trial for children with heart failure, of 93 children with DCM, 59 (63%) had better New York Heart Association or Ross heart failure stage at 6-month follow-up (16).
Not surprisingly, our rate of cardiac improvement, defined as normal LV size and function by echocardiographic assessment, is lower than that of studies defining cardiac improvement as the resolution of heart failure symptoms. In an earlier single-center retrospective series by Lewis et al., of 63 children with idiopathic DCM, 25% had normal LV function at follow-up (3). This study is similar to ours in that children with myocarditis and other known causes of DCM were excluded; all included patients had both depressed LV function and LV dilation at presentation, and the endpoint was recovery of LV function by echocardiographic measurement. In a recent report from the Australian National Population-based Study of Childhood Cardiomyopathy, the incidence of recovering normal echocardiographic LV function and size in children with DCM due to a variety of causes was 33% at 15 years (20). While both of the aforementioned studies show a higher rate of normalization, this difference may be due to the longer duration of follow-up with a mean follow-up duration of 7 years and 15 years, respectively (3, 20). Additionally, when those with a known cause for DCM are excluded, the rate of echocardiographic normalization in the Australian cohort is consistent with our finding, with normalization occurring in approximately 20% at 2 years among the 27 children with idiopathic DCM (20).
Younger age at diagnosis, specifically prior to age 10 years, predicted recovery of normal echocardiographic function and size within 2 years of presentation. Younger age has predicted cardiac recovery in other studies of pediatric DCM as well. Lewis et al. found that children who recovered normal function were younger than those with persistently abnormal function (mean [SD], 2.1 [±1.8] years old compared to 4.5 [±5.9] years old, respectively) (3).
Less LV dilation at presentation was the other independent predictor for recovering normal LV size and function within 2 years of presentation. Although at diagnosis our normalized group had a mean LVEDD z-score that was closer to normal than those of the other groups, the LV was, nevertheless, markedly dilated at presentation, with a mean z-score of 4.6 (1.9). Previously reported single-center series have had relatively small numbers of patients and have not found any echo parameters at presentation predictive for recovery, which may be due to limited power (3, 18). Our analysis contributes new information.
A previous PCMR report found that a higher LVEDD z-score at diagnosis was associated with transplant but not with mortality in DCM from all causes (7). Knowing that heart transplant affects the natural history of idiopathic DCM and competing outcomes, our finding of normal LV size and function in 22% of children by 2 years draws attention to the possibility for myocardial recovery even in children with marked LV dilation at presentation. Nevertheless, longer follow-up is necessary because some of these children (n = 9) later died or underwent heart transplant for recurrent heart failure.
This analysis was not designed to address the need for long-term follow-up or continued medical therapy in children recovering LV size and function. Most of these children were still receiving anti-congestive medications at the time of the follow-up echocardiogram. The impact of the withdrawal of these medications on outcome could not be ascertained from this observational dataset.
An area of study is to identify children with full cardiac recovery in terms of normal diastolic function, normal cardiac magnetic resonance imaging results without residual inflammation or fibrosis, absence of an arrhythmogenic substrate, and normal LV size and function. In our study, some of the children with normal LV size and function at follow-up did later undergo heart transplant, and 10 of 51 normalized cases that had follow-up data past two years had later abnormal LV size or function without recurring normalization. In the Australian report, none of those with normal LV size and function at follow-up had subsequent decreased function, death, or transplant (20). Thus, the question remains as to who requires ongoing follow-up or medical therapy. Are these children at risk for recurrent heart failure, or is it only a subset of children at risk who have persistent abnormalities? How much change over time in LV size and function is natural variation or due to a change in ventricular loading or geometry with alterations in medical therapy? Understanding this cardiac recovery versus remission further and predicting the impact of genotype or gene modifiers on prognosis, response to medical therapy, and response to ventricular-assist support in children with the potential for cardiac recovery are key goals of future research.
Limitations of the Study
The strength of the PCMR is the collaboration of 98 pediatric cardiac centers across the United States and Canada to collect data on children with DCM over 20 years. Importantly, serial echocardiograms collected during this observational study made this analysis possible. Nevertheless, the lack of standardized follow-up and time at which echocardiograms were obtained is a limitation. The echocardiographic data collected were abstracted annually, recording the latest echocardiogram findings from each year. Thus, the timing was left-censored with some children classified as having a normal echocardiogram at 2-year follow-up, but the normalization could have occurred earlier and time to recovery values in this report may be overestimates. However, our sensitivity analysis indicated that our findings were robust to reclassification of patients who had additional follow-up data, and in practice, many patients may not undergo echocardiograms more than annually.
Another limitation is that 15% of subjects known to have abnormal LV function and dilation at diagnosis were excluded from analysis due to insufficient follow-up information. We found that these 127 patients were slightly older at diagnosis than the 741 analyzed. Therefore, because younger age at diagnosis was associated with earlier time to echo normalization, our reported recovery rates may be mildly overestimated with respect to true rates in the pediatric idiopathic DCM population.
Information on the presence of tricuspid or mitral regurgitation and family history of sudden death or heart disease was available for only a few children, so these variables could not be included as candidate predictors in the multivariable model. Lastly, information related to medication use, such as beta blockers and angiotensin-converting enzyme inhibitors, was insufficient for analysis of the association between medical therapy and recovery of normal echocardiographic function; or between discontinuation of therapy and outcome.
Conclusions
Despite marked LV dilation at presentation, 22% of children with idiopathic DCM recovered normal LV size and function within 2 years. Younger age and less LV dilation at diagnosis independently predicted normalization within 2 years of presentation. This improvement in cardiac function and size was not sustained in all children, which suggests that other indicators of cardiac recovery need to be explored, longer follow-up may be required for some children, or medical therapy may need to be continued beyond the recovery of normal LV size and function. Investigations related to predictors for cardiac recovery, such as gene associations and outcome, markers for cardiac recovery versus remission, and the impact of long-term medical therapy or ventricular unloading with ventricular assist devices in children with idiopathic DCM are important next steps.
Acknowledgments
Grant support: Supported by grants from the National Heart, Lung, and Blood Institute (HL 53392 and NHLBI R01 087000) and the Children’s Cardiomyopathy Foundation.
ABBREVIATIONS
- BSA
body surface area
- CI
confidence interval
- DCM
dilated cardiomyopathy
- EDD
end-diastolic dimension
- EF
ejection fraction
- ESD
end-systolic dimension
- FS
fractional shortening
- LV
left ventricular
- SD
standard deviation
Footnotes
The authors have no financial disclosures
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Eng J Med. 1994;331:1564–75. doi: 10.1056/NEJM199412083312307. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies. Circulation. 2006;113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Lewis AB. Late recovery of ventricular function in children with idiopathic dilated cardiomyopathy. Am Heart J. 1999;138:334–8. doi: 10.1016/s0002-8703(99)70121-3. [DOI] [PubMed] [Google Scholar]
- 4.Foerster SR, Canter CE, Cinar A, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the Pediatric Cardiomyopathy Registry. Circ Heart Fail. 2010;3:689–97. doi: 10.1161/CIRCHEARTFAILURE.109.902833. [DOI] [PubMed] [Google Scholar]
- 5.Matsumura Y, Hoshikawa-Nagai E, Kubo T, et al. Left ventricular reverse remodeling in long-term (>12 years) survivors with idiopathic dilated cardiomyopathy. Am J Cardiol. 2013;111:106–10. doi: 10.1016/j.amjcard.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 6.Redfield MM, Gersh BJ, Bailey KR, Ballard DJ, Rodeheffer RJ. Natural history of idiopathic dilated cardiomyopathy: effect of referral bias and secular trend. J Am Coll Cardiol. 1993;22:1921–6. doi: 10.1016/0735-1097(93)90780-5. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez JA, Orav EJ, Wilkinson JD, et al. Competing risks for death and cardiac transplantation in children with dilated cardiomyopathy: results from the Pediatric Cardiomyopathy Registry. Circulation. 2011;124:814–23. doi: 10.1161/CIRCULATIONAHA.110.973826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh TP, Sleeper LA, Lipshultz S, et al. Association of left ventricular dilation at listing for heart transplant with postlisting and early posttransplant mortality in children with dilated cardiomyopathy. Circ Heart Fail. 2009;2:591–8. doi: 10.1161/CIRCHEARTFAILURE.108.839001. [DOI] [PubMed] [Google Scholar]
- 9.Azevedo VM, Santos MA, Albanesi Filho FM, Castier MB, Tura BR, Amino JG. Outcome factors of idiopathic dilated cardiomyopathy in children- a long-term follow-up review. Cardiol Young. 2007;17:175–84. doi: 10.1017/S1047951107000170. [DOI] [PubMed] [Google Scholar]
- 10.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99(2):445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 11.Friedman RA, Moak JP, Garson A., Jr Clinical course of idiopathic dilated cardiomyopathy in children. J Am Coll Cardiol. 1991;18:152–6. doi: 10.1016/s0735-1097(10)80233-5. [DOI] [PubMed] [Google Scholar]
- 12.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 13.Grenier MA, Osganian SK, Cox GF, et al. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139(2 Pt 3):S86–95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statistical Assoc. 1999;94:496–509. [Google Scholar]
- 16.Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298(10):1171–9. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 17.Daubeney PEF, Nugent AW, Chondros P, et al. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation. 2006;114:2671–8. doi: 10.1161/CIRCULATIONAHA.106.635128. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan JJ, Roche SL, Crossland DS, et al. Recovery of heart function in children with acute severe heart failure. Transplantation. 2008;85(7):975–9. doi: 10.1097/TP.0b013e318168fe3c. [DOI] [PubMed] [Google Scholar]
- 19.Taliercio CP, Seward JB, Driscoll DJ, et al. Idiopathic dilated cardiomyopathy in the young: clinical profile and natural history. J Am Coll Cardiol. 1985;6:1126–31. doi: 10.1016/s0735-1097(85)80319-3. [DOI] [PubMed] [Google Scholar]
- 20.Alexander PMA, Daubeney PEF, Nugent AW, et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: results from a national population-based study of childhood cardiomyopathy. Circulation. 2013 Sep 13; doi: 10.1161/CIRCULATIONAHA.113.002767. Epub ahead of print. [DOI] [PubMed] [Google Scholar]